Abstract
Anaemia is a global public health problem affecting both developing and developed countries with major consequences for human health as well as social and economic development. It occurs at all stages of the life cycle, but is more prevalent in pregnant women and young children. Iron deficiency anaemia (IDA) impairs thyroid metabolism in animals and human and may negatively affect growth and develpment of children. On the other hand both overt and subclinical hypothyroidism are associated with anemia and adding iron to thyroxine therapy improves both conditions compared to thyroxine therapy alone. In addition patients with chronic hemolytic anemia requiring repeated blood transfusion have high prevalence of hypothalamic-pituitary thyroid axis. Both primary hypothyroidism and central hypothyroidism occur in these patients with increasing prevalence with age, severity of the anemia and higher ferritin concentration denoting poor chelation. Proper blood transfusion and intensive chelation appears to prevent deterioration of thyroid function and in many cases can reverse thyroid pathology. Physicians treating these forms of anemia should be aware of thyroid disorders in these patients for early screening, prevention and proper management of any thyroid dysfunction. (www.actabiomedica.it)
Keywords: iron, iron deficiency anemia (IDA), thalassemia, sickle cell disease, thyroid, free thyroxine, TSH
Introduction
Anemia affects one-quarter of the world’s population and is concentrated in preschool-aged children and women, making it a global public health problem. Over 90% of affected individuals live in developing countries (1). Only 50% of anemia is caused by iron deficiency, the remainder is caused by vitamin A, B12, folate deficiencies, malaria, HIV, other infectious diseases, sickle cell disease and other inherited anemia (2).
Anaemia is defined as a haemoglobin level of 110 gm/L or less in women and 130 gm/L or less in men (3). Normal thyroid status is dependent on the presence of many trace elements e.g., iron, iodine, selenium, and zinc for both the synthesis and metabolism of thyroid hormones. Deficiencies of these elements can impair thyroid functions (4).
The aim of the present study was to review studies in animals and humans in relation to thyroid hormone function.
1. Anemia and thyroid function animal studies
Studies in animals and humans have shown that iron deficiency anemia (IDA) impairs thyroid metabolism (5-8).
Beard et al. reported decreased triiodothyronine (T3) production and disturbed norepinephrine metabolism in age-matched male Sprague-Dawley rats depleted of iron by dietary means. Repletion with iron dextran produced 80% correction of hematocrit and liver T3 production in 7 days. In addition, acute correction of iron deficiency anemia (IDA) using isovolemic exchange transfusion reversed the alterations in T3 production (7-9).
Male weanling Sprague-Dawley rats fed low iron food showed significant decrease in TPO activity to 33%, 45 and 56 % of normal with decreasing the amount of iron in the feeds (10, 11) Iron deficiency decreased plasma concentrations of T3 and T4 and increased in vitro hepatic rT3 deiodination suggesting that iron-deficient animals tend to metabolize thyroid hormone via a deactivating pathway (12).
Mathur et al. (13) evaluated the peri-partum changes in the level of thyroid-stimulating hormone (TSH) and thyroid function, due to dietary iron deficiency anaemia in the female albino rats of Wister strain that were fed on iron deficient diets [30,15,7.2 mg Fe/kg of diet] and control diets [50 mg Fe/kg of diet]. Significant differences in the levels of the hemoglobin (Hb), packed cell volume (PCV), and TSH were observed (p<0.05). The two way analysis of variance (ANOVA) before pregnancy, during pregnancy and after delivery showed a significant rise in the levels of TSH.
The severely iron deficient mothers showed postpartum hypothyroidism and increased preterm delivery. In addition, the females with severe IDA (7.2 mg Fe/kg of diet) could not lactate and failed to conceive months after their first premature deliveries.
In another experiment, pregnant rat dams were rendered IDA from early gestation through weaning. Serum total thyroxine (T4) and total T3, and brain T3 levels, were subsequently measured in postnatal d 12 pups (P12). Iron (Fe) deficiency (ID) reduced serum T3 by 43%, serum T4 by 67%, and whole-brain T3 by 25% at P12. Brain mRNA analysis revealed that expression of several thyroid hormone (TH) -responsive genes were altered in IDA neonates, suggesting that reduced TH concentrations were sensed by the low dietary iron (FeD) neonatal brain (14). Similarly, pregnant Sprague Dawley rats rendered Fe-deficient from early gestation through postnatal d 10 (P10). Fe deficiency significantly lowered P10 serum total T3 (45%), serum T4 (52%), whole brain T3 (14%), and hippocampal T3 (18%) concentrations, producing a mild thyroid deficiency.
Fe deficiency lowered Pvalb, Enpp6, and Mbp mRNA levels in the P10 hippocampus and also altered Hairless, Dbm, and Dio2 mRNA levels in the P10 cerebral cortex. These results suggest that some of the brain defects associated with ID may be mediated through altered thyroidal status and the concomitant alterations in TH-responsive gene transcription (15).
Smith et al. (16) showed that thyroid hormone binding by nuclear receptors tended to be lower in ID rats when compared to normal controls, that suggest that the metabolism of T4 is altered in ID.
Combining ID with an additional mild thyroidal perturbation (6-propyl-2-thiouracil [PTU]) during development more severely impairs neonatal thyroid status and brain TH-responsive gene expression than either deficiency alone (7, 8, 15, 17).
Bastian et al. (18) showed that fetal and neonatal ID exacerbates mild thyroid hormone insufficiency due to its effect on thyroid hormone levels and brain thyroid hormone responsive gene expression.
2. Human studies
Ipek et al. (19) studied thyroid function in 90 children (age 1-14 years) with IDA and 38 normal children. They reported that T3 and T4 values were statistically lower in the anemic group (p=0.002, p<0.001, respectively). However, they did not find difference between the groups in terms of freeT4 (fT4) and freeT3 (fT3) levels. There was a positive correlation between TT3 and ferritin and between T3 and transferrin saturation levels.
Tienboon and Unachak (20), found no difference in T4, T3, fT4, fT3, thyroxine-binding globulin (TBG), TSH levels in children with IDA anemia before versus after iron treatment. However, they observed that before iron therapy the response of TSH to an intravenous bolus of thyrotropin releasing hormone (TRH) was lower in the children with IDA with longer time to reach the peak compared to control children. Upon resolution of the anemia, the difference was not significant.
Beard et al. (8) found that anemic women had lower rectal temperatures than did control women and a lower rate of oxygen consumption at 100 min of cold exposure. Plasma T4 and T3 concentrations were significantly (p<0.002) lower in anemic than in control women at baseline and during cold exposure. Iron supplementation corrected the anemia and significantly improved rectal temperature at 100 min and partially normalized plasma thyroid hormone concentrations.
Azizi et al. (21) found a relation between the frequency of goitre and serum ferritin level in school children in Iran and reported that the frequency of goitre was related to ID. Reffat (22) reported higher prevalence of IDA in 52 out of 118 non-pregnant females with abnormal thyroid (44%) compared to euthyroid pregnant females (14.3%). These findings were supported by those of Chandel et al. (23).
Martinez-Torres et al. (24) reported 10% lower T3 levels in human subjects with moderate to severe iron deficiency anemia and ID without anemia when compared to iron-replete subjects, and Beard et al.(25) showed that in iron-deficient subjects, serum T3 and T4 levels significantly decreased. These results were supported by others (5, 8, 9).
A cross-sectional study was conducted among 227 school children aged 6-12 years living in hilly regions of eastern Nepal (26). The cohort comprised euthyroid (80.6%, n=183), overt hypothyroid (1.3%, n=3), subclinical hypothyroid (16.3%, n=37) and subclinical hyperthyroid (1.8%, n=4) children, respectively. About 35.2% (n=80) children were anemic, 43.6% (n=99) were ID. Hypothyroidism (overt and subclinical) was common in anemic and iron deficient children. The relative risk of having hypothyroidism (overt and subclinical) in anemic and ID children was 5.5 and 1.9, respectively, as compared to non-anemic and iron sufficient children. Authors reported significant negative correlation between TSH and Hb levels (26). In a larger cohort (n=759) of Nepali school children aged 6-13 y it was shown that low urinary iodine excretion was common in children with iron deficiency and anemia (27).
Another cross-sectional study was carried out in Lar Province in the South of Iran (28, 29). By a stepwise random sampling from all 94 iron deficient high schools girls and urinary iodine and serum ferritin, iron, total iron binding capacity (TIBC), TSH, T4, T3, FT4, FT3, T3 Resin Uptake (T3RU), reverse triiodothyronine (rT3) were measured. There was a positive correlation between plasma T4 and serum ferritin (p<0.001). Subjects with low serum ferritin had a higher T3/T4 ratio (p<0.001). Using a stepwise regression analysis, it was found that ferritin contributed significantly to rT3 concentration (p<0.004). They concluded that ID may impair thyroid hormone status (28, 29).
TSH concentrations were measured in the basal state and in response to an intravenous bolus of thyrotropin releasing hormone (TRH) in nine children one to three years of age with IDA before and after treatment with oral iron. Compared to the control children, the TSH response over time to TRH, TSH area under the curve (TSH-AUC), and the peak TSH value after stimulation were all lower in the IDA children both before and after resolution of anaemia. However, normal thyroid function was preserved in these children with iron deficiency anaemia (20).
The mechanism by which Fe status influences thyroid and iodine metabolism is still not clear. IDA could impair thyroid metabolism through anemia and lowered oxygen transport (30, 31), V may alter central nervous system control of thyroid metabolism (32) and nuclear T3 binding (20). Thyroid peroxidase (TPO) is a heme-containing enzyme catalyzing the two initial steps in thyroid hormone synthesis.
In summary, IDA decreases serum T4 and T3 concentrations, reduces peripheral conversion of T4 to T3, decreases T3 metabolism (turn over), decreases hepatic T4-5’-deiodinase and may increase circulating TSH activities (5-8).
3. Anemia in primary hypothyroid subjects
Anemia is a common finding in patients with hypothyroidism. Normochromic normocytic (anemia of chronic disease), hypochromic microcytic and megaloblastic types are all reported by different authors (33-37).
Chu et al.(34) reported anemia in 65% of children and adolescents with hypothyroidism. Erdogan et al. (35) studied 100 patients with overt hypothyroid, 100 patients with subclinical hypothyroid, and 200 healthy controls and reported an anemia prevalence of 43% in the overt hypothyroid group, 39% in the subclinical hypothyroid group, and 26% in the control group (p=0.0003 and p=0.02, respectively). They concluded that the frequency of anemia in subclinical hypothyroidism is as high as that in overt hypothyroidism. Das et al. (36) studied 60 adult nonpregnant untreated primary hypothyroid patients with anemia without any obvious cause and reported IDA in 43.3% of them. There was no difference between the hypothyroid groups in terms of anemia. Vitamin B12, Fe, and folic acid were similar between these groups. Therefore, suspicion of hypothyroidism should be considered in anemias with uncertain etiology.
Franzese et al. (37) studied newborns with congenital hypothyroidism diagnosed by neonatal screening. They reported that anemia was a frequent finding in infants with congenital hypothyroidism and was dependent on the degree of neonatal hypothyroidism and implied that hypothyroidism during development may produce persisting changes even after thyroid replacement has begun.
Normocytic anemia, is due to thyroid hormones deficit itself not followed by nutritive deficit. Lack of stimulation of erythroid colony development by thyroid hormones, reduction in oxygen distribution to tissues and diminution of erythropoietin level in the absence of thyroid hormones leads to this normocytic anemia.
Normocytic anemia is characterized by reticulopenia, hypoplasia of erythroid lineage, decreased level of erythropoietin, mainly regular erythrocyte survival. Acanthocytosis findings in cytologic blood smear suggest hypothyroidism in about 90% of cases. This “uncomplicated” anemia secondary to hypothyroidism responded to thyroid replacement therapy alone (33-36). Microcytic anemia may occur due to menorrhagia occurring as a result of various hormonal instability and malabsorption observed in hypothyroidism. Macrocytic anemia is caused by malabsorption of vitamin B12, folic acid, pernicious anemia and inadequate nutrition. Pernicious anemia occurs 20 times more frequently in patients with hypothyroidism than generally. Macrocytosis is found in up to 55% patients with hypothyroidism and may result from the insufficiency of the thyroid hormones themselves without nutritive deficit (36, 38, 39).
Therefore, anemia in hypothyroid needs to be properly evaluated because treatment will depend on the causes of anemia.
4. Iron therapy: effect on thyroid hormones in normal subjects
Ninety four iron-deficient adolescent girls were randomly assigned to one of four groups and treated with a single oral dose of 190 mg iodine plus 300 mg ferrous sulphate 5 times/week (n=24), 300 mg ferrous sulphate 5 times/week (n=23), a single oral dose of 190 mg iodine (n=25), or a placebo (n=22) for 12 weeks. After the intervention, thyroid indices tT4, tT3 and T3RU increased and reverse RT3 decreased in the iron+iodine group (10 vs 8.9 mug/dl, p<0.001; 143 vs 138 mug/dl, p<0.05; 32.3 vs 28.4%, p<0.001 and 24.8 vs 44.2 ng/dl, p<0.001, respectively) and in the iron group. These results indicate that improvement of iron status was accompanied by an improvement in some indices of thyroid hormones (40).
Zimmermann et al. (41) showed that iron supplementation improved the efficacy of iodized salt in goitrous children with iron deficiency.
Gökdeniz et al. (42) investigated the effect of iron deficiency anemia and iron treatment on the thyroid functions in 42 patients with IDA and 38 healthy individuals. Before iron treatment, secondary hypothyroidism (35.7%) and subclinical hypothyroidism (16.6%) were found in patient group. Before the treatment TSH levels were higher, fT4 was lower in the IDA group and fT3 levels were not different. After treatment with iron, fT4 values significantly increased. Authors concluded that secondary and subclinical hypothyroidism occurs in patients with IDA and are reversible with iron supplementation.
Beard et al.(8) showed that in 10 women with IDA, iron supplementation corrected the anemia, significantly (p=0.03) improved rectal temperature at 100 min, and partially normalized plasma thyroid hormone concentrations. This experiment demonstrated a functional consequence of iron-deficiency anemia in the balance of heat production and loss and suggested that thyroid-hormone metabolism may be responsible.
In contrast, the study of Tienboon and Unachak (20) showed that there was no statistical difference in thyroid hormones in iron-deficient-anemic children prior to resolution of anaemia as compared to after its resolution. Erdal et al. (43) showed that patients with subclinical hypothyroidism had significantly lower serum iron levels than euthyroid controls and levothyroxine (L-T4) replacement alone resulted in reversal of the iron deficiency.
5. Iron therapy: effect on thyroid hormones in subclinical hypothyroid subjects
Subclinical hypothyroidism is a common endocrine disorder, affecting approximately 4.3% of the U.S. population (44). The study of Cinemre et al. (45) examined the effect of L-T4 used jointly with iron supplementation on the hematologic parameters of patients with subclinical hypothyroidism (SH) and iron deficiency anemia. They showed that IDA in patients with subclinical hypothyroidism benefits substantially by the addition of low L-T4 dose to iron replacement, as compared with iron replacement alone. Patients in the combination group had significant improvements in their TSH after 3 months of treatment. In addition, the combined intervention revealed significant improvement in levels of Hb over iron therapy alone, rendering the majority of patients euthyroid while simultaneously reversing the anemia.
In a randomized, double-blind, active-controlled trial, 60 patients with subclinical hypothyroidism plus IDA received Iron salt plus placebo (20 patients), L-T4 plus placebo (20 patients), or L-T4 plus iron salt (20 patients) for 3 months. The increase from baseline in Hb and ferritin in the L-T4 plus iron group was superior to the other groups. The decrease in TSH in the 2 groups that received L-T4 was superior to the group treated with iron salt (46).
6. Thyroid disorders in thalassemia major (TM)
The reported thyroid dysfunction seen in patients with TM includes primary hypothyroidism-caused by abnormalities of the thyroid gland, subclinical hypothyroidism as well as secondary hypothyroidism. The frequency of hypothyroidism shows a discrepancy depending on the region, quality of management, and treatment protocols. The reported frequency of thyroid dysfunctions ranges between 13% and 60% in different studies and occurs after 10 years of age regardless of difference in the rate of prevalence, largely as in the form of subclinical hypothyroidism (47-52).
Primary hypothyroidism is characterized by an elevated TSH level and low T4. Secondary or central hypothyroidism characterized by decreased T4 and low TSH (47-52)
Longitudinal studies proved worsening of thyroid function in thalassemic patients with advancing age. The lack of proper increase of TSH in response to low circulating levels of fT4 of these patients indicated a defective pituitary thyrotrophic function added to the primary dysfunction of the thyroid (53, 54). Thyroid echographic data showed features of dishomogeneity of the thyroid parenchyma with different degrees of severity, with the highest score observed in patients with hypothyroidism (53). Pitrolo et al. (55) reported a reduced echogenicity in 47% of TM and a diffuse spotty echogenicity in 33% of them, indicative of thyroid dysfunction.
Thyroid dysfunction appears to be primarily due to the toxicity of the excess unbound iron, within cells or in plasma which generates reactive oxygen species, leading to lipid peroxidation. The result of lipid peroxidation, under conditions of iron overload, leads to generation of both unsaturated (malondialdehyde and hydroxynonenal) and saturated (hexanal) aldehydes. Both have been implicated in cellular dysfunction, cytotoxicity and cell death (56, 57).
Certain tissues are particularly susceptible to excess iron incorporation when Non-Transferrin-Bound Iron (NTBI) is present. Thyrotropin (TSH) - releasing hormone stimulates TSH beta promoter activity by two distinct mechanisms involving calcium influx through L type Ca2 + channels (LTCCs) and protein kinase C (57). The most recent evidence suggests that LTCCs are the front-runners for mediating NTBI transport in iron overload conditions. LTCCs are moderately in thyrotrophs that appear to be at the greatest risk in iron overload. In addition, protein kinase C has been shown to be regulated by iron with possible deleterious effect of excess iron on its function. Both mechanisms appear to be affected by iron overload and can explain the defective TSH secretion in response to low FT4 in thalassemic patients (57). The deposition of iron in the pituitary gland and its deleterious effects on pituitary size and functions has been reported in many studies and review (48-54, 58).
After combined chelation that lead to significant decrease in total body iron overload 14/18 who had subclinical or compensated hypothyroidism presented a significant increase in mean fT4, fT3 (p<0.001) and an decrease in TSH. Among them, 10/18 (56%) discontinued thyroxin therapy (p<0.001) and 4/18 (22%) reduced their thyroxin dose. Out of the remaining 4 who had overt hypothyroidism, 2 converted to compensated hypothyroidism (58).
7. Thyroid function in sickle-cell disease (SCD)
The reports of thyroid assessment in patients with SCD have been inconsistent. The prevalence of hypothyroidism ranged between 2-6% of patients in different studies. Other reported increased TSH response to TSH-releasing hormone in SCD compared with controls and thus were suggestive of primary thyroid failure. In one study, children and adolescents with SCD showed increased incidence of hypothyroidism (6%) of both central and primary hypothyroidism. However, other studies reported normal thyroid function in children with SCD (59-63).
The etiology of thyroid dysfunction in SCD is not clear; however, most affected patients have received multiple transfusions consistent with severe iron overload. Autopsy reports in some patients have shown significant iron deposition in the thyroid gland, suggesting that the etiology of the primary thyroid failure might well be transfusional hemosiderosis and subsequent cellular damage to the thyroid gland (59-63).
Investigators propose that thyroid dysfunctions in SCD patients may be caused by iron overload due to recurrent blood transfusions or disruptions of tissue vitalization during vaso-occlusive crisis and inflammatory mediators. Some of the patients had histopatho-logical examination that have indicated iron deposition and concluded that iron may lead to cellular damage and, eventually, thyroid dysfunction. Evident extensive fibrosis of the thyroid gland as well as extensive deposition of iron in the cells lining the thyroid follicles was reported in transfusion dependent SCD. Even in SCD who are not transfusion dependent, thyroid doppler evaluation in SCD patients showed significantly higher resistance index and pulsatility index values, and lower thyroid volume compared with control group. This may be due to impaired thyroidal microcirculation. Iron overload and toxicity as well as diminished perfusion to the pituitary gland can explain cases with central hypothyroidism (64-71).
Conclusions
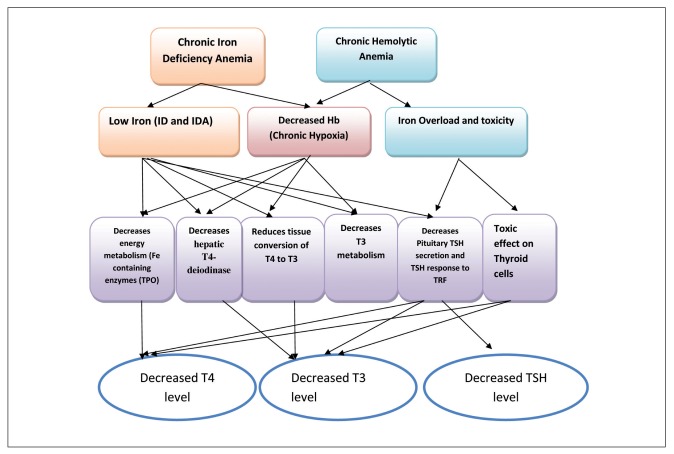
Anemia and ID seems to be associated with thyroid dysfunction particularly hypothyroidism. Future studies should be done in large samples and be directed toward finding the reasons for low thyroid hormones in anemic and iron deficient children (Figure 1).
Figure 1.
Mechanisms of thyroid dysfunction in chronic anemias
Legend: ID = iron deficiency, IDA = iron deficiency anemia, T3 = triiodothyronine, T4 = thyroxine, TSH = thyrotrophic hormone, TPO = thyroid peroxidase
IDA in patients with subclinical hypothyroidism benefits substantially by the addition of low-dose L-T4 to iron replacement, which yields greater improvement in hematologic parameters than iron replacement alone. Subclinical hypothyroidism should be treated in patients with iron deficiency anemia when the two conditions coexist. This would provide a desired therapeutic response to oral iron replacement and prevent ineffective iron therapy.
In transfusion dependent patients with chronic hemolytic anemia proper correction of the Hb level and intensive chelation can prevent deterioration of thyroid function and may reverse thyroid dysfunction in some cases.
In view of the significant impact of chronic anemias on thyroid function pediatricians, hematologists and endocrinologists should advocate primary prevention and screening for these forms of anemia according to their prevalence. Adequate correction of the anemia, sound nutrition, early diagnosis and management of thyroid and other endocrine dysfunctions can markedly improve the final outcome of these patients.
References
- 1.American Institute of Nutrition. Second report of the ad hoc committee on standards for nutritional studies. J Nutr. 1980;110:1726–1732. doi: 10.1093/jn/107.7.1340. [DOI] [PubMed] [Google Scholar]
- 2.Yip R. Iron deficiency: contemporary scientific issues and international programmatic approaches. J Nutr. 1994;124:1479S–1490S. doi: 10.1093/jn/124.suppl_8.1479S. [DOI] [PubMed] [Google Scholar]
- 3.WHO/UNU/UNICEF. Geneva, World Health Organization. 2001. Iron deficiency anaemia. Assessment, prevention and control. A guide for programme managers. [Google Scholar]
- 4.Eftekhari MH, Eshraghian MR, Mozaffari-Khosravi H, Saadat N, Shidfar F. Effect of iron repletion and correction of iron deficiency on thyroid function in iron-deficient Iranian adolescent girls. Pak J Biol Sci. 2007;10:255–60. doi: 10.3923/pjbs.2007.255.260. [DOI] [PubMed] [Google Scholar]
- 5.Beard J, Green W, Miller L, Finch C. Effect of iron-deficiency anemia on hormone levels and thermoregulation during cold exposure. Am J Physiol. 1984;247:R114–R119. doi: 10.1152/ajpregu.1984.247.1.R114. [DOI] [PubMed] [Google Scholar]
- 6.Tang F, Wong TM, Loh TT. Effects of cold exposure of TRH on the serum TSH levels in the iron-deficient rat. Horm Metab Res. 1988;20:616–619. doi: 10.1055/s-2007-1010899. [DOI] [PubMed] [Google Scholar]
- 7.Beard J, Tobin B, Green W. Evidence of thyroid hormone deficiency in iron-deficient anemic rats. J Nutr. 1989;119:772–778. doi: 10.1093/jn/119.5.772. [DOI] [PubMed] [Google Scholar]
- 8.Beard JL, Borel MJ, Derr J. Impaired thermoregulation and thyroid function in iron-deficiency anemia. Am J Clin Nutr. 1990;52:813–819. doi: 10.1093/ajcn/52.5.813. [DOI] [PubMed] [Google Scholar]
- 9.Beard JL, Tobin BW, Smith SM. Effects of iron repletion and correction of anemia on norepinephrine turnover and thyroid metabolism in iron deficiency. Proc Soc Exp Biol Med. 1990;193:306–312. doi: 10.3181/00379727-193-43040. [DOI] [PubMed] [Google Scholar]
- 10.Hess SY, Zimmermann MB, Arnold M, Langhans W, Hurrell RF. Iron deficiency anemia reduces thyroid peroxidase activity in rats. J Nutr. 2002;132:1951–1955. doi: 10.1093/jn/132.7.1951. [DOI] [PubMed] [Google Scholar]
- 11.Dillman E, Gale C, Green W, Johnson DG, Mackler B, Finch C. (1980) Hypothermia in iron deficiency due to altered triiodothyronine metabolism. Am J Physiol. 1980;239:R377–R381. doi: 10.1152/ajpregu.1980.239.5.R377. [DOI] [PubMed] [Google Scholar]
- 12.Smith SM, Johnson PE, Lukaski HC. In vitro hepatic thyroid hormone deiodination in iron-deficient rats: effect of dietary fat. Life Sci. 1993;53:603–609. doi: 10.1016/0024-3205(93)90268-8. [DOI] [PubMed] [Google Scholar]
- 13.Mathur N, Joshi SC, Mathur S. Effect of dietary iron deficiency anaemia on TSH and peripartum thyroid function. Endocrine Abs. 2006;12:P123. [Google Scholar]
- 14.Bastian TW, Prohaska JR, Georgieff MK, Anderson GW. Perinatal iron and copper deficiencies alter neonatal rat circulating and brain thyroid hormone concentrations. Endocrinology. 2010;151:4055–4065. doi: 10.1210/en.2010-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bastian TW, Anderson JA, Fretham SJ, Prohaska JR, Georgieff MK, Anderson GW. Fetal and Neonatal Iron Deficiency Reduces Thyroid Hormone-Responsive Gene mRNA Levels in the Neonatal Rat Hippocampus and Cerebral Cortex. Endocrinology. 2012;153:5668–5680. doi: 10.1210/en.2012-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith SM, Finley J, Johnson LK, Lukaski HC. Indices of in vivo and in vitro thyroid hormone metabolism in iron-deficient rats. Nutr Res. 1994;14:729–739. [Google Scholar]
- 17.Bernal J. Action of thyroid hormone in brain. J Endocrinol Invest. 2002;25:268–288. doi: 10.1007/BF03344003. [DOI] [PubMed] [Google Scholar]
- 18.Bastian TW, Prohaska JR, Georgieff MK, Anderson GW. Fetal and neonatal iron deficiency exacerbates mild thyroid hormone insufficiency effects on male thyroid hormone levels and brain thyroid hormone-responsive gene expression. Endocrinology. 2014;155:1157–1167. doi: 10.1210/en.2013-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ipek IÖ, Kaçmaz E, Bozaykut A, Gönül Sezer R, Seren L, Paketçi C. The effect of iron deficiency anemia on plasma thyroid hormone levels in childhood. Turk Arch Ped. 2011;46:122–125. [Google Scholar]
- 20.Tienboon P, Unachak K. Iron deficiency anaemia in childhood and thyroid function. Asia Pac J Clin Nutr. 2003;12:198–202. [PubMed] [Google Scholar]
- 21.Azizi F, Mirmiran P, Sheikholeslam R, Hedayati M, Rastmanesh R. The relation between serum ferritin and goitre, urinary iodine and thyroid hormone. Int J Vitam Nutr Res. 2002;72:296–299. doi: 10.1024/0300-9831.72.5.296. [DOI] [PubMed] [Google Scholar]
- 22.Refaat B. Prevalence and Characteristics of Anemia Associated with Thyroid Disorders in Non-pregnant Saudi Women during the Childbearing Age: A Cross-sectional Study. Biomed J. 2015;38:307–316. doi: 10.4103/2319-4170.151032. [DOI] [PubMed] [Google Scholar]
- 23.Chandel RS, Chatterjee G, Abichandani LG. Impact of subclinical hypothyroidism on iron status and hematological parameters. Ann Pathol Lab Med. 2015;2:A21–A25. [Google Scholar]
- 24.Martinez-Torres C, Cubeddu L, Dillman E, Brengelmann GR, Leets I, Layrisse M, Johnson DG, Finch C. Effect of exposure to low temperature on normal and iron - deficient subjects. Am J Physiol. 1984;246:R380–R383. doi: 10.1152/ajpregu.1984.246.3.R380. [DOI] [PubMed] [Google Scholar]
- 25.Beard JL, Tobin BW, Smith SM. Effects of iron repletion and correction of anemia on norepinephrine turnover and thyroid metabolism in iron deficiency. Proc Soc Exp Biol Med. 1990;193:306–312. doi: 10.3181/00379727-193-43040. [DOI] [PubMed] [Google Scholar]
- 26.Khatiwada S, Gelal B, Baral N, Lamsal M. Association between iron status and thyroid function in Nepalese children. Thyroid Res. 2016;9:2. doi: 10.1186/s13044-016-0031-0. doi: 10.1186/s13044-016-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khatiwada S, Lamsal M, Gelal B, Gautam S, Nepal AK, Brodie D, Baral N. Anemia, Iron Deficiency and Iodine Deficiency among Nepalese School Children. Indian J Pediatr. 2016;83:617–621. doi: 10.1007/s12098-015-1924-y. [DOI] [PubMed] [Google Scholar]
- 28.Eftekhari MH, Keshavarz SA, Jalali M, Saadat N, Seyasi F, Eshraghian MR. Thyroid Hormones Status in Iron Deficient Adolescent Girls. Iran J Med Sci. 2003;28:161–165. [Google Scholar]
- 29.Eftekhari MH, Keshavarz SA, Jalali M, Saadat N, Seyasi F, Eshraghian MR, Elguero E. The relationship between iron status and thyroid hormone concentration in iron-deficient adolescent Iranian girls. Asia Pac J Clin Nutr. 2006;15:50–55. [PubMed] [Google Scholar]
- 30.Surks MI. Effects of thyrotropin and thyroidal iodine metabolism during hypoxia. Am J Physiol. 1969;216:436–439. doi: 10.1152/ajplegacy.1969.216.2.436. [DOI] [PubMed] [Google Scholar]
- 31.Galton VA. Some effects of altitude on thyroid function. Endocrinology. 1972;91:1393–1403. doi: 10.1210/endo-91-6-1393. [DOI] [PubMed] [Google Scholar]
- 32.Beard JL, Brigham DE, Kelley SK, Green MH. Plasma thyroid hormone kinetics are altered in iron-deficient rats. J Nutr. 1998;128:1401–1408. doi: 10.1093/jn/128.8.1401. [DOI] [PubMed] [Google Scholar]
- 33.Larson SO. Anemia and iron metabolism in hypothyroidism. Acta Med Scand. 1957;157:339–363. doi: 10.1111/j.0954-6820.1957.tb14445.x. [DOI] [PubMed] [Google Scholar]
- 34.Chu JY, Monteleone JA, Peden VH, Graviss ER, Vernava AM. Anemia in children and adolescents with hypothyroidism. Clin Pediatr (Phila) 1981;20:696–699. doi: 10.1177/000992288102001102. [DOI] [PubMed] [Google Scholar]
- 35.Erdogan M, Kösenli A, Ganidagli S, Kulaksizoglu M. Characteristics of anemia in subclinical and overt hypothyroid patients. Endocr J. 2012;59:213–220. doi: 10.1507/endocrj.ej11-0096. [DOI] [PubMed] [Google Scholar]
- 36.Das C, Sahana PK, Sengupta N, Giri D, Roy M, Mukhopadhyay P. Etiology of anemia in primary hypothyroid subjects in a tertiary care center in Eastern India. Ind J Endocrinol Metab. 2012;16(Suppl 2):S361–S363. doi: 10.4103/2230-8210.104093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Franzese A, Salerno M, Argenziano A, Buongiovanni C, Limauro R, Tenore A. Anemia in infants with congenital hypothyroidism diagnosed by neonatal screening. J Endocrinol Invest. 1996;19:613–619. doi: 10.1007/BF03349027. [DOI] [PubMed] [Google Scholar]
- 38.Antonijevic N, Nesovic M, Trbojevic B, Milosevic R. Anemia in hypothyroidism. Med Pregl. 1999;52:136–140. [PubMed] [Google Scholar]
- 39.Chu JY, Monteleone JA, Peden VH, Graviss ER, Vernava AM. Anemia in children and adolescents with hypothyroidism. Clin Pediatr (Phila) 1981;20:696–699. doi: 10.1177/000992288102001102. [DOI] [PubMed] [Google Scholar]
- 40.Eftekhari MH. Effects of administration of iron, iodine and simultaneous iron-plus-iodine on the thyroid hormone profile in iron-deficient adolescent Iranian girls. Eur J Clin Nutr. 2006;60:545–552. doi: 10.1038/sj.ejcn.1602349. [DOI] [PubMed] [Google Scholar]
- 41.Zimmermann M, Adou P, Torresani T, Zeder T, Hurrell R. Iron supplementation in goitrous, iron-deficient children improves their response to oral iodized oil. Eur J Endocrinol. 2000;142:217–223. doi: 10.1530/eje.0.1420217. [DOI] [PubMed] [Google Scholar]
- 42.Gökdeniz E, Demir C, Dilek I. The effects of iron deficiency anemia on the thyroid functions. J Clin Exp Invest. 2010;1:156–160. [Google Scholar]
- 43.Erdal M, Sahin M, Hasimi A, et al. Trace element levels in hashimoto thyroiditis patients with subclinical hypothyroidism. Biol Trace Elem Res. 2008;123:1–7. doi: 10.1007/s12011-008-8117-8. [DOI] [PubMed] [Google Scholar]
- 44.Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, Braverman LE. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994) J Clin Endocrinol Metab. 2002;87:489–499. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- 45.Cinemre H, Bilir C, Gokosmanoglu F, Bahcebasi T. Hematologic effects of levothyroxine in iron-deficient subclinical hypothyroid patients: a randomized, double-blind, controlled study. J Clin Endocrinol Metab. 2009;94:151–156. doi: 10.1210/jc.2008-1440. [DOI] [PubMed] [Google Scholar]
- 46.Ravanbod M, Asadipooya K, Kalantarhormozi M, Nabipour I, Omrani GR. Treatment of iron-deficiency anemia in patients with subclinical hypothyroidism. Am J Med. 2013;126:420–424. doi: 10.1016/j.amjmed.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 47.Kurtoglu AU, Kurtoglu E, Temizkan AK. Effect of iron overload on endocrinopathies in patients with beta-thalassaemia major and intermedia. Endokrynol Pol. 2012;63:260–263. [PubMed] [Google Scholar]
- 48.Shamshirsaz AA, Bekheirnia MR, Kamgar M, Pourzahedgilani N, Bouzari N, Habibzadeh M, Hashemi R, Shamshirsaz AA, Aghakhani S, Homayoun H, Larijani B. Metabolic and endocrinologic complications in beta-thalassemia major: A multicenter study in Tehran. BMC Endocr Disord. 2003;3:4. doi: 10.1186/1472-6823-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Malik SA, Syed S, Ahmed N. Frequency of hypothyroidism in patients of beta-thalassaemia. J Pak Med Assoc. 2010;60:17–20. [PubMed] [Google Scholar]
- 50.Zervas A, Katopodi A, Protonotariou A, Livadas S, Karagiorga M, Politis C, Tolis G. Assessment of thyroid function in two hundred patients with beta-thalassemia major. Thyroid. 2002;12:151–154. doi: 10.1089/105072502753522383. [DOI] [PubMed] [Google Scholar]
- 51.Najafipour F, Aliasgarzadeh A, Aghamohamadzadeh N, Bahrami A, Mobasri M, Niafar M, Mobasri M, Niafar M, Khoshbaten M. A cross-sectional study of metabolic and endocrine complications in beta-thalassemia major. Ann Saudi Med. 2008;28:361–366. doi: 10.5144/0256-4947.2008.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Landau H, Matoth I, Landau-Cordova Z, Goldfarb A, Rachmilewitz EA, Glaser B. Cross-sectional and longitudinal study of the pituitary-thyroid axis in patients with thalassaemia major. Clin Endocrinol (Oxf) 1993;38:55–61. doi: 10.1111/j.1365-2265.1993.tb00973.x. [DOI] [PubMed] [Google Scholar]
- 53.Filosa A, Di Maio S, Aloj G, Acampora C. Longitudinal study on thyroid function in patients with thalassemia major. J Pediatr Endocrinol Metab. 2006;19:1397–1404. doi: 10.1515/jpem.2006.19.12.1397. [DOI] [PubMed] [Google Scholar]
- 54.Soliman AT, Al Yafei F, Al-Naimi L, Almarri N, Sabt A, Yassin M, De Sanctis V. Longitudinal study on thyroid function in patients with thalassemia major: High incidence of central hypothyroidism by 18 years. Indian J Endocr Metab. 2013;17:1090–1095. doi: 10.4103/2230-8210.122635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pitrolo L, Malizia G, Lo Pinto C, Malizia V, Capra M. Ultrasound thyroid evaluation in thalassemic patients: correlation between the aspects of thyroidal stroma and function. Pediatr Endocrinol Rev. 2004;2:313–315. [PubMed] [Google Scholar]
- 56.Gabutti V, Piga A. Results of long-term iron-chelating therapy. Acta Haematol. 1996;95:26–36. doi: 10.1159/000203853. [DOI] [PubMed] [Google Scholar]
- 57.Shupnik MA, Weck J, Hinkle PM. Thyrotropin (TSH)-releasing hormone stimulates TSH beta promoter activity by two distinct mechanisms involving calcium influx through L type Ca2+channels and protein kinase C. Mol Endocrinol. 1996;10:90–99. doi: 10.1210/mend.10.1.8838148. [DOI] [PubMed] [Google Scholar]
- 58.Farmaki K. Hypothyroidism in thalassemia. Publisher, INTECH Open Access Publisher, 2012, ISBN, 9535100211, 9789535100218, http://cdn.intechopen.com/pdfs-wm/27826.pdf . [Google Scholar]
- 59.Phillips G Jr, Becker B, Keller VA, Hartman J. Hypothyroidism in adults with sickle cell anemia. Am J Med. 1992;92:567–570. doi: 10.1016/0002-9343(92)90757-3. [DOI] [PubMed] [Google Scholar]
- 60.Fung EB, Harmatz PR, Lee PD, Milet M, Bellevue R, Jeng MR, Kalinyak KA, Hudes M, Bhatia S, Vichinsky EP. Multi-Centre Study of Iron Overload Research Group. Increased prevalence of iron-overload associated endocrinopathy in thalassaemia versus sickle cell disease. Br J Haematol. 2006;135:574–582. doi: 10.1111/j.1365-2141.2006.06332.x. [DOI] [PubMed] [Google Scholar]
- 61.Parshad O, Stevens MC, Hudson C, Rosenthal J, Melville GN, Dunn DT, Serjeant GR. Abnormal thyroid hormone and thyrotropin levels in homozygous sickle cell disease. Clin Lab Haematol. 1989;11:309–315. doi: 10.1111/j.1365-2257.1989.tb00228.x. [DOI] [PubMed] [Google Scholar]
- 62.Özen S, Ünal S, Erçetin N, Taşdelen B. Frequency and Risk Factors of Endocrine Complications in Turkish Children and Adolescents with Sickle Cell Anemia. Turkish Journal of Hematology. 2013;30:25–31. doi: 10.4274/tjh.2012.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Evliyaoğlu N, Kilinç Y, Sargin O. Thyroid functions in mild and severe forms of sickle cell anemia. Acta Paediatr Jpn. 1996;38:460–453. doi: 10.1111/j.1442-200x.1996.tb03527.x. [DOI] [PubMed] [Google Scholar]
- 64.Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet. 2010;376:2018–2031. doi: 10.1016/S0140-6736(10)61029-X. [DOI] [PubMed] [Google Scholar]
- 65.Smiley D, Dagogo-Jack S, Umpierrez G. Therapy insight: metabolic and endocrine disorders in sickle cell disease. Nat Clin Pract Endocrinol Metab. 2008;4:102–109. doi: 10.1038/ncpendmet0702. [DOI] [PubMed] [Google Scholar]
- 66.El-Hazmi MA, Bahakim HM, al-Fawaz I. Endocrine functions in sickle cell anaemia patients. J Trop Pediatr. 1992;38:307–313. doi: 10.1093/tropej/38.6.307. [DOI] [PubMed] [Google Scholar]
- 67.Al-Saqladi AW, Cipolotti R, Fijnvandraat K, Brabin BJ. Growth and nutritional status of children with homozygous sickle cell disease. Ann Trop Paediatr. 2008;28:165–189. doi: 10.1179/146532808X335624. [DOI] [PubMed] [Google Scholar]
- 68.Karazincir S1, Balci A, Yonden Z, Gali E, Daplan T, Beyoglu Y, Kaya H, Egilmez E. Thyroid Doppler indices in patients with sickle cell disease. Clin Imaging. 2013;37(5):852–5. doi: 10.1016/j.clinimag.2013.05.008. doi: 10.1016/j.clinimag.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 69.Phillips G Jr, Becker B, Keller VA, Hartman J. Hypothyroidism in adults with sickle cell anemia. Am J Med. 1992;92:567–570. doi: 10.1016/0002-9343(92)90757-3. [DOI] [PubMed] [Google Scholar]
- 70.Soliman AT, Yassin M, Bedai S. Pituitary abnormalities in short adolescents and young adults with sickle-cell disease and recurrent vaso-occlusive crisis. Endocrine Abs. 2014;35:P859. [Google Scholar]
- 71.Soliman AT, Darwish A, Mohammed SH, Bassiony MR, El Banna N, Asfour M. Circulating growth hormone (GH), insulin-like growth factor-I (IGF-I) and free thyroxine, GH response to clonidine provocation and CT scanning of the hypothalamic-pituitary area in children with sickle cell disease. J Trop Pediatr. 1995;41:285–289. doi: 10.1093/tropej/41.5.285. [DOI] [PubMed] [Google Scholar]