Abstract
Anaemia is a global public health problem affecting both developing and developed countries with major consequences for human health as well as social and economic development. It occurs at all stages of the life cycle, but is more prevalent in pregnant women and young children. Iron deficiency anaemia (IDA) was considered to be among the most important contributing factors to the global burden of disease. Prolonged and/or chronic anemia has a negative effect on linear growth especially during the rapid phases (infancy and puberty). Additionally infants with chronic IDA have delayed cognitive, motor, and affective development that may be long-lasting. In view of the significant impact of chronic anemias on growth, pediatricians endocrinologists and hematologists should advocate primary prevention and screening for growth disturbance in these forms of anemias. The extent of the negative effect of different forms of chronic anemias on linear growth and its possible reversibilty is addressed in this review. The possible mechanisms that may impair growth in the different forms of anemias are addressed with special attention to their effect on the growth hormone (GH) – insulin like growth factor -I (IGF-I). (www.actabiomedica.it)
Keywords: iron, iron deficiency anemia (IDA); Thalassemia; sickle cell disease; growth; height (Ht); height standard deviation score (HtSDS); growth hormone (GH); insulin-like growth factor-I (IGF-I)
Introduction
Anaemia is a condition in which the number of red blood cells (and consequently their oxygen-carrying capacity) is insufficient to meet the body’s physiologic needs. Anemia is defined as a hemoglobin level of less than the 5th percentile for age (<11 g/dL in children aged 6-59 months, <11.5 g/dl in children aged 5-11 years and 12 g/dl in older children (aged 1214). Severe anaemia is defined as blood haemoglobin concentration <7 g/dL for children (1-3).
Children (6-59 months) for 2011 showed a high global prevalence of anemia (42%). Prevalence included African region (32%), regions of the Americas (56%), South east Asia (41%), European region (54%), Eastern Mediterranean region (38%) and Western Pacific region (64%) (4).
Anemia may result from a number of causes. Approximately 50% of cases or more are due to iron deficiency (ID). However, the prevalence of iron deficiency anemia (IDA) varies among population groups and in different areas of the world (5-8). Other causes of anemia include: micronutrient deficiencies, acute and chronic infections, and inherited or acquired disorders that affect hemoglobin synthesis, red blood cell production or red blood cell survival (e.g. hemoglobinopathies).
Approximately 5% of the worldwide population has a variation in the a or p chain of the hemoglobin molecule, although not all of these are symptomatic and some are known as silent carriers. Sickle cell disease (SCD) is more common in people of Central African origin while β-thalassaemias are more common in Mediterranean, Middle Eastern and Southeast Asian populations (9, 10). Only 1.7% of the global population has signs as a result of the gene mutations, known as a thalassemia trait. However, particular ethnic groups are more likely to be affected and 5-30% of the population may be symptomatic among these groups (11-14).
ID and IDA are associated with many acute and chronic complications because iron is essential for all tissues of the developing body. In infants and young children, severe chronic anemia may lead to delayed growth and long term effects on neurodevelopment and behavior. The pathogenesis of these changes includes negative effect on neurotransmitter myelination and monoamine metabolism in striatum and the hippocampus and impaired energy metabolism.
Anemia with iron deficiency versus anemia with iron excess
Iron performs vital functions including carrying of oxygen from lung to tissues, transport of electrons within cells, acting as co-factor for essential enzymatic reactions, including synthesis of steroid hormones and neurotransmission. Mitochondria supply cells with adenosine triphosphate, heme, and iron-sulfur clusters (ISC). Mitochondrial energy metabolism involves both heme-and ISC-dependent enzymes. Mitochondrial iron supply and function require iron regulatory proteins that control messenger RNA translation and stability and iron is positively correlated with mitochondrial oxidative capacity. Ferritin is the stored form of iron used by the cells, and is a better measure of available iron levels than serum iron. Iron deficiency and IDA can negatively affect these functions. (15-17).
On the other hand excess iron accumulation causes organ dysfunction through the production of reactive oxygen species. As there is no passive excretory mechanism of iron, iron is easily accumulated when exogenous iron is loaded by hereditary factors, repeated transfusions, and other diseased conditions. The free irons, non-transferrin-bound iron, and labile plasma iron in the circulation, and the labile iron pool within the cells, are responsible for iron toxicity. There is a sophisticated balance of body iron metabolism of storage and transport, which is regulated by several factors including the peptide hepcidin. The characteristic features of advanced iron overload are failure of vital organs such as liver and heart in addition to endocrine dysfunctions (18).
Effect of antenatal and infant anemia on growth
Early ID appears to have specific effects on the central nervous system. In the rat, a brief period of ID during the brain growth spurt (10-28 days) causes a lasting deficit in brain iron, which persists into adulthood despite correction of the anemia. ID alters neurotransmitter function in the brains of ID rats. The activity of monoamine oxidase and aldehyde oxidase, enzymes and the functional activity of dopamine Dd2 receptors are reversibly diminished. Many dopaminemediated behaviors are modified (19-21). Pregnant rats on Fe restricted diet produced litters with a significant reduction in the physical growth indexes (body weight, body length, tail length, and head length) compared with the control group. These results suggest that adequate Fe is essential during both intrauterine and neonatal life (22).
Transfer of iron from the mother to the fetus is supported by a substantial increase in maternal iron absorption during pregnancy and is regulated by the placenta. Most iron transfer to the fetus occurs after week 30 of gestation, which corresponds to the time of peak efficiency of maternal iron absorption. A placental iron transfer system regulates iron transport to the fetus. When maternal iron status is poor, the number of placental transferrin receptors increases so that more iron is taken up by the placenta. Excessive iron transport to the fetus may be prevented by the placental synthesis of ferritin. Evidence is accumulating that the capacity of this system may be inadequate to maintain iron transfer to the fetus when the mother is iron deficient (23).
In human studies, the incidence of low birth weight babies is significantly more in mothers who are anemic in their third trimester. Preterm deliveries occur more frequently in mothers who are anemic in their second and third trimesters. In one study, a doubling of low birth weight rate and 2 to 3 fold increase in the perinatal mortality rates is seen when the maternal Hb is <8 g/dL. In another study, a U-shape relationship was found between Hb concentration and the risk of preterm delivery and low birth weight. Severe anemia and high hemoglobin concentration were both associated with increased risk of preterm deliveries and low birth weight (24-30).
In a multivariate regression analysis of data from 691 women in rural Nepal, the odds for low birth weight were increased across the range of anemia increasing with lower hemoglobin in an approximately dose-related manner (31). On the other hand a Brazilian study assessed the association between iron status at birth and growth of 95 preterm infants. At birth no association was found between markers of iron status and gestational age, weight, and length. Both crude and adjusted analyses showed no effect of iron status on infantile postnatal growth (32).
Effect of anemia and iron supplementation on growth of children with and without IDA
Few controlled studies have investigated the effect of IDA, and the effect of treatment with iron, on growth in children. One study showed that treatment of children with IDA with oral iron for 2 months resulted in a considerably greater increase in weight velocity compared to the placebo group (33). Other studies supported these observations, and also suggested that the correction of anemia is associated with a reduction in the increased morbidity (fever, respiratory tract infections, diarrhea) frequently seen in children with IDA (34, 35).
Bhatia et al. assessed the growth status of 117 anemic (Hb 7-10 g/dl) and 53 normal (11 g/dl) children (3-5 years). The anemic children had significantly lower body weight, height and weight for age (36). Soliman et al. measured growth and parameters in 40 children (aged 17.2±12.4 months) with IDA before and for 6 months after iron therapy in comparison with normal controls. Before treatment children with IDA were significantly shorter and had slower growth velocity (GV) compared with age-matched controls. After treatment, their GV, length standard deviation scores (LSDS) and body mass index (BMI) increased significantly (significant catch-up of growth). Their GV was correlated significantly with mean Hb concentration (36).
In school children Bandhu et al. found that those with IDA had significantly lower mid-arm circumference (MAC) (boys and girls), head circumference (HC) (g irls) and Ht (boys), when compared to the normal group. Iron supplementation improved their hematological parameters which was associated with significant improvement of Ht, Wt and MAC. Growth of anemic children supplemented with iron was superior to that of anemic placebo-treated children as indicated by a better weight gain and a significantly higher weight for height (38).
In summary, IDA in children, especially during the infancy and early childhood can significantly impairs growth that can be corrected by adequate iron therapy.
The effect of blind iron supplementation on linear growth in infants and children
A systematic review analyzed 25 randomized controlled trials (RCTs) that evaluated the effect of iron supplementation on physical growth in children (interventions included oral or parenteral iron supplementation, or iron-fortified formula milk or cereals). The pooled estimates did not document a statistically significant positive effect of iron supplementation on any anthropometric variable including weight [Wt]-for-age, Wt-for-height [Ht],Ht-for-age, mid-arm circumference [MAC] and skinfold thickness. However, greater Wt-for-age in supplemented children in malaria hyper-endemic regions and greater Wt-for-Ht for children above 5 years of age were noted. On the other hand a negative effect on linear growth was observed in developed countries and with supplementation for 6 months or longer (39).
A meta-analysis of 21 RCTs examining iron (supplementation) interventions in children and adolescents aged <18 years found that the iron-supplementation had no significant effect on growth (40). Another meta-analysis studied the effect of iron intervention using iron-fortified foods, iron-fortified formula, or iron supplements on growth (Ht, Wt, MAC) during gestation, infancy, childhood, and adolescence. The overall pooled result showed no significant effects of iron intervention on any of the parameters measured. When results were stratified according to dose of iron, duration of intervention, age, and baseline iron status, only doses of 40-66 mg of supplemental iron and intervention in children ≥6 years of age showed a slight but significant association with weight and MAC (41).
A multicentre pragmatic controlled trial studied the effect of providing multiple micronutrients (MNP) in powder through primary healthcare on anemia and growth of young Brazilian Children (n=512). MNP effectively reduced anemia and improved growth and micronutrient status among this cohort of Brazilian children (42). In addition, iron supplementation appeared to have a positive effect on the physical performance of children, as evaluated through the post exercise heart rate in anemic subjects, blood lactate levels and treadmill endurance time. Blood lactate levels were significantly lower (p<0.05) in iron supplemented group in comparison to placebo both before and after exercise. Treadmill endurance time was significantly better in iron supplemented group when compared with placebo in one study (43).
Effect of iron deficiency anemia and iron treatment on growth hormone-insulin-like growth factor-I axis
Endocrine paths have been proposed to explain the effect of IDA on growth. Anemia imposes a hypoxic condition on hepatocytes which inhibits protein synthesis. In vitro, low oxygen conditions inhibit insulin-like growth factor-I (IGF-I) action by increasing IGF binding protein -1 (IGFBP-1), especially phosphorylated IGFBP-1, which inhibits IGF-I action. Moreover, hypoxia inhibits the IGF-I-induced cell proliferation (36, 44-47). In-vitro, hypoxic conditions lead to increasing hypoxia induced factor (HIF-1a) availability which decreases hGH RNA levels and is accompanied by recruitment of HIF-1a to the hGH1 promoter in situ (48).
Transferrin (Tf) is the major circulating iron binding protein. In addition to its function as the Fe3+-carrier protein in serum, it has a unique ability to bind IGFs and to interact with IGFBP-3. Tf can abolish IGFBP-3-induced cell proliferation and apoptosis in different cell lines. On the other hand, the Fe3±Tf complex might facilitate the transport of IGFs across the capillary wall by receptor-mediated transcytosis. Therefore, increased Tf during IDA may adversely affect the integrity of IGF-I system (47).
Effect of anemia on GH-IGF-I axis (Animal studies)
Calves with IDA were found to have low plasma IGF-I concentrations. After recombinant growth hormone (GH) administration, increments in IGF-I in IDA calves were reduced despite high plasma GH levels. This suggested decreased sensitivity (partial resistance) to GH during anemia (49, 50).
In Wistar rats, dietary ID decreased hematocrit and Hb concentrations, IGF-I, 1,25-dihydroxycholecalciferol, and osteocalcin concentrations and bone mineral density of the femur and vertebrae compared with control rats. Bone histomorphometric parameters showed that the bone formation rate and osteoclast surface in the lumbar vertebra were significantly reduced in the ID group compared with the control group (50-53).
Gestational ID in rats attenuates postnatal hippocampal IGF signaling and results in markedly suppressed hippocampal IGF activation and protein kinase B signaling. Early postnatal iron treatment of gestational ID reactivates the IGF system and promotes neurogenesis and differentiation in the hippocampus (54, 55).
Effect of anemia on GH-IGF-I axis in children, adolescents and adults
Isguven et al. studied 25 prepubertal children with IDA and 25 healthy controls. IGF-1, ghrelin, and insulin levels were significantly lower in the ID group (56). They suggested that low ghrelin and insulin levels might be the cause of the appetite loss in IDA. In addition, low Ghrelin (a GH secretagogue) may decrease GH and subsequently IGF-I secretion and related growth delay both to low IGF-1 secretion and appetite loss (56).
In 40 infants and young children with IDA (Hb = 8.2±1.2 g/dL) treated for 6 months with iron therapy, circulating IGF-I increased significantly, along with acceleration of GV and increased length SDS and BMI (56).
In adolescents, Choi and Kim reported significant correlation between Hb concentration and serum iron on the one hand and IGF-I concentration on the other hand (57).
In a large cohort (n= 1,093) of adults the association of IGF-I with Hb concentration was studied. Anemic adults exhibited significantly lower IGF-I compared with non-anemic controls (58, 59).
Effect of thalassemia on growth and growth hormone-insulin-like growth factor-I axis
Thalassemia and growth are linked by different, multifactorial mechanisms. Growth retardation occurs almost invariably in homozygous p-thalassemia. Significant size retardation is observed in stature, sitting height, weight, biacromial (shoulder), and bicristal (iliac crest) breadths. After the age of 4 years, the longitudinal growth patterns display rates consistently behind those of normal controls. Growth retardation becomes markedly severe with the failure of the pubertal growth spurt. With the introduction of high transfusion regimes and efficient iron chelation in thalassemia management, prepubertal linear growth has improved markedly. However, abnormal growth is still observed in the majority of patients during late childhood and adolescence (46). Hemosiderosis (secondary to repeated packed cell transfusion) induced damage of the endocrine glands (pituitary, thyroid, gonads, and pancreas), liver, and growth plate. All these factors appear to contribute to slow growth in these children and adolescents (60-62).
Many studies done on children with thalassemia have shown a variable prevalence of defective GH secretion in response to different stimuli (clonidine, glucagon, Insulin hypoglycemia, and GH-releasing hormone). Some of the short thalassemic children with normal GH secretion, have neurosecretory dysfunction of GH secretion. In addition, IGF-I concentrations have been shown to be low in the majority of children and adults with thalassemia, with or without GH deficiency (63-67). However, other important factors also contribute to this growth delay including repeated lowering of Hb (anemic hypoxia), hepatic siderosis and toxicity of chelation therapy (68, 69).
One-day-IGF-1 generation tests have shown lower IGF-I generation in thalassemic children compared with normal short children and those with GHD. Defective GH secretion and hepatic siderosis are major causes of low IGF-I secretion (67, 69). Acute correction of anemia, by packed cell transfusion, significantly increases the serum concentration of IGF-I but does not affect GH secretion or IGF-I in response to GH stimulation. Some acceleration of linear growth can be achieved by GH therapy; however this growth response appears inferior to the response of non-thalassemic children with GH deficiency. In addition, increasing caloric intake and improving nutrition has been shown to increase IGF-1 and growth in these patients (63-66).
Effect of Sickle cell Disease (SCD) on growth and growth hormone-insulin-like growth factor-I axis
Sickle-cell disease is the most prevalent genetic hematologic condition in the United States. Numerous studies have demonstrated poor growth and delayed maturation in children with homozygous SCD; however, the pathophysiology remains inadequately understood.
At birth, affected children have normal weight and length. However, around 6 months of age their growth patterns begin to diverge from normal. The growth deficits experienced by these children remain a problem with clinical significance. The severity of the disease, microcirculation disorder, the affection of GH-IGF-I axis, delayed puberty, defective nutrition and hypermetabolic status are important factors affecting growth in these patients (70, 71).
Soliman et al, studied a large cohort of children and adolescents with SCD (n=110) and thalassaemia (n=72) receiving nearly the same protocol of transfusion and chelation, and compared them with those for 200 normal age-matched children, 30 children with constitutional delay of growth (CSS), and 25 children with growth hormone deficiency (GHD). Before transfusion, Hb in concentration had not been less than 9 g/dl for 7 years; desferrioxamine was administered for 7-10 years. The height standard deviation score (HtSDS), growth velocity (GV) (cm/yr), and GV standard deviation score (GVDSD) of children and adolescents with SCD and thalassaemia were significantly decreased compared to normal children (p<0.01). Fifty one % of children with SCD had GVS-DS less than -1. The GV of thalassaemic children was significantly slower than that for children with SCD. Children with thalassaemia and SCD had HtSDS and GVSDS comparable to those for children with CSS but higher than those for patients with GHD.
The MAC and triceps skin fold thickness were significantly smaller in children with thalassaemia and SCD compared to normal children. The upper/lower segment ratio was significantly lower in thalassaemic and SCD patients than in normal children. Serum ferritin concentration was correlated negatively with the linear GV in all patients (r=-0.45, p<0.001). Short children with thalassaemia and SCD had significantly decreased serum insulin-like growth factor 1 (IGF-1) concentrations compared to children with CSS. Collectively, these data confirm the high prevalence of impaired growth and pubertal delay/failure in children and adolescents with SCD (72).
A survey of 63 children with SCD in Baltimore reported that 25% were lower than the 5th percentile for either height for age, weight for age, or weight for height (73).
A prospective longitudinal study reported the effects of SCD severity and nutritional status on growth for 4 years. In 148 children with SCD (78 females), growth in height, weight, or BMI declined in 84% of subjects; 38% fell below the 5th percentile in one or more measures. For all children, the percentage of subjects who exhibited growth failure (≤5th percentile) at any time during the study was 26%, 22%, and 24%, for weight, height, and BMI, respectively. Puberty was delayed 1 to 2 y, and median age at menarche was 13.2 y. Skeletal age was delayed by 0.7±1.4 y overall and by 1.3±1.5 y in children 10 to 15 y old. Height status declined over time and was positively associated with advancing puberty and hematological measures in girls, and nutritional status in girls and boys. The longitudinal regression models consistently indicated that for unknown reason girls experienced some growth recovery with the onset and progression through puberty, whereas for boys, there was no positive effect of puberty on growth (74-77).
In the Stroke Prevention Trial for Sickle Cell Anemia Study (STOP Trial), a well-controlled longterm transfusion therapy protocol, height, weight, and BMI Z scores improved significantly in those receiving transfusions, whereas there were no changes in growth status in the control group. After 24 mo of treatment, children in the transfusion group approached normal height for age and weight for age Z scores (78, 79).
Soliman et al. in two studies found that 9 of 21 and 8 of 15 children with SCD had a defective GH response to both clonidine and glucagon provocation (peak<10 micrograms/L). These children differed from the other 12 children with SCD in having slower linear growth velocity (GV and GVSDS), lower circulating concentrations of IGF-I and IGFBP-3. These patients had partial or complete empty sellae in CT scans of the hypothalamic-pituitary area. The two groups with SCD did not differ significantly in dietary intake, body mass index (BMI), midarm circumferences, skinfold thickness, serum albumin concentration, or intestinal absorption of D-xylose. A single injection of GH produced a smaller increase in circulating IGF-I in children with SCD with or without defective GH secretion versus 10 age-matched children with idiopathic short stature (ISS) and 11 children with isolated GH deficiency (GHD), suggesting partial GH resistance in the SCD group (80,81).
Collett-Solberg et al, demonstrated that children with SCD have abnormalities in the IGF-I axis, which worsen with age (82). Nunlee-Bland et al, reported high incidence of GH deficiency in children with SCD(SS). They treated 5 patients with GH for 3 or more years and demonstrated significant improvement in their height SDS (83).
Defective GH release, and consequently low IGF-I production and slow growth velocity in children with SCD might be secondary to hypoxic-vascular insults to their hypothalamic-pituitary axis during one or more of the sickling episodes (80, 81, 83). In support of these findings, Smiley et al found that children with SCD whose height is below the 25 th percentile for age have significantly decreased serum IGF-I concentrations compared with children with constitutional short stature. The authors suggested that decreased synthesis of IGF-I may be secondary to a disturbed GH-IGF-1 axis, undernutrition, or the hypermetabolic state of the disease (84).
Luporini et al. studied growth parameters and plasma concentrations of growth hormone (GH), IGF-1, and IGFBP-3 in 41 children with SCD whose haplotypes were defined. Results showed that plasma concentrations of IGF-I (total, free, and free/total fraction) and IGFBP-3 were significantly reduced in all patients with sickle cell anemia compared with the healthy children. Patients with the CAR/CAR haplotype had significantly lower mean growth velocity compared with those with Ben/Ben. When the GH/IGF axis elements were compared in relation with the different haplotypes, total IGF-I levels in CAR/CAR patients were significantly lower compared with levels in patients with Ben/Ben. A positive relationship was found between hematocrit and fetal hemoglobin percentages on the one hand and total IGF-I, free/total IGF-I, and IGFBP-3 on the other hand in patients with SCD. Authors suggested that delayed growth of these patients may be linked to intrinsic factors of the disease and assumed that decrease of total IGF-I concentrations in patients with CAR/CAR haplotype is secondary to the severity of the disease (85). In support of this view, Brazilian studies also found a correlation between SCD clinical manifestations and Hb F and the beta S-globin haplotype, including more vaso-occlusive crises, more infections and slower growth (86).
In addition, abnormalities in gonadotropin secretion patterns (elevated luteinizing hormone (LH) and depressed follicle-stimulating hormone (FSH) in early puberty) in boys and girls with SCD and poor testosterone response to gonadotrophin-releasing hormone in some boys suggest impairments in the regulatory feedback mechanisms of the hypothalamic-pituitarygonadal axis leading to sexual maturity (87, 88).
Moreover, nutritional studies of children with SCD have identified numerous deficits that likely contribute to growth failure. Increased energy requirements have been reported for children, teenagers, and adults with SCD in their usual state of health. Increased protein turnover adds an additional nutritional burden. Nutrient deficiencies based on biomarkers have been reported for vitamins B6, D, and E, retinol and zinc. In a 12-mo trial, zinc supplementation was shown to increase linear growth in children with SCD-SS. However, there are remarkably few supplementation or general nutrition intervention studies aimed at improving growth or nutritional status in children with SCD (89-99).
Conclusions
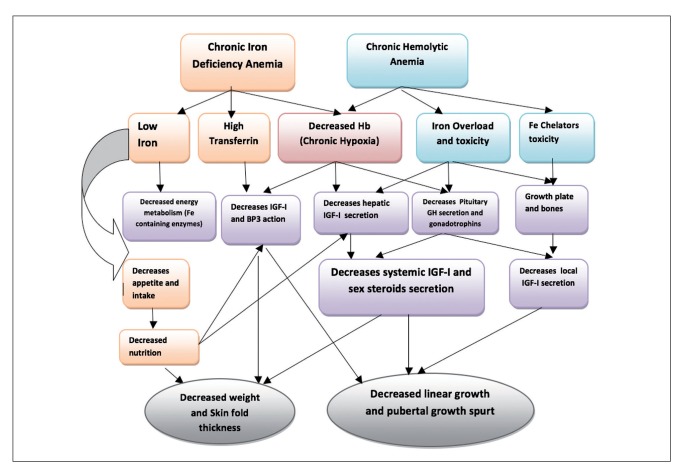
It appears that all forms of chronic anemia have a negative effect on linear growth during all stages of growth (infancy, childhood and adolescence). Although infants with chronic IDA may have delayed cognitive, motor, and affective development that may be long-lasting, it appears that growth abnormalities can be entirely corrected. Defective growth in chronic anemias is mediated partially through defective GH-IGF-I secretion. Correction of all forms of anemia is associated with partial or complete catch-up growth and a significant increase in IGF-I secretion (Figure 1).
Figure 1.
Mechanisms of growth impairment in chronic anemias
In view of the significant impact of chronic anemias on growth and pubertal development, endocrinologists should advocate primary prevention and screening for growth abnormalities in these forms of anemia according to their prevalence. Adequate correction of the anemia, sound nutrition, early diagnosis and management of dysfunction of growth and pubertal axes can markedly improve the final outcome of these children.
References
- 1.United Nations Children’s Fund, United Nations University, World Health Organization. Iron deficiency anaemia assessment, prevention, and control: a guide for programme managers. Geneva: World Health Organization; 2001. [accessed 7 May 2015]. WHO/NHD/01.3; http://www.who.int/nutrition/publications/en/ida_assessment_prevention_control.pdf .
- 2.Stoltzfus RJ, Mullany L, Black RE. In: Ezzati M, Lopez Ad, Rodgers A, Murray CJL, editors. Iron deficiency anaemia. Comparative quantification of health risks: global and regional burden of disease attributable to selected major risk factors. [accessed 20 May 2015];Geneva: World Health Organization. 2004 163210 http://www.who.int/publications/cra/chapters/volume 1/0163-0210.pdf?ua=1 . [Google Scholar]
- 3.Balarajan Y, Ramakrishnan U, Ozaltin E, Shankar AH, Subramanian SV. Anaemia in low-income and middle-income countries. Lancet. 2011;378:2123–2135. doi: 10.1016/S0140-6736(10)62304-5. [DOI] [PubMed] [Google Scholar]
- 4.The global prevalence of anaemia in 2011. WHO Library Cataloguing-in-Publication Data. ISBN 978 92 4 1564960 (NLM classification: WH 155). http://apps.who.int/iris/bitstream/10665/177094/1/9789241564960_eng.pdf?ua=1&ua=1 .
- 5.World Health Organization. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Geneva: World Health Organization. 2011. Available from: (WHO/NMH/NHD/MNM/11.1) http://www.who.int/vmnis/indicators/haemoglobin .
- 6.Shafir T, Angulo-Barroso R, Calatroni A, Jimenez E, Lozoff B. Effects of iron deficiency in infancy on patterns of motor development over time. Hum Mov Sci. 2006;25:821–838. doi: 10.1016/j.humov.2006.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waugh EJ, Polivy J, Ridout R, Hawker GA. A prospective investigation of the relations among cognitive dietary restraint, subclinical ovulatory disturbances, physical activity, and bone mass in healthy young women. Am J Clin Nutr. 2007;86:1791–801. doi: 10.1093/ajcn/86.5.1791. [DOI] [PubMed] [Google Scholar]
- 8.Rouault TA. The role of iron regulatory proteins in mammalian iron homeostasis and disease. Nat Chem Biol. 2006;2:406–414. doi: 10.1038/nchembio807. [DOI] [PubMed] [Google Scholar]
- 9.Williams TN, Weatherall DJ. World distribution, population genetics, and health burden of the hemoglobinopathies. Cold Spring Harb Perspect Med. 2012;2:a011692. doi: 10.1101/cshperspect.a011692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weatherall DJ. The inherited diseases of hemoglobin are an emerging global health burden. Blood. 2010;115:4331–4336. doi: 10.1182/blood-2010-01-251348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. http://www.ironhealthalliance.com/disease-states/thalassemia/epidemiology-and-pathophysiologyjsp .
- 12.Soliman AT, elZalabany M, Amer M, Ansari BM. Growth and pubertal development in transfusion-dependent children and adolescents with thalassaemia major and sickle cell disease: a comparative study. J Trop Pediatr. 1999;45:23–30. doi: 10.1093/tropej/45.1.23. [DOI] [PubMed] [Google Scholar]
- 13.Rhodes M, Akohoue SA, Shankar SM, Fleming I, Qi An A, Yu C, Acra S, Buchowski MS. Growth Patterns in Children with Sickle Cell Anemia during Puberty. Pediatr Blood Cancer. Pediatr Blood Cancer. 2009;53:635–641. doi: 10.1002/pbc.22137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esezobor CI, Akintan P, Akinsulie A, Temiye E, Adeyemo T. Wasting and stunting are still prevalent in children with sickle cell anaemia in Lagos, Nigeria. Ital J Pediatr. 2016 May 4;42(1):45. doi: 10.1186/s13052-016-0257-4. doi: 10.1186/s13052-016-0257-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rouault TA. The role of iron regulatory proteins in mammalian iron homeostasis and disease. Nat Chem Biol. 2006;2:406–414. doi: 10.1038/nchembio807. [DOI] [PubMed] [Google Scholar]
- 16.Galy B, Ferring-Appel D, Sauer SW, Kaden S, Lyoumi S, Puy H, Kölker S, Gröne HJ, Hentze MW. Iron regulatory proteins secure mitochondrial iron sufficiency and function. Cell Metab. 2010;12:194–201. doi: 10.1016/j.cmet.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 17.Zhang DL, Ghosh MC, Rouault TA. The physiological functions of iron regulatory proteins in iron homeostasis - An update. Front Pharmacol. 2014;5:124. doi: 10.3389/fphar.2014.00124. doi: 10.3389/fphar.2014.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kohgo Y, Ikuta K, Ohtake T, Torimoto Y, Kato J. Body iron metabolism and pathophysiology of iron overload. Int J Hematol. 2008;88:7–15. doi: 10.1007/s12185-008-0120-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Callahan LS, Thibert KA, Wobken JD, Georgieff MK. Early-life iron deficiency anemia alters the development and long-term expression of parvalbumin and perineuronal nets in the rat hippocampus. Dev Neurosci. 2013;35:427–436. doi: 10.1159/000354178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hubbard AC, Bandyopadhyay S, Wojczyk BS, Spitalnik SL, Hod EA, Prestia KA. Effect of dietary iron on fetal growth in pregnant mice. Comp Med. 2013;63:127–135. [PMC free article] [PubMed] [Google Scholar]
- 21.Guidi GC, Lechi Santonastaso C. Advancements in anemias related to chronic conditions. Clin Chem Lab Med. 2010;48:1217–1226. doi: 10.1515/CCLM.2010.264. [DOI] [PubMed] [Google Scholar]
- 22.Woodall SM, Johnston BM, Breier BH, Gluckman PD. Chronic maternal undernutrition in the rat leads to delayed postnatal growth and elevated blood pressure of offspring. Pediatr Res. 1996;40:438–443. doi: 10.1203/00006450-199609000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Allen HL. Anemia and iron deficiency: effects on pregnancy outcome 1,2,3. Am J Clin Nutr. 2000;71(Suppl. 5):1280s–1284s. doi: 10.1093/ajcn/71.5.1280s. [DOI] [PubMed] [Google Scholar]
- 24.Murphy JF, O’Riordan J, Newcombe RG, Coles EC, Pearson JF. Relations of hemoglobin levels in first and second trimesters to outcome of pregnancy. Lancet. 1986;1:992–995. doi: 10.1016/s0140-6736(86)91269-9. [DOI] [PubMed] [Google Scholar]
- 25.Steer P, Alam MA, Wadsworth J, Welch A. Relation between maternal hemoglobin concentration and birth weight in different ethnic groups. BMJ. 1995;310:489–491. doi: 10.1136/bmj.310.6978.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar KJ, Asha N, Murthy DS, Sujatha M, Manjunath V. Maternal Anemia in Various Trimesters and its Effect on Newborn Weight and Maturity: An Observational Study. Int J Prev Med. 2013;4:193–199. [PMC free article] [PubMed] [Google Scholar]
- 27.Moghaddam Tabrizi F, Barjasteh S. Maternal Hemoglobin Levels during Pregnancy and their Association with Birth Weight of Neonates. Iran J Pediatr Hematol Oncol. 2015;5:211–217. [PMC free article] [PubMed] [Google Scholar]
- 28.Kalaivani K. Prevalence and consequences of anaemia in pregnancy. Indian J Med Res. 2009;130:627–633. [PubMed] [Google Scholar]
- 29.Wang J, Ren AG, Ye RW, Zheng JC, Li S, Liu JM, Yang RL, Zhang FR, Zhang T, Zhang JB, Li Z. Study on the third trimester hemoglobin concentrations and the risk of low birth weight and preterm delivery. Zhonghua Liu Xing Bing Xue Za Zhi. 2007;28:15–18. [PubMed] [Google Scholar]
- 30.Yip R. Significance of an abnormally low or high hemoglobin concentration during pregnancy: special consideration of iron nutrition. Am J Clin Nutr. 2000;72:272s–279s. doi: 10.1093/ajcn/72.1.272S. [DOI] [PubMed] [Google Scholar]
- 31.Dreyfuss M. PhD dissertation. Baltimore: Johns Hopkins University; 1998. Anemia and iron deficiency during pregnancy: etiologies and effects on birth outcomes in Nepal. [Google Scholar]
- 32.Sichieri R, Fonseca VM, Hoffman D, Trugo NM, Moura AS. Lack of association between iron status at birth and growth of preterm infants. Rev Saude Publica. 2006;40:641–647. doi: 10.1590/s0034-89102006000500013. [DOI] [PubMed] [Google Scholar]
- 33.Angeles IT, Schultink WJ, Matulssi P, Gross R, Sastroamidjoj S. Increased rate of stunting among anaemic Indonesian pre-school children through iron supplementation. Am J Clin Nutr. 1993;58:339–342. doi: 10.1093/ajcn/58.3.339. [DOI] [PubMed] [Google Scholar]
- 34.Chowang L, Soemantri AG, Pollitt E. Iron supplementation and physical growth or rural Indonsian children. Am J Clin Nutr. 1988;47:496–501. doi: 10.1093/ajcn/47.3.496. [DOI] [PubMed] [Google Scholar]
- 35.Beard JL. Iron biology in immune function, muscle metabolism and neuronal functioning. J Nutr. 2001;131:568S–579S. doi: 10.1093/jn/131.2.568S. [DOI] [PubMed] [Google Scholar]
- 36.Bhatia D, Seshadri S. Growth performance in anemia and following iron supplementation. Indian Pediatr. 1993;30:195–200. [PubMed] [Google Scholar]
- 37.Soliman AT, Al Dabbagh MM, Habboub AH, Adel A, Humaidy NA, Abushahin A. Linear growth in children with iron deficiency anemia before and after treatment. J Trop Pediatr. 2009;55:324–327. doi: 10.1093/tropej/fmp011. [DOI] [PubMed] [Google Scholar]
- 38.Bandhu R, Shankar N, Tandon OP. Effect of iron on growth in iron deficient anemic school going children. Indian J Physiol Pharmacol. 2003;47:59–66. [PubMed] [Google Scholar]
- 39.Sachdev H, Gera T, Nestel P. Effect of iron supplementation on physical growth in children: Systematic review of randomized controlled trials. Public Health Nutr. 2006;9:904–920. doi: 10.1017/phn2005918. [DOI] [PubMed] [Google Scholar]
- 40.Ramakrishnan U, Aburto N, McCabe G, Martorell R. Multimicronutrient interventions but not vitamin a or iron interventions alone improve child growth: Results of 3 metaanalyses. J Nutr. 2004;134:2592–602. doi: 10.1093/jn/134.10.2592. [DOI] [PubMed] [Google Scholar]
- 41.Vucic V, Berti C, Vollhardt C, Fekete K, Cetin I, Koletzko B, Gurinovic M, van’t Veer P. Effect of iron intervention on growth during gestation, infancy, childhood, and adolescence: A systematic review with meta-analysis. Nutr Rev. 2013;71:386–401. doi: 10.1111/nure.12037. [DOI] [PubMed] [Google Scholar]
- 42.Cardoso MA, Augusto RA, Bortolini GA, Oliveira CS, Tietzman DC, Sequeira LA, Hadler MC, Peixoto Mdo R, Muniz PT, Vitolo MR, Lira PI, Jaime PC. ENFAC Working Group. Effect of Providing Multiple Micronutrients in Powder through Primary Healthcare on Anemia in Young Brazilian Children: A Multicentre Pragmatic Controlled Trial. PLoS One. 2016 Mar 14;11(3):e0151097. doi: 10.1371/journal.pone.0151097. doi: 10.1371/journal.pone.0151097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gera T1, Sachdev HP, Nestel P. Effect of iron supplementation on physical performance in children and adolescents: systematic review of randomized controlled trials. Indian Pediatr. 2007;44:15–24. [PubMed] [Google Scholar]
- 44.Chhagan MK, Van den Broeck J, Luabeya KK, Mpontshane N, Tomkins A, Bennish ML. Effect on longitudinal growth and anemia of zinc or multiple micronutrients added to vitamin A: a randomized controlled trial in children aged 6-24 months. BMC Public Health. 2010 Mar 18;10:145. doi: 10.1186/1471-2458-10-145. doi: 10.1186/1471-2458-10-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Preedy VR, Smith DM, Sugden PH. The effects of 6 hours of hypoxia on protein synthesis in rat tissues in vivo and in vitro. Biochem J. 1985;228:179–185. doi: 10.1042/bj2280179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsunawaki T, Sakai K, Momomura M, Wachi Y, Matsuzawa Y, Iwashita M. Hypoxia alters phosphorylation status of insulin-like growth factor (IGF)-binding protein-1 and attenuates biological activities of IGF-I in HepG2 cell cultures. J Obstet Gynaecol Res. 2013;39:1367–1373. doi: 10.1111/jog.12078. [DOI] [PubMed] [Google Scholar]
- 47.Kajimura S, Aida K, Duan C. Insulin-like growth factor-binding protein-1 (IGFBP-1) mediates hypoxia-induced embryonic growth and developmental retardation. Proc Natl Acad Sci. 2005;102:1240–1245. doi: 10.1073/pnas.0407443102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Storch S, Kubler B, Honing S, Ackmann M, Zapf J, Blum W, et al. Transferrin binds insulin-like growth factors and affects binding properties of insulin-like growth factor binding protein-3. FEBS Lett. 2001;509:395–398. doi: 10.1016/s0014-5793(01)03204-5. [DOI] [PubMed] [Google Scholar]
- 49.Vakili H, Jin Y, Cattini PA. Negative Regulation of Human Growth Hormone Gene Expression by Insulin Is Dependent on Hypoxia-inducible Factor Binding in Primary Nontumor Pituitary Cells. The J Biol Chem. 2012;287:3328233292. doi: 10.1074/jbc.M112.380949. Epub 2012 Jul 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ceppi A, Blum JW. Effects of growth hormone on growth performance, haematology, metabolites and hormones in iron-deficient veal calves. Zentralbl Veterinarmed A. 1994;41:443–458. doi: 10.1111/j.1439-0442.1994.tb00111.x. [DOI] [PubMed] [Google Scholar]
- 51.Blum JW1, Hammon H. Endocrine and metabolic aspects in milk-fed calves. Domest Anim Endocrinol. 1999;17:219–230. doi: 10.1016/s0739-7240(99)00039-9. [DOI] [PubMed] [Google Scholar]
- 52.Katsumata S, Katsumata-Tsuboi R, Uehara M, Suzuki K. Severe iron deficiency decreases both bone formation and bone resorption in rats. J Nutr. 2009;139:238–243. doi: 10.3945/jn.108.093757. [DOI] [PubMed] [Google Scholar]
- 53.Medeiros DM, Stoecker B, Plattner A, Jennings D, Haub M. Iron deficiency negatively affects vertebrae and femurs of rats independently of energy intake and body weight. J Nutr. 2004;134:3061–3067. doi: 10.1093/jn/134.11.3061. [DOI] [PubMed] [Google Scholar]
- 54.Katsumata S, Tsuboi R, Uehara M, Suzuki K. Dietary iron deficiency decreases serum osteocalcin concentration and bone mineral density in rats. Biosci Biotechnol Biochem. 2006;70:2547–2550. doi: 10.1271/bbb.60221. [DOI] [PubMed] [Google Scholar]
- 55.Tran PV, Fretham SJ, Wobken J, Miller BS, Georgieff MK. Gestational-neonatal iron deficiency suppresses and iron treatment reactivates IGF signaling in developing rat hippocampus. Am J Physiol Endocrinol Metab. 2012;302:E316–324. doi: 10.1152/ajpendo.00369.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Isguven P, Arslanoglu I, Erol M, Yildiz M, Adal E, Erguven M. Serum levels of ghrelin, leptin, IGF-I, IGFBP-3, insulin, thyroid hormones and cortisol in prepubertal children with iron deficiency. Endocr J. 2007;54:985–990. doi: 10.1507/endocrj.k07-031. [DOI] [PubMed] [Google Scholar]
- 57.Soliman AT, Eldabbagh M, Adel A, Sabt A. Linear growth and circulating IGF-I concentrations in children with Iron deficiency anemia after treatment. Arch Dis Child. 2012;97:A220. [Google Scholar]
- 58.Choi JW, Kim SK. Association of serum insulin-like growth factor-I and erythropoiesis in relation to body iron status. Ann Clin Lab Sci. 2004;34:324–328. [PubMed] [Google Scholar]
- 59.Succurro E, Arturi F, Caruso V, Rudi S, Sciacqua A, Andreozzi F, et al. Low insulin-like growth factor-1 levels are associated with anaemia in adult non-diabetic subjects. Thromb Haemost 201. 105:365–370. doi: 10.1160/TH10-06-0379. [DOI] [PubMed] [Google Scholar]
- 60.De Sanctis V, Soliman AT, Elsedfy H, Skordis N, Kattamis C, Angastiniotis M, et al. Growth and endocrine disorders in thalassemia: The international network on endocrine complications in thalassemia (I-CET) position statement and guidelines. Indian J Endocrinol Metab. 2013;17:8–18. doi: 10.4103/2230-8210.107808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.De Sanctis V, Katz M, Vullo C, Bagni B, Ughi M, Wonke B. Effect of different treatment regimes on linear growth and final height in beta-thalassaemia major. Clin Endocrinol (Oxf) 1994;40:791–798. doi: 10.1111/j.1365-2265.1994.tb02514.x. [DOI] [PubMed] [Google Scholar]
- 62.De Sanctis V. Growth and puberty and its management in thalassaemia. Horm Res. 2002;58(Suppl 1):72–79. doi: 10.1159/000064766. [DOI] [PubMed] [Google Scholar]
- 63.Kyriakou A, Skordis N. Thalassaemia and aberrations of growth and puberty. Mediterr J Hematol Infect Dis. 2009 Jul 27;1(1):e2009003. doi: 10.4084/MJHID.2009.003. doi: 10.4084/MJHID.2009.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Soliman AT, Abushahin A, Abohezeima K, Khalafallah H, Adel A, Elawwa A, et al. Age related IGF-I changes and IGF-I generation in thalassemia major. Pediatr Endocrinol Rev. 2011;8(Suppl 2):278–283. [PubMed] [Google Scholar]
- 65.Soliman AT, Khalafallah H, Ashour R. Growth and factors affecting it in thalassemia major. Hemoglobin. 2009;33(Suppl 1):S116–126. doi: 10.3109/03630260903347781. [DOI] [PubMed] [Google Scholar]
- 66.Soliman AT, El Banna N, Ansari BM. GH response to provocationand circulating IGF-I and IGF-binding protein-3 concentrations, the IGF-I generation test and clinical response to GH therapy in children with beta-thalassaemia. Eur J Endocrinol. 1998;138:394–400. doi: 10.1530/eje.0.1380394. [DOI] [PubMed] [Google Scholar]
- 67.Soliman AT, El Banna N, AbdEl Fattah M, ElZalabani MM, Ansari BM. Bone mineral density in prepubertal children with beta-thalassemia: Correlation with growth and hormonal data. Metabolism. 1998;47:541–548. doi: 10.1016/s0026-0495(98)90237-2. [DOI] [PubMed] [Google Scholar]
- 68.Soliman AT, elZalabany MM, Mazloum Y, Bedair SM, Ragab MS, Rogol AD, Ansari BM. Spontaneous and provoked growth hormone (GH) secretion and insulin-like growth factor I (IGF-I) concentration in patients with beta thalassaemia and delayed growth. J Trop Pediatr. 1999;45:327–337. doi: 10.1093/tropej/45.6.327. [DOI] [PubMed] [Google Scholar]
- 69.Soliman A, De Sanctis V, Elsedfy H, Yassin M, Skordis N, Karimi M, et al. Growth hormone deficiency in adults with thalassemia: An overview and the I-CET recommendations. Georgian Med News. 2013:79–88. [PubMed] [Google Scholar]
- 70.Soliman AT, El-Matary W, Fattah MM, Nasr IS, El Alaily RK, Thabet MA. The effect of high-calorie diet on nutritional parameters of children with beta-thalassaemia major. Clin Nutr. 2004;23:1153–1158. doi: 10.1016/j.clnu.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 71.Bennett EL. Understanding growth failure in children with homozygous sickle-cell disease. J Pediatr Oncol Nurs. 2011;28:67–74. doi: 10.1177/1043454210382421. [DOI] [PubMed] [Google Scholar]
- 72.Soliman AT, elZalabany M, Amer M, Ansari BM. Growth and pubertal development in transfusion-dependent children and adolescents with thalassaemia major and sickle cell disease: a comparative study. J Trop Pediatr. 1999;45:23–30. doi: 10.1093/tropej/45.1.23. [DOI] [PubMed] [Google Scholar]
- 73.Henderson RA, Saavedra JM, Dover GJ. Prevalence of impaired growth in children with homozygous sickle cell anemia. Am J Med Sci. 1994;307:405–407. doi: 10.1097/00000441-199406000-00004. [DOI] [PubMed] [Google Scholar]
- 74.Zemel BS, Kawchak DA, Ohene-Frempong K, Schall JI, Stallings VA. Effects of delayed pubertal development, nutritional status, and disease severity on longitudinal patterns of growth failure in children with sickle cell disease. Pediatr Res. 2007;61:607–613. doi: 10.1203/pdr.0b013e318045bdca. [DOI] [PubMed] [Google Scholar]
- 75.Singhal A, Thomas P, Cook R, Wierenga K, Serjeant G. Delayed adolescent growth in homozygous sickle cell disease. Arch Dis Child. 1994;71:404–408. doi: 10.1136/adc.71.5.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Olambiwonnu NO, Penny R, Frasier SD. Sexual maturation in subjects with sickle cell anemia: studies of serum gonadotropin concentration, height, weight and skeletal age. J Pediatr. 1975;87:459–464. doi: 10.1016/s0022-3476(75)80662-7. [DOI] [PubMed] [Google Scholar]
- 77.Platt OS, Rosenstock W, Espeland MA. Influence of sickle hemoglobinopathies on growth and development. N Engl J Med. 1984;311:7–12. doi: 10.1056/NEJM198407053110102. [DOI] [PubMed] [Google Scholar]
- 78.Stevens MC, Maude GH, Cupidore L, Jackson H, Hayes RJ, Serjeant GR. Prepubertal growth and skeletal maturation in children with sickle cell disease. Pediatrics. 1986;78:124–132. [PubMed] [Google Scholar]
- 79.Wang WC, Morales KH, Scher CD, Styles L, Olivieri N, Adams R, Brambilla D. Effect of long-term transfusion on growth in children with sickle cell anemia: results of the STOP trial. J Pediatr. 2005;147:244–247. doi: 10.1016/j.jpeds.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 80.Soliman AT, El Banna N, alSalmi I, De Silva V, Craig A, Asfour M. Growth hormone secretion and circulating insulin-like growth factor-I (IGF-I) and IGF binding protein-3 concentrations in children with sickle cell disease. Metabolism. 1997;46:1241–1245. doi: 10.1016/s0026-0495(97)90224-9. [DOI] [PubMed] [Google Scholar]
- 81.Soliman AT, Darwish A, Mohammed SH, Bassiony MR, El Banna N, Asfour M. Circulating growth hormone (GH), insulin-like growth factor-I (IGF-I) and free thyroxine, GH response to clonidine provocation and CT scanning of the hypothalamic-pituitary area in children with sickle cell disease. J Trop Pediatr. 1995;41:285–289. doi: 10.1093/tropej/41.5.285. [DOI] [PubMed] [Google Scholar]
- 82.Collett-Solberg PF1, Fleenor D, Schultz WH, Ware RE. Short stature in children with sickle cell anemia correlates with alterations in the IGF-I axis. J Pediatr Endocrinol Metab. 2007;20:211–218. doi: 10.1515/jpem.2007.20.2.211. [DOI] [PubMed] [Google Scholar]
- 83.Nunlee-Bland G, Rana SR, Houston-Yu PE, Odonkor W. Growth hormone deficiency in patients with sickle cell disease and growth failure. J Pediatr Endocrinol Metab. 2004;17:601–606. doi: 10.1515/jpem.2004.17.4.601. [DOI] [PubMed] [Google Scholar]
- 84.Smiley D, Dagogo-Jack S, Umpierrez G. Therapy insight: Metabolic and endocrine disorders in sickle cell disease. Nat Clin Pract Endocrinol Metab. 2008 Feb;4(2):102–9. doi: 10.1038/ncpendmet0702. doi: 10.1038/ncpendmet0702. [DOI] [PubMed] [Google Scholar]
- 85.Luporini SM, Bendit I, Manhani R, Bracco OL, Manzella L, Giannella-Neto D. Growth hormone and insulin-like growth factor I axis and growth of children with different sickle cell anemia haplotypes. J Pediatr Hematol Oncol. 2001;23:357–363. doi: 10.1097/00043426-200108000-00007. [DOI] [PubMed] [Google Scholar]
- 86.Filho IL, Leite AC, Moura PG, Ribeiro GS, Cavalcante AC, Azevedo FC, Andrada-Serpa MJ. Genetic polymorphisms and cerebrovascular disease in children with sickle cell anemia from Rio de Janeiro. Brazil. Arq Neuropsiquiatr. 2011;69:431–435. doi: 10.1590/s0004-282x2011000400004. [DOI] [PubMed] [Google Scholar]
- 87.Olambiwonnu NO, Penny R, Frasier SD. Sexual maturation in subjects with sickle cell anemia: studies of serum gonadotropin concentration, height, weight and skeletal age. J Pediatr. 1975;87:459–464. doi: 10.1016/s0022-3476(75)80662-7. [DOI] [PubMed] [Google Scholar]
- 88.Singhal A, Gabay L, Serjeant GR. Testosterone deficiency and extreme retardation of puberty in homozygous sicklecell disease. West Indian Med J. 1995;44:20–23. [PubMed] [Google Scholar]
- 89.Badaloo A, Jackson AA, Jahoor F. Whole body protein turnover and resting metabolic rate in homozygous sickle cell disease. Clin Sci. 1989;77:93–97. doi: 10.1042/cs0770093. [DOI] [PubMed] [Google Scholar]
- 90.Borel MJ, Buchowski MS, Turner EA, Peeler BB, Goldstein RE, Flakoll PJ. Alterations in basal nutrient metabolism increase resting energy expenditure in sickle cell disease. Am J Physiol. 1998;274:E357–E364. doi: 10.1152/ajpendo.1998.274.2.E357. [DOI] [PubMed] [Google Scholar]
- 91.Barden EM, Zemel BS, Kawchak DA, Goran MI, Ohene-Frempong K, Stallings VA. Total and resting energy expenditure in children with sickle cell disease. J Pediatr. 2000;136:73–79. doi: 10.1016/s0022-3476(00)90053-2. [DOI] [PubMed] [Google Scholar]
- 92.Nelson MC, Zemel BS, Kawchak DA, Barden EM, Frongillo EA, Coburn SP, Ohene-Frempong K, Stallings VA. Vitamin B6 status of children with sickle cell disease. J Pediatr Hematol Oncol. 2002;24:463–469. doi: 10.1097/00043426-200208000-00011. [DOI] [PubMed] [Google Scholar]
- 93.Buison AM, Kawchak DA, Schall J, Ohene-Frempong K, Stallings VA, Zemel BS. Low vitamin D status in children with sickle cell disease. J Pediatr. 2004;145:622–627. doi: 10.1016/j.jpeds.2004.06.055. [DOI] [PubMed] [Google Scholar]
- 94.Natta C, Machlin L. Plasma levels of tocopherol in sickle cell anemia subjects. Am J Clin Nutr. 1979;32:1359–1362. doi: 10.1093/ajcn/32.7.1359. [DOI] [PubMed] [Google Scholar]
- 95.Natta CL, Machlin LJ, Brin M. A decrease in irreversibly sickled erythrocytes in sickle cell anemia patients given vitamin E. Am J Clin Nutr. 1980;33:968–971. doi: 10.1093/ajcn/33.5.968. [DOI] [PubMed] [Google Scholar]
- 96.Schall JI, Zemel BS, Kawchak DA, Ohene-Frempong K, Stallings VA. Vitamin A status, hospitalizations, and other outcomes in young children with sickle cell disease. J Pediatr. 2004;145:99–106. doi: 10.1016/j.jpeds.2004.03.051. [DOI] [PubMed] [Google Scholar]
- 97.Leonard MB, Zemel BS, Kawchak DA, Ohene-Frempong K, Stallings VA. Plasma zinc status, growth, and maturation in children with sickle cell disease. J Pediatr. 1998;132:467–471. doi: 10.1016/s0022-3476(98)70022-8. [DOI] [PubMed] [Google Scholar]
- 98.Zemel BS, Kawchak DA, Fung EB, Ohene-Frempong K, Stallings VA. Effect of zinc supplementation on growth and body composition in children with sickle cell disease. Am J Clin Nutr. 2002;75:300–307. doi: 10.1093/ajcn/75.2.300. [DOI] [PubMed] [Google Scholar]
- 99.Phebus CK, Maciak BJ, Gloninger MF, Paul HS. Zinc status of children with sickle cell disease: relationship to poor growth. Am J Hematol. 1988;29:67–73. doi: 10.1002/ajh.2830290203. [DOI] [PubMed] [Google Scholar]