Abstract
Aims
Treatment ‘effects’ are often inferred from non-randomized and observational studies. These studies have inherent biases and limitations, which may make therapeutic inferences based on their results unreliable. We compared the conflicting findings of these studies to those of prospective randomized controlled trials (RCTs) in relation to pharmacological treatments for heart failure (HF).
Methods and results
We searched Medline and Embase to identify studies of the association between non-randomized drug therapy and all-cause mortality in patients with HF until 31 December 2017. The treatments of interest were: angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, beta-blockers, mineralocorticoid receptor antagonists (MRAs), statins, and digoxin. We compared the findings of these observational studies with those of relevant RCTs. We identified 92 publications, reporting 94 non-randomized studies, describing 158 estimates of the ‘effect’ of the six treatments of interest on all-cause mortality, i.e. some studies examined more than one treatment and/or HF phenotype. These six treatments had been tested in 25 RCTs. For example, two pivotal RCTs showed that MRAs reduced mortality in patients with HF with reduced ejection fraction. However, only one of 12 non-randomized studies found that MRAs were of benefit, with 10 finding a neutral effect, and one a harmful effect.
Conclusion
This comprehensive comparison of studies of non-randomized data with the findings of RCTs in HF shows that it is not possible to make reliable therapeutic inferences from observational associations. While trials undoubtedly leave gaps in evidence and enrol selected participants, they clearly remain the best guide to the treatment of patients.
Keywords: Heart failure , Pharmacotherapy , Associations , Observational studies , Randomized controlled trials
Introduction
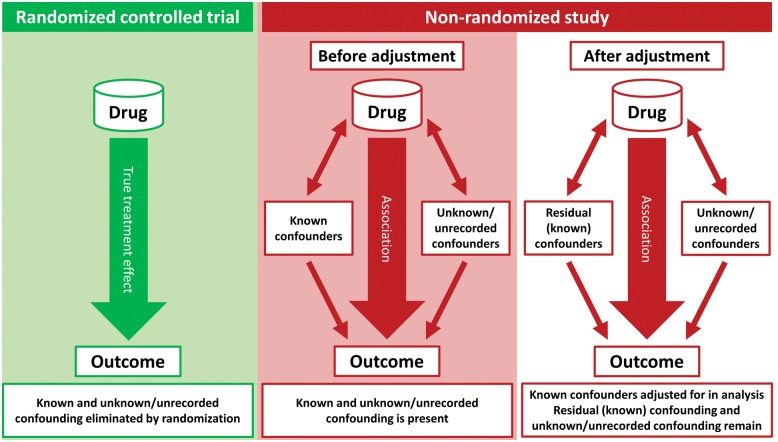
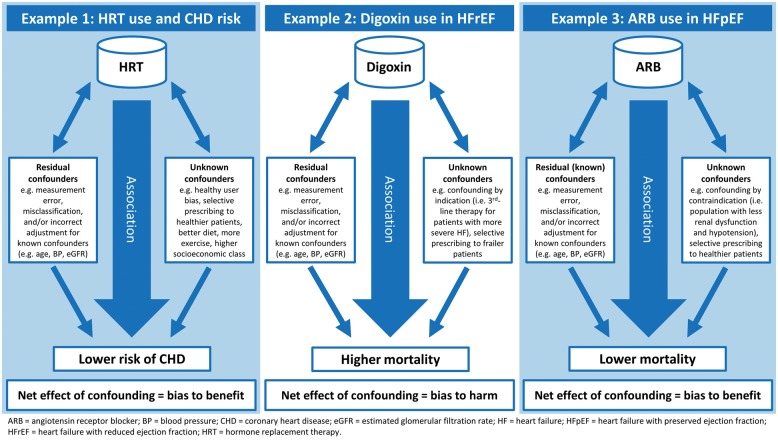
Randomized controlled trials (RCTs) are widely acknowledged to be the gold standard test of whether or not a drug is beneficial.1–4 Although the biases and limitations of non-randomized, observational studies have been recognized for decades (Figure 1), studies of this type purporting to describe the effects of treatment continue to be published, even in high-impact journals.5–10 Indeed, the ‘comparative effectiveness’ and ‘big data’ movements have given non-randomized studies a new respectability in some peoples’ eyes.11–13 Advocates point to the use of more sophisticated analytical techniques than in the past and increasingly larger ‘real-world’ datasets.14–17 If the findings of observational studies could validly determine the effect of treatments, such information would clearly be of considerable value. On the other hand, if such analyses are inherently flawed they serve only to cause confusion, e.g. the association between hormone replacement therapy and decreased risk of coronary heart disease (CHD)18,19 (Figure 2), and maybe worse, e.g. lead to discontinuation of effective therapy by physicians or patients misled by the findings.20
Figure 1.
Confounding in non-randomized studies.
Figure 2.
Examples of confounding in non-randomized studies.
There is a particularly strong evidence base for pharmacological treatments in heart failure (HF), making it an appropriate condition in which to compare treatment effects established in RCTs with those reported in non-randomized studies. We have, therefore, compared the conflicting results of non-randomized studies of the ‘effect’ of pharmacological treatments with those of RCTs using the same therapies for HF. Although many publications of this type have used the word ‘effect’, more correctly they have actually described associations between treatments and outcomes.
Methods
Search strategy and eligibility criteria
We conducted a comprehensive search of the electronic databases Medline and Embase to identify observational studies examining the association between non-randomized drug therapy and all-cause mortality in patients with HF. The drugs of interest were those included in all major HF guidelines: angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), beta-blockers, mineralocorticoid receptor antagonists (MRAs), statins (3-hydroxy-3-methyl-glutaryl-coenzyme A reductase inhibitors), and digoxin, where the effect on all-cause mortality had been tested in at least one large RCT.21,22 The term ‘heart failure’ was searched in title and keywords relating to outcome data and pharmacotherapy were searched in title or abstract to retrieve all potentially relevant articles (see Supplementary material online, Figures S1–S5). The search, updated until 31 December 2017, was limited to studies of adults, published in the English language, with more than 100 participants in both the study drug and control groups, with a minimum follow-up period of six months. Studies of patients with left ventricular systolic dysfunction and/or HF after myocardial infarction were not included. We also excluded studies describing only subgroups of patients with HF, e.g. those with HF and chronic kidney disease, HF and diabetes etc. Bibliographies of meta-analyses, guidelines, reviews, and manuscripts identified through the search strategy were also hand-searched for additional eligible studies. The review was conducted according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines.23
Non-randomized studies were considered for inclusion in this review if the following requirements were met:
Inclusion of patients with HF
Report of the ‘effect’ of the drug of interest on all-cause mortality
Estimate of treatment ‘effect’ provided as a multivariate-adjusted hazard ratio (HR), risk ratio/relative risk, or odds ratio
Data extraction, synthesis, and risk of bias
Data from the manuscripts identified through the search criteria were abstracted and tabulated by one reviewer (C.J.R.). The data were independently verified by a second reviewer (R.T.C.), with a third reviewer (J.J.M.) resolving any discrepancies. The articles retrieved were categorized according to HF phenotype, based on ejection fraction (EF), and drug class for comparison with the relevant randomized trials. For studies that reported more than one multivariable-adjusted ‘effect’ estimate, the estimate which had been adjusted for most confounders was used. A two-tailed P-value of 0.05 was considered significant.
The quality of each study was assessed with the Cochrane Collaboration Risk of Bias tool for RCTs and the Risk of Bias Assessment tool for Non-randomized Studies (RoBANS) tool for observational studies (see Supplementary material online, Tables S1 and S2).24,25 Studies judged as having a low risk of bias have been presented separately from those with a high or unclear risk of bias in the Supplementary material online, Figures S6–S19.
Results
We identified 92 publications reporting 94 non-randomized studies.26–117 Together, these described 158 estimates of the ‘effect’ of the six treatments of interest on all-cause mortality. These six treatments had been tested in 25 RCTs.118–147 The results of our analyses are summarized in Table 1 and described in detail in Tables 2–6. The forest plots in the Supplementary material online, Figures S6–S19 illustrate the treatment effects/association between treatment and outcomes in the trials and observational studies, respectively, reported in Tables 2–6 and include a quality assessment of these trials/studies.
Table 1.
Summary of the concordance between the effect of treatment on mortality in randomized controlled trials and the association between non-randomized use of the same treatments and mortality in observational studies in HF
| Treatment | Randomized controlled trials | Observational studies |
||
|---|---|---|---|---|
| Benefit | Neutral | Harm | ||
| HFrEF | ||||
| ACEI/ARB | Benefit | 5 | 2 | 0 |
| Beta-blocker | Benefit | 16 | 2 | 0 |
| MRA | Benefit | 1 | 10 | 1 |
| Statin | Neutral | 14 | 3 | 0 |
| Digoxin | Neutral | 1 | 4 | 5 |
| HFpEF | ||||
| ACEI/ARB | Neutral | 5 | 7 | 0 |
| Beta-blocker | Neutral | 9 | 4 | 0 |
| MRA | Neutral | 1 | 2 | 0 |
| Statin | — | — | — | — |
| Digoxin | Neutral | 1 | 3 | 0 |
| Mixed/unspecified HF phenotype | ||||
| ACEI/ARB | Neutral | 8 | 2 | 0 |
| Beta-blocker | Neutral | 17 | 2 | 0 |
| MRA | — | 2 | 3 | 0 |
| Statin | Neutral | 11 | 1 | 0 |
| Digoxin | Neutral | 2 | 7 | 7 |
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; MRA, mineralocorticoid receptor antagonist.
Table 2.
All-cause mortality in randomized and non-randomized ACEI/ARB HF studies
| First author, country, year of publication (study name) | Study design | Study period | Region | Mean follow -up (months) | Patients (n) | Study (n) | Control (n) | All-cause mortality—unadjusted HR (95% CI) | All-cause mortality—adjusted HR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| HFrEF (ACEI) | |||||||||
| Randomized controlled trials—beneficial treatment effect | |||||||||
| SOLVD Investigators, USA, 1991 (SOLVD-Treatment)118 | RCT | 1986–1989 | USA, Canada, Belgium | 41 | 2569 | 1285 | 1284 | RR: 0.84 (0.74–0.95; P < 0.004) | — |
| Jong, Canada, 2003 (X-SOLVD Overall)119 | RCT | 1986–1990 | USA, Canada, Belgium | 134–145a | 6797 | 3396 | 3401 | 0.90 (0.84–0.95; P < 0.0003) | — |
| Jong, Canada, 2003 (X-SOLVD-Prevention)119 | RCT | 1986–1990 | USA, Canada, Belgium | 134a | 4228 | 2111 | 2117 | 0.86 (0.79–0.93; P < 0.001) | — |
| Randomized controlled trials—neutral treatment effect | |||||||||
| SOLVD Investigators, USA, 1992 (SOLVD-Prevention)120 | RCT | 1986–1990 | USA, Canada, Belgium | 37 | 4228 | 2111 | 2117 | RR: 0.92 (0.79–1.08; P < 0.30) | — |
| Jong, Canada, 2003 (X-SOLVD-Treatment)119 | RCT | 1986–1990 | USA, Canada, Belgium | 145a | 2569 | 1285 | 1284 | 0.93 (0.85–1.01; P < 0.01) | — |
| Observational studies—beneficial treatment effect | |||||||||
| Masoudi, USA, 2004 (NHC)26 | Retrospective cohort study (≥65 years) | 1998–1999, 2000–2001 | USA | 12 | 17 456 | 12 069 | 13 600 | RR: 0.78 (0.75–0.81; P < 0.0001) | RR: 0.86 (0.82–0.90) |
| HFrEF (ARB) | |||||||||
| Randomized controlled trials—neutral treatment effect | |||||||||
| Granger, USA, 2003 (CHARM-Alternative)121 | RCT | 1999–2001 | Multiregional | 34a | 2028 | 1013 | 1015 | 0.87 (0.74–1.03; P < 0.11) | 0.83 (0.70–0.99; P < 0.033) |
| HFrEF (ACEI + ARB) | |||||||||
| Observational studies—beneficial treatment effect | |||||||||
| Sanam, USA, 2016 (Alabama HF Project)27 | Retrospective cohort study (PSM) (≥65 years) | 1998–2001 | USA | 12 | 954 | 477 | 477 | — | 0.77 (0.62–0.96; P < 0.020) |
| Liu, China, 201428 | Prospective cohort study | 2005–2010 | China | 52a | 2154 | 1421 | 733 | — | 0.43 (0.33–0.57; P < 0.001) |
| Lund, Sweden, 2012 (Swedish HF Registry)29 | Registry (PSM) | 2000–2011 | Sweden | 12 | 4010 | 2005 | 2005 | — | 0.80 (0.74–0.86; P < 0.001) |
| Masoudi, USA, 2004 (NHC)26 | Retrospective cohort study (≥65 years) | 1998–1999, 2000–2001 | USA | 12 | 17 456 | 13 600 | 3856 | — | RR: 0.83 (0.79–0.88) |
| Observational studies—neutral treatment effect | |||||||||
| Ushigome, Japan, 2015 (1. CHART-1)30 | Prospective cohort study | 2000–2005 | Japan | 36 | 543 | 385 | 158 | — | 0.67 (0.40–1.12; P < 0.128) |
| Ushigome, Japan, 2015 (2. CHART-2)30 | Prospective cohort study | 2006–2010 | Japan | 36 | 1360 | 1061 | 299 | — | 0.83 (0.60–1.15; P < 0.252) |
| HFpEF (ACEI) | |||||||||
| Randomized controlled trials—neutral treatment effect | |||||||||
| Cleland, UK, 2006 (PEP-CHF)122 | RCT (≥70 years) | 2000–2003 | Multiregional | 26 | 850 | 424 | 426 | 1.09 (0.75–1.58; P < 0.665) | — |
| Observational studies—beneficial treatment effect | |||||||||
| Gomez-Soto, Spain, 201031 | Prospective cohort study (propensity score adjusted) | 2001–2005 | Spain | 30a | 1120 | 255 | 865 | RR: 0.34 (0.23–0.46; P < 0.001) | 0.67 (0.52–0.71) |
| Shah, USA, 2008 (NHC)32 | Retrospective cohort study (≥65 years) | 1998–1999, 2000–2001 | USA | 36 | 13 533 | 6413 | 7120 | — | RR: 0.93 (0.89–0.98) |
| Tribouilloy, France, 200833 | Prospective cohort study (PSM) | 2000 | France | 60 | 240 | 120 | 120 | 0.61 (0.43–0.87; P < 0.006) | 0.58 (0.40–0.82; P < 0.002) |
| Grigorian Shamagian, Spain, 200634 | Prospective cohort study | 1991–2002 | Spain | 31 | 416 | 210 | 206 | 0.56 (0.40–0.79; P < 0.001) | 0.63 (0.44–0.90; P < 0.012) |
| Observational studies—neutral treatment effect | |||||||||
| Mujib, USA, 2013 (OPTIMIZE-HF)35 | Registry (PSM) (≥65 years) | 2003–2004 | USA | 29a | 2674 | 1337 | 1337 | — | 0.96 (0.88–1.05; P < 0.373) |
| Dauterman, USA, 2001 (Medicare)36 | Retrospective cohort study (≥65 years) | 1993–1994, 1996 | USA | 12 | 430 | 206 | 224 | — | 1.15 (0.79–1.67; P < 0.46) |
| Philbin, USA, 2000 (MISCHF)37 | Registry | 1995, 1996–1997 | USA | 6 | 302 | 137 | 165 | OR: 0.72 (0.38–1.39) | OR: 0.61 (0.30–1.25) |
| Philbin, USA, 1997 (MISCHF)38 | Registry | 1995 | USA | 6 | 350 | 190 | 160 | — | OR: 0.63 (P < 0.15–95% CI not reported) |
| HFpEF (ARB) | |||||||||
| Randomized controlled trials—neutral treatment effect | |||||||||
| Massie, USA, 2008 (I-PRESERVE)123 | RCT | 2002–2005 | Multiregional | 50 | 4128 | 2067 | 2061 | 1.00 (0.88–1.14; P < 0.98) | — |
| Yusuf, Canada, 2003 (CHARM-Preserved)124 | RCT | 1999–2000 | Multiregional | 37a | 3023 | 1514 | 1509 | 1.02 (0.85–1.22; P < 0.836) | — |
| Observational studies—neutral treatment effect | |||||||||
| Patel, USA, 2012 (OPTIMIZE-HF)39 | Registry (PSM) (≥65 years) | 2003–2004 | USA | 72 | 592 | 296 | 296 | 0.93 (0.76–1.14; P < 0.509) | — |
| HFpEF (ACEI + ARB) | |||||||||
| Observational studies—beneficial treatment effect | |||||||||
| Lund, Sweden, 2012 (Swedish HF Registry)29 | Registry (PSM) | 2000–2011 | Sweden | 12 | 6658 | 3329 | 3329 | — | 0.91 (0.85–0.98; P < 0.008) |
| Observational studies—neutral treatment effect | |||||||||
| Ushigome, Japan, 2015 (1. CHART-1)30 | Prospective cohort study | 2000–2005 | Japan | 36 | 463 | 304 | 159 | — | 0.86 (0.51–1.47; P < 0.592) |
| Ushigome, Japan, 2015 (2. CHART-2)30 | Prospective cohort study | 2006–2010 | Japan | 36 | 2316 | 1619 | 697 | — | 1.01 (0.77–1.32; P < 0.924) |
| Mixed/unspecified HF phenotype (ACEI) | |||||||||
| Randomized controlled trials—beneficial treatment effect | |||||||||
| Cohn, USA, 1991 (V-HeFT-II)125 | RCT | 1986–1990 | USA | 24 | 804 | 403 | 401 (H-ISDN) | RR: 0.72 (P < 0.016–95% CI not reported) | — |
| CONSENSUS Trial Study Group, Sweden, 1987 (CONSENSUS)126 | RCT | 1985–1986 | Sweden, Norway, Finland | 12 | 245 | 127 | 126 | RR: 0.69 (P < 0.001–95% CI not reported) | — |
| Observational studies—beneficial treatment effect | |||||||||
| Keyhan, Canada, 2007 (1. female cohort)40 | Retrospective cohort study (≥65 years) | 1998–2003 | Canada | 12 | 14 693 | 9801 | 4892 | 0.75 (0.71–0.78) | 0.80 (0.76–0.85) |
| Keyhan, Canada, 2007 (2. male cohort)40 | Retrospective cohort study (≥65 years) | 1998–2003 | Canada | 12 | 13 144 | 9419 | 3725 | 0.62 (0.59–0.65) | 0.71 (0.67–0.75) |
| Tandon, Canada, 2004 (75% HFrEF, 25% HFpEF)41 | Prospective cohort study | 1989–2001 | Canada | 32a | 1041 | 878 | 163 | — | OR: 0.60 (0.39–0.91) |
| Pedone, Italy, 2004 (GIFA)42 | Prospective cohort study (≥65 years) | 1998 | Italy | 10 | 818 | 550 | 268 | 0.56 (0.41–0.78) | 0.60 (0.42–0.88) |
| Ahmed, USA, 2003 (Medicare)43 | Retrospective cohort study (PSM) | 1994 | USA | 36 | 1090 | 528 | 562 | 0.77 (0.66–0.91) | 0.81 (0.69–0.97) |
| Sin, Canada, 2002 (19% HFrEF, 36% HFpEF, 45% unknown)44 | Retrospective cohort study (≥65 years) (propensity score adjusted) | 1994–1998 | Canada | 21a | 11 942 | 4908 | 7034 | — | 0.59 (0.55–0.62) |
| Mixed/unspecified HF phenotype (ARB) | |||||||||
| Randomized controlled trials—neutral treatment effect | |||||||||
| Pfeffer, USA, 2003 (CHARM Overall Programme) (60% HFrEF, 40% HFpEF)127 | RCT | 1999–2001 | Multiregional | 40a | 7599 | 3803 | 3796 | 0.91 (0.83–1.00; P < 0.055) | 0.90 (0.82–0.99; P < 0.032) |
| Mixed/unspecified HF phenotype (ACEI + ARB) | |||||||||
| Observational studies—beneficial treatment effect | |||||||||
| Gastelurrutia, Spain, 2012 (75% HFrEF, 25% HFrEF)45 | Prospective cohort study | 2001–2008 | Spain | 44a | 960 | 846 | 114 | — | 0.52 (0.39–0.69; P < 0.001) |
| Teng, Australia, 2010 (WAHMD) (24% HFrEF, 30% HFpEF, 46% unknown)46 | Retrospective cohort study | 1996–2006 | Australia | 12 | 944 | 701 | 243 | — | 0.71 (0.57–0.89; P < 0.003) |
| Observational studies—neutral treatment effect | |||||||||
| Ushigome, Japan, 2015 (1. CHART-1) (54% HFrEF, 46% HFpEF)30 | Prospective cohort study | 2000–2005 | Japan | 36 | 1006 | 689 | 317 | — | 0.79 (0.55–1.14; P < 0.208) |
| Ushigome, Japan, 2015 (2. CHART-2) (37% HFrEF, 63% HFpEF)30 | Prospective cohort study | 2006–2010 | Japan | 36 | 3676 | 2677 | 999 | — | 0.94 (0.76–1.15; P < 0.534) |
Median.
—, Not reported; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CHARM, Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity; CHART, Chronic Heart Failure Analysis and Registry in the Tohoku district; CI, confidence interval; CONSENSUS, Cooperative North Scandinavian Enalapril Survival Study; GIFA, Gruppo Italiano di Farmacovigilanza nell'Anziano; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; H-ISDN, hydralazine-isosorbide dinitrate; HR, hazard ratio; I-PRESERVE, Irbesartan in Patients with Heart Failure and Preserved Ejection Fraction; MISCHF, Management to Improve Survival in Congestive Heart Failure; NHC, National Heart Care; OPTIMIZE-HF, Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure; OR, odds ratio; PEP-CHF, Perindopril in Elderly People with Chronic Heart Failure; PSM, propensity score matched study; RCT, randomized controlled trial; RR, risk ratio/relative risk; SOLVD, Studies of Left Ventricular Dysfunction; V-HeFT-II, Vasodilator Heart Failure Trial II; WAHMD, Western Australia Hospital Morbidity Data; X-SOLVD, Extended follow-up of the SOLVD trials.
Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers
Heart failure with reduced ejection fraction
Two landmark randomized trials in heart failure with reduced ejection fraction (HFrEF) demonstrated a reduction in mortality with an ACEI118–120 and one further trial showed a consistent benefit with an ARB.121 We identified one non-randomized study showing lower mortality in patients with HFrEF treated with an ACEI.26 Most studies, however, examined patients treated with either an ACEI or ARB. Of six such studies, four reported an association between ACEI/ARB use and lower mortality,26–29 whereas two did not.30 Overall, therefore, in HFrEF five non-randomized estimates of treatment ‘effect’ found that use of an ACEI or ARB was associated with lower mortality and two did not (Table 2).
Heart failure with preserved ejection fraction
One moderately large randomized trial showed no effect of perindopril on mortality, although the estimate of treatment effect was not robust because of limited power.122 However, two large RCTs showed no effect of irbesartan123 and candesartan (in Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity—CHARM)124 on mortality. Of eight observational studies examining ACEI use and outcome in heart failure with preserved ejection fraction (HFpEF), four suggested that use of this treatment was associated with a lower mortality,31–34 whilst four did not not35–38 (Table 2). We identified one observational study of ARB use in patients with HFpEF which suggested no mortality benefit.39 A further three non-randomized studies reported estimates of a treatment ‘effect’ for use of either an ACEI or ARB in HFpEF. One study found an association between ACEI/ARB use and better survival29 and two studies did not.30 Overall, therefore, in HFpEF, five non-randomized studies found that use of an ACEI or ARB was associated with lower mortality and seven did not (Table 2).
Mixed/unspecified heart failure phenotype
The CHARM Programme showed a neutral effect of candesartan on mortality in patients with HFpEF and HFrEF combined.127 Nine non-randomized studies were identified, which reported 10 estimates of a ‘treatment-effect’ for use of either an ACEI or ARB in patients with HFrEF or HFpEF (i.e. both major HF phenotypes). Of these analyses, eight suggested a benefit40–46 and two reported a neutral effect30 (Table 2).
Beta-blockers
Heart failure with reduced ejection fraction
Several landmark RCTs demonstrated significant mortality benefit with the use of beta-blockers in HFrEF.128–131 Seventeen non-randomized studies reported 18 estimates of beta-blocker ‘treatment-effect’. Sixteen of these suggested beta-blocker use was associated with a lower mortality28,30,46–58 and two did not30,59 (Table 3).
Table 3.
All-cause mortality in randomized and non-randomized beta-blocker HF studies
| First author, country, year of publication (study name) | Study design | Study period | Region | Mean follow-up (months) | Patients (n) | Study (n) | Control (n) | All-cause mortality—unadjusted HR (95% CI) | All-cause mortality—adjusted HR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| HFrEF | |||||||||
| Randomized controlled trials—beneficial treatment effect | |||||||||
| Packer, USA, 2001 (COPERNICUS)128 | RCT | 1997–2000 | Multiregional | 10 | 2289 | 1156 | 1133 | RR: 0.65 (0.52–0.81; P < 0.00013) | — |
| MERIT-HF Study Group, Sweden, 1999 (MERIT-HF)129 | RCT | 1997–1998 | Europe, USA | 12 | 3991 | 1990 | 2001 | RR: 0.66 (0.53–0.81; P < 0.0001) | — |
| CIBIS Investigators, UK, 1999 (CIBIS-II)130 | RCT | — | Europe | 16 | 2647 | 1327 | 1320 | 0.66 (0.54–0.81; P < 0.0001) | — |
| Packer, USA, 1996 (US Carvedilol HF Study Group)131 | RCT | 1993–1995 | USA | 7 | 1094 | 696 | 398 | RR: 0.35 (0.20–0.61; P < 0.001) | — |
| Randomized controlled trials—neutral treatment effect | |||||||||
| van Veldhuisen, Netherlands, 2009 (SENIORS)132 | Pre-specified subgroup analysis of RCT (EF <35%) (≥70 years) | 2000–2002 | Europe | 21 | 1359 | 678 | 681 | 0.84 (0.66–1.08) | — |
| BEST Investigators, USA, 2001 (BEST)133 | RCT | 1995–1998 | USA, Canada | 24 | 2708 | 1354 | 1354 | 0.90 (0.78–1.02; P > 0.10) | — |
| ANZ HF Research Collaborative Group, New Zealand, 1997 (ANZ)134 | RCT (IHD) | — | Australia, New Zealand | 19 | 415 | 207 | 208 | RR: 0.76 (0.42–1.36; P > 0.1) | — |
| CIBIS Investigators, France, 1994 (CIBIS-I)135 | RCT | 1989–1992 | Europe | 23 | 641 | 320 | 321 | — | RR: 0.80 (0.56–1.15) |
| Observational studies—beneficial treatment effect | |||||||||
| Cadrin-Tourigny, Canada, 2017 (AF-CHF)47 | Post hoc analysis of RCT (PSM) (AF) | 2001–2005 | Multiregional | 37a | 655 | 426 | 229 | — | 0.72 (0.55–0.95; P < 0.018) |
| Bhatia, USA, 2015 (Alabama HF Project)48 | Retrospective cohort study (PSM) (≥65 years) | 1998–2001 | USA | 48 | 760 | 380 | 380 | — | 0.81 (0.67–0.98) |
| Ushigome, Japan, 2015 (2. CHART-2)30 | Prospective cohort study | 2006–2010 | Japan | 36 | 1360 | 870 | 490 | — | 0.59 (0.44–0.81; P < 0.001) |
| Del Carlo, Brazil, 201449 | Retrospective cohort study | 1992, 1994, 1996, 1999, 2005–2006 | Brazil | 12 | 333 | 199 | 134 | 0.3 (0.2–0.5; P < 0.001) | 0.3 (0.2–0.5; P < 0.001) |
| Liu, China, 201428 | Prospective cohort study | 2005–2010 | China | 52a | 2154 | 1471 | 683 | — | 0.75 (0.57–0.999; P < 0.049) |
| Lund, Sweden, 2014 (Swedish HF Registry)50 | Registry (PSM) | 2005–2012 | Sweden | 23a | 6081 | 4054 | 2027 | — | 0.89 (0.82–0.97; P < 0.005) |
| El-Refai, USA, 201351 | Retrospective cohort study | 2000–2008 | USA | 25a | 1094 | 927 | 167 | — | 0.26 (0.17–0.40; P < 0.001) |
| Xu, China, 201352 | Retrospective cohort study | 2007–2012 | China | 31a | 685 | 555 | 130 | — | 0.69 (0.50–0.95; P < 0.021) |
| Teng, Australia, 2010 (WAHMD)46 | Retrospective cohort study | 1996–2006 | Australia | 12 | 225 | 100 | 125 | — | 0.53 (0.32–0.87; P < 0.011) |
| Hernandez, USA, 2009 (OPTIMIZE-HF)53 | Registry (≥65 years) | — | USA | 12 | 3001 | 1800 | 1201 | 0.65 (0.57–0.73) | 0.77 (0.68–0.87) |
| Miyagishima, Japan, 200954 | Retrospective cohort study | 2000–2004 | Japan | 36 | 431 | 297 | 134 | — | 0.48 (0.32–0.73) |
| Fauchier, France, 2009 (41% HFrEF)55 | Retrospective cohort study (AF) | 2000–2004 | France | 29 | 1269 | 449 | 820 | — | RR: 0.60 (0.40–0.89; P < 0.01) |
| Pascual-Figal, Spain, 200856 | Registry (>70 years) | 2002–2003 | Spain | 31a | 272 | 139 | 133 | 0.45 (0.31–0.65; P < 0.001) | 0.53 (0.34–0.80; P < 0.003) |
| Jost, Germany, 2005 (Ludwigshafen HF Registry) (1. ‘Trial patients’)57 | Registry | 1995–2004 | Germany | 31 | 278 | 166 | 112 | — | 0.57 (0.38–0.86) |
| Jost, Germany, 2005 (Ludwigshafen HF Registry) (2. ‘Non-trial patients’)57 | Registry | 1995–2004 | Germany | 31 | 397 | 204 | 193 | — | 0.72 (0.53–0.97) |
| Bobbio, Italy, 2003 (BRING-UP)58 | Prospective cohort study | 1998 | Italy | 12 | 2843 | 1582 | 1261 | RR: 0.46 (0.38–0.57) | 0.64 (0.48–0.86) |
| Observational studies—neutral treatment effect | |||||||||
| Ushigome, Japan, 2015 (1. CHART-1)30 | Prospective cohort study | 2000–2005 | Japan | 36 | 543 | 184 | 359 | — | 0.87 (0.50–1.50; P < 0.610) |
| Huan Loh, UK, 200759 | Retrospective cohort study | — | UK | 36a | 900 | 738 | 162 | 0.54 (0.40–0.73; P < 0.001) | 0.73 (0.53–1.02; P < 0.067) |
| HFpEF | |||||||||
| Randomized controlled trials—neutral treatment effect | |||||||||
| Yamamoto, Japan, 2013 (J-DHF)136 | PROBE | 2004–2009 | Japan | 38 | 245 | 120 | 125 | 0.99 (0.53–1.86; P < 0.975) | — |
| van Veldhuisen, Netherlands, 2009 (SENIORS)132 | Pre-specified subgroup analysis of RCT (EF >35%) (≥70 years) | 2000–2002 | Europe | 21 | 752 | 380 | 372 | 0.91 (0.62–1.33; P < 0.718) | — |
| Observational studies—beneficial treatment effect | |||||||||
| Ruiz, Spain, 201660 | Prospective cohort study (PSM) | 2006–2015 | Spain | 22a | 1970 | 985 | 985 | RR: 0.76 (0.70–0.83; P < 0.001) | 0.78 (0.71–0.85; P < 0.001) |
| Lund, Sweden, 2014 (Swedish HF Registry)50 | Registry (PSM) | 2005–2012 | Sweden | 23a | 8244 | 5496 | 2748 | — | 0.93 (0.86–0.996; P < 0.04) |
| El-Refai, USA, 201351 | Retrospective cohort study | 2000–2008 | USA | 25a | 741 | 570 | 171 | — | 0.43 (0.27–0.68; P < 0.001) |
| Nevzorov, Israel, 201261 | Retrospective cohort study | 2001–2005 | Israel | 24 | 345 | 154 | 191 | — | 0.69 (0.47–0.99; P < 0.046) |
| Gomez-Soto, Spain, 201162 | Prospective cohort study (propensity score adjusted) | 2001–2005 | Spain | 30a | 1085 | 378 | 707 | RR: 0.37 (0.21–0.50; P < 0.001) | 0.72 (0.58–0.84) |
| Teng, Australia, 2010 (WAHMD)46 | Retrospective cohort study | 1996–2006 | Australia | 12 | 284 | 101 | 183 | — | 0.62 (0.39–0.99; P < 0.048) |
| Fauchier, France, 2009 (35% HFpEF)55 | Retrospective cohort study (AF) | 2000–2004 | France | 29 | 1269 | 449 | 820 | — | RR: 0.45 (0.26–0.80; P < 0.006) |
| Shah, USA, 2008 (NHC)32 | Retrospective cohort study (≥65 years) | 1998–1999, 2000–2001 | USA | 36 | 13 533 | 4562 | 8971 | — | RR: 0.92 (0.87–0.97) |
| Dobre, Netherlands, 200763 | Prospective cohort study (propensity score adjusted) | 2000–2005 | Netherlands | 25 | 443 | 227 | 216 | — | 0.57 (0.37–0.88; P < 0.01) |
| Observational studies—neutral treatment effect | |||||||||
| Ushigome, Japan, 2015 (1. CHART-1)30 | Prospective cohort study | 2000–2005 | Japan | 36 | 463 | 104 | 359 | — | 0.89 (0.45–1.75; P < 0.734) |
| Ushigome, Japan, 2015 (2. CHART-2)30 | Prospective cohort study | 2006–2010 | Japan | 36 | 2316 | 1018 | 1298 | — | 0.94 (0.73–1.22; P < 0.654) |
| Patel, USA, 2014 (OPTIMIZE-HF)64 | Registry (PSM) (≥65 years) | 2003–2004 | USA | 72 | 2198 | 1099 | 1099 | — | 0.99 (0.90–1.10; P < 0.897) |
| Hernandez, USA, 2009 (OPTIMIZE-HF)53 | Registry (≥65 years) | — | USA | 12 | 4153 | 1621 | 2532 | 0.87 (0.77–0.97) | 0.94 (0.84–1.07) |
| Mixed/unspecified HF phenotype | |||||||||
| Randomized controlled trials—neutral effect | |||||||||
| Flather, UK, 2005 (SENIORS) (65% HFrEF, 35% HFpEF)137 | RCT (≥70 years) | 2000–2002 | Multiregional | 21 | 2128 | 1067 | 1061 | 0.88 (0.71–1.08; P < 0.21) | — |
| Observational studies—beneficial treatment effect | |||||||||
| Katz, Israel, 2016 (HFSIS) (38% HFrEF, 15% HFmrEF, 22% HFpEF, 26% unknown)65 | Prospective cohort study | 2003 | Israel | 120 | 2402 | 1481 | 921 | — | 0.83 (0.77–0.89; P < 0.001) |
| Maison, France, 201366 | Registry (propensity score adjusted) | 2000 | France | 96 | 281 | 101 | 180 | — | 0.54 (0.34–0.84) |
| Gastelurrutia, Spain, 2012 (75% HFrEF, 25% HFrEF)45 | Prospective cohort study | 2001–2008 | Spain | 44a | 960 | 776 | 184 | — | 0.51 (0.39–0.66; P < 0.001) |
| Marijon, France, 2010 (EVADEF)67 | Prospective cohort study (ICD) | 2001–2003 | France | 22 | 1030 | 721 | 309 | 0.53 (0.30–0.91; P < 0.02) | 0.56 (0.32–0.98; P < 0.04) |
| Teng, Australia, 2010 (WAHMD) (24% HFrEF, 30% HFpEF, 46% unknown)46 | Retrospective cohort study | 1996–2006 | Australia | 12 | 944 | 318 | 626 | — | 0.68 (0.53–0.86; P < 0.002) |
| Fauchier, France, 2009 (41% HFrEF, 35% HFpEF, 24% unknown)55 | Retrospective cohort study (AF) | 2000–2004 | France | 29 | 1269 | 449 | 820 | 0.59 (0.45–0.78; P < 0.0002) | 0.60 (0.43–0.84; P < 0.003) |
| Jordán, Spain, 2009 (BADAPIC) (77% HFrEF, 23% HFpEF)68 | Registry | 2000–2002 | Spain | 35 | 3162 | 2242 | 920 | — | RR: 0.82 (0.47–0.95) |
| Dobre, Netherlands, 2007 (55% HFrEF, 45% HFpEF)69 | Prospective cohort study (propensity score adjusted) | 2000–2004 | Netherlands | 22 | 625 | 308 | 317 | 0.55 (0.39–0.78; P < 0.001) | |
| Keyhan, Canada, 2007 (1. female cohort)70 | Retrospective cohort study (≥65 years) | 1998–2003 | Canada | 30 | 14 693 | 7584 | 7109 | 0.67 (0.64–0.70) | 0.79 (0.75–0.83) |
| Keyhan, Canada, 2007 (2. male cohort)70 | Retrospective cohort study (≥65 years) | 1998–2003 | Canada | 30 | 13 144 | 6499 | 6645 | 0.64 (0.61–0.67) | 0.76 (0.72–0.80) |
| Chan, USA, 2005 (CHS) (19% HFrEF, 36% HFpEF, 45% unknown)71 | Prospective cohort study (≥65 years) | 1989–2000 | USA | 120 | 950 | 157 | 793 | 0.74 (0.56–0.98) | 0.74 (0.56–0.98) |
| Tandon, Canada, 2004 (75% HFrEF, 25% HFpEF)41 | Prospective cohort study | 1989–2001 | Canada | 32a | 1041 | 475 | 566 | — | OR: 0.52 (0.39–0.70) |
| Maggioni, Italy, 2003 (BRING-UP) (1. no BB vs. continued BB)72 | Registry | 1998 | Italy | 12 | 2226 | 771 | 1455 | — | 0.74 (0.55–0.99; P < 0.045) |
| Maggioni, Italy, 2003 (BRING-UP) (2. no BB vs. initiated BB)72 | Registry | 1998 | Italy | 12 | 2320 | 865 | 1455 | — | 0.60 (0.45–0.80; P < 0.0003) |
| McCullough, USA, 2003 (REACH)73 | Retrospective cohort study | 1995–1998 | USA | 12 | 1317 | 647 | 670 | — | OR: 0.75 (0.57–0.98; P < 0.04) |
| Sin, Canada, 2002 (19% HFrEF, 36% HFpEF, 45% unknown)44 | Retrospective cohort study (≥65 years) (propensity score adjusted) | 1994–1998 | Canada | 21a | 11 942 | 1162 | 10 780 | — | 0.72 (0.65–0.80) |
| McAlister, Canada, 1999 (78% HFrEF, 22% HFpEF)74 | Prospective cohort study | 1989–1995 | Canada | 17 | 566 | 147 | 419 | — | OR: 0.5 (P < 0.006–95% CI not reported) |
| Observational studies—neutral treatment effect | |||||||||
| Ushigome, Japan, 2015 (1. CHART-1) (54% HFrEF, 46% HFpEF)30 | Prospective cohort study | 2000–2005 | Japan | 36 | 1006 | 288 | 718 | — | 0.96 (0.63–1.44; P < 0.829) |
| Ushigome, Japan, 2015 (1. CHART-2) (37% HFrEF, 63% HFpEF)30 | Prospective cohort study | 2006–2010 | Japan | 36 | 3676 | 1886 | 1790 | — | 0.82 (0.68–1.00; P < 0.055) |
Median.
—, Not reported; AF, atrial fibrillation cohort; AF-CHF, Atrial Fibrillation and Congestive Heart Failure; ANZ, Australia/New Zealand; BADAPIC, Registry of the Working Group on Heart Failure, Heart Transplantation and Other Therapeutic Alternatives of the Spanish Society of Cardiology; BB, beta-blocker; BEST, Beta-blocker Evaluation in Survival Trial; BRING-UP: Beta-Blockers in Patients With Congestive Heart Failure: Guided Use in Clinical Practice; CHS, Cardiovascular Health Study; CHART, Chronic Heart Failure Analysis and Registry in the Tohoku district; CI, confidence interval; CIBIS, Cardiac Insufficiency Bisoprolol Study; COPERNICUS, Carvedilol Prospective Randomized Cumulative Survival; EF, ejection fraction; EVADEF: Évaluation Médico-Économique du Défibrillateur Automatique Implantable; HF, heart failure; HFmrEF, heart failure with mid-range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HFSIS, National Heart Failure Survey in Israel; HR, hazard ratio; ICD, implantable cardioverter defibrillator cohort; IHD, ischaemic heart disease cohort; J-DHF, Japanese Diastolic Heart Failure; MERIT-HF, Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure; NHC, National Heart Care; OPTIMIZE-HF, Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure; OR, odds ratio; PROBE, prospective randomized open blind endpoint study; PSM, propensity score matched study; RCT, randomized controlled trial; REACH, Resource Utilization Among Congestive Heart Failure; RR, risk ratio/relative risk; SENIORS, Study of the Effects of Nebivolol Intervention on Outcomes and Rehospitalisation in Seniors with Heart Failure; ‘Trial patients’, patients meeting the inclusion criteria of the MERIT-HF trial; ‘'Non-trial patients’, patients not meeting the inclusion criteria of the MERIT-HF trial; WAHMD, Western Australia Hospital Morbidity Data.
Heart failure with preserved ejection fraction
The effect of beta-blockers on mortality was examined in one small randomized trial136 and a pre-specified subgroup analysis of a randomized trial which included patients with both HFrEF and HFpEF.132 Overall, we identified 13 non-randomized studies of beta-blockers in HFpEF, of which nine reported an association between beta-blocker use and better survival,32,46,50,51,55,60–63 whereas four did not30,53,64 (Table 3).
Mixed/unspecified heart failure phenotype
One moderately large RCT evaluated the effects of nebivolol in patients with both HFrEF and HFpEF, demonstrating a neutral effect on mortality.137 We identified 17 observational studies reporting 19 estimates of the ‘effect’ of treatment, with 17 suggesting benefit,41,44–46,55,65–74 and two reporting no difference in outcome between those treated with and not treated with a beta-blocker30 (Table 3).
Mineralocorticoid receptor antagonists
Heart failure with reduced ejection fraction
Two pivotal RCTs in HFrEF demonstrated the mortality and hospitalization benefits of MRAs.138,139 In contrast, of 12 non-randomized studies only one concluded MRAs were of benefit,75 with 10 finding a neutral effect,30,54,76–82 and one suggesting a harmful effect83 (Table 4).
Table 4.
All-cause mortality in randomized and non-randomized MRA HF studies
| First author, country, year of publication (study name) | Study design | Study period | Region | Mean follow-up (months) | Patients (n) | Study (n) | Control (n) | All-cause mortality—unadjusted HR (95% CI) | All-cause mortality—adjusted HR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| HFrEF | |||||||||
| Randomized controlled trials—beneficial treatment effect | |||||||||
| Zannad, USA, 2011 (EMPHASIS-HF)138 | RCT | 2006–2010 | Multiregional | 21a | 2737 | 1364 | 1373 | 0.78 (0.64–0.95; P < 0.01) | 0.76 (0.62–0.93; P < 0.008) |
| Pitt, USA, 1999 (RALES)139 | RCT | 1995–1996 | Multiregional | 24 | 1663 | 822 | 841 | RR: 0.70 (0.60–0.82; P < 0.001) | — |
| Observational studies—beneficial treatment effect | |||||||||
| Hamaguchi, Japan, 2010 (JCARE-CARD)75 | Prospective cohort study | 2004–2005 | Japan | 26 | 946 | 435 | 511 | 0.75 (0.54–1.04; P < 0.078) | 0.62 (0.41–0.93; P < 0.02) |
| Observational studies—neutral treatment effect | |||||||||
| Lam, USA, 2017 (Alabama HF Project)76 | Retrospective cohort study (PSM) | 1998–2001 | USA | 12 | 648 | 324 | 324 | — | 1.11 (0.83–1.49; P < 0.483) |
| Ushigome, Japan, 2015 (1. CHART-1)30 | Prospective cohort study | 2000–2005 | Japan | 36 | 543 | 116 | 427 | — | 1.39 (0.80–2.43; P < 0.247) |
| Ushigome, Japan, 2015 (2. CHART-2)30 | Prospective cohort study | 2006–2010 | Japan | 36 | 1360 | 493 | 867 | — | 1.23 (0.91–1.66; P < 0.172) |
| Frankenstein, Norway, 2013 (Norwegian HF Registry)77 | Registry (PSM) | — | Norway, Germany | 44 | 4832 | 1565 | 3267 | 1.08 (0.97–1.22; P < 0.17) | 1.03 (0.88–1.20; P < 0.74) |
| Lee, USA, 2013 (KPNC)78 | Retrospective cohort study | 2006–2008 | USA | 30a | 2358 | 521 | 1837 | — | 0.93 (0.60–1.44) |
| Lund, Sweden, 2013 (Swedish HF Registry)79 | Registry (PSM) | 2000–2012 | Sweden | 27a | 18 852 | 6551 | 12 301 | 1.10 (1.04–1.15; P < 0.001) | 1.05 (1.00–1.11; P < 0.054) |
| Pascual-Figal, Spain, 2013 (MUSIC)80 | Prospective cohort study (PSM) | 2003–2004 | Spain | 38a | 362 | 181 | 181 | 1.25 (0.81–1.94; P < 0.318) | 1.46 (0.84–2.55; P < 0.185) |
| Hernandez, USA, 2012 (GWTG-HF/Medicare)81 | Registry | 2005–2009 | USA | 36 | 5887 | 1070 | 4817 | 0.98 (0.90–1.06; P < 0.58) | 1.05 (0.97–1.15; P < 0.23) |
| Miyagishima, Japan, 200954 | Retrospective cohort study | 2000–2004 | Japan | 36 | 431 | 312 | 119 | — | 0.83 (0.54–1.30) |
| Ouzounian, Canada, 2007 (ICONS)82 | Prospective cohort study | 1997–2001 | Canada | 24 | 7816 | 644 | 7172 | — | OR: 0.97 (0.79–1.20) |
| Observational studies—harmful treatment effect | |||||||||
| O'Meara, Canada, 2012 (AF-CHF)83 | Post hoc analysis of RCT (AF) | 2001–2005 | Multiregional | 37 | 1376 | 616 | 760 | — | 1.40 (1.10–1.80; P < 0.005) |
| HFpEF | |||||||||
| Randomized controlled trials—neutral treatment effect | |||||||||
| Pfeffer, USA, 2015 (TOPCAT-Americas subgroup)140 | Post hoc analysis of RCT | 2006–2012 | USA, Canada, Brazil, Argentina | 35 | 1767 | 886 | 881 | 0.83 (0.68–1.02; P < 0.08) | — |
| Pfeffer, USA, 2015 (TOPCAT-Russia/Georgia subgroup)140 | Post hoc analysis of RCT | 2006–2012 | Russia, Georgia | 44 | 1678 | 836 | 842 | 1.12 (0.80–1.55; P < 0.51 | — |
| Pitt, USA, 2014 (TOPCAT)141 | RCT | 2006–2012 | Multiregional | 40 | 3445 | 1722 | 1723 | 0.91 (0.77–1.08; P < 0.295) | 0.88 (0.74–1.05; P < 0.151) |
| Observational studies—beneficial treatment effect | |||||||||
| Bonsu, Malaysia, 201784 | Retrospective cohort study | 2009–2013 | Ghana | 60 | 878 | 227 | 651 | — | 0.66 (0.49–0.89; P < 0.006) |
| Observational studies—neutral treatment effect | |||||||||
| Ushigome, Japan, 2015 (2. CHART-2)30 | Prospective cohort study | 2006–2010 | Japan | 36 | 2316 | 491 | 1825 | — | 0.96 (0.72–1.29; P < 0.808) |
| Patel, USA, 2013 (OPTIMIZE-HF)85 | Registry (PSM) (≥65 years) | 2002–2008 | USA | 29 | 974 | 487 | 487 | — | 1.03 (0.89–1.20; P < 0.693) |
| Mixed/unspecified HF phenotype | |||||||||
| Observational studies—beneficial treatment effect | |||||||||
| Bonsu, Malaysia, 2017 (23% HFrEF, 18% HFmrEF, 59% HFpEF)84 | Retrospective cohort study | 2009–2013 | Ghana | 60 | 1488 | 417 | 1071 | — | 0.81 (0.65–0.99; P < 0.049) |
| Sligl, Canada, 2004 (75% HFrEF, 25% HFpEF)86 | Prospective cohort study | 1989–2001 | Canada | 32a | 1037 | 136 | 901 | — | RR: 0.13 (0.04–0.42) |
| Observational studies—neutral treatment effect | |||||||||
| Ushigome, Japan, 2015 (1. CHART-1) (54% HFrEF, 46% HFpEF)30 | Prospective cohort study | 2000–2005 | Japan | 36 | 1006 | 182 | 824 | — | 1.36 (0.89–2.07; P < 0.154) |
| Ushigome, Japan, 2015 (2. CHART-2) (37% HFrEF, 63% HFpEF)30 | Prospective cohort study | 2006–2010 | Japan | 36 | 3676 | 984 | 2692 | — | 1.14 (0.93–1.39; P < 0.223) |
| Teng, Australia, 2010 (34% HFrEF, 19% HFpEF, 47% unknown)46 | Retrospective cohort study | 1996–2006 | Australia | 12 | 944 | 154 | 790 | — | 0.87 (0.64–1.20; P < 0.390) |
Median.
—, Not reported; AF, atrial fibrillation cohort; AF-CHF, Atrial Fibrillation and Congestive Heart Failure; CHART, Chronic Heart Failure Analysis and Registry in the Tohoku district; CI, confidence interval; EMPHASIS-HF, Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure; GWTG-HF, Get With The Guidelines Heart Failure; HF, heart failure; HFmrEF, heart failure with mid-range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HR, hazard ratio; ICONS, Improving Cardiovascular Outcomes in Nova Scotia; JCARE-CARD, Japanese Cardiac Registry of Heart Failure in Cardiology; KPNC, Kaiser Permanente Northern California; MRA, mineralocorticoid receptor antagonist; MUSIC, Multi-Sensor Monitoring in Congestive Heart Failure; OPTIMIZE-HF, Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure; OR, odds ratio; PSM, propensity score matched study; RALES, Randomized Aldactone Evaluation Study; RCT, randomized controlled trial; RR, risk ratio/relative risk; TOPCAT, Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist Trial.
Heart failure with preserved ejection fraction
One large RCT showed no effect of spironolactone on mortality in patients with HFpEF.141 Two observational studies also found a neutral effect,30,85 but a further non-randomized study reported an association between MRA use and lower mortality84 (Table 4).
Mixed/unspecified heart failure phenotype
Of five studies of patients with a mixed HF phenotype, two suggested benefit,84,86 and three reported a neutral effect30,46 (Table 4).
Statins
Heart failure with reduced ejection fraction
Two large RCTs showed a neutral effect of rosuvastatin on mortality in HFrEF (one trial included a small number of patients with HFpEF).142,144 Sixteen non-randomized studies reported 17 estimates of the ‘effect’ of statin treatment in HFrEF. Of these, 14 reported an association between statin use and better outcome,28,59,87–97 whereas only three found no association30,59,98 (Table 5).
Table 5.
All-cause mortality in randomized and non-randomized statin HF studies
| First author, country, year of publication (study name) | Study design | Study period | Region | Mean follow-up (months) | Patients (n) | Study (n) | Control (n) | All-cause mortality—unadjusted HR (95% CI) | All-cause mortality—adjusted HR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| HFrEF | |||||||||
| Randomized controlled trials—neutral treatment effect | |||||||||
| Kjekshus, Norway, 2007 (CORONA)142 | RCT | 2003–2005 | Europe, Russia, South Africa | 33a | 5011 | 2514 | 2497 | 0.95 (0.86–1.05; P < 0.31) | — |
| Takano, Japan, 2013 (PEARL)143 | PROBE | 2006–2008 | Japan | 36a | 574 | 288 | 286 | — | 0.73 (0.44–1.20; P < 0.211) |
| Observational studies—beneficial treatment effect | |||||||||
| Alehagen, Sweden, 2015 (Swedish HF Registry)87 | Registry (PSM) | 2000–2012 | Sweden | 47a | 10 762 | 5381 | 5381 | — | 0.81 (0.76–0.86; P < 0.001) |
| Liu, China, 201428 | Prospective cohort study | 2005–2010 | China | 52a | 2154 | 936 | 1218 | — | 0.50 (0.37–0.67; P < 0.001) |
| Gomez-Soto, Spain, 2010 (56% HFrEF)88 | Prospective cohort study (propensity score adjusted) | 2001–2005 | Spain | 34 | 2573 | 1343 | 1230 | — | 0.20 (0.09–0.31; P < 0.001) |
| Sumner, USA, 2009 (COMPANION)89 | Post hoc analysis of RCT (CRT) | 2000–2002 | USA | 15–16a | 1520 | 603 | 917 | 0.85 (0.67–1.07; P < 0.15) | 0.77 (0.61–0.97; P < 0.03) |
| Coleman, USA, 200890 | Retrospective cohort study (ICD) | 1997–2007 | USA | 31 | 1204 | 642 | 562 | — | 0.67 (0.53–0.85; P < 0.001) |
| Dickinson, USA, 2007 (SCD-HeFT)91 | Post hoc analysis of RCT | 1997–2001 | North America, New Zealand | 46 | 2521 | 965 | 1556 | — | 0.70 (0.58–0.83; P < 0.001) |
| Huan Loh, UK, 2007 (1. no statin vs. initiated statin)59 | Retrospective cohort study | — | UK | 36a | 479 | 102 | 377 | 0.52 (0.32–0.84) | 0.50 (0.30–0.83) |
| Krum, Australia, 2007 (CIBIS-II)92 | Post hoc analysis of RCT | — | Europe | 16 | 2647 | 226 | 2421 | 0.57 (0.37–0.94) | 0.60 (0.39–0.94); P < 0.02 |
| Krum, Australia, 2007 (Val-HeFT)93 | Post hoc analysis of RCT | 1997–1999 | Multiregional | 23 | 5010 | 1602 | 3408 | — | 0.81 (0.70–0.94; P < 0.005) |
| Anker, UK, 2006 (1. ELITE-II)94 | Post hoc analysis of RCT | 1997–1998 | Multiregional | 18a | 3132 | 2734 | 398 | 0.61 (0.45–0.83; P < 0.0007) | 0.61 (0.44–0.84; P < 0.003) |
| Anker, UK, 2006 (2. European Centres Study)94 | Retrospective cohort study | 1992–2000 | Europe | 24a | 2068 | 705 | 1363 | 0.59 (0.49–0.72; P < 0.0001) | 0.58 (0.44–0.77; P < 0.0001) |
| Goldberger, USA, 2006 (DEFINITE)95 | Post hoc analysis of RCT (non-ischaemic DCM) | 1998–2002 | USA | 29 | 458 | 110 | 348 | 0.22 (0.09–0.55; P < 0.001) | 0.23 (0.09–0.58; P < 0.04) |
| Ray, Canada, 200596 | Retrospective cohort study (66–85 years) | 1995–2001 | Canada | 24 | 28 828 | 1146 | 27 682 | 0.50 (0.43–0.59) | 0.67 (0.57–0.78) |
| Mozaffarian, USA, 2004 (PRAISE)97 | Post hoc analysis of RCT | 1992–1994 | USA | 15 | 1153 | 134 | 1019 | 0.38 (0.23–0.64) | 0.44 (0.26–0.75) |
| Observational studies—neutral treatment effect | |||||||||
| Ushigome, Japan, 2015 (CHART-2)30 | Prospective cohort study | 2006–2010 | Japan | 36 | 1360 | 515 | 845 | — | 0.84 (0.60–1.17; P < 0.299) |
| Ouzounian, Canada, 2009 (EFFECT) (23% HFrEF)98 | Retrospective cohort study | 1999–2001 | Canada | 60 | 6451 | 5330 | 1121 | — | 0.84 (0.70–1.02; P < 0.07) |
| Huan Loh, UK, 2007 (2. no statin vs. continued statin)59 | Retrospective cohort study | — | UK | 36a | 760 | 377 | 383 | 0.74 (0.52–1.05) | 0.82 (0.55–1.23) |
| Mixed/unspecified HF phenotype | |||||||||
| Randomized controlled trials—neutral treatment effect | |||||||||
| Tavazzi, Italy, 2008 (GISSI-HF Rosuvastatin) (90% HFrEF, 10% HFpEF)144 | RCT (≥60 years) | 2002–2005 | Italy | 47a | 4574 | 2285 | 2289 | 1.03 (95.5% CI 0.92–1.15; P < 0.660) | 1.00 (95.5% CI 0.90–1.12; P < 0.943) |
| Observational studies—beneficial treatment effect | |||||||||
| Bonsu, Malaysia, 2017 (23% HFrEF, 18% HFmrEF, 59% HFpEF)99 | Retrospective cohort study (IPTW) | 2009–2013 | Ghana | 60 | 1488 | 552 | 936 | — | 0.79 (0.65–0.96; P < 0.019) |
| Ballo, Italy, 2016100 | Retrospective cohort study | — | Italy | 12 | 2088 | 643 | 1445 | — | 0.65 (0.51–0.83; P < 0.001) |
| Gastelurrutia, Spain, 2012 (75% HFrEF, 25% HFrEF)45 | Prospective cohort study | 2001–2008 | Spain | 44a | 960 | 591 | 369 | 0.45 (0.37–0.54; P < 0.001) | 0.66 (0.53–0.83; P < 0.001) |
| Gomez-Soto, Spain, 2010 (56% HFrEF, 44% HFpEF)88 | Prospective cohort study (propensity score adjusted) | 2001–2005 | Spain | 34 | 2573 | 1343 | 1230 | — | 0.71 (0.59–0.83) |
| Jordán, Spain, 2009 (BADAPIC) (77% HFrEF, 23% HFpEF)68 | Registry | 2000–2002 | Spain | 35 | 3162 | 1305 | 1857 | — | RR: 0.73 (0.45–0.88; P < 0.001) |
| Nevzorov, Israel, 2009 (61% HFrEF, 39% HFpEF)101 | Retrospective cohort study (IHD) | 2001–2005 | Israel | 12 | 656 | 238 | 418 | OR: 0.63 (0.40–0.87; P < 0.006) | 0.66 (0.40–0.97; P < 0.035) |
| Ouzounian, Canada, 2009 (EFFECT)98 | Retrospective cohort study (PSM) | 1999–2001 | Canada | 60 | 1442 | 721 | 721 | — | 0.85 (0.72–1.00; P < 0.05) |
| Ryan, UK, 2009 (THIN) (1. statin before HF diagnosis)102 | Retrospective cohort study | 1995–2004 | UK | 24 | 10 914 | 2185 | 8239 | — | 0.53 (0.40–0.70; P < 0.001) |
| Ryan, UK, 2009 (THIN) (2. statin after HF diagnosis)102 | Retrospective cohort study | 1995–2004 | UK | 24 | 8729 | 191 | 8538 | — | 0.68 (0.46–0.99; P < 0.047) |
| Foody, USA, 2006 (NHC) (48% HFrEF, 52% HFpEF)103 | Retrospective cohort study (≥65 years) | 1998–1999, 2000–2001 | USA | 36a | 54 960 | 9163 | 45 797 | 0.67 (0.65–0.69; P < 0.001) | 0.82 (0.79–0.85; P < 0.001) |
| Go, USA, 2006 (KPNC) (25% HFrEF, 26% HFpEF, 49% unknown)104 | Retrospective cohort study (propensity score adjusted) | 1996–2004 | USA | 29a | 24 598 | 12 648 | 11 960 | — | 0.76 (0.72–0.80; P < 0.001) |
| Observational studies—neutral treatment effect | |||||||||
| Ushigome, Japan, 2015 (CHART-2) (37% HFrEF, 63% HFpEF)30 | Prospective cohort study | 2006–2010 | Japan | 36 | 3676 | 1332 | 2344 | — | 0.81 (0.65–1.02; P < 0.068) |
Median.
—, Not reported; BADAPIC, Registry of the Working Group on Heart Failure, Heart Transplantation and Other Therapeutic Alternatives of the Spanish Society of Cardiology; CHART, Chronic Heart Failure Analysis and Registry in the Tohoku district; CI, confidence interval; CIBIS-II, Cardiac Insufficiency Bisoprolol Study II; COMPANION, Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure; CORONA, Controlled Rosuvastatin Multinational Trial in Heart Failure; CRT, cardiac resynchronization therapy cohort; DCM, dilated cardiomyopathy cohort; DEFINITE, Defibrillators in Non-Ischaemic Cardiomyopathy Treatment Evaluation; EFFECT, Enhanced Feedback for Effective Cardiac Treatment; ELITE-II, Evaluation of Losartan in the Elderly II; GISSI-HF, Gruppo Italiano per lo Studio della Sopravvivenza nell'Insuffi cienza cardiaca Heart Failure; HF, heart failure; HFmrEF, heart failure with mid-range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HR, hazard ratio; ICD, implantable cardioverter defibrillator cohort; IHD, ischaemic heart disease cohort; IPTW, inverse-probability-of-treatment weighted study; KPNC, Kaiser Permanente Northern California; NHC, National Heart Care; OR, odds ratio; PEARL, Pitavastatin Heart Failure study; PRAISE, Prospective Randomized Amlodipine Survival Evaluation; PROBE, prospective randomized open blind endpoint study; PSM, propensity score matched study; RCT, randomized controlled trial; RR, risk ratio/relative risk; SCD-HeFT, Sudden Cardiac Death in Heart Failure Trial; THIN, The Health Improvement Network; Val-HeFT, Valsartan Heart Failure Trial.
Heart failure with preserved ejection fraction
The use of statins has not been evaluated in a randomized trial in patients with HFpEF, therefore, no relevant non-randomized studies were identified.
Mixed/unspecified heart failure phenotype
One large statin trial included patients with both HFrEF and HFpEF and showed no effect of treatment on mortality.144 Eleven observational studies reported 12 estimates of the ‘effect’ of a statin in patients with a mixture of HFrEF and HFpEF phenotypes, or where EF was not specified. Of these, 11 reported an association between statin use and better outcome,45,68,88,98–104 with only one describing no relationship between treatment and mortality30 (Table 5).
Digoxin
Heart failure with reduced ejection fraction
A single RCT, the Digitalis Investigators Group (DIG) trial, showed that, in sinus rhythm, digoxin had a neutral effect on death but reduced the risk of HF hospitalization.145 Nine non-randomized studies reported 10 estimates of the ‘effect’ of digoxin treatment in HFrEF, with five concluding digoxin was harmful,107–110 four reporting a neutral effect,30,55,106 and one suggesting digoxin was beneficial105 (Table 6).
Table 6.
All-cause mortality in randomized and non-randomized digoxin HF studies
| First author, country, year (study name) | Study design | Study period | Region | Mean follow-up (months) | Patients (n) | Study (n) | Control (n) | All-cause mortality—unadjusted HR (95% CI) | All-cause mortality—adjusted HR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| HFrEF | |||||||||
| Randomized controlled trials—neutral treatment effect | |||||||||
| Digoxin Investigation Group, USA, 1997 (DIG Main Trial)145 | RCT (SR) | 1991–1993 | USA, Canada | 37 | 6800 | 3397 | 3403 | RR: 0.99 (0.91–1.07; P < 0.80) | — |
| Observational studies—beneficial treatment effect | |||||||||
| Andrey, Spain, 2011 (51% HFrEF)105 | Prospective cohort study (PSM) (SR/AF) | 2001–2008 | Spain | 46a | 2842 | 1421 | 1421 | — | 0.92 (0.89–0.95; P < 0.005) |
| Observational studies—neutral treatment effect | |||||||||
| Ushigome, Japan, 2015 (1. CHART-1)30 | Prospective cohort study (SR/AF) | 2000–2005 | Japan | 36 | 543 | 229 | 314 | — | 0.99 (0.61–1.61; P < 0.978) |
| Ushigome, Japan, 2015 (2. CHART-2)30 | Prospective cohort study (SR/AF) | 2006–2010 | Japan | 36 | 1360 | 586 | 774 | — | 1.10 (0.80–1.51; P < 0.558) |
| Fauchier, France, 2009 (41% HFrEF)55 | Retrospective cohort study (AF) | 2000–2004 | France | 29 | 1269 | 591 | 678 | — | RR: 0.79 (0.54–1.16; P < 0.23) |
| Dhaliwal, USA, 2008106 | Retrospective cohort study (SR/AF) | 2002–2004 | USA | 10a | 347 | 155 | 192 | 1.15 (0.85–1.55; P < 0.371) | 1.11 (0.81–1.53; P < 0.521) |
| Observational studies—harmful treatment effect | |||||||||
| Al-Khateeb, Saudi Arabia, 2017107 | Retrospective cohort study (PSM) (SR/AF) | 2000–2015 | Saudi Arabia | 43a | 1075 | 325 | 750 | 1.81 (1.33–2.45; P < 0.001) | 1.74 (1.20–2.38; P < 0.0001) |
| Freeman, USA, 2013 (KPNC)108 | Retrospective cohort study (SR/AF) | 2006–2008 | USA | 30a | 2891 | 529 | 2362 | — | 1.72 (1.25–2.36) |
| Butler, USA, 2010 (Val-HeFT)109 | Post hoc analysis of RCT (SR/AF) | — | Multiregional | 23 | 5010 | 1636 | 3374 | 1.46 (1.23–1.64; P < 0.001) | 1.28 (1.05–1.57; P < 0.02) |
| Domanski, USA, 2005 (SOLVD) (1. female cohort)110 | Post hoc analysis of RCT (SR/AF) | 1986–1989 | USA, Canada, Belgium | 39 | 988 | 370 | 618 | 1.48 (1.10–2.00; P < 0.01) | 1.36 (1.03–1.80; P < 0.03) |
| Domanski, USA, 2005 (SOLVD) (2. male cohort)110 | Post hoc analysis of RCT (SR/AF) | 1986–1989 | USA, Canada, Belgium | 39 | 5809 | 1874 | 3935 | 1.37 (1.20–1.56; P < 0.0001) | 1.42 (1.26–1.61; P < 0.0001) |
| HFpEF | |||||||||
| Randomized controlled trials—neutral treatment effect | |||||||||
| Ahmed, USA, 2006 (DIG Ancillary Trial)146 | RCT (SR) | 1991–1993 | USA, Canada | 37 | 988 | 492 | 496 | 0.99 (0.76–1.28; P < 0.925) | — |
| Observational studies—beneficial treatment effect | |||||||||
| Andrey, Spain, 2011 (49% HFpEF)105 | Prospective cohort study (PSM) (SR/AF) | 2001–2008 | Spain | 46a | 2842 | 1421 | 1421 | — | 0.86 (0.79–0.92; P < 0.008) |
| Observational studies—neutral treatment effect | |||||||||
| Ushigome, Japan, 2015 (1. CHART-1)30 | Prospective cohort study (SR/AF) | 2000–2005 | Japan | 36 | 463 | 249 | 214 | — | 0.92 (0.55–1.54; P < 0.764) |
| Ushigome, Japan, 2015 (2. CHART-2)30 | Prospective cohort study (SR/AF) | 2006–2010 | Japan | 36 | 2316 | 335 | 1981 | — | 1.07 (0.81–1.41; P < 0.632) |
| Fauchier, France, 2009 (35% HFpEF)55 | Retrospective cohort study (AF) | 2000–2004 | France | 29 | 1269 | 591 | 678 | — | RR: 1.21 (0.77–1.89; P < 0.42) |
| Mixed/unspecified HF phenotype | |||||||||
| Randomized controlled trials—neutral treatment effect | |||||||||
| Rich, USA, 2001 (DIG Overall)147 | RCT (SR) | 1991–1993 | USA, Canada | 37 | 7788 | 3889 | 3899 | RR: 0.99 (0.92–1.07; P < 0.7815) | — |
| Observational studies—beneficial treatment effect | |||||||||
| Ahmed, USA, 2014 (Alabama HF Project) (57% HFrEF, 25% HFpEF, 18% unknown)111 | Retrospective cohort study (PSM) (SR/AF) | 1998–2001 | USA | 12 | 1842 | 921 | 921 | — | 0.83 (0.70–0.98) |
| Andrey, Spain, 2011 (51% HFrEF, 49% HFpEF)105 | Prospective cohort study (PSM) (SR/AF) | 2001–2008 | Spain | 46a | 2842 | 1421 | 1421 | — | 0.90 (0.84–0.97) |
| Observational studies—neutral treatment effect | |||||||||
| Ushigome, Japan, 2015 (1. CHART-1) (54% HFrEF, 46% HFpEF)30 | Prospective cohort study (SR/AF) | 2000–2005 | Japan | 36 | 1006 | 478 | 528 | — | 0.97 (0.69–1.38; P < 0.875) |
| Ushigome, Japan, 2015 (2. CHART-2) (37% HFrEF, 63% HFpEF)30 | Prospective cohort study (SR/AF) | 2006–2010 | Japan | 36 | 3676 | 921 | 2755 | — | 1.06 (0.87–1.31; P < 0.555) |
| Flory, USA, 2012 (THIN) (1. female cohort)112 | Retrospective cohort study (SR/AF) | 1986–2008 | UK | — | 30 035 | 10 808 | 19 227 | — | 1.00 (0.96–1.06) |
| Flory, USA, 2012 (THIN) (2. male cohort)112 | Retrospective cohort study (SR/AF) | 1986–2008 | UK | — | 27 194 | 9487 | 17 707 | — | 1.00 (0.95–1.06) |
| Fauchier, France, 2009 (41% HFrEF, 35% HFpEF, 24% unknown)55 | Retrospective cohort study (AF) | 2000–2004 | France | 29 | 1269 | 591 | 678 | — | 0.90 (0.66–1.24; P < 0.53) |
| Hallberg, Sweden, 2007 (RIKS-HIA) (58% HFrEF, 42% HFpEF) (1. AF cohort)113 | Registry (propensity score adjusted) | 1995–2003 | Sweden | 12 | 16 960 | 7758 | 9202 | RR: 1.07 (1.01–1.14) | RR: 1.00 (0.94–1.06) |
| Pedone, Italy, 2004 (GIFA)42 | Prospective cohort study (SR/AF) | 1998 | Italy | 10 | 818 | 539 | 279 | — | 0.75 (0.51–1.10) |
| Observational studies—harmful treatment effect | |||||||||
| Eisen, USA, 2017 (ENGAGE AF-TIMI 48) (41% HFrEF, 34% HFpEF, 24% unknown)114 | Post hoc analysis of RCT (IPTW) (AF) | 2008–2010 | Multiregional | 34a | 8102 | 4051 | 4051 | — | 1.29 (1.15–1.44) |
| Katz, Israel, 2016 (HFSIS) (38% HFrEF, 15% HFmrEF, 22% HFpEF, 26% unknown)65 | Prospective cohort study (SR/AF) | 2003 | Israel | 120 | 2402 | 380 | 2022 | — | 1.27 (1.16–1.42; P < 0.001) |
| Madelaire, Denmark, 2016115 | Retrospective cohort study (PSM) (SR) | 1996–2012 | Denmark | 32a | 15 981 | 5327 | 10 654 | — | 1.19 (1.15–1.24; P < 0.001) |
| Shah, Canada, 2014116 | Retrospective cohort study (PSM) (≥65 years) (AF) | 1998–2012 | Canada | 37 | 27 972 | 13 986 | 13 986 | 1.14 (1.11–1.17) | 1.14 (1.10–1.17) |
| Whitbeck, USA, 2013 (AFFIRM)117 | Post hoc analysis of RCT (AF) | — | Multiregional | 42 | 1076 | — | — | — | 1.41 (1.09–1.84; P < 0.01) |
| Hallberg, Sweden, 2007 (RIKS-HIA) (58% HFrEF, 42% HFpEF) (2. SR cohort)113 | Registry (propensity score adjusted) | 1995–2003 | Sweden | 12 | 22 345 | 3796 | 18 549 | RR: 1.35 (1.26–1.44) | RR: 1.11 (1.04–1.19) |
| Tandon, Canada, 2004 (75% HFrEF, 25% HFpEF)41 | Prospective cohort study (SR/AF) | 1989–2001 | Canada | 32a | 1041 | 671 | 370 | — | OR: 1.51 (1.10–2.07) |
Median.
—, Not reported; AF, atrial fibrillation cohort; AFFIRM, Atrial Fibrillation Follow-up Investigation of Rhythm Management; CHART, Chronic Heart Failure Analysis and Registry in the Tohoku district; CI, confidence interval; DIG, Digitalis Investigation Group; ENGAGE AF-TIMI 48, Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation - Thrombolysis in Myocardial Infarction 48; GIFA, Gruppo Italiano di Farmacovigilanza nell'Anziano; HF, heart failure; HFmrEF, heart failure with mid-range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HFSIS, National Heart Failure Survey in Israel; HR, hazard ratio; KPNC, Kaiser Permanente Northern California; IPTW, inverse-probability-of-treatment weighted study; OR, odds ratio; PSM, propensity score matched study; RCT, randomized controlled trial; RIKS-HIA, Registry of Information and Knowledge about Swedish Heart Intensive Care Admissions; RR, risk ratio/relative risk; SOLVD, Studies of Left Ventricular Dysfunction; SR, sinus rhythm cohort; SR/AF, sinus rhythm and atrial fibrillation cohort; THIN, The Health Improvement Network; Val-HeFT, Valsartan Heart Failure Trial.
Heart failure with preserved ejection fraction
A single randomized trial of modest size, the DIG ancillary trial in HFpEF (n = 988), showed no effect of digoxin on mortality in patients with HFpEF in sinus rhythm, although the estimate of the effect of treatment was not robust because of limited power.146 Four observational studies were identified, one suggesting that non-randomized digoxin treatment was beneficial,105 and three showing a neutral association between treatment and mortality30,55 (Table 6).
Mixed/unspecified heart failure phenotype
The combined main and ancillary DIG trials showed a neutral effect of digoxin on mortality.147 Fourteen observational studies reported effect estimates for digoxin in patients with HFrEF and HFpEF in combination, or where EF was not specified. These studies reported 16 estimates of ‘treatment-effect’. Seven found an association between the use of digoxin and a higher mortality,41,65,113–117 seven were neutral,30,42,55,112,113 and two suggested better outcomes associated with digoxin use105,111 (Table 6).
Discussion
There is a particularly strong evidence base for the treatment of HF, making it an appropriate condition in which to compare treatment effects established in RCTs with those estimated in non-randomized and observational studies.
Looking first at patients with HFrEF, six observational studies (reporting seven ‘effect’ estimates) fulfilled our inclusion criteria, and examined the association between treatment with an ACEI/ARB and mortality. Of these, five showed a lower mortality in patients receiving treatment of this type,26–29 whereas two did not,30 i.e. there was relatively good concordance between these non-randomized studies and the pivotal RCTs. However, the same concordance was not found in studies in HFpEF (see below).
The non-randomized analyses of beta-blockers in HFrEF also showed good agreement with the RCTs, with 16 of 18 analyses concordant.28,30,46–59 However, this was not the case in observational studies of patients with a mixed HF phenotype, where the Study of the Effects of Nebivolol Intervention on Outcomes and Rehospitalisation in Seniors with Heart Failure (SENIORS) trial had shown a neutral effect on mortality.137 Of the 19 non-randomized analyses, 17 showed a lower mortality among patients of this type treated with a beta-blocker.30,41,44–46,55,65–74
However, the picture was quite different for MRAs, which reduce mortality in HFrEF. Of 12 observational studies, one reported lower mortality in patients treated with a MRA,75 10 did not find a better or worse outcome (i.e. were neutral),30,54,76–82 and one found a higher mortality (worse outcome) in the MRA treated patients.83 It is worth exploring this discordance in more detail. By far the largest study included 18 852 patients from Sweden and is worth examining in detail.79 The authors of this study used matching of spironolactone treated (n = 6551) and untreated (n = 12 301) patients. The authors also attempted to adjust for residual confounding in several different ways. Despite these statistical approaches, the multivariate HR for all-cause mortality with spironolactone vs. no spironolactone was 1.05 [95% confidence interval (CI) 1.00–1.11; P = 0.054] in the model adjusted for propensity score and 1.10 (95% CI 1.02–1.19; P = 0.020) in a 1:1 matched model. These findings stand in stark contrast to two separate trials of MRAs in HFrEF. The authors of the above observational study argued that the severity of HF symptoms and concomitant use of beta-blockers might explain the difference between their findings and the Randomized Aldactone Evaluation Study (RALES) trial, which used spironolactone in severely symptomatic patients, few of which were treated with a beta-blocker.139 However, patients with mild symptoms, the large majority of which were treated with a beta-blocker, were enrolled in the Eplerenone in Mild Patients Hospitalization And Survival Heart Failure (EMPHASIS-HF) trial, which demonstrated a clear mortality benefit of the MRA eplerenone.138 As an alternative explanation for their discrepant findings, the authors postulated that trial inclusion/exclusion criteria select patients more likely to benefit and less likely to experience harm pointing out, for example, the younger average age of patients in RALES (65 years) compared with the Swedish registry (71 years); however, the average age in EMPHASIS-HF was 69 years. In any case (and counterintuitively), the authors own analysis showed a significant treatment-by-age interaction whereby older (rather than younger) patients did better with MRA treatment.79 Several other of the authors’ subgroup analyses (e.g. significantly better outcome with an MRA in patients without diabetes compared to with diabetes) are directly contradicted by independent but consistent subgroup analyses from RALES and EMPHASIS-HF. The authors of the Swedish study also speculated that patients in the ‘real-world’ treated with a MRA maybe at greater risk of harm because of less careful monitoring of renal function and potassium.
Another notable example of a discrepancy between observational data and randomized trials does address issues of safety and generalisability. All but three of a remarkable 17 observational ‘effect’ estimates suggested that statins have a mortality benefit in HFrEF,28,30,59,87–98 yet two large independent RCTs showed no effect of this type of treatment on death.142,144 In patients with the mixed/unspecified HF phenotype, a further 11 of 12 analyses reported an association of statin use with mortality benefit.30,45,68,88,98–104 Again, it is instructive to examine one of the observational studies in detail. Go et al.104 used a Kaiser Permanente dataset with almost 25 000 patients to conduct careful propensity score-adjusted analyses of outcome related to statin treatment; the authors also used time-varying covariate adjustment for statin initiation during follow-up. The adjusted HR for all-cause mortality in patients treated with a statin (compared with those who were not) was 0.66 (95% CI 0.61–0.71) in individuals with CHD and 0.60 (95% CI 0.54–0.67) in those without CHD. Apart from the improbably large ‘reduction’ in mortality (34–40%), the similar ‘effect’ in patients with and without CHD seems unlikely given everything we know about the actions of statins. Moreover, the prior arguments made about generalisability and safety would need to be inverted here as the observational datasets included broad populations of patients with HF, presumably, receiving less intense monitoring than in the clinical trials.
Even in HFpEF, there are clearly discrepant findings between a large observational dataset and two randomized trials with an ARB123,124 and one trial with an ACEI.122 Once again, the most obvious example involves the Swedish HF registry.29 As previously, the authors of this study used an age- and propensity score-matched cohort. The adjusted HR for all-cause mortality in patients treated with an ACEI or ARB, compared with those not treated with one of these agents, was 0.90 (95% CI 0.85–0.96; P = 0.001). The authors also described a ‘dose–response’ relationship whereby the HR for high-dose treatment compared with no treatment was 0.85 (95% CI 0.78–0.83) and compared with low-dose treatment was 0.94 (95% CI 0.87–1.02). For this study, the authors used the issue of generalisability to explain why they saw benefit compared with the prior trials, in contradistinction to the case for MRAs where the opposite argument was made. Specifically, in this case, with ACEIs and ARBs, they argued that the broader, older and higher-risk population in the registry responded favourably to treatment compared with the more selected participants enrolled in the trials.
Much has been written recently in relation to the safety of digoxin in atrial fibrillation. Indeed, in a very illustrative example of the unreliability of observational data, Bavendiek et al.148 highlighted how in three separate and independent post hoc analyses of the same dataset, digoxin treatment was variably associated with increased all-cause mortality, was not associated with increased mortality and, in the third analysis, was associated with decreased in mortality in patients with an EF less than 30%. In HF, there is the same type of discrepancy between observational data and the single large RCT in HFrEF, an ancillary trial in HFpEF, and the combined analysis of the effect of digoxin in both HF phenotypes.145–147 In each of these analyses, digoxin had a neutral effect on all-cause mortality. A total of 30 observational analyses variously show better, worse, and neutral outcomes.30,41,42,55,65,105–117
Why the non-randomized analyses of outcomes related to use of ACEI/ARB and beta-blockers in HFrEF were generally (but not absolutely) concordant with the RCTs, in contrast to the other treatments examined, is an interesting question. There may be less confounding by indication, i.e. ACEIs/ARBs and beta-blockers are recommended in essentially all patients with HFrEF, whereas digoxin and, at least until recently, MRAs were reserved for patients with more advanced HF. There may also have been particularly strong publication bias making it difficult to report studies suggesting that use of ACEIs/ARBs or beta-blockers is not associated with better outcomes (or even associated with worse outcomes). Of course, with both treatments there is also a strong selection bias whereby the sickest patients are least likely to be prescribed (and to tolerate) these therapies. The opposite consideration may apply to the non-randomized studies showing an association between treatments such as statins and lower mortality, with the possibility of other biases such as the ‘healthy-user effect’ not fully adjusted for.
Although our analyses show that the findings of non-randomized studies of the association between treatment use and outcomes are frequently inconsistent, they do not mean observational studies/registries are of no value. Registry-based analyses may be all that is available where randomized trials are not possible, such as in rare diseases or for rare outcomes. The latter forms the basis of pharmaco-epidemiological surveillance for rare adverse effects of drugs not identified in clinical trials. Non-randomized analyses may provide information on under-studied groups or subgroups excluded from clinical trials. However, the results of such analyses must be interpreted with caution, especially if the results of different analyses of this type conflict. Registries serve an important function in describing the use (or under-use) of evidence-based therapies in the ‘real-world’, often leading to initiatives to improve prescribing. Perhaps the greatest value of registries is the potential they offer to conduct more ‘real-world’ randomized trials, i.e. to randomize patients in a registry to treatment and follow their outcomes within the registry. This approach has been pioneered in a study of thrombus aspiration in ST-segment elevation myocardial infarction using the Swedish Coronary Angiography and Angioplasty Registry149 and a similar approach is now being used to conduct the Spironolactone Initiation Registry Randomized Interventional Trial in Heart Failure with Preserved Ejection Fraction (SPIRRIT-HFpEF)150 in the Swedish HF Registry [NCT02901184].
Our study has a number of strengths and limitations. The strengths include the robust evidence base in HF, with often more than one randomized trial supporting the use or avoidance of specific therapies. There is a specific limitation in relation to the effect of MRAs in HFpEF. In the single, prospective, RCT, ineligible patients were included, and study drug was not administered, at certain investigative sites.141 As a result, the integrity of the trial has been questioned, as has the overall treatment effect observed.151 Examination of the effect of therapy in regions where the trial is thought to have been conducted as intended suggested possible benefit of spironolactone, compared with placebo.140 Consequently, the effect of spironolactone in this RCT and in the one observational analysis which suggested no benefit from MRA therapy may not be in agreement.
Conclusion
This comprehensive comparison of the robust evidence base in HF with an increasing number of non-randomized data shows that it is not possible to make reliable therapeutic inferences from observational associations. While trials undoubtedly leave gaps in evidence and enrol selected participants, they clearly remain the best guide to the treatment of patients.
Conflict of interest: P.S.J. reports having received consulting fees from Novartis, research funding from Boehringer Ingelheim and serving on an advisory board for Vifor Pharma, all outside the submitted work. J.J.V.M. reports payments for trial-related activities to the University of Glasgow from Novartis, Cardiorentis, Amgen, Oxford University/Bayer, GlaxoSmithKline, Theracos, Abbvie, DalCor, Pfizer, Merck, AstraZeneca, Bristol Myers Squibb, and Kidney Research UK (KRUK)/Kings College Hospital, London/Vifor-Fresenius Pharma, all outside the submitted work.
Supplementary Material
References
- 1. Collins R, MacMahon S.. Reliable assessment of the effects of treatment on mortality and major morbidity, I: clinical trials. Lancet 2001;357:373–380. [DOI] [PubMed] [Google Scholar]
- 2. Barton S. Which clinical studies provide the best evidence? The best RCT still trumps the best observational study. BMJ 2000;321:255–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sacks H, Chalmers TC, Smith H.. Randomized versus historical controls for clinical trials. Am J Med 1982;72:233–240. [DOI] [PubMed] [Google Scholar]
- 4. Byar DP, Simon RM, Friedewald WT, Schlesselman JJ, DeMets DL, Ellenberg JH, Gail MH, Ware JH.. Randomized clinical trials. Perspectives on some recent ideas. N Engl J Med 1976;295:74–80. [DOI] [PubMed] [Google Scholar]
- 5. Wang MT, Bolland MJ, Gamble G, Grey A.. Media coverage, journal press releases and editorials associated with randomized and observational studies in high-impact medical journals: a cohort study. PLoS One 2015;10:e0145294.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kaplan RM, Chambers DA, Glasgow RE.. Big data and large sample size: a cautionary note on the potential for bias. Clin Transl Sci 2014;7:342–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Freemantle N, Marston L, Walters K, Wood J, Reynolds MR, Petersen I.. Making inferences on treatment effects from real world data: propensity scores, confounding by indication, and other perils for the unwary in observational research. BMJ 2013;347:f6409–f6409. [DOI] [PubMed] [Google Scholar]
- 8. Wang PS, Schoenbaum M.. Invited Commentary: assessing treatment effects by using observational analyses–opportunities and limitations. Am J Epidemiol 2009;170:286–287; discussion 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Grimes DA, Schulz KF.. Bias and causal associations in observational research. Lancet 2002;359:248–252. [DOI] [PubMed] [Google Scholar]
- 10. Rosenbaum PR. Discussing hidden bias in observational studies. Ann Intern Med 1991;115:901–905. [DOI] [PubMed] [Google Scholar]
- 11. King SB. Big trials or big data. JACC Cardiovasc Interv 2016;9:869–870. [DOI] [PubMed] [Google Scholar]
- 12. Lyman GH, Levine M.. Comparative effectiveness research in oncology: an overview. J Clin Oncol 2012;30:4181–4184. [DOI] [PubMed] [Google Scholar]
- 13. Greenfield S, Kaplan SH.. Observational studies versus randomized trials: squaring off. J Comp Eff Res 2012;1:385–386. [DOI] [PubMed] [Google Scholar]
- 14. Streeter AJ, Lin NX, Crathorne L, Haasova M, Hyde C, Melzer D, Henley WE.. Adjusting for unmeasured confounding in nonrandomized longitudinal studies: a methodological review. J Clin Epidemiol 2017;87:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rubin DB. The design versus the analysis of observational studies for causal effects: parallels with the design of randomized trials. Stat Med 2007;26:20–36. [DOI] [PubMed] [Google Scholar]
- 16. D’Agostino RB, D’Agostino RB.. Estimating treatment effects using observational data. JAMA 2007;297:314–316. [DOI] [PubMed] [Google Scholar]
- 17. D’Agostino RB. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 1998;17:2265–2281. [DOI] [PubMed] [Google Scholar]
- 18. Grodstein F, Manson JE, Colditz GA, Willett WC, Speizer FE, Stampfer MJ.. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med 2000;133:933–941. [DOI] [PubMed] [Google Scholar]
- 19. Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SAA, Howard BV, Johnson KC, Kotchen JM, Ockene J; Writing Group for the Women’s Health Initiative Investigators . Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002;288:321–333. [DOI] [PubMed] [Google Scholar]
- 20. Matthews A, Herrett E, Gasparrini A, Staa T, Van, Goldacre B, Smeeth L, Bhaskaran K.. Impact of statin related media coverage on use of statins: interrupted time series analysis with UK primary care data. BMJ 2016;i3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2016;37:2129–2200.27206819 [Google Scholar]
- 22. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJV, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WHW, Tsai EJ, Wilkoff BL; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62:e147–e239. [DOI] [PubMed] [Google Scholar]
- 23. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JAC; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim SY, Park JE, Lee YJ, Seo HJ, Sheen SS, Hahn S, Jang BH, Son HJ.. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J Clin Epidemiol 2013;66:408–414. [DOI] [PubMed] [Google Scholar]
- 26. Masoudi FA, Rathore SS, Wang Y, Havranek EP, Curtis JP, Foody JM, Krumholz HM.. National patterns of use and effectiveness of angiotensin-converting enzyme inhibitors in older patients with heart failure and left ventricular systolic dysfunction. Circulation 2004;110:724–731. [DOI] [PubMed] [Google Scholar]
- 27. Sanam K, Bhatia V, Bajaj NS, Gaba S, Morgan CJ, Fonarow GC, Butler J, Deedwania P, Prabhu SD, Wu WC, White M, Love TE, Aronow WS, Fletcher RD, Allman RM, Ahmed A.. Renin-angiotensin system inhibition and lower 30-day all-cause readmission in Medicare beneficiaries with heart failure. Am J Med 2016;129:1067–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu X, Yu H, Pei J, Chu J, Pu J, Zhang S.. Clinical characteristics and long-term prognosis in patients with chronic heart failure and reduced ejection fraction in China. Australian and New Zealand Society of Cardiac and Thoracic Surgeons (ANZSCTS) and the Cardiac Society of Australia and New Zealand (CSANZ). Hear Lung Circ 2014;23:818–826. [DOI] [PubMed] [Google Scholar]
- 29. Lund LH, Benson L, Dahlström U, Edner M.. Association between use of renin-angiotensin system antagonists and mortality in patients with heart failure and preserved ejection fraction. JAMA 2012;308:2108.. [DOI] [PubMed] [Google Scholar]
- 30. Ushigome R, Sakata Y, Nochioka K, Miyata S, Miura M, Tadaki S, Yamauchi T, Sato K, Onose T, Tsuji K, Abe R, Oikawa T, Kasahara S, Takahashi J, Shimokawa H.. Temporal trends in clinical characteristics, management and prognosis of patients with symptomatic heart failure in Japan. Report from the CHART studies. Circ J 2015;79:2396–2407. [DOI] [PubMed] [Google Scholar]
- 31. Gomez-Soto FM, Romero SP, Bernal JA, Escobar MA, Puerto JL, Andrey JL, Almenara J, Gomez F.. Mortality and morbidity of non-systolic heart failure treated with angiotensin-converting enzyme inhibitors: a propensity-adjusted case-control study. Int J Cardiol 2010;139:276–282. [DOI] [PubMed] [Google Scholar]
- 32. Shah R, Wang Y, Foody JM.. Effect of statins, angiotensin-converting enzyme inhibitors, and beta blockers on survival in patients ≥65 years of age with heart failure and preserved left ventricular systolic function. Am J Cardiol 2008;101:217–222. [DOI] [PubMed] [Google Scholar]
- 33. Tribouilloy C, Rusinaru D, Leborgne L, Peltier M, Massy Z, Slama M.. Prognostic impact of angiotensin-converting enzyme inhibitor therapy in diastolic heart failure. Am J Cardiol 2008;101:639–644. [DOI] [PubMed] [Google Scholar]
- 34. Grigorian Shamagian L, Roman AV, Ramos PM, Veloso PR, Bandin Dieguez MA, Gonzalez-Juanatey JR.. Angiotensin-converting enzyme inhibitors prescription is associated with longer survival among patients hospitalized for congestive heart failure who have preserved systolic function: a long-term follow-up study. J Card Fail 2006;12:128–133. [DOI] [PubMed] [Google Scholar]
- 35. Mujib M, Patel K, Fonarow GC, Kitzman DW, Zhang Y, Aban IB, Ekundayo OJ, Love TE, Kilgore ML, Allman RM, Gheorghiade M, Ahmed A.. Angiotensin-converting enzyme inhibitors and outcomes in heart failure and preserved ejection fraction. Am J Med 2013;126:401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dauterman KW, Go AS, Rowell R, Gebretsadik T, Gettner S, Massie BM.. Congestive heart failure with preserved systolic function in a statewide sample of community hospitals. J Card Fail 2001;7:221–228. [DOI] [PubMed] [Google Scholar]
- 37. Philbin EF, Rocco TA, Lindenmuth NW, Ulrich K, Jenkins PL.. Systolic versus diastolic heart failure in community practice: clinical features, outcomes, and the use of angiotensin-converting enzyme inhibitors. Am J Med 2000;109:605–613. [DOI] [PubMed] [Google Scholar]
- 38. Philbin EF, Rocco TA.. Use of angiotensin-converting enzyme inhibitors in heart failure with preserved left ventricular systolic function. Am Heart J 1997;134:188–195. [DOI] [PubMed] [Google Scholar]
- 39. Patel K, Fonarow GC, Kitzman DW, Aban IB, Love TE, Allman RM, Gheorghiade M, Ahmed A.. Angiotensin receptor blockers and outcomes in real-world older patients with heart failure and preserved ejection fraction: a propensity-matched inception cohort clinical effectiveness study. Eur J Heart Fail 2012;14:1179–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Keyhan G, Chen SF, Pilote L.. Angiotensin-converting enzyme inhibitors and survival in women and men with heart failure. Eur J Heart Fail 2007;9:594–601. [DOI] [PubMed] [Google Scholar]
- 41. Tandon P, McAlister FA, Tsuyuki RT, Hervas-Malo M, Dupuit R, Ezekowitz J, Cujec B, Armstrong PW.. The use of β-blockers in a tertiary care heart failure clinic. Arch Intern Med 2004;164:769.. [DOI] [PubMed] [Google Scholar]
- 42. Pedone C, Pahor M, Carosella L, Bernabei R, Carbonin P.. Use of angiotensin-converting enzyme inhibitors in elderly people with heart failure: prevalence and outcomes. J Gerontol A Biol Sci Med Sci 2004;59:716–721. [DOI] [PubMed] [Google Scholar]
- 43. Ahmed A, Maisiak R, Allman RM, DeLong JF, Farmer R.. Heart failure mortality among older Medicare beneficiaries: association with left ventricular function evaluation and angiotensin-converting enzyme inhibitor use. South Med J 2003;96:124–129. [DOI] [PubMed] [Google Scholar]
- 44. Sin DD, McAlister FA.. The effects of beta-blockers on morbidity and mortality in a population-based cohort of 11,942 elderly patients with heart failure. Am J Med 2002;113:650–656. [DOI] [PubMed] [Google Scholar]
- 45. Gastelurrutia P, Lupón J, de Antonio M, Urrutia A, Díez C, Coll R, Altimir S, Bayes-Genis A.. Statins in heart failure: the paradox between large randomized clinical trials and real life. Mayo Clin Proc 2012;87:555–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Teng TH, Hung J, Finn J.. The effect of evidence-based medication use on long-term survival in patients hospitalised for heart failure in Western Australia. Med J Aust 2010;192:306–310. [DOI] [PubMed] [Google Scholar]
- 47. Cadrin-Tourigny J, Shohoudi A, Roy D, Talajic M, Tadros R, Mondésert B, Dyrda K, Rivard L, Andrade JG, Macle L, Guerra PG, Thibault B, Dubuc M, Khairy P.. Decreased mortality with beta-blockers in patients with heart failure and coexisting atrial fibrillation: an AF-CHF substudy. JACC Hear Fail 2017;5:99–106. [DOI] [PubMed] [Google Scholar]
- 48. Bhatia V, Bajaj NS, Sanam K, Hashim T, Morgan CJ, Prabhu SD, Fonarow GC, Deedwania P, Butler J, Carson P, Love TE, Kheirbek R, Aronow WS, Anker SD, Waagstein F, Fletcher R, Allman RM, Ahmed A.. Beta-blocker use and 30-day all-cause readmission in Medicare beneficiaries with systolic heart failure. Am J Med 2015;128:715–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Del Carlo CH, Cardoso JN, Ochia ME, Oliveira MT Jr, Ramires JA, Pereira-Barretto AC, Temporal variation in the prognosis and treatment of advanced heart failure—before and after 2000. Arq Bras Cardiol 2014;495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lund LH, Benson L, Dahlström U, Edner M, Friberg L.. Association between use of β-blockers and outcomes in patients with heart failure and preserved ejection fraction. JAMA 2014;312:2008–2018. [DOI] [PubMed] [Google Scholar]
- 51. El-Refai M, Peterson EL, Wells K, Swadia T, Sabbah HN, Spertus JA, Williams LK, Lanfear DE.. Comparison of β-blocker effectiveness in heart failure patients with preserved ejection fraction versus those with reduced ejection fraction. J Card Fail 2013;19:73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Xu Y, Shi Y, Zhu Z, Cui C, Li B, Chen F, Li D, Chen S, Guo Y.. Prognosis of patients with heart failure and reduced ejection fraction in China. Exp Ther Med 2013;6:1437–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hernandez AF, Hammill BG, O'Connor CM, Schulman KA, Curtis LH, Fonarow GC.. Clinical effectiveness of beta-blockers in heart failure. Findings from the OPTIMIZE-HF (Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure) Registry. J Am Coll Cardiol 2009;53:184–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Miyagishima K, Hiramitsu S, Kimura H, Mori K, Ueda T, Kato S, Kato Y, Ishikawa S, Iwase M, Morimoto S, Hishida H, Ozaki Y.. Long term prognosis of chronic heart failure: reduced vs preserved left ventricular ejection fraction. Circ J 2009;73:92–99. [DOI] [PubMed] [Google Scholar]
- 55. Fauchier L, Grimard C, Pierre B, Nonin E, Gorin L, Rauzy B, Cosnay P, Babuty D, Charbonnier B.. Comparison of beta blocker and digoxin alone and in combination for management of patients with atrial fibrillation and heart failure. Am J Cardiol 2009;103:248–254. [DOI] [PubMed] [Google Scholar]
- 56. Pascual-Figal DA, Redondo B, Caro C, Manzano S, Garrido IP, Ruipérez JA, Valdés M.. Comparison of late mortality in hospitalized patients >70 years of age with systolic heart failure receiving beta blockers versus those not receiving beta blockers. Am J Cardiol 2008;102:1711–1717. [DOI] [PubMed] [Google Scholar]
- 57. Jost A, Rauch B, Hochadel M, Winkler R, Schneider S, Jacobs M, Kilkowski C, Kilkowski A, Lorenz H, Muth K, Zugck C, Remppis A, Haass M, Senges J.. Beta-blocker treatment of chronic systolic heart failure improves prognosis even in patients meeting one or more exclusion criteria of the MERIT-HF study. Eur Heart J 2005;26:2689–2697. [DOI] [PubMed] [Google Scholar]
- 58. Bobbio M, Ferrua S, Opasich C, Porcu M, Lucci D, Scherillo M, Tavazzi L, Maggioni AP.. Survival and hospitalization in heart failure patients with or without diabetes treated with β-blockers. J Card Fail 2003;9:192–202. [DOI] [PubMed] [Google Scholar]
- 59. Huan Loh P, Windram JD, Tin L, Reddy P, Velavan P, Rigby AS, Atkin P, Nikitin NP, Clark AL, Cleland JGF.. The effects of initiation or continuation of statin therapy on cholesterol level and all-cause mortality after the diagnosis of left ventricular systolic dysfunction. Am Heart J 2007;153:537–544. [DOI] [PubMed] [Google Scholar]
- 60. Ruiz G, Andrey JL, Puerto JL, Escobar MA, Romero SP, Aranda R, Pedrosa MJ, Gomez F.. Prognosis of heart failure with preserved ejection fraction treated with β-blockers: a propensity matched study in the community. Int J Cardiol 2016;222:594–602. [DOI] [PubMed] [Google Scholar]
- 61. Nevzorov R, Porath A, Henkin Y, Kobal SL, Jotkowitz A, Novack V.. Effect of beta blocker therapy on survival of patients with heart failure and preserved systolic function following hospitalization with acute decompensated heart failure. Eur J Intern Med 2012;23:374–378. [DOI] [PubMed] [Google Scholar]
- 62. Gomez-Soto FM, Romero SP, Bernal JA, Escobar MA, Puerto JL, Andrey JL, Almenara J, Gomez F.. Mortality and morbidity of newly diagnosed heart failure with preserved systolic function treated with β-blockers: a propensity-adjusted case-control populational study. Int J Cardiol 2011;146:51–55. [DOI] [PubMed] [Google Scholar]
- 63. Dobre D, van Veldhuisen DJ, DeJongste MJL, Lucas C, Cleuren G, Sanderman R, Ranchor AV, Haaijer-Ruskamp FM.. Prescription of beta-blockers in patients with advanced heart failure and preserved left ventricular ejection fraction. Clinical implications and survival. Eur J Heart Fail 2007;9:280–286. [DOI] [PubMed] [Google Scholar]
- 64. Patel K, Fonarow GC, Ekundayo OJ, Aban IB, Kilgore ML, Love TE, Kitzman DW, Gheorghiade M, Allman RM, Ahmed A.. Beta-blockers in older patients with heart failure and preserved ejection fraction: class, dosage, and outcomes. Int J Cardiol 2014;173:393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Katz A, Maor E, Leor J, Klempfner R.. Addition of beta-blockers to digoxin is associated with improved 1- and 10-year survival of patients hospitalized due to decompensated heart failure. Int J Cardiol 2016;221:198–204. [DOI] [PubMed] [Google Scholar]
- 66. Maison P, Desamericq G, Hemery F, Elie N, Del’Volgo A, Dubois-Randé JL, Hittinger L, Macquin-Mavier I.. Relationship between recommended chronic heart failure treatments and mortality over 8 years in real-world conditions: a pharmacoepidemiological study. Eur J Clin Pharmacol 2013;69:901–908. [DOI] [PubMed] [Google Scholar]
- 67. Marijon E, Trinquart L, Otmani A, Leclercq C, Fauchier L, Chevalier P, Klug D, Defaye P, Lellouche N, Mansourati J, Deharo JC, Sadoul N, Anselme F, Maury P, Davy JM, Extramiana F, Hidden-Lucet F, Probst V, Bordachar P, Mansour H, Chauvin M, Jouven X, Lavergne T, Chatellier G, Le Heuzey JY.. Predictors for short-term progressive heart failure death in New York Heart Association II patients implanted with a cardioverter defibrillator-the EVADEF study. Am Heart J 2010;159:659.. [DOI] [PubMed] [Google Scholar]
- 68. Jordán AJ, Anguita MP; BADAPIC Study Researchers. Effect of statin treatment on mortality in a large cohort of heart failure patients. Rev Esp Cardiol 2009;62:323–327. [DOI] [PubMed] [Google Scholar]
- 69. Dobre D, DeJongste MJL, Lucas C, Cleuren G, van Veldhuisen DJ, Ranchor AV, Haaijer-Ruskamp F.. Effectiveness of beta-blocker therapy in daily practice patients with advanced chronic heart failure; is there an effect-modification by age? Br J Clin Pharmacol 2007;63:356–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Keyhan G, Chen SF, Pilote L.. The effectiveness of β-blockers in women with congestive heart failure. J Gen Intern Med 2007;22:955–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Chan JD, Rea TD, Smith NL, Siscovick D, Heckbert SR, Lumley T, Chaves P, Furberg CD, Kuller L, Psaty BM.. Association of beta-blocker use with mortality among patients with congestive heart failure in the Cardiovascular Health Study (CHS). Am Heart J 2005;150:464–470. [DOI] [PubMed] [Google Scholar]
- 72. Maggioni AP, Sinagra G, Opasich C, Geraci E, Gorini M, Gronda E, Lucci D, Tognoni G, Balli E, Tavazzi L.. Treatment of chronic heart failure with beta adrenergic blockade beyond controlled clinical trials: the BRING-UP experience. Heart 2003;89:299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. McCullough PA, Philbin EF, Spertus JA, Sandberg KR, Kaatz S.. Angiotensin converting enzyme inhibitors and beta-blockers in African Americans with heart failure. Ethn Dis 2003;13:331–336. [PubMed] [Google Scholar]
- 74. McAlister FA, Teo KK, Taher M, Montague TJ, Humen D, Cheung L, Kiaii M, Yim R, Armstrong PW.. Insights into the contemporary epidemiology and outpatient management of congestive heart failure. Am Heart J 1999;138:87–94. [DOI] [PubMed] [Google Scholar]
- 75. Hamaguchi S, Kinugawa S, Tsuchihashi-Makaya M, Goto K, Goto D, Yokota T, Yamada S, Yokoshiki H, Takeshita A, Tsutsui H.. Spironolactone use at discharge was associated with improved survival in hospitalized patients with systolic heart failure. Am Heart J 2010;160:1156–1162. [DOI] [PubMed] [Google Scholar]
- 76. Lam PH, Dooley DJ, Inampudi C, Arundel C, Fonarow GC, Butler J, Wu W-C, Blackman MR, Anker MS, Deedwania P, White M, Prabhu SD, Morgan CJ, Love TE, Aronow WS, Allman RM, Ahmed A.. Lack of evidence of lower 30-day all-cause readmission in Medicare beneficiaries with heart failure and reduced ejection fraction discharged on spironolactone. Int J Cardiol 2017;227:462–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Frankenstein L, Katus HA, Grundtvig M, Hole T, de Blois J, Schellberg D, Atar D, Zugck C, Agewall S; Norwegian Heart Failure Registry Steering Committee. Association between spironolactone added to beta-blockers and ACE inhibition and survival in heart failure patients with reduced ejection fraction: a propensity score-matched cohort study. Eur J Clin Pharmacol 2013;69:1747–1755. [DOI] [PubMed] [Google Scholar]
- 78. Lee KK, Shilane D, Hlatky MA, Yang J, Steimle AE, Go AS.. Effectiveness and safety of spironolactone for systolic heart failure. Am J Cardiol 2013;112:1427–1432. [DOI] [PubMed] [Google Scholar]
- 79. Lund LH, Svennblad B, Melhus H, Hallberg P, Dahlström U, Edner M.. Association of spironolactone use with all-cause mortality in heart failure: a propensity scored cohort study. Circ Heart Fail 2013;6:174–183. [DOI] [PubMed] [Google Scholar]
- 80. Pascual-Figal DA, Caballero L, Bayes-Genis A, Gonzalez-Juanatey JR, Vazquez R, Bayes de Luna A, Cinca J; MUSIC Investigators. Spironolactone in mild chronic heart failure: insights from a propensity-matched analysis of the MUSIC study cohort. Int J Cardiol 2013;168:4525–4527. [DOI] [PubMed] [Google Scholar]
- 81. Hernandez AF, Mi X, Hammill BG, Hammill SC, Heidenreich PA, Masoudi FA, Qualls LG, Peterson ED, Fonarow GC, Curtis LH.. Associations between aldosterone antagonist therapy and risks of mortality and readmission among patients with heart failure and reduced ejection fraction. JAMA 2012;308:2097–2107. [DOI] [PubMed] [Google Scholar]
- 82. Ouzounian M, Hassan A, Cox JL, Johnstone DE, Howlett J; Improving Cardiovascular Outcomes in Nova Scotia Study Investigators. The effect of spironolactone use on heart failure mortality: a population-based study. J Card Fail 2007;13:165–169. [DOI] [PubMed] [Google Scholar]
- 83. O'Meara E, Khairy P, Blanchet MC, de Denus S, Pedersen OD, Levesque S, Talajic M, Ducharme A, White M, Racine N, Rouleau J-L, Tardif J-C, Roy D; AF-CHF investigators. Mineralocorticoid receptor antagonists and cardiovascular mortality in patients with atrial fibrillation and left ventricular dysfunction: insights from the Atrial Fibrillation and Congestive Heart Failure trial. Circ Heart Fail 2012;5:586–593. [DOI] [PubMed] [Google Scholar]
- 84. Bonsu KO, Owusu IK, Buabeng KO, Reidpath DD, Kadirvelu A.. Clinical characteristics and prognosis of patients admitted for heart failure: a 5-year retrospective study of African patients. Int J Cardiol 2017;238:128–135. [DOI] [PubMed] [Google Scholar]
- 85. Patel K, Fonarow GC, Kitzman DW, Aban IB, Love TE, Allman RM, Gheorghiade M, Ahmed A.. Aldosterone antagonists and outcomes in real-world older patients with heart failure and preserved ejection fraction. JACC Hear Fail 2013;1:40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Sligl W, McAlister FA, Ezekowitz J, Armstrong PW.. Usefulness of spironolactone in a specialized heart failure clinic. Am J Cardiol 2004;94:443–447. [DOI] [PubMed] [Google Scholar]
- 87. Alehagen U, Benson L, Edner M, Dahlström U, Lund LH.. Association between use of statins and outcomes in heart failure with reduced ejection fraction: prospective propensity score matched cohort study of 21,864 patients in the Swedish Heart Failure Registry. Circ Heart Fail 2015;8:252–260. [DOI] [PubMed] [Google Scholar]
- 88. Gomez-Soto FM, Romero SP, Bernal JA, Escobar MA, Puerto JL, Andrey JL, Ruiz P, Gomez F.. Mortality and morbidity of newly diagnosed heart failure treated with statins: a propensity-adjusted cohort study. Int J Cardiol 2010;140:210–218. [DOI] [PubMed] [Google Scholar]
- 89. Sumner AD, Boehmer JP, Saxon LA, Carson P, Feldman AM, Galle E, Bristow MR.. Statin use is associated with improved survival in patients with advanced heart failure receiving resynchronization therapy. Congest Heart Fail 2009;15:159–164. [DOI] [PubMed] [Google Scholar]
- 90. Coleman CI, Kluger J, Bhavnani S, Clyne C, Yarlagadda R, Guertin D, White CM.. Association between statin use and mortality in patients with implantable cardioverter-defibrillators and left ventricular systolic dysfunction. Heart Rhythm 2008;5:507–510. [DOI] [PubMed] [Google Scholar]
- 91. Dickinson MG, Ip JH, Olshansky B, Hellkamp AS, Anderson J, Poole JE, Mark DB, Lee KL, Bardy GH; SCD-HeFT Investigators. Statin use was associated with reduced mortality in both ischemic and nonischemic cardiomyopathy and in patients with implantable defibrillators: mortality data and mechanistic insights from the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT). Am Heart J 2007;153:573–578. [DOI] [PubMed] [Google Scholar]
- 92. Krum H, Bailey M, Meyer W, Verkenne P, Dargie H, Lechat P, Anker S.. Impact of statin therapy on clinical outcomes in chronic heart failure patients according to beta-blocker use: results of CIBIS II. Cardiology 2007;108:28–34. [DOI] [PubMed] [Google Scholar]
- 93. Krum H, Latini R, Maggioni AP, Anand I, Masson S, Carretta E, Ingrillì F, Pettinati G, Glazer R, Tognoni G, Cohn J.. Statins and symptomatic chronic systolic heart failure: a post-hoc analysis of 5010 patients enrolled in Val-HeFT. Int J Cardiol 2007;119:48–53. [DOI] [PubMed] [Google Scholar]
- 94. Anker SD, Clark AL, Winkler R, Zugck C, Cicoira M, Ponikowski P, Davos CH, Banasiak W, Zardini P, Haass M, Senges J, Coats AJS, Poole-Wilson PA, Pitt B.. Statin use and survival in patients with chronic heart failure–results from two observational studies with 5200 patients. Int J Cardiol 2006;112:234–242. [DOI] [PubMed] [Google Scholar]
- 95. Goldberger JJ, Subacius H, Schaechter A, Howard A, Berger R, Shalaby A, Levine J, Kadish AH; DEFINITE Investigators. Effects of statin therapy on arrhythmic events and survival in patients with nonischemic dilated cardiomyopathy. J Am Coll Cardiol 2006;48:1228–1233. [DOI] [PubMed] [Google Scholar]
- 96. Ray JG, Gong Y, Sykora K, Tu JV.. Statin use and survival outcomes in elderly patients with heart failure. Arch Intern Med 2005;165:62–67. [DOI] [PubMed] [Google Scholar]
- 97. Mozaffarian D, Nye R, Levy WC.. Statin therapy is associated with lower mortality among patients with severe heart failure. Am J Cardiol 2004;93:1124–1129. [DOI] [PubMed] [Google Scholar]
- 98. Ouzounian M, Tu JV, Austin PC, Chong A, Liu PP, Lee DS.. Statin therapy and clinical outcomes in heart failure: a propensity-matched analysis. J Card Fail 2009;15:241–248. [DOI] [PubMed] [Google Scholar]
- 99. Bonsu KO, Owusu IK, Buabeng KO, Reidpath DD, Kadirvelu A.. Statin treatment and clinical outcomes of heart failure among Africans: an inverse probability treatment weighted analysis. J Am Heart Assoc 2017;6:e004706.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ballo P, Balzi D, Barchielli A, Turco L, Franconi F, Zuppiroli A.. Gender differences in statin prescription rates, adequacy of dosing, and association of statin therapy with outcome after heart failure hospitalization: a retrospective analysis in a community setting. Eur J Clin Pharmacol 2016;72:311–319. [DOI] [PubMed] [Google Scholar]
- 101. Nevzorov R, Novack V, Henkin Y, Kobal SL, Jotkowitz A, Porath A.. Discrepancy between results of randomized control studies and retrospective analysis: the case of statin therapy effect on one-year mortality in patients with decompensated heart failure. Eur J Intern Med 2009;20:494–498. [DOI] [PubMed] [Google Scholar]
- 102. Ryan RP, McManus RJ, Mant J, Macleod JAA, Hobbs FDR.. Statins in heart failure: retrospective cohort study using routine primary care data. Ann Med 2009;41:490–496. [DOI] [PubMed] [Google Scholar]
- 103. Foody JM, Shah R, Galusha D, Masoudi FA, Havranek EP, Krumholz HM.. Statins and mortality among elderly patients hospitalized with heart failure. Circulation 2006;113:1086–1092. [DOI] [PubMed] [Google Scholar]
- 104. Go AS, Lee WY, Yang J, Lo JC, Gurwitz JH.. Statin therapy and risks for death and hospitalization in chronic heart failure. JAMA 2006;296:2105–2111. [DOI] [PubMed] [Google Scholar]
- 105. Andrey JL, Romero S, García-Egido A, Escobar MA, Corzo R, Garcia-Dominguez G, Lechuga V, Gómez F.. Mortality and morbidity of heart failure treated with digoxin. A propensity-matched study. Int J Clin Pract 2011;65:1250–1258. [DOI] [PubMed] [Google Scholar]
- 106. Dhaliwal AS, Bredikis A, Habib G, Carabello BA, Ramasubbu K, Bozkurt B.. Digoxin and clinical outcomes in systolic heart failure patients on contemporary background heart failure therapy. Am J Cardiol 2008;102:1356–1360. [DOI] [PubMed] [Google Scholar]
- 107. Al-Khateeb M, Qureshi WT, Odeh R, Ahmed AM, Sakr S, Elshawi R, Bdeir MB, Al-Mallah MH.. The impact of digoxin on mortality in patients with chronic systolic heart failure: a propensity-matched cohort study. Int J Cardiol 2017;228:214–218. [DOI] [PubMed] [Google Scholar]
- 108. Freeman JV, Yang J, Sung SH, Hlatky MA, Go AS.. Effectiveness and safety of digoxin among contemporary adults with incident systolic heart failure. Circ Cardiovasc Qual Outcomes 2013;6:525–533. [DOI] [PubMed] [Google Scholar]
- 109. Butler J, Anand IS, Kuskowski MA, Rector T, Carson P, Cohn JN; Val-HeFT Investigators. Digoxin use and heart failure outcomes: results from the Valsartan Heart Failure Trial (Val-HeFT). Congest Heart Fail 2010;16:191–195. [DOI] [PubMed] [Google Scholar]
- 110. Domanski M, Fleg J, Bristow M, Knox S.. The effect of gender on outcome in digitalis-treated heart failure patients. J Card Fail 2005;11:83–86. [DOI] [PubMed] [Google Scholar]
- 111. Ahmed A, Bourge RC, Fonarow GC, Patel K, Morgan CJ, Fleg JL, Aban IB, Love TE, Yancy CW, Deedwania P, van Veldhuisen DJ, Filippatos GS, Anker SD, Allman RM.. Digoxin use and lower 30-day all-cause readmission for Medicare beneficiaries hospitalized for heart failure. Am J Med 2014;127:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Flory JH, Ky B, Haynes K, M Brunelli S, Munson J, Rowan C, Strom BL, Hennessy S.. Observational cohort study of the safety of digoxin use in women with heart failure. BMJ Open 2012;2:e000888.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Hallberg P, Lindbäck J, Lindahl B, Stenestrand U, Melhus H; RIKS-HIA Group. Digoxin and mortality in atrial fibrillation: a prospective cohort study. Eur J Clin Pharmacol 2007;63:959–971. [DOI] [PubMed] [Google Scholar]
- 114. Eisen A, Ruff CT, Braunwald E, Hamershock RA, Lewis BS, Hassager C, Chao T, Le Heuzey JY, Mercuri M, Rutman H, Antman EM, Giugliano RP.. Digoxin use and subsequent clinical outcomes in patients with atrial fibrillation with or without heart failure in the ENGAGE AF‐TIMI 48 trial. J Am Heart Assoc 2017;6:e006035.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Madelaire C, Schou M, Nelveg-Kristensen KE, Schmiegelow M, Torp-Pedersen C, Gustafsson F, Køber L, Gislason G.. Use of digoxin and risk of death or readmission for heart failure and sinus rhythm: a nationwide propensity score matched study. Int J Cardiol 2016;221:944–950. [DOI] [PubMed] [Google Scholar]
- 116. Shah M, Avgil Tsadok M, Jackevicius CA, Essebag V, Behlouli H, Pilote L.. Relation of digoxin use in atrial fibrillation and the risk of all-cause mortality in patients ≥65 years of age with versus without heart failure. Am J Cardiol 2014;114:401–406. [DOI] [PubMed] [Google Scholar]
- 117. Whitbeck MG, Charnigo RJ, Khairy P, Ziada K, Bailey AL, Zegarra MM, Shah J, Morales G, Macaulay T, Sorrell VL, Campbell CL, Gurley J, Anaya P, Nasr H, Bai R, Di Biase L, Booth DC, Jondeau G, Natale A, Roy D, Smyth S, Moliterno DJ, Elayi CS.. Increased mortality among patients taking digoxin–analysis from the AFFIRM study. Eur Heart J 2013;34:1481–1488. [DOI] [PubMed] [Google Scholar]
- 118. SOLVD Investigators, Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN.. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991;325:293–302. [DOI] [PubMed] [Google Scholar]
- 119. Jong P, Yusuf S, Rousseau MF, Ahn SA, Bangdiwala SI.. Effect of enalapril on 12-year survival and life expectancy in patients with left ventricular systolic dysfunction: a follow-up study. Lancet 2003;361:1843–1848. [DOI] [PubMed] [Google Scholar]
- 120. SOLVD Investigators, Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN.. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med 1992;327:685–691. [DOI] [PubMed] [Google Scholar]
- 121. Granger CB, McMurray JJV, Yusuf S, Held P, Michelson EL, Olofsson B, Ostergren J, Pfeffer MA, Swedberg K; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced eft-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet 2003;362:772–776. [DOI] [PubMed] [Google Scholar]
- 122. Cleland JGF, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J; PEP-CHF Investigators. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J 2006;27:2338–2345. [DOI] [PubMed] [Google Scholar]
- 123. Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A; I-PRESERVE Investigators. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med 2008;359:2456–2467. [DOI] [PubMed] [Google Scholar]
- 124. Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJV, Michelson EL, Olofsson B, Ostergren J; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet 2003;362:777–781. [DOI] [PubMed] [Google Scholar]
- 125. Cohn JN, Johnson G, Ziesche S, Cobb F, Francis G, Tristani F, Smith R, Dunkman WB, Loeb H, Wong M, Bhat G, Goldman S, Fletcher RD, Doherty J, Hughes CV, Carson P, Cintron G, Shabetai R, Haakenson C.. A comparison of enalapril with hydralazine–isosorbide dinitrate in the treatment of chronic congestive heart failure. N Engl J Med 1991;325:303–310. [DOI] [PubMed] [Google Scholar]
- 126. CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. N Engl J Med 1987;316:1429–1435. [DOI] [PubMed] [Google Scholar]
- 127. Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJV, Michelson EL, Olofsson B, Ostergren J, Yusuf S, Pocock S; CHARM Investigators and Committees. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet 2003;362:759–766. [DOI] [PubMed] [Google Scholar]
- 128. Packer M, Coats AJS, Fowler MB, Katus HA, Krum H, Mohacsi P, Rouleau JL, Tendera M, Castaigne A, Roecker EB, Schultz MK, Staiger C, Curtin EL, DeMets DL.. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 2001;344:1651–1658. [DOI] [PubMed] [Google Scholar]
- 129. MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999;353:2001–2007. [PubMed] [Google Scholar]
- 130. Dargie HJ, Lechat P.. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet 1999;353:9–13. [PubMed] [Google Scholar]
- 131. Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, Shusterman NH.. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. N Engl J Med 1996;334:1349–1355. [DOI] [PubMed] [Google Scholar]
- 132. van Veldhuisen DJ, Cohen-Solal A, Böhm M, Anker SD, Babalis D, Roughton M, Coats AJS, Poole-Wilson PA, Flather MD; SENIORS Investigators. Beta-blockade with nebivolol in elderly heart failure patients with impaired and preserved left ventricular ejection fraction: data from SENIORS (Study of Effects of Nebivolol Intervention on Outcomes and Rehospitalization in Seniors With Heart Failure). J Am Coll Cardiol 2009;53:2150–2158. [DOI] [PubMed] [Google Scholar]
- 133. Beta-Blocker Evaluation of Survival Trial Investigators, Eichhorn EJ, Domanski MJ, Krause-Steinrauf H, Bristow MR, Lavori PW.. A trial of the beta-blocker bucindolol in patients with advanced chronic heart failure. N Engl J Med 2001;344:1659–1667. [DOI] [PubMed] [Google Scholar]
- 134. Australia/New Zealand Heart Failure Research Collaborative Group. A randomised, placebo-controlled trial of carvedilol in patients with congestive heart failure due to ischaemic heart disease. Lancet 1997;349:375–380. [PubMed] [Google Scholar]
- 135. CIBIS Investigators and Committees. A randomized trial of beta-blockade in heart failure. The Cardiac Insufficiency Bisoprolol Study (CIBIS). Circulation 1994;90:1765–1773. [DOI] [PubMed] [Google Scholar]
- 136. Yamamoto K, Origasa H, Hori M; J-DHF Investigators. Effects of carvedilol on heart failure with preserved ejection fraction: the Japanese Diastolic Heart Failure study (J-DHF). Eur J Heart Fail 2013;15:110–118. [DOI] [PubMed] [Google Scholar]
- 137. Flather MD, Shibata MC, Coats AJS, Van Veldhuisen DJ, Parkhomenko A, Borbola J, Cohen-Solal A, Dumitrascu D, Ferrari R, Lechat P, Soler-Soler J, Tavazzi L, Spinarova L, Toman J, BöHm M, Anker SD, Thompson SG, Poole-Wilson PA; SENIORS Investigators. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur Heart J 2005;26:215–225. [DOI] [PubMed] [Google Scholar]
- 138. Zannad F, McMurray JJV, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011;364:11–21.21073363 [Google Scholar]
- 139. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J.. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999;341:709–717. [DOI] [PubMed] [Google Scholar]
- 140. Pfeffer MA, Claggett B, Assmann SF, Boineau R, Anand IS, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Heitner JF, Lewis EF, O'Meara E, Rouleau J-L, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, McKinlay SM, Pitt B.. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation 2015;131:34–42. [DOI] [PubMed] [Google Scholar]
- 141. Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O'Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM; TOPCAT Investigators. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014;370:1383–1392. [DOI] [PubMed] [Google Scholar]
- 142. Kjekshus J, Apetrei E, Barrios V, Böhm M, Cleland JGF, Cornel JH, Dunselman P, Fonseca C, Goudev A, Grande P, Gullestad L, Hjalmarson A, Hradec J, Jánosi A, Kamenský G, Komajda M, Korewicki J, Kuusi T, Mach F, Mareev V, McMurray JJV, Ranjith N, Schaufelberger M, Vanhaecke J, van Veldhuisen DJ, Waagstein F, Wedel H, Wikstrand J; CORONA Group. Rosuvastatin in older patients with systolic heart failure. N Engl J Med 2007;357:2248–2261. [DOI] [PubMed] [Google Scholar]
- 143. Takano H, Mizuma H, Kuwabara Y, Sato Y, Shindo S, Kotooka N, Fujimatsu D, Kobayashi Y, Inoue T, Node K, Komuro I; PEARL Study Investigators. Effects of pitavastatin in Japanese patients with chronic heart failure: the Pitavastatin Heart Failure study (PEARL study). Circ J 2013;77:917–925. [DOI] [PubMed] [Google Scholar]
- 144. Tavazzi L, Maggioni AP, Marchioli R, Barlera S, Franzosi MG, Latini R, Lucci D, Nicolosi GL, Porcu M, Tognoni G; GISSI-HF Investigators. Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet 2008;372:1231–1239. [DOI] [PubMed] [Google Scholar]
- 145. Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med 1997;336:525–533. [DOI] [PubMed] [Google Scholar]
- 146. Ahmed A, Rich MW, Fleg JL, Zile MR, Young JB, Kitzman DW, Love TE, Aronow WS, Adams KF, Gheorghiade M.. Effects of digoxin on morbidity and mortality in diastolic heart failure: the ancillary Digitalis Investigation Group trial. Circulation 2006;114:397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Rich MW, McSherry F, Williford WO, Yusuf S; Digitalis Investigation Group. Effect of age on mortality, hospitalizations and response to digoxin in patients with heart failure: the DIG study. J Am Coll Cardiol 2001;38:806–813. [DOI] [PubMed] [Google Scholar]
- 148. Bavendiek U, Aguirre Davila L, Koch A, Bauersachs J.. Assumption versus evidence: the case of digoxin in atrial fibrillation and heart failure. Eur Heart J 2017;38:2095–2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Fröbert O, Lagerqvist B, Olivecrona GK, Omerovic E, Gudnason T, Maeng M, Aasa M, Angerås O, Calais F, Danielewicz M, Erlinge D, Hellsten L, Jensen U, Johansson AC, Kåregren A, Nilsson J, Robertson L, Sandhall L, Sjögren I, Östlund O, Harnek J, James SK; TASTE Trial. Thrombus aspiration during ST-segment elevation myocardial infarction. N Engl J Med 2013;369:1587–1597. [DOI] [PubMed] [Google Scholar]
- 150. Lund LH, Oldgren J, James S.. Registry-based pragmatic trials in heart failure: current experience and future directions. Curr Heart Fail Rep 2017;14:59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. de Denus S, O'Meara E, Desai AS, Claggett B, Lewis EF, Leclair G, Jutras M, Lavoie J, Solomon SD, Pitt B, Pfeffer MA, Rouleau JL.. Spironolactone metabolites in TOPCAT - new insights into regional variation. N Engl J Med 2017;376:1690–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.