Abstract
Aim: This article attempts to describe the aging process of the vocal folds and the main features of the aged voice. Background: In the world ageing population era, aging diseases and aging disorders are crucial. Voice disorders (presbyphonia) are common in the elderly and have a significant impact on communication and quality of life. Some of these disorders depend on the vocal folds, which consist of an extracellular matrix (ECM), fibrous proteins, interstitial proteins, and glycosaminoglycans. The density and spatial arrangement of these elements are important, as changes in their deposition can alter the biomechanical properties and vibratory function of the vocal folds. Discussion: The aging voice process is analyzed in detail from mechanical factors like pulmonary bellows alteration, to hormonal factors and life style. Conclusions: The elderly people undergoe mechanical, anatomical and functional changes: alterations of the pulmonary bellows, systemic changes like hormonal disregulation, and laryngeal changes, that resulting in hoarseness, which is difficult to treat. (www.actabiomedica.it)
Keywords: presbiphonia, aging voice, young voice, elderly voice, vocal tract
Background
Age-related vocal fold’s alterations in the deposition of extracellular matrix (ECM) have been reported in humans, including excessive accumulation of collagen, dense collagen bundles, reduced elastin, and decreased hyaluronic acid (HA), and it has been suggested that the vocal folds undergo an age-dependent slowdown in the synthesis and degradation of collagen strictly related to a slowdown in the expression of genes coding for the matrix and degrading enzymes. The result is the over accumulation of ECM collagen observed in elderly humans vocal folds (1, 2).
Many factors influence the changes in voice that occur throughout life: changes in the volume and shape of the vocal tract and rib cage, steroid hormones, the biochemical-histological morphology of the vocal fold, and many other circumstantial factors.
This article attempts to describe the aging process of the vocal folds and the main features of the aged voice.
Discussion
Alterations of the pulmonary bellows
The rib chest is a group of structures whose main role is to allow gaseous exchange between air and blood and only secondarily produce the air flow needed for phonation. The pulmonary bellows is made up of many structures, in particular the thorax skeleton, with its twelve dorsal vertebrae, the ribs and the sternum; the main inspiratory muscles (diaphragm, external intercostal muscles), the secondary inspiratory muscles (sternocleidomastoid muscle, scalene muscles); the expiratory muscles (internal intercostal muscles, abdominal muscles) and of course the lungs. During respiratory acts the ribs and sternum perform complex movements with two components: one vertical and one horizontal. During inspiration the upper part of the thorax elevates and the lower part expands (Fig. 1).
Figure 1.
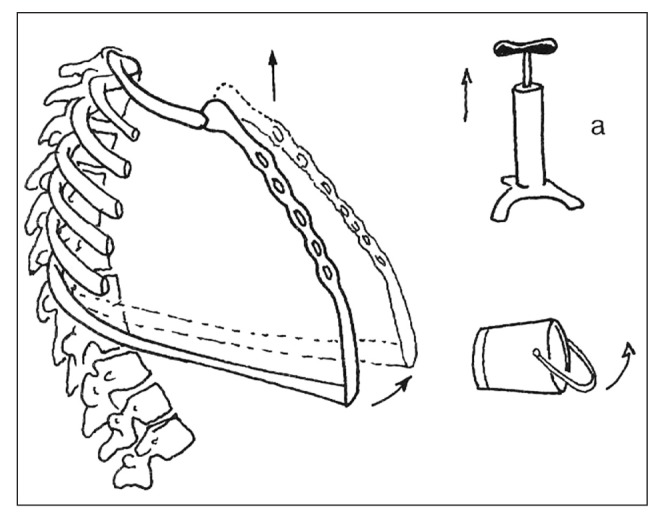
Expansion to “pump handle” and “bucket handle”
Elderly people often lean forward (elderly person’s cyphoscolosis) and the respiratory muscles are weaker. In this case, the rib chest and the spinal column are modified (cyphoscoliosis) and the lung bellows is weaker. Overlapping comorbidities like Chronic Obstructive Pulmonary Disease and emphysema can also contribute to a deficit in the airflow necessary for good speech. These factors lead to reduced efficiency of the bellows breathing, which, in the elderly, leads to the phenomenon known as phonasthenia (3).
Changes in the vocal tract
Changes in the vocal tract throughout life play a very significant role in the voice’s aging process. In infants, the larynx is high-lying and this allows the epiglottis and soft palate to overlap and the pharyngeal lumen to open directly in the nasopharynx; the larynx is intranarinal. This anatomy allows sucking and breathing simultaneously. An infant’s nasal breathing and swallowing occurs only in a “safe” part of the breathing-swallowing cycle.
At this stage of life, nutrition and breathing are the main purpose of human beings and communication is nonverbal; facial expressions and crying being the main performances. The vocal tract is not completely developed and phonemes are articulated in a very rudimentary way. In the infant the protection of the airway is further enhanced by the shape of the epiglottis, by the aryepiglottic-folds, by their thickness and by the introversion of the epiglottis to the glottic level of the arytenoids.
The oropharynx begins to increase in volume at two years of age. The descent of the hyoid-mandibular complex coincides with the eruption of the deciduous teeth, expression of the maturation of the complex mechanisms for coordination of the neuromuscular control of swallowing, and coincides with the beginning of the spoken word.
In adult humans, unlike in infants, the airway and the digestive tracts are not completely separated, but intersecting, so it is necessary to close the larynx during swallowing and achieve perfect neuromuscular coordination of the swallowing mechanism. Verbal expression becomes predominant and it has probably influenced the shape and position of the larynx and vocal tract, from an evolutionary point of view (4) (Fig. 2).
Figure 2.
Larynx in childhood and adult larynx
In the elderly, the vocal tract undergoes further significant changes: the nose falls, teeth can be missing, and there is often weight gain with the accumulation of fat in the neck and in the parapharyngeal space.
Hormonal changes
Hormonal changes can profoundly influence the aging voice. The voice is a secondary sexual characteristic, influenced by sex hormones. Their concentrations have a crucial impact on phonation and on the morphology of the human larynx, in which a significant number of receptors for these hormones can be found (5). The phono-articulatory function should be considered an integrated system: neurological, motor and psychological, involving the larynx and the vocal tract, and within this system, sex hormones play a pivotal role (6). The human voice is characterized by two independent acoustic components, both influenced by the hormonal balance: the Fundamental frequency (F0), determined by the vibration of the vocal folds, and Formant frequencies (or resonant): determined by the size and shape of the vocal tract.
In post-menopausal women, estrogen levels fall and androgen levels increase relatively; this leads to an increase in the thickness of the vocal cords, which in turn leads to a deeper tone of voice: refered to as virilization of the voice (7). After menopause, the agility in speech decreases and the breaks increase: the speech is slower. This phenomenon is called verbal diadochokinesis and seems to be closely linked to neurophysiological, metabolic and structural mechanisms associated with the decrease of estrogen, and it improves with hormone replacement therapy (6). These phenomena do not necessarily make the voice less pleasant.
A complementary phenomenon occurs in men when the male andropause occurs after a fall in the level of androgens and a relatively rise in the estrogen/androgen ratio with consequent thinning of the vocal folds; the vocal timbre becomes more acute and feminization of the voice occurs (8). In the male, the fundamental frequency of the voice is “a call” to the female, but also an “alert” for rivals; in both cases it is a “signal” of the physical-reproductive features of the subject and then plays the role of a secondary sexual characteristic.
Before puberty there is little difference between male and female voices. During puberty, the male and female larynxes undergo changes that stabilize different patterns that affect the F0 (the average F0 in an adult man is about 1oo Hz, while that in an adult woman is about 213 Hz, Figure 3). In old age, the climacteric and menopause tend to reduce the differences between the sexes. It has been shown that the correlation between blood levels of testosterone and F0 is very significant. This finding emphasizes the importance of testosterone in the development of the male larynx. On the other hand, the vocal tract, which determines the formant frequencies, seems to be more influenced by other factors, such as Growth Hormone (GH) (6).
Figure 3.
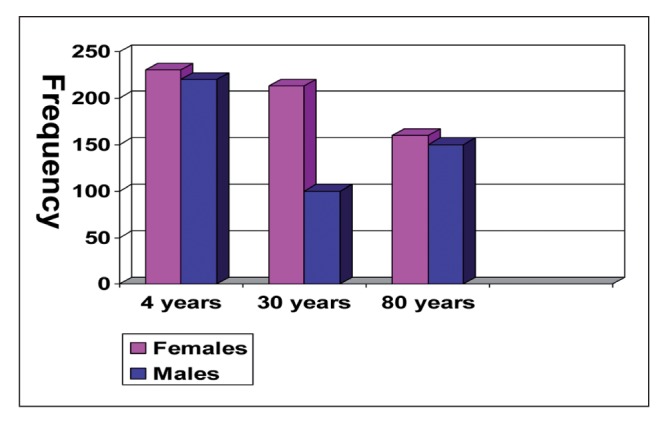
Fundamental frequencies are very different in male and females during reproductive life, but they are very similar in childhood and old age ( non reproductive life)
Circumstantial factors
The main circumstantial factors that influence the aging process include lifestyle, especially smoking (3-4 benzopyrene, anthracene and other hydrocarbons), alcohol abuse, diet (folate deficiency), the workplace (inhalation of irritating dust-gas-vapors), certain digestive diseases (pharyngolaryngeal reflux/gastroesophageal reflux), some general and local-regional diseases (chronic laringopathy), infectious causes (HPV, HIV, chronic candidiasis) and thesaurismosis (amyloidosis).
Changes in the histological-biochemical morphology of the vocal fold
Changes in histological-biochemical morphology of the vocal fold play a crucial role in the development of presbyphonia, and the maculae flavae seem to be mainly responsible for these variations (9). The maculae flavae are dense masses of cells and extracellular matrix that are located at the anterior and posterior commissures. The cellular component consists of stellate cells (immature) and fibroblasts. The extracellular component is made up of protein fibrils: collagen fibers, reticular and elastic, glycoproteins and hyaluronic acid (HA) (10). HA plays a biomechanical role tied to the three-dimensional arrangement of its macromolecules; it forms large macromolecular aggregates in a large porous mesh. The pores of HA contain fibrous proteins, collagen and elastic fibers. HA is concentrated in the middle layer of the lamina propria and in particular at the lower face of the vocal cord (infrafold area). This structure damps down the vibratory vocal fold and protects it from trauma caused by the forcefull expulsion of air necessary for phonation. Its presence is strongly affected by vitamin A, stored in stellate cells (11). The stellate cells of children constantly synthesize extracellular matrix, which is essential for the viscoelastic properties of the lamina propria of the future adult, and become fibroblasts.
The maculae flavae in infants, although immature, are crucial structures for the growth and development of the vocal ligament. In the infant, the maculae flavae produce collagen and elastic fibers to create an immature ligament. In children, the fibroblasts in the maculae flavae begin to get longer and produce extracellular matrix (12). Fibroblasts synthesize collagen and elastic fibers in the lamina propria of the vocal ligament and in turn are produced and controlled in the maculae flavae.
The changes that occur at the level of the vocal folds are related to the structure and function of fibroblasts, and they influence the development of histological components of the vocal ligament. The vocal ligament keeps the vocal folds stretched and plays a complex and still not fully understood role; it is absent in the infants of other mammals, (it develops with age) and mute individuals (so it is a dynamic element). Cytological studies performed on fibroblasts of the superficial layer of the lamina propria and maculae flavae show significant morphological and functional changes with aging (13). Adult human fibroblasts in the maculae flavae produce collagen and elastic fibers that continually renew the ligament.
In the elderly the activity of the fibroblasts in the maculae flavae decreases, and less hyaluronic acid is produced. Elastic fibers tend to atrophy and instead collagen fibers tend to increase in density. The stiffness of the vocal ligament increases and the increase in collagen concentration reduces the viscoelastic properties of the mucosa (14). So in the elderly there is a thinning of the surface layer of the lamina propria and the glottis becomes oval with consequent glottic failure.
Conclusions
In summary the aging of the vocal cords is charaterized by many factors: mechanical factors, structural factors, systemic (hormonal) factors and laryngeal factors.
The principal mechanisms that lead to hoarsness are alterations of the pulmonary bellows, wich becomes stiffner and weaker; alterations of the vocal tract that is high-lying in infants, then decends in the neck subsequently; hormonal changes: the voice is a secondary sexual characteristic, influenced by sex hormones, that vary through life depending on the reproductive needs, indeed fundamental frequencies are very different in male and females during reproductive life, but they are very similar in childhood and old age ( non reproductive life); circumstantial factors like smoking, workplace, digestive disease, some general and localregional disease and infectious causes; changes in the histological-biochemical morphology of the vocal fold that leads to mucosal and lamina propria atrophy, resulting in hoarseness, which is difficult to treat.
Basic Fibroblast Growth Factor (bFGF) is a growth factor that acts as a catalyst: it stimulates the regeneration of fibroblasts and extracellular matrix through the involvement of the macula flavae as potential banks of fibroblasts and extracellular matrix (15).
Future perspective: some prospective animal studies have shown that Hepatocyte Growth Factor (HGF) can increase the amount of hyaluronic acid and decrease the concentration of type I collagen (16, 17). One study investigates the potential of the decellularized human umbilical vein (HUV) as an allogeneic, acellular extracellular matrix (ECM) scaffold for engineering the vocal fold lamina propria in vitro (18). Thus the maculae flavae can be interpreted as the true stem cells of the vocal folds.
Acknowledgment
We thank Leona Bassein for revising the English and contributing to the organization of this review.
References
- 1.Ohno T, Hirano S, Rousseau B. Age-Associated Changes in the Expression and Deposition of Vocal Fold Collagen and Hyaluronan. Ann Otol Rhinol Laryngol. 2009;118(10):735–41. doi: 10.1177/000348940911801009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ding H, Gray SD. Senescent expression of genes coding collagens, collagen-degrading metalloproteinases, and tissue inhibitors of metalloproteinases in rat vocal folds: comparison with skin and lungs. J Gerontol A Biol Sci Med Sci. 2001;56(4):B145–52. doi: 10.1093/gerona/56.4.b145. [DOI] [PubMed] [Google Scholar]
- 3.Bergamini G, Fustos R, Casolino D. Relazione ufficiale del LXXXIX Congresso Nazionale SIO, San Benedetto del Tronto, 22-25 maggio 2002. Vol. 46. Pisa: Pacini editore; 2002. Anatomo fisiologia dell’apparato pneumo-fonatorioIn: Casolino D, editor. Le disfonie: fisiopatologia, clinica ed aspetti medico legali. [Google Scholar]
- 4.Laitman JT, Reidenberg JS. The Human Aerodigestive Tract and Gas Reflux: An Evolutionary Perspective. The American Journal of Medicine. 1997;24:103(5A). doi: 10.1016/s0002-9343(97)00313-6. [DOI] [PubMed] [Google Scholar]
- 5.Butler JE, Hammond TH, Gray SD. Gender-related differences of hyaluronic acid distribution in the human vocal fold. Laryngoscope. 2001;111(5):907–11. doi: 10.1097/00005537-200105000-00029. [DOI] [PubMed] [Google Scholar]
- 6.Meurer EM, Wender MCO, Corleta HvE, Capp E. Female suprasegmental speech parameters in reproductive age and postmenopause. Maturitas. 2004;48(1):71–7. doi: 10.1016/j.maturitas.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Laureano JM, Sa MFS, Ferriani RA, Romao GS. Variation on jitter and shimmer among women in menacme and postmenopausal women. Journal of Voice. 1997;23(6):687–6. doi: 10.1016/j.jvoice.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Evans S, Neave N. The relationship between testosterone and vocal frequencies in human males. Delia Wakelin and Colin Hamilton Physiology & Behavior. 2008;93(4-5):783–8. doi: 10.1016/j.physbeh.2007.11.033. [DOI] [PubMed] [Google Scholar]
- 9.Sato K, Hirano M, Nakashima T. 3D Structure of the Macula Flava in the Human Vocal Fold. Acta Oto-laryngologica. 2003;123(2):269–73. doi: 10.1080/00016480310001123. [DOI] [PubMed] [Google Scholar]
- 10.Fuja TJ, Ostrem EM, Probst-Fuja MN, Titze IR. Differential cell adhesion to vocal fold extracellular matrix constituents. Matrix Biology. 2006;25:240–51. doi: 10.1016/j.matbio.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Sato K, Nakashima T. Stellate cells in the human child vocal fold macula flava. The Laryngoscope. 2009;119(1):203–10. doi: 10.1002/lary.20010. [DOI] [PubMed] [Google Scholar]
- 12.Awd Allah R S, Dkhil M A, Farhoud E. Fibroblasts in the human vocal fold mucosa an ultrastructural study of different age groups. Singapore Med J. 2009;50(2):201. [PubMed] [Google Scholar]
- 13.Sato K, Hirano M. Age-related changes of elastic fibers in the superficial layer of the lamina propria of vocal folds. Ann Otol Rhinol Laryngol. 1997;106:44–8. doi: 10.1177/000348949710600109. [DOI] [PubMed] [Google Scholar]
- 14.Sato K, Hirano M, Nakashima T. Age-related changes of collagenous fibers in the human vocal fold mucosa. Ann Otol Rhinol Laryngol. 2002;111:15–20. doi: 10.1177/000348940211100103. [DOI] [PubMed] [Google Scholar]
- 15.Hirano S, Kishimoto Y, Suehiro A, Kanemaru S, Ito J. Regeneration of aged vocal fold: first human case treated with fibroblast growth factor. Laryngoscope. 2008;118(12):2254–9. doi: 10.1097/MLG.0b013e3181845720. [DOI] [PubMed] [Google Scholar]
- 16.Ohno T, French LC, Hirano S, Ossoff RH, Rousseau B. Effect of Hepatocyte Growth Factor on Gene Expression of Extracellular Matrix During Wound Healing of the Injured Rat Vocal Fold. Ann Otol Rhinol Laryngol. 2008;117:696–702. doi: 10.1177/000348940811700912. [DOI] [PubMed] [Google Scholar]
- 17.Suehiro A, Wright H, Rousseau B. Optimal Concentration of Hepatocyte Growth Factor for Treatment of the Aged Rat Vocal Fold. Laryngoscope. 2011;121(8):1726–34. doi: 10.1002/lary.21816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan RW, Rodriguez ML, McFetridge PS. The Human Umbilical Vein with Wharton’s Jelly as an Allogeneic, Acellular Construct for Vocal Fold Restoration. Tissue Engineering. 2009;15(11) doi: 10.1089/ten.tea.2009.0064. Part A. [DOI] [PMC free article] [PubMed] [Google Scholar]


