Abstract
Aim: Many aspects of the surgical management of multiple sporadic colorectal cancer syndrome, either synchronous and metachronous, remain to be cleared, in particular the prognostic influence of the extent of surgical resection. Method: A retrospective review was performed of patients diagnosed with multiple colorectal cancer from 1982 to May 2010. Clinical and pathologic data were collected and reviewed. Survival analysis was performed. Results: We identified 23 patients with multiple sporadic colorectal cancers, of which 8 had synchronous (SC) and 15 metachronous cancers (MC). Of the MC patients, 2 (13%) had the second cancer within 2 years, 4 (27%) in the time period of 2-5 years and 9 (60%) after 5 years. Twenty-one patients underwent multiple segmental resections; 2 patients underwent subtotal colectomy. The 5-year overall survival rate of SC and MC patients was 100% and 87% (p<0.001) respectively. The 5-year overall survival rate of multiple segmental resection patients and subtotal colectomy was 94% and 75% (p=0.655) respectively. Conclusion: Either synchronous and metachronous MSCRC patients showed good prognosis independently from to the extent of resection. Our results support a less aggressive biological behaviour allowing a more conservative management. Multiple segmental colorectal resections seem appropriate from an oncologic point of view in MSCRC patients. (www.actabiomedica.it)
Keywords: multiple colorectal cancer, synchronous colorectal cancer, metachronous colorectal cancer, subtotal colectomy, multiple segmental resections
Introduction
Multiple primary colorectal cancers account for 2-5% of all colorectal cancers. The incidence of multiple primary colorectal cancer increases to up to 20% in patients diagnosed with heredo-familial syndrome (1, 2). About 25% of patients with colorectal cancer have a family history of colorectal cancer that suggests a hereditary contribution, common exposures among family members, or a combination of both, whereas the majority of patients have a sporadic disease with no apparent evidence of having inherited the disorder. Multiple colorectal cancer can occur in the absence of a defined heredo-familial syndrome, presenting as metachronous carcinomas (MC) in patients with a history of sporadic colorectal cancer or two or more sporadic synchronous carcinomas (SC) at the diagnosis. A personal history of colorectal cancer is a well-known risk factor for developing a second colorectal cancer and it is estimated that in patients undergoing resection of a single colorectal cancer, metachronous colorectal cancer develops in 1.5% to 3% of cases within the first 5 years postoperatively. The risk remains high for up to ten years in some patients (3, 4). Over one-half of second primary colorectal cancers arise within 24 months of the initial resection and may represent synchronous cancers that were missed initially (5, 6). The prognosis of multiple colorectal cancer remains controversial, and although postoperative surveillance is highly recommended for detecting metachronous cancers or polyps, the optimal frequency and benefits of postoperative colonoscopy are still under debate (7-10). Moreover, while in younger patients affected by heredo-familial colorectal cancer syndromes a prophylactic total colectomy is recommended instead of multiple segmental colorectal resections, in multiple sporadic colorectal cancer this indication seems unclear and not supported by current evidence (11).
The purpose of the present study is to better define the prognostic influence of the surgical management of multiple sporadic colorectal cancer patients.
Methods
Clinical and pathological data
The medical records of patients with the diagnosis of multiple synchronous or metachronous colorectal cancer treated at our Institution between 1982 and 2010 were retrospectively reviewed. A database of patients with a histological diagnosis of multiple colorectal adenocarcinoma was created. Follow-up data were obtained from the Parma Cancer Registry and patients’ clinical charts.
Demographic and clinical data (age, gender, history of cancer, history of colonic polyposis, type of surgery, post-operative morbidity and mortality), pathological data (location of tumours, interval time between tumours, TNM Staging according to the American Joint Committee on Cancer, number of lymph nodes examined, number of lymph nodes positive, grade, histologic type) and data on adjuvant chemotherapy and radiotherapy were collected. If not clearly reported by clinical charts and registries, anamnestic data were additionally collected by telephone interview.
In order to focus the analysis only on sporadic colorectal cancers, familial adenomatous polyposis and Lynch syndrome cases, screened by Amsterdam II, Bethesda and Revised Bethesda guidelines for hereditary non-polyposis CRC were considered as specific exclusion criteria (12).
A metachronous cancer was defined as a second primary colorectal cancer occurring more than 6 months after the index cancer without evidence of local recurrence (13, 14).
In order to summarize the location of the tumours, the large intestine was divided into 3 sectors based on the main feeding vessels: Right-section (R) from the caecum to the proximal transverse colon fed by the ileo-colic and the right colic vessels, Transverse-section (T) from the middle transverse colon to the splenic flexure fed by the middle colic vessels and Left-section (L) from the descending colon to the rectum fed by the inferior mesenteric vessels. Thus, the location of MSCRC was classified into 3 groups based on the combination of the two colorectal sections involved for each patient: right and left sections (RL), right and transverse sections (RT), left and transverse sections (LT).
Since there were no cases of total colectomy, the extent of surgical resection was classified into two categories: multiple segmental colorectal resections in the case of preservation of at least one main pedicle with all its branches, and subtotal colectomy when one branch of the only preserved pedicle was ligated. Reconstruction of the bowel transit was achieved through 5 different procedures: ileo-transverse colo-rectal anastomosis (ITR), ileo-descending colonic anastomosis (ID), ileosigmoid colonic anastomosis (IS), ascending colo-rectal anastomosis (AR), or anti-peristaltic caeco-rectal anastomosis (aCR) (15-17).
Statistical analysis
Data were analyzed using SPSS software (version 14.0; SPSS, Inc., Chicago, IL, USA). The MannWhitney U test was used to compare medians between the numeric variables. Pearson chi-square test was used to compare proportions and nominal variables. Survival analysis was performed utilizing the KaplanMeyer method. Follow up of metachronous CRC patients was considered as starting at the time of the first colorectal resection or alternatively as starting at the time of the second colorectal resection, when judged appropriate. Possible prognostic factors influencing survival were first evaluated by univariate analysis (logrank test). Only parameters which showed significance in univariate analysis were further analyzed by multivariate analysis (Cox proportional hazards test, forward-conditional method). Statistical significance was determined by ap value of less than .05.
Results
Population characteristics
We identified 23 patients diagnosed with multiple sporadic colorectal cancers. The median age at the time of diagnosis was 72 years (range: 63-80) for SC patients and 71 years (range: 50-85) and 78 years for index and metachronous cancers respectively in MC patients, with a mean interval time between the diagnosis of the first and the second cancer of 106±97 months (range: 8-360). The majority of MC patients developed the second cancer after 5 years (60%), 2 (13%) within 2 years and 4 (27%) in the time period of 2-5 years. The male-to-female ratio of SC and MC groups was 1.00 and 2.75 respectively, whereas the ratio of controls was 0.97.
Location
Eight, 11 and 4 patients were reported in group RL, LT and RT respectively. There were 5 patients with SC in the RL group, 1 in the LT group and 1 in the RT group. Only one patient, classified as LT, had three synchronous cancers, located at rectum, sigmoid colon and splenic flexure. Twelve out of the 15 patients with MC (80%) had the first tumour located distally to the splenic flexure, 5 at the descending colon and 7 at the rectum; 3 of these subsequently developed a second cancer at the right colon, 8 at the transverse colon and 1 at the rectum. The remaining 3 MC patients had the first tumour located proximally to the hepatic flexure, 1 at the ascending colon and 2 at the hepatic flexure; these patients subsequently developed a second cancer at the transverse colon.
Surgical resection and clinical outcomes
We performed a multiple segmental colorectal resection in 19 patients (83%). A subtotal colectomy was performed in 4 patients, 2 preserving the sigmoid colon fed by the sigmoid vessels and 2 preserving the caecum fed by the ileo-colic vessels, all due to a blood supply of the remnant colon judged as insufficient after multiple segmental colorectal resections. Bowel reconstruction was achieved through 9 AR (39%), 8 ITR (35%), 2 ID (9%), 2 IS (9%) and 2 aCR (9%) anastomoses. All the 8 patients with RL cancer location underwent ITR anastomosis with middle colic vessel preservation; the left colic vessels were preserved in 2 of those patients. The 4 patients with RT cancer location underwent 2 ID and 2 IS anastomoses respectively; the left colic vessels were consistently preserved in the 2 ID anastomoses while the middle and left colic vessels were both ligated in the 2 patients undergoing IS anastomosis. Nine out of the 11 patients with LT cancer location underwent AR; in 4 patients the middle colic vessels were preserved. The remaining 2 patients underwent aCR anastomosis.
Overall postoperative morbidity and mortality were 26% and 0% respectively. No difference in terms of morbidity and mortality between multiple segmental resection and subtotal/total colectomy was found (27% vs. 50%; p=0.231).
Pathological findings
Stage, grade and histotype are reported in Table 1. Mucinous histotype was found in 12 tumours with an overall prevalence of 26%. SC patients showed a higher prevalence of cancers with mucinous histotype, albeit not significant (31% vs. 23%;p=0.726). Four patients had both mucinous tumours whereas 4 patients had only one mucinous tumour.
Table 1.
Demographic, clinical, pathological and molecular data of multiple sporadic colorectal cancer patients
| Syn/Met | Gender | Age | Stage | Grade | Mucinous tumour |
|---|---|---|---|---|---|
| Syn | M | 69 | II - I | G2 - G3 | Both |
| Syn | F | 68 | I - I | G2 - G1 | None |
| Syn | M | 74 | II - III | G2 - G3 | Both |
| Syn | F | 70 | I - I | G2 - G3 | None |
| Met | M | 69 | III - I | G3 - G2 | None |
| Met | M | 79 | III - II | G2 - G3 | Second |
| Met | M | 71 | III - III | G3 - G3 | Both |
| Syn | F | 80 | II - I | G2 - G3 | None |
| Met | M | 48 | I - III | G3 - G3 | None |
| Met | M | 73 | II - II | G3 - G3 | First |
| Syn* | M | 79 | IV - IV | G2 - G2 | First |
| Met | F | 54 | I - I | G3 - G2 | None |
| Met | M | 76 | I - I | G1 - G2 | Second |
| Met | M | 57 | II - II | G3 - G3 | Both |
| Met | M | 84 | I - II | G2 - G2 | None |
| Met | F | 56 | I - I | G2 - G2 | None |
| Met | F | 85 | III - III | G3 - G3 | None |
| Syn | F | 63 | III - I | G2 - G3 | None |
| Met | M | 75 | III - I | G3 - G2 | None |
| Met | F | 60 | III - III | G2 - G2 | None |
| Syn | M | 73 | I - II | G2 - G2 | None |
| Met | M | 76 | I - II | G2 - G2 | None |
| Met | M | 51 | II - II | G2 - G2 | None |
patient diagnosed with three synchronous colorectal tumours
Survival analysis
The median follow-up of SC and MC patients was 78 and 132 months respectively. The 5-year and 10-year overall actuarial survival rates were 100% and 80% in SC patients, 87% and 78% from the first cancer in MC patients respectively (p=0,976). (Fig. 1) Interval time between the index and second cancer was not associated with the overall survival after the second cancer in MC patients (p=0.284). In fact, the 5-year overall survival after the second cancer of MC patients developing the second cancer before or after the 5-year-surveillance was 67% and 64% respectively (p=0.883). A recurrence was documented in 6 patients (26%). The first site of recurrence was loco-regional in 1 SC patient and distant in 5 patients: 3 MC patients and 2 SC patients. The site of distant failure was the liver in 2 patients, the lung in 2 patients and the peritoneum in 1 patient.
Figure 1.
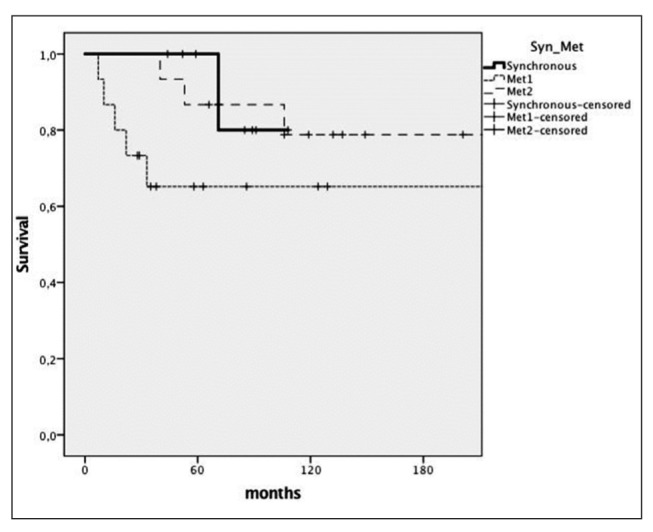
Kaplan-Meyer survival curves comparing overall survival of synchronous cancer patients (Synch), metachronous cancer patients from the first cancer (Met1), metachronous cancer patients from the second cancer (Met2)
Table 2.
Variable influencing survival in multiple sporadic colorectal cancer patients
| Univariate analysis | |
|---|---|
| Gender | 0.586 |
| Age | 0.041* |
| Interval (within 5 yrs) | 0.052* |
| Location | 0.660 |
| Extent of surgical resection | 0.655 |
| Stage | 0.281 |
| Grade | 0.730 |
| Nodal involvement | 0.064* |
| Mucinous histotype | 0.875 |
| Adjuvant therapy in stage III | 0.868 |
Univariate analysis demonstrated that later age at the first cancer diagnosis (p=0.002) and nodal involvement (p=0.064) were associated with a significant decrease in overall survival (Tab. 2). The Cox proportional hazards test, performed on the MSCRC patients, failed to identify independent predictors of prognosis.
Discussion and conclusions
MSCRC is a relatively rare disease. The reported incidence of multiple primary colorectal cancers is considerably variable, ranging from 0.6 to 10.6%; the probable reasons for this variability are the low incidence, the lack of routine screening for heredo-familial colorectal cancer syndromes and, most of all, the lack of distinction between sporadic and non-sporadic multiple colorectal cancer (18, 19).
The most relevant data of our series arose from survival analysis: both synchronous and metachronous multiple sporadic colorectal cancer patients showed excellent prognosis independently from stage and grade, despite a significantly higher median age.
Survival data suggest the hypothesis of a different pathogenesis of MSCRC, possibly resulting in a less aggressive biologic behaviour. The comparison of pathologic features of multiple cancers in each patient revealed a tendency of SC patients to develop cancers of differing stage and grade, whereas MC patients tend to develop cancers of similar stage and grade in the vast majority of cases. Mucinous histotype as a pathologic feature appears to have a similar distribution among SC and MC patients to that of the general population. Those data are surely not sufficient to account for such a difference in survival rate; only a thorough pathological and genetic analysis on larger samples could yield more exhaustive answers (20-23).
From a clinical/therapeutic point of view, while SC patients represent a distinct subgroup of cancer patients with a possibly better prognosis, MC patients could be considered as a subgroup of patients with a new sporadic colorectal cancer after a curative treatment of the first one, thus reflecting both a higher tendency to develop colonic cancer and a positive response to oncologic treatment. Only larger cohort studies will be able to confirm these hypotheses. Many authors reported an extremely varying average of interval to detection of a metachronous colorectal cancer (10, 13, 19, 24, 25). In our series 63% of the MC patients developed the second cancer after the 5 years of surveillance and the interval time did not influence the prognosis of the second cancer.
Concerning the surgical approach, in our series it was constantly conservative, aimed at the preservation of bowel function, subtotal and total colectomy being performed only in the case of suboptimal or insufficient blood supply of the remnant colon (15). Supported by our short- and long-term results, segmental or regional colonic resections seem appropriate in the elective treatment of MSCRC, and the indication for a total colectomy should not be based on oncologic purposes in either SC or MC patients. We have no data on the influence of the laparoscopic approach. Multiple segmental colorectal resection for synchronous or metachronous cancer could be technically more difficult, although the progressive advances in mini-invasive surgical techniques could make the laparoscopic approach the gold-standard even in these patients (26, 27).
In conclusion, MSCRC showed specific clinical features with a better prognosis than single sporadic colorectal cancer, both for SC and for MC patients. Segmental or regional colorectal resections seem appropriate from an oncologic point of view in the elective treatment of MSCRC, and the indication for a total colectomy should not be based on oncologic purposes in these patients. Future research should confirm these results on larger series, possibly identifying a tailored therapeutic approach and surveillance for this subgroup of oncologic patients.
Conflict of Interest Statement:
Dr Stefano Cecchini is currently receiving a grant co-financed by the University of Parma and the LILT onlus National Association (Italian League Against Cancer) - Parma Section for a Research Fellowship named “Association between clinical-pathologic features, genetics and oncologic outcomes in patients diagnosed with multiple colorectal cancer” from Nov 2011 to Nov 2015. For the remaining authors none were declared.
References
- 1.Taylor DP, Burt RW, Williams MS, Haug PJ, Cannon-Albright LA. Population-based family history-specific risks for colorectal cancer: a constellation approach. Gastroenterology. 2010;138:877–85. doi: 10.1053/j.gastro.2009.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fearon ER. Molecular genetics of colorectal cancer. Annu Rev Pathol. 2011;6:479–507. doi: 10.1146/annurev-pathol-011110-130235. [DOI] [PubMed] [Google Scholar]
- 3.Schoemaker D, Black R, Giles L, Toouli J. Yearly colonoscopy, liver CT, and chest radiography do not influence 5-year survival of colorectal cancer patients. Gastroenterology. 1998;114:7. doi: 10.1016/s0016-5085(98)70626-2. [DOI] [PubMed] [Google Scholar]
- 4.Ringland CL, Arkenau HT, O’Connell DL, Ward RL. Second primary colorectal cancers (SPCRCs): experiences from a large Australian Cancer Registry. Ann Oncol. 2010;21:92. doi: 10.1093/annonc/mdp288. [DOI] [PubMed] [Google Scholar]
- 5.Barillari P, Ramacciato G, Manetti G, Bovino A, Sammartino P, Stipa V. Surveillance of colorectal cancer: effectiveness of early detection of intraluminal recurrences on prognosis and survival of patients treated for cure. Dis Colon Rectum. 1996;39:388. doi: 10.1007/BF02054052. [DOI] [PubMed] [Google Scholar]
- 6.Green RJ, Metlay JP, Propert K, Catalano PJ, Macdonald JS, Mayer RJ, et al. Surveillance for second primary colorectal cancer after adjuvant chemotherapy: an analysis of Intergroup 0089. Ann Intern Med. 2002;136:261. doi: 10.7326/0003-4819-136-4-200202190-00005. [DOI] [PubMed] [Google Scholar]
- 7.Bekdash B, Harris S, Broughton CI, Caffarey SM, Marks CG. Outcome after multiple colorectal tumours. Br J Surg. 1997;84:1442–4. [PubMed] [Google Scholar]
- 8.Wang HZ, Huang XF, Wang Y, Ji JF, Gu J. Clinical features, diagnosis, treatment and prognosis of multiple primary colorectal carcinoma. World J Gastroenterol. 2004;10(14):2136–9. doi: 10.3748/wjg.v10.i14.2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruinvels DJ, Stiggelbout AM, Kievit J, van Houwelingen HC, Habbema JD, van de Velde CJ. Follow-up of patients with colorectal cancer. A meta-analysis. Ann Surg. 1994;219:174. doi: 10.1097/00000658-199402000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramsey SD, Howlader N, Etzioni R, Brown ML, Warren JL, Newcomb P. Surveillance endoscopy does not improve survival for patients with local and regional stage colorectal cancer. Cancer. 2007;109:2222. doi: 10.1002/cncr.22673. [DOI] [PubMed] [Google Scholar]
- 11.Maeda T, Cannom RR, Beart RW Jr, Etzioni DA. Decision model of segmental compared with total abdominal colectomy for colon cancer in hereditarynonpolyposis colorectal cancer. J Clin Oncol 2010 Mar. 1;28(7):1175–80. doi: 10.1200/JCO.2009.25.9812. [DOI] [PubMed] [Google Scholar]
- 12.Umar A, Boland CR, Terdiman JP, Syngal S, de la Chapelle A, Ruschoff J, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004 Feb 18;96(4):261–8. doi: 10.1093/jnci/djh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fajobi O, Yiu CY, Sen-Gupta SB, Boulos PB. Metachronous colorectal cancers. British Journal of Surgery. 1998;85:897–901. doi: 10.1046/j.1365-2168.1998.00800.x. [DOI] [PubMed] [Google Scholar]
- 14.Park IJ, Yu CS, Kim HC, Jung YH, Han KR, Kim JC. Metachronous colorectal cancer. Colorectal Dis. 2006 May;8(4):323–7. doi: 10.1111/j.1463-1318.2006.00949.x. [DOI] [PubMed] [Google Scholar]
- 15.Violi V, Costi R, Marchesi F, Cecchini S, Sarli L, Roncoroni L. Anti-peristaltic ileocolonproctoplasty: a salvage procedure in extensive resective colorectal surgery. Int J Colorectal Dis. 2007 Oct;22(10):1277–81. doi: 10.1007/s00384-007-0320-0. [DOI] [PubMed] [Google Scholar]
- 16.Marchesi F, Sarli L, Percalli L, Sansebastiano GE, Veronesi L, Di Mauro D, Porrini C, Ferro M, Roncoroni L. Subtotal colectomy with antiperistaltic cecorectal anastomosis in the treatment of slow-transit constipation: long-term impact on quality of life. World J Surg. 2007 Aug;31(8):1658–64. doi: 10.1007/s00268-007-9111-6. [DOI] [PubMed] [Google Scholar]
- 17.Marchesi F, Percalli L, Pinna F, Cecchini S, Riccò M, Roncoroni L. Laparoscopic subtotal colectomy with antiperistaltic cecorectal anastomosis: a new step in the treatment of slow-transit constipation. Surg Endosc. 2012 Jun;26(6):1528–33. doi: 10.1007/s00464-011-2092-4. [DOI] [PubMed] [Google Scholar]
- 18.Cuniffe WJ, Hasieton PS, Tweedle DE, Schofield PF. Incidence of synchronous and metachronous colorectal carcinoma. Br J Surg. 1984;71:941–3. doi: 10.1002/bjs.1800711210. [DOI] [PubMed] [Google Scholar]
- 19.Kaibara N, Koga S, Jinnai D. Synchronous and metachronous malignancies of the colon and rectum in Japan with special reference to a coexisting early cancer. Cancer. 1984;54:1870–4. doi: 10.1002/1097-0142(19841101)54:9<1870::aid-cncr2820540917>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 20.Azzoni C, Bottarelli L, Cecchini S, Silini EM, Bordi C, Sarli L. Sporadic colorectal carcinomas with low-level microsatellite instability: a distinct subgroup with specific clinicopathological and molecular features. Int J Colorectal Dis. 2011 Apr;26(4):445–53. doi: 10.1007/s00384-011-1133-8. doi: 10.1007/s00384-011-1133-8. [DOI] [PubMed] [Google Scholar]
- 21.Sarli L, Bottarelli L, Azzoni C, Campanini N, Di Cola G, Barilli AL, Marchesi F, Mazzeo A, Salvemini C, Morari S, Di Mauro D, Donadei E, Necchi F, Roncoroni L, Bordi C. Loss of p27 expression and microsatellite instability in sporadic colorectal cancer. Surg Oncol. 2006 Aug;15(2):97–106. doi: 10.1016/j.suronc.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Roncoroni L, Costi R, Marchesi F, Bottarelli L, Sarli L, Violi V, Bordi C. Second anastomotic recurrence after radical left hemicolectomy: report of a case. Dis Colon Rectum. 2004 Sep;47(9):1547–9. doi: 10.1007/s10350-004-0617-9. [DOI] [PubMed] [Google Scholar]
- 23.Costi R, Azzoni C, Marchesi F, Bottarelli L, Violi V, Bordi C. Repeated anastomotic recurrence of colorectal tumors: genetic analysis of two cases. World J Gastroenterol. 2011 Aug 28;17(32):3752–8. doi: 10.3748/wjg.v17.i32.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang HZ, Huang XF, Wang Y, Ji JF, Gu J. Clinical features, diagnosis, treatment and prognosis of multiple primary colorectal carcinoma. World J Gastroenterol. 2004;10(14):2136–9. doi: 10.3748/wjg.v10.i14.2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bulow S, Svendsen LB, Mellemgaard A. Metachronous colorectal carcinoma. Br J Surg. 1990;77:502–5. doi: 10.1002/bjs.1800770509. [DOI] [PubMed] [Google Scholar]
- 26.Marchesi F, Pinna F, Percalli L, Cecchini S, Riccò M, Costi R, Pattonieri V, Roncoroni L. Totally laparoscopic right colectomy: theoretical and practical advantages over the laparo-assisted approach. J Laparoendosc Adv Surg Tech A. 2013 May;23(5):418–24. doi: 10.1089/lap.2012.0420. doi: 10.1089/lap.2012.0420. [DOI] [PubMed] [Google Scholar]
- 27.Sarli L, Rollo A, Cecchini S, Regina G, Sansebastiano G, Marchesi F, Veronesi L, Ferro M, Roncoroni L. Impact of obesity on laparoscopic-assisted left colectomy in different stages of the learning curve. Surg Laparosc Endosc Percutan Tech. 2009 Apr;19(2):114–7. doi: 10.1097/SLE.0b013e31819f2035. [DOI] [PubMed] [Google Scholar]


