Abstract
Objective
Hypoxic-ischemic white mater brain injury commonly occurs in neonates with critical congenital heart disease (CHD). Recent work has shown that longer time-to-surgery is associated with increased risk for this injury. Here we investigate changes in perinatal cerebral hemodynamics during the transition from fetal to neonatal circulation to ascertain mechanisms that may underlie this risk.
Methods
Neonates with either transposition of the great arteries (TGA) or hypoplastic left heart syndrome (HLHS) were recruited for pre-operative non-invasive optical monitoring of cerebral oxygen saturation (ScO2), cerebral oxygen extraction fraction (OEF), and cerebral blood flow (CBF) using diffuse optical spectroscopy and diffuse correlation spectroscopy, two non-invasive optical techniques. Measurements were acquired daily from day of consent until the morning of surgery. Temporal trends in these measured parameters during the preoperative period were assessed with a mixed-effects model.
Results
Forty-eight neonates with TGA or HLHS were studied. ScO2 was significantly and negatively correlated with time, and OEF was significantly and positively correlated with time. CBF did not significantly change with time during the pre-operative period.
Conclusions
In neonates with TGA or HLHS, increasing cerebral oxygen extraction combined with an abnormal cerebral blood flow response during the time between birth and heart surgery leads to a progressive decrease in cerebral tissue oxygenation. The results support and help explain the physiological basis for recent studies that show longer time-to-surgery increases the risk of acquiring white matter injury.
Graphical abstract
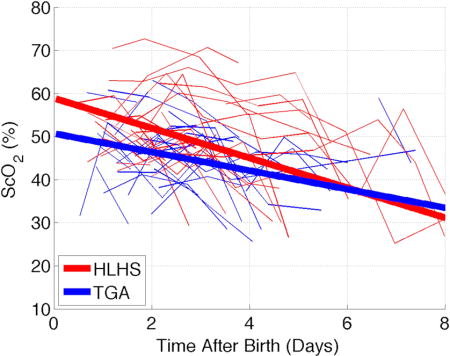
Introduction
Approximately 30,000 children are born each year in the United States with congenital heart disease (CHD). Nearly one third of these children are born with critical CHD, defined as lesions that require cardiac surgery in the neonatal period.1 In the last 3 decades, survival of neonates with CHD has improved dramatically, with the majority of these patients now reaching school age.2 The focus of research has shifted from survival beyond the neonatal period to addressing the neurodevelopment disabilities seen among long-term survivors. Nearly half of the school-age survivors exhibit neurobehavioral symptoms, such as inattention, hyperactivity, and impaired executive function.3–5
Increasing evidence suggests that underlying these neurobehavioral symptoms is hypoxic-ischemic white matter injury (WMI) seen on brain magnetic resonance imaging (MRI) termed periventricular leukomalacia (PVL).6–9 We have previously reported that longer time between birth and surgery was associated with an increased risk for WMI/PVL in infants with transposition of the great arteries (TGA) and hypoplastic left heart syndrome (HLHS).10, 11 Due to this common risk of increased time-to-surgery, the objective of this study was to investigate pre-operative cerebral physiology and hemodynamics in neonates with HLHS and TGA to elucidate the pathology underlying the increased risk for brain injury with longer waiting times for surgery. Diffuse optical and diffuse correlation spectroscopies (DOS and DCS, respectively) were utilized for non-invasive bedside quantification of pre-operative cerebral hemodynamics12, 13, and the time course of these parameters during the pre-operative period explored.
Materials and Methods
Patient Population
All term (37-42 weeks gestation) newborns with pre- or post-natally diagnosed critical CHD admitted to the cardiac intensive care unit (CICU) at The Children’s Hospital of Philadelphia were screened for study inclusion and approached for participation as early as possible. Exclusion criteria included: birth weight less than 2 kg, a history of neonatal depression (e.g., 5 minute APGAR<5, cord blood pH<7.0, sepsis, or birth asphyxia), perinatal seizures, evidence of end-organ injury, pre-operative cardiac arrest, and significant pre-operative intracerebral hemorrhage such as grade 3 or 4 intraventricular hemorrhage. Infants with identified or suspected genetic syndromes were not excluded.
Study Protocol
All procedures were approved by the Institutional Review Board at the Children’s Hospital of Philadelphia. Patient demographic data were recorded. Daily DOS/DCS measurements of cerebral tissue oxygen saturation (ScO2), oxygen extraction fraction (OEF), and cerebral blood flow (CBF) were made as soon as consent was obtained, with the last measurement made on the morning of surgery prior to induction of anesthesia. Vitals data was captured continuously (CNS Technology, LLC, Ambler, PA), and daily measurements of peripheral capillary oxygen saturation (SpO2) from pulse-oximetry are reported from the time that the daily DOS/DCS measurements were performed. Preoperative blood gases were acquired per clinical protocol, and arterial hemoglobin concentration (Hgb) on day of birth and day of surgery are reported.
DOS/DCS Measurements
Diffuse optical spectroscopy (DOS) and diffuse correlation spectroscopy (DCS) utilize near-infrared light to noninvasively probe the static and dynamic properties of cortical brain tissue. Our custom-made optical instrument combines these two techniques on a mobile cart that can be used in the MRI suite, the operating room, and peri-operatively at the bedside.14–16
DOS (also known as frequency-domain near-infrared spectroscopy or fd-NIRS) is a widely accepted method to quantify tissue oxygenation. Multi-separation frequency domain DOS, employed in this study, is capable of accurate quantification of cerebral tissue oxygen saturation (ScO2), i.e., in contrast to, commercial oximeters that employ continuous-wave NIRS to monitor trends in cerebral oxygen saturation.17–19 DOS uses photon diffusion theory to relate the measured amplitude attenuation and phase shift of modulated and multiply scattered light detected on the tissue surface to the wavelength-dependent tissue absorption (μa) and scattering (μa′) properties. The wavelength- and time-dependent absorption coefficient, μa (λ, t), depends linearly on oxy-([HbO2]) and deoxy-hemoglobin ([Hb]) concentration; thus measurements at multiple wavelengths yields these two parameters. From [HbO2] and [Hb], we derive total hemoglobin concentration (THC=[HbO2]+[Hb]) and cerebral tissue oxygen saturation (ScO2=[HbO2]/THC). Cerebral oxygen extraction fraction (OEF) can be calculated from ScO2 and peripheral capillary oxygen saturation (SpO2) measured clinically from a pulse-oximeter using the formula , where γ is the fraction of blood in the venous compartment, assumed to be 0.75.14, 20 Cerebral blood volume (CBV, mL/100g of tissue) can be calculated from THC. The DOS device employed in the present study (Imagent, ISS Inc., Champaign, IL) is amplitude modulated at 110 MHz and employs source lasers at 2 wavelengths, λ=688 and 830 nm.
DCS uses near-infrared light to non-invasively monitor CBF. DCS measures the temporal fluctuations of the light intensity emerging in remission at the tissue surface; these fluctuations are caused primarily by moving red blood cells.12, 21–25 Correlation diffusion theory is then employed to convert these temporal fluctuations into a blood flow index (BFI, measured in units of cm2/s).12 Although this index does not have traditional physiological units of cerebral blood flow (CBF), recent studies have shown that BFI correlates strongly with other gold standard measures of CBF.15, 16, 26–30 Specifically, Jain et al. validated BFI against CBF measured in the superior sagittal sinus with phase contrast MRI in a similar population of infants with critical CHD.16 Further, BFI can be combined with OEF, calculated using the equation above to give an index of cerebral metabolic rate of oxygen consumption (CMRO2,i) using the formula CMRO2,i = OEF × BFI × CaO2 where CaO2 is the arterial oxygen concentration which can be approximated as CaO2 ≈ 1.39 × SpO2 × Hgb.14, 15
DOS and DCS measurements were conducted once daily from the day of consent until the morning of surgery. The time of the measurements was recorded with respect to the subject’s time of birth. Measurements were made noninvasively over both the right and left frontal cortex. At each location, four repetitions of the basic measurement were acquired in order to account for local inhomogeneities under the optical probe. These eight repetitions were then averaged to derive a global measure of ScO2 and BFI.
Brain MRI
In a subset of patients (n=33), a brain MRI was obtained immediately prior to surgery as part of a different study protocol.11, 31 All images were acquired using a 1.5T Avanto MRI system (Siemens Medical Systems, Malvern, Pa) using a 12-channel head coil. The studies included T1-weighted magnetization-prepared rapid acquisition gradient echo and T2-weighted sampling perfection with application-optimized contrasts using different flip angle evolution sequences acquired in the axial plane. Two independent observers, who were unaware of the clinical data, evaluated the total brain maturation score (TMS) using axial T1- and T2-weighted images.32, 33
Statistical Analysis
For analysis purposes, patients were grouped according to cardiac diagnosis. Continuous variables were summarized by standard descriptive statistics (mean, standard deviation (SD), or median, interquartile range (IQR) as appropriate), and frequencies and percentages were used for categorical variables.
The pre-operative temporal trends in cerebral hemodynamics were examined using a linear mixed-effects model implemented within the SAS mixed procedure, a method commonly used for analyzing correlated data such as repeated measures or clustered data. The model was used to predict the mean outcome variable (i.e., ScO2, OEF, BFI, CMRO2,i, and SpO2) as a function of time and cardiac diagnosis. In this study, since a single measurement was taken on the same patient at multiple time points, these these measures (i.e., ScO2, OEF, BFI, CMRO2,i, and SpO2) are correlated with each other; we refer to this as “within-subject correlation”. Specifically, subject-specific random effects were included in the model to account for within-subject correlations resulting from the repeated measures. Random intercept and slope were assumed in the linear mixed-effects model to capture potential difference in the baselines and trajectories among individuals. For the covariance structure, we compared the compound symmetry (CS) with the autoregressive (1) (AR(1)), and we used the Akaike information criterion (AIC) to determine the optimal covariance matrices. CS assumes that correlations between all pairs of measures within the same subject are the same, while AR(1) assumes that correlations between two measures decrease exponentially with distance (i.e., decrease with the time between the two measures). Normality of the outcome variables was examined graphically and statistically using Shapiro-Wilk test. In the mixed-effects model, we considered both linear and non-linear temporal trends by including a term quadratic in time into the model. All analyses were performed using SAS 9.4 statistical software (SAS Institute Inc, Cary, NC). Statistical significance was declared for P values <0.05.
Results
From March 2013 to March 2016, a total of N=70 neonates with complex CHD were recruited. Cardiac diagnoses included HLHS (n=24), TGA (n=24), tetralogy of Fallot (n=6), hypoplastic arch (n=3), interrupted aortic arch (n=3), aortic atresia (n=3), double inlet left ventricle (n=2), truncus arteriosis (n=2), coarctation of the aorta (n=2), and double outlet right ventricle (n=1). Due to challenges in grouping patients with shared physiology, and small numbers in certain groups, our analysis was limited to only patients with TGA and HLHS.
Patient demographics for the subset of patients with TGA or HLHS (n=48) are summarized in Table 1. All patients were full-term with an average gestational age of 38.9±0.7 weeks and an average time-to-surgery of 4.3±2.5 days. In this cohort, patients with a diagnosis of TGA had on average an older gestational age than those with a diagnosis of HLHS. No differences in birth weight, time-to-surgery, head circumference or brain maturation were observed between diagnoses.
Table 1.
Patient demographics and initial optical measurements.
| All (n=48) | HLHS (n=24) | TGA (n=24) | P value | |
|---|---|---|---|---|
| Demographics | ||||
| Female, n (%) | 17 (35.4) | 9 (37.5) | 8 (33.3) | 0.76 |
| Time-to-surgery, day | 4.3 ± 2.5 | 4.8 ± 3.0 | 3.9 ± 1.7 | 0.42 |
| Gest. Age, wk | 38.9 ± 0.7 | 39.2 ± 0.7 | 38.7 ± 0.6 | 0.03 |
| Birth weight, kg | 3.4 ± 0.5 | 3.5 ± 0.4 | 3.3 ± 0.5 | 0.31 |
|
| ||||
| Head circumference, cm | 34.3 ± 1.6 | 34.5 ± 1.2 | 34.1 ± 1.9 | 0.18 |
| Total Brain Maturation Score* | 10.2 ± 1.1 | 10.0 ± 1.2 | 10.5 ± 1.0 | 0.23 |
|
| ||||
| Optical measurements | ||||
| Number of measurements | 4.0 ± 2.4 | 4.6 ± 3.1 | 3.3 ± 1.3 | 0.12 |
| Initial ScO2 (%) | 49.4 ± 8.4 | 52.0 ± 7.2 | 46.8 ± 8.9 | 0.04 |
| Initial OEF | 0.59 ± 0.13 | 0.57 ± 0.11 | 0.62 ± 0.15 | 0.20 |
| Initial BFI (10−8 cm2/s) | 1.7 ± 0.7 | 1.5 ± 0.6 | 1.9 ± 0.7 | 0.07 |
| Initial CMRO2,i (10−7 ml/dl × cm2/s) | 1.9 ± 0.9 | 1.7 ± 0.9 | 2.1 ± 0.9 | 0.10 |
ScO2, cerebral tissue oxygen saturation; OEF, oxygen extraction fraction; BFI, blood flow index; CMRO2,i, cerebral metabolic rate of oxygenation; TGA, Transposition of the Great Arteries; HLHS: Hypoplastic Left Heart Syndrome.
Total Brain Maturation Score measured only for a subset of patients (HLHS: n=20; TGA: n=13)
Initial measurements of ScO2, OEF, BFI, and CMRO2,i are reported in Table 1. The first measured ScO2 was lower on average (p=0.04) in patients with TGA (46.8±8.9%) compared to patients with HLHS (52.0±7.2%). The average initial ScO2 amongst all subjects was 49.4±8.4%. Daily measurements of ScO2, OEF, BFI, and CMRO2,i for all subjects are shown in Figure 1. Preoperative measurements of Hgb and daily measurements of SpO2 are shown in Figure 2.
Figure 1.
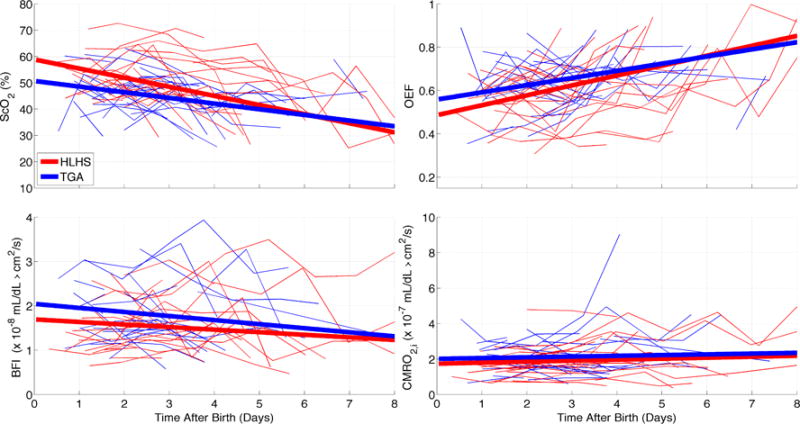
Time profiles of ScO2 (a), OEF (b), BFI (c) and CMRO2,i (d). Each thin line represents measurements for a single subject with either hypoplastic left heart syndrome (red) or transposition of the great arteries (blue). Thick lines represent linear trends derived from a mixed effects model reported in Table 2.
Figure 2.
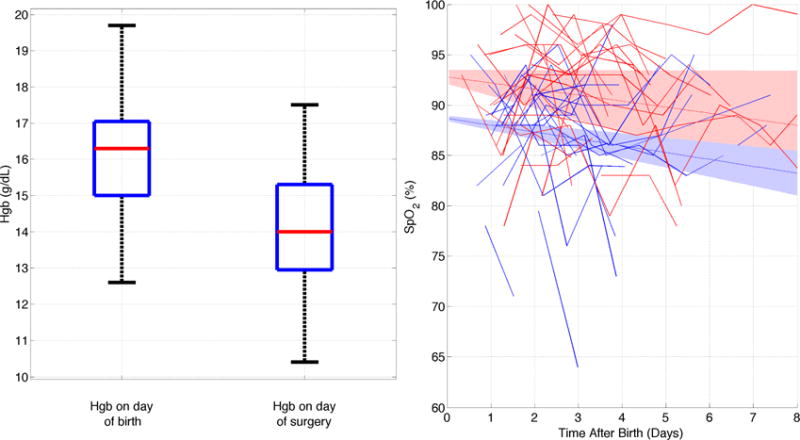
Left: Boxplot demonstrating preoperative hemoglobin on day of birth and on day of surgery. Right: Time profiles of SpO2. Each thin line represents measurements for a single subject with either a hypoplastic left heart syndrome (red) or transposition of the great arteries (blue). Thick lines represent linear trends derived from a mixed effects model reported in Table 3 and shaded area represents the 95% confidence interval of the model.
We performed a linear mixed-effects model to assess the effects of time and cardiac diagnosis on the daily measurements of ScO2, OEF, BFI and CMRO2,i (Table 2, Figure 1) and on daily measurements of SpO2 (Table 3, Figure 2). Since preoperative ScO2 was different between the two cohorts, cardiac diagnosis was included in the mixed-effects model as a covariate. Only the results from a linear model are reported because higher order of effects of time were not found to be significant. Time from birth was a significant predictor of pre-operative ScO2 (p=0.02), and OEF (p=0.01). Cardiac diagnosis (HLHS or TGA) significantly predicts pre-operative ScO2 (p<0.01), BFI (p=0.05), and SpO2 (p=0.01). The interaction between cardiac diagnosis and time was not a significant predictor of any of the outcome variables. We also performed a linear mixed-effects model on the subset (n=33) of subjects who received brain MRIs on the morning of surgery to assess the effect of brain maturation on preoperative CMRO2,i (Table 4); the latter was computed using a simple steady-state model described in the Methods section. Total brain maturation score did not affect the temporal trends of CMRO2,i but was inversely related to baseline measurements of CMRO2,i (p=0.03).
Table 2.
Coefficients and standard error of the linear mixed-effects model for the optically derived outcome variables ScO2, OEF, BFI, and CMRO2,i.
| Outcome Variable | Estimate (SE) of Coefficient | P value |
|---|---|---|
| ScO2, % | ||
| Intercept | 50.7 (2.2) | <0.0001 |
| Time, hr | −0.09 (0.04) | 0.02 |
| Cardiac Dx | 8.2 (2.8) | <0.01 |
| Time×Cardiac Dx | −0.05 (0.05) | 0.24 |
| OEF | ||
| Intercept | 0.56 (0.03) | <0.0001 |
| Time, hr | 0.0014 (0.0005) | 0.01 |
| Cardiac Dx | −0.07 (0.04) | 0.09 |
| Time×Cardiac Dx | 0.0005 (0.0007) | 0.45 |
| BFI, 10−8 cm2/s | ||
| Intercept | 2.0 (0.1) | <0.0001 |
| Time, hr | −0.003 (0.003) | 0.16 |
| Cardiac Dx | −0.35 (0.18) | 0.05 |
| Time×Cardiac Dx | 0.001 (0.003) | 0.69 |
| CMRO2,i, 10−7 ml/dl × cm2/s | ||
| Intercept | 2.0 (0.2) | <0.0001 |
| Time, hr | 0.002 (0.004) | 0.68 |
| Cardiac Dx | −0.28 (0.27) | 0.30 |
| Time×Cardiac Dx | 0.0006 (0.006) | 0.91 |
For each outcome variable, the table reports the y-intercept for the linear model as well as the coefficients for the 3 variables: time after birth, cardiac diagnosis (HLHS and TGA), and an interaction term between diagnosis and time. For cardiac diagnosis, TGA was used as the reference group. The linear model for each outcome variable is y = β0 + β1 × Time + β2 × Cardiac Diagnosis + β3 × Time × Cardiac Diagnosis.
Table 3.
Coefficients and standard error of the linear mixed-effects model for the clinically derived outcome variable SpO2.
| Outcome Variable | Estimate (SE) of Coefficient | P value |
|---|---|---|
| SpO2, % | ||
| Intercept | 88.7 (1.2) | <0.0001 |
| Time, hr | −0.03 (0.02) | 0.19 |
| Cardiac Dx | 4.1 (1.6) | 0.01 |
| Time×Cardiac Dx | 0.003 (0.029) | 0.91 |
Table reports the y-intercept for the linear model as well as the coefficients for the 3 variables: time after birth, cardiac diagnosis (HLHS and TGA), and an interaction term between diagnosis and time. For cardiac diagnosis, TGA was used as the reference group. The linear model for each outcome variable is y = β0 + β1 × Time + β2 × Cardiac Diagnosis + β3 × Time × Cardiac Diagnosis.
Table 4.
Coefficients and standard error from the linear mixed-effect model using time after birth, cardiac diagnosis (HLHS and TGA), total brain maturation score (TMS), and an interaction term between TMS and time as predictors for optically derived CMRO2,I on a subset of patients who received a pre-operative brain MRI (n=33). For cardiac diagnosis, TGA was used as the reference group.
| Outcome Variable | Estimate (SE) of Coefficient | P value |
|---|---|---|
| CMRO2,i, 10−7 ml/dl × cm2/s | ||
| Intercept | 5.1 (1.2) | <0.0001 |
| Time, hr | −0.01 (0.03) | 0.61 |
| Cardiac Dx | −0.75 (0.23) | <0.01 |
| TMS | −0.25 (0.11) | 0.03 |
| Time×TMS | 0.001 (0.003) | 0.62 |
The linear model for each outcome variable is y = β0 + β1 × Time + β2 × Cardiac Diagnosis + β3 × TMS + β4 × Time × TMS
Discussion
This investigation is the first report on longitudinal monitoring of pre-operative cerebral hemodynamics in neonates with critical CHD. Previous studies have looked at cross-sectional trends in pre-operative saturations in these two patient populations independently.10, 11 Our group previously used the same non-invasive optical techniques (DOS/DCS) to measure ScO2 immediately prior to surgery in infants with HLHS.11 In our cross-sectional analysis, preoperative ScO2 was negatively correlated with a longer time between birth and surgery, suggesting that ScO2 decreases between birth and surgery. This initial observation is further supported by the present study as daily measurements of preoperative ScO2 were found to negatively correlate with time. In both the previous11 and current study, the differences in timing of surgery between subjects were a result of “modifiable” logistics including availability of the requested surgeon and the day of the week on which the patient was born. No patients went to surgery early (e.g., for pulmonary over-circulation) or had surgery delayed for medical reasons (e.g., for infection, bleeding, seizures).
Although pre-operative cerebral hemodynamics have been previously studied using DOS/DCS in neonates with TGA, the relationship to time-to-surgery has not been previously investigated. In 2009, Petit et al. reported that preoperative brain injury in neonates with transposition of the great arteries was associated with systemic hypoxemia and longer time-to-surgery, again suggesting that increased risk of WMI/PVL with longer time-to-surgery could be due to decreasing ScO2 during the preoperative period.10 With the present study we were able to confirm this finding and were able to demonstrate longitudinal decrease in ScO2 similar to the decreases in ScO2 measured in neonates with HLHS. Since the increase in pre-operative oxygen extraction exists independent of cardiac diagnosis, our findings suggest a commonality in the mechanism behind the previously reported increased risk for WMI/PVL with longer time-to-surgery in both populations.
The decreases in ScO2 measured in this patient population are larger in magnitude than what would be expected for a healthy neonate. Franceschini et al. similarly used diffuse optical spectroscopy to study trends in ScO2 in healthy infants over the first year of life.34 They reported an average decrease of 10 percentage points in ScO2 from 0 to 6 weeks, without corresponding changes in cerebral blood volume or CMRO2; this change was attributed to the decrease in hematocrit during the transition from fetal to adult hemoglobin. This relatively minimal change in ScO2 is not sufficient to explain the large decrease in ScO2 of −2.2% per day observed in this CHD population. Additionally, Franceschini et al. report an average ScO2 of 67% in healthy neonates measured during the first week of life, whereas the average initial ScO2 measured in this cohort of infants with CHD is 49.4 ± 8.4%. Therefore, it appears that infants with complex CHD are born with a lower than normal cerebral tissue oxygen saturation and also experience a greater than normal drop in saturation during the first week of life.
Cerebral oxygen extraction is related to oxygen delivery and metabolism through the equation CMRO2,i = OEF × BFI × CaO2, valid in steady state, where CaO2 is the arterial oxygen concentration which can be approximated as CaO2 ≈ 1.39 × SpO2 × Hgb.14, 15 Therefore, an increase in OEF could be caused by an increase in CMRO2,i, a decrease in BFI, a decrease in SpO2, a decrease in Hgb, or a combination of these factors. In this study, it is reasonable to speculate that the observed increase in OEF after birth is due to a decrease in oxygen carrying capacity caused by decreased hemoglobin after repeated blood draws. However, we did not observe a statistically significant difference between Hgb measured on day of birth and Hgb measured on day-of-surgery (Figure 2). A decrease in arterial oxygen saturation would also explain an increase in oxygen extraction, but we did not observe a decrease in SpO2 during the preoperative period. Furthermore, the expected physiological response to a decrease in oxygen carrying capacity caused by a decrease in hemoglobin, would be to increase CBF to keep oxygen supply constant; CBF did not exhibit this increase in our mixed effects model results from the whole study population. This absence of CBF compensation suggests a failure of compensatory mechanisms, for example a failure of cerebrovascular resistance. Further, the mismatch between increased oxygen extraction and stagnant oxygen delivery explains the association of longer time-to-surgery with increased risk for WMI/PVL before surgery in TGA and after surgery in HLHS.
Another possible cause for increased oxygen extraction during the preoperative period is an increased oxygen demand during the newborn period. However, CMRO2,i was not found to be increasing within error in this study. Previously, CMRO2 has been shown to increase during the first few weeks of life in premature infants with similar brain maturation as full term infants with complex CHD.33, 35, In the present study baseline CMRO2,i was found to be inversely related to brain maturation, thus supporting previous research that suggests brain immaturity is correlated with cerebral oxygen metabolism.35 An increase in oxygen demand would be expected to cause a corresponding increase in oxygen delivery, but again an increase in CBF was not observed indicating a failure of compensatory mechanisms.
Understanding the fetal circulation is salient to understanding the failure of CBF compensation to meet increasing pre-operative oxygen extraction. Prenatal studies using Doppler ultrasound have shown fetuses with HLHS have lower than normal cerebral vascular resistance (CVR).36, 37 Lower fetal CVR is likely due to decreased oxygen delivery to the brain caused by the altered anatomy that results from ductal dependent CBF.36, 38 Doppler ultrasound in fetuses with TGA also demonstrates a decrease in fetal CVR, reflecting a deficit in oxygen delivery that is due to the lower oxygen saturations caused by the transposed great vessels.38, 39 Sustained decrease in CVR during fetal life could exhaust the compensatory mechanisms that are needed to increase CBF in response to increasing OEF.
The significance of longer time-to-surgery has been previously demonstrated.10, 11, 40 Further, we have shown that in patients with HLHS there exists a strong correlation between lower cerebral oxygen saturations and longer time-to-surgery and that these factors increased the risk for both the prevalence and severity of WMI/PVL. The current results provide insight into the underlying cause of this increased risk. These findings demonstrate the critical role of the preoperative period in the development of risk for WMI/PVL. If further corroborated, these observations could lead to a shift in the emphasis of neuroprotective strategies from the operative period to the preoperative period. However, as both of the aforementioned studies were conducted at a single center, it is imperative to investigate the preoperative period and risk for WMI/PVL with age at time of Norwood procedure at different centers with different perioperative and operative clinical care strategies. A larger, multicenter study between the Children’s Hospital of Philadelphia and Texas Children’s Hospital is currently being pursued to further investigate the changing cerebral hemodynamics during the preoperative period and the effect on risk for white matter injury.
Study Limitations
A significant limitation of the present study was that measurements of preoperative cerebral hemodynamics were taken only once per day starting with the day of study inclusion when consent was obtained. Thus, the timing of the first measurement differed between subjects. Furthermore, measurements were performed at times of convenience rather than a standardized to time of day. Because of this relatively low frequency of data acquisition, analysis with a mixed effects model was limited to low order models, and although we found that BFI and CMRO2,i did not significantly change with time, the uncertainties associated with these measurements were relatively large. Thus, it is possible that CBF and oxygen metabolism were changing during the preoperative period, but the sensitivity of our measurements could have been too low to discern these changes. We note that application of simple steady-state models to our whole data set, relating OEF, CBF and oxygen metabolism (based on the mixed effects model results) are consistent within the measurement error bars. Higher frequency of data acquisition is needed to fit higher order models to these preoperative variables and to decrease uncertainty, and these improvements will be necessary to begin to elicit an “optimal” timing of surgery with respect to cerebral hemodynamics. Additionally, the current study protocol did not permit for blood gas measurements at the same time as optical measurements, limiting analysis of the effect that changes in hemoglobin may have to the measured cerebral hemodynamics.
Another significant limitation in the present study is that it was conducted at a single site. Since preoperative care, specifically time from birth until surgery, varies between different institutions, the results presented here may be less applicable to other sites. Further investigation into different preoperative management strategies, operative strategies, and their effect on development of brain injury and trends in cerebral hemodynamics is necessary to further understand the relationship between clinical care and neurodevelopmental outcomes. The multicenter study described above aims to accomplish this goal.
Conclusion
We investigated the pre-operative trends in cerebral hemodynamics in neonates with TGA or HLHS awaiting surgery. We observed that cerebral tissue oxygenation decreases during the pre-operative period in all patients, regardless of cardiac diagnosis. This decrease in oxygenation was associated with an increase in oxygen extraction, but not with a corresponding increase in cerebral blood flow. The observed increase in oxygen demand without compensating oxygen delivery could lead to the previously reported increase in risk for white matter brain injury with longer time-to-surgery.
Supplementary Material
Video 1.
Demonstration of optical measurements of cerebral blood flow and oxygen saturation.
Acknowledgments
We acknowledge invaluable assistance from Thomas Riley, Wesley Baker, Steven Schenkel, Michael Friedman, the staff of the cardiac intensive care unit at the Children’s Hospital of Philadelphia, and most importantly the patients and their families.
Funding Sources:
This study was supported by the NIH Grant Nos. NS-072338, NS-60653, HL-007954, HL-007915, P41-EB015893, the Thrasher Research Foundation, and the June and Steve Wolfson Family foundation.
Abbreviations and Acronyms
- BFI
blood flow index
- CBF
cerebral blood flow
- CBV
cerebral blood volume
- CHD
congenital heart disease
- CICU
cardiac intensive care unit
- CMRO2
cerebral metabolic rate of oxygenation
- DCS
diffuse correlation spectroscopy
- DOS
diffuse optical spectroscopy
- Hb
deoxyhemoglobin, measured DOS
- HbO2
oxyhemoglobin, measured with DOS
- Hct
hematocrit, measured from blood gas
- Hgb
hemoglobin, measured from blood gas
- HLHS
hypoplastic left heart syndrome
- OEF
cerebral oxygen extraction fraction
- SaO2
arterial oxygen saturation
- SpO2
peripheral capillary oxygen saturation
- ScO2
cerebral tissue oxygen saturation
- TGA
transposition of the great arteries
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
Authors have nothing to disclose with regard to commercial support.
References
- 1.Hoffman JIE, Kaplan S. The incidence of congenital heart disease. Journal of the American College of Cardiology. 2002;39:1890–1900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- 2.Graham EM, Zyblewski SC, Phillips JW, Shirali GS, Bradley SM, Forbus GA, Bandisode VM, Atz AM. Comparison of Norwood Shunt Types: Do the Outcomes Differ 6 Years Later? Ann Thorac Surg. 2010;90:31–35. doi: 10.1016/j.athoracsur.2010.03.078. [DOI] [PubMed] [Google Scholar]
- 3.Bellinger D, Wypij D, Rivkin M, DeMaso D, Robertson R, Dunbar-Masterson C, Rappaport L, Wernovsky G, Jonas R, Newburger J. Adolescents With d-Transposition of the Great Arteries Corrected With the Atrial Switch Procedure. Pediatric Cardiology. 2011;124:1361–1369. doi: 10.1161/CIRCULATIONAHA.111.026963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marino B, Lipkin P, Newburger J, Peacock G, Gerdes M, Gaynor J, Mussatto K, Uzark K, Goldberg C, Johnson W, Li J, Smith S, Bellinger D, Mahle W, Committee AHACHD, Young CoCDit, Nursing CoC and Council S Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management: a scientific statement from the American Heart Association. Circulation. 2012;126:1143–1172. doi: 10.1161/CIR.0b013e318265ee8a. [DOI] [PubMed] [Google Scholar]
- 5.Shillingford A, Glanzman M, Ittenbach R, Clancy R, Gaynor J, Wernovsky G. Inattention, hyperactivity, and school performance in a population of school-age children with complex congenital heart disease. Pediatrics. 2008;121:759–767. doi: 10.1542/peds.2007-1066. [DOI] [PubMed] [Google Scholar]
- 6.Mahle W, Tavani F, Zimmerman R, Nicolson S, Galli K, Ganor J, Clancy R, Montenegro L, Spray T, Chiavacci R, Wernovsky G, Kurth C. An MRI study on neurological injury before and after congenital heart surgery. Circulation. 2002;106:109–114. [PubMed] [Google Scholar]
- 7.Imamura T, Ariga H, Kaneko M, Watanabe M, Shibukawa Y, Fukuda Y, Nagasawa K, Goto A, Fujiki T. Neurodevelopmental outcomes of children with periventricular leukomalacia. Pediatrics & Neonatology. 2013;54:367–372. doi: 10.1016/j.pedneo.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Fawer CL, Diebold P, Calame A. Periventricular leucomalacia and neurodevelopmental outcome in preterm infants. Archives of Disease in Childhood. 1987;62:30–36. doi: 10.1136/adc.62.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller SP, Ferriero DM, Leonard C, Piecuch R, Glidden DV, Partridge C, Perez M, Mukherjee P, Vigneron DB, Barkovich AJ. Early brain injury in premature newborns detected with magnetic resonance imaging is associated with adverse early neurodevelopmental outcome. Journal of Pediatrics. 2005;147:609–616. doi: 10.1016/j.jpeds.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 10.Petit CJ, Rome JJ, Wernovsky G, Mason SE, Shera DM, Nicolson SC, Montenegro LM, Tabbutt S, Zimmerman RA, Licht DJ. Preoperative brain injury in transposition of the great arteries is associated with oxygenation and time to surgery, not balloon atrial septostomy. Circulation. 2009;119:709–716. doi: 10.1161/CIRCULATIONAHA.107.760819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lynch JM, Buckley EM, Schwab PJ, McCarthy AL, Winters ME, Busch DR, Xiao R, Goff DA, Nicolson SC, Montenegro LM, Fuller S, Gaynor JW, Spray TL, Yodh AG, Naim MY, Licht DJ. Time-to-Surgery and Pre-operative Cerebral Hemodynamics Predict Post-operative White Matter Injury in Neonates with Hypoplastic Left Heart Syndrome. The Journal of Thoracic and Cardiovascular Surgery. 2014;148:2181–2188. doi: 10.1016/j.jtcvs.2014.05.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durduran T, Choe R, Baker WB, Yodh AG. Diffuse optics for tissue monitoring and tomography. Reports on Progress in Physics. 2010;73 doi: 10.1088/0034-4885/73/7/076701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goff DA, Buckley EM, Durduran T, Wang J, Licht DJ. Noninvasive Cerebral Perfusion Imaging in High-Risk Neonates. Seminars in Perinatology. 2010;34:46–56. doi: 10.1053/j.semperi.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buckley EM, Lynch JM, Goff DA, Schwab PJ, Baker WB, Durduran T, Busch DR, Nicolson SC, Montenegro LM, Naim R, Xiao TL, Spray TL, Yodh AG, Gaynor JW, Licht DJ. Early Post-Operative Changes in Cerebral Oxygen Metabolism Following Neonatal Cardiac Surgery: Effects of Surgical Duration. The Journal of Thoracic and Cardiovascular Surgery. 2012;145:196–205. doi: 10.1016/j.jtcvs.2012.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Durduran T, Zhou C, Buckley EM, Kim MN, Yu G, Choe R, Gaynor JW, Spray TL, Durning SM, Mason Se, Montenegro LM, Nicolson SC, Zimmerman RA, Putt ME, Wang J, Greenberg JH, Detre JA, Yodh AG, Licht DJ. Optical measurement of cerebral hemodynamics and oxygen metabolism in neonates with congenital heart defects. Journal of Biomedical Optics. 2010;15:037004. doi: 10.1117/1.3425884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jain V, Buckley EM, Licht DJ, Lynch JM, Schwab PJ, Naim MY, Lavin N, Nicolson SC, Montenegro LM, Yodh AG, Wehrli FW. Cerebral Oxygen Metabolism in Neonates with Congenital Heart Disease Quantified by MRI and Optics. Journal of Cerebral Blood Flow and Metabolism. doi: 10.1038/jcbfm.2013.214. Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hueber DM, Franceschini MA, Ma HY, Zhang Q, Ballesteros JR, Fantini S, Wallace D, Ntziachristos V, Chance B. Non-invasive and quantitiative near-infared haemoglobin spectrometry in the piglet brain during hypoxic stress, using a frequency-domain multidistance instrument. Physics in Medicine and Biology. 2000;46 doi: 10.1088/0031-9155/46/1/304. [DOI] [PubMed] [Google Scholar]
- 18.Kurth C, Thayer W. A multiwavelength frequency-domain near-infrared cerebral oximeter. Physics in Medicine and Biology. 1999;44:727. doi: 10.1088/0031-9155/44/3/015. [DOI] [PubMed] [Google Scholar]
- 19.Watzman HM, Kurth CD, Montenegro LM, Rome J, Steven JM, Nicolson SC. Arterial and Venous Contributions to Near-Infrared Cerebral Oximetry. Anesthesiology. 2000;93:947–953. doi: 10.1097/00000542-200010000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Culver JP, Durduran T, Furuya D, Cheung C, Greenberg JH, Yodh AG. Diffuse optical tomography of cerebral blood flow, oxygenation, and metabolism in rat during focal ischemia. Journal of Cerebral Blood Flow and Metabolism. 2003;23:911–924. doi: 10.1097/01.WCB.0000076703.71231.BB. [DOI] [PubMed] [Google Scholar]
- 21.Boas DA, Yodh AG. Spatially varying dynamical properties of turbid media probed with diffusing temporal light correlation. Journal of the Optical Society of America A. 1997;14:192–215. [Google Scholar]
- 22.Pine D, Weitz D, Chaikin P, E H. Diffusing wave spectroscopy. Physical Review Letters. 1988;60:1134–1137. doi: 10.1103/PhysRevLett.60.1134. [DOI] [PubMed] [Google Scholar]
- 23.Weitz D, Pine D. Diffusing Wave Spectroscopy. In: Brown W, editor. Dynamic Light Scattering: The Method and Some Applications. Oxford: Oxford University Press; 1993. pp. 652–720. [Google Scholar]
- 24.Maret G, Wolf P. Multiple light scattering from disordered media. The effect of brownian motion of scatterers. Zeitschrift für Physik B - Condensed Matter. 1987;65:409–413. [Google Scholar]
- 25.Boas DA, Campbell LE, Yodh AG. Scattering and imaging with diffusing temporal field correlations. Physical Review Letters. 1995;75:1855–1858. doi: 10.1103/PhysRevLett.75.1855. [DOI] [PubMed] [Google Scholar]
- 26.Buckley EM, Cook NM, Durduran T, Kim MN, Zhou C, Choe R, Yu G, Schultz S, Sehgal CM, Licht DJ, Arger PH, Putt ME, Hurt HH, Yodh AG. Cerebral hemodynamics in preterm infants during positional intervention measured with diffuse correlation spectroscopy and transcranial Doppler ultrasound. Optics Express. 2009;17:12571–12581. doi: 10.1364/oe.17.012571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buckley EM, Hance D, Pawlowski T, Lynch JM, Wilson FB, Mesquita RC, Durduran T, Diaz LK, Putt ME, Licht DJ, Fogel MA, Yodh A. Validation of diffuse correlation spectroscopic measurement of cerebral blood flow using phase-encoded velocity mapping magnetic resonance imaging. Journal of Biomedical Optics. 2012;17:037007. doi: 10.1117/1.JBO.17.3.037007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diop M, Migueis M, Lee T, Lawrence KS. Comparison of time-resolved and continuous-wave near-infrared techniques for measuring cerebral blood flow in piglets. Journal of Biomedical Optics. 2010;15:057004–057004. doi: 10.1117/1.3488626. [DOI] [PubMed] [Google Scholar]
- 29.Kim MN, Durduran T, Frangos S, Edlow BL, Buckley EM, Moss HE, Zhou C, Yu G, Choe R, Maloney-Wilensky E, Wolf RL, Grady MS, Greenberg JH, Levine JM, Yodh AG, Detre JA, Kofke WA. Noninvasive measurement of cerebral blood flow and blood oxygenation using near-infrared and diffuse correlation spectroscopies in critically brain-injured adults. 2010 doi: 10.1007/s12028-009-9305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou C, Eucker SA, Durduran T, Yu G, Ralston J, Friess SH, Ichord RN, Marguiles SS, Yodh AG. Diffuse optical monitoring of hemodynamic changes in piglet brain with closed head injury. Journal of Biomedical Optics. 2009;14:034015. doi: 10.1117/1.3146814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jain V, Buckley EM, Licht DJ, Lynch JM, Schwab PJ, Naim MY, Lavin N, Nicolson SC, Montenegro LM, Yodh AG, Wehrli FW. Cerebral Oxygen Metabolism in Neonates with Congenital Heart Disease Quantified by MRI and Optics. Journal of Cerebral Blood Flow and Metabolism. 2014;34:380–388. doi: 10.1038/jcbfm.2013.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Childs AM, Ramenghi LA, Cornete L, Tanner SF, Arthur RJ, Martinez D, Levene MI. Cerebral maturation in premature infants: quantitative assessment using MR imaging. American Journal of Neuroradiology. 2001;22:1577–1582. [PMC free article] [PubMed] [Google Scholar]
- 33.Licht DJ, Shera DM, Clancy R, Wernovsky G, Montenegro LM, Nicolson SC, Zimmerman RA, Spray TL, Gaynor JW, Vossough A. Brain maturation is delayed in infants with complex congenital heart defects. The Journal of Thoracic and Cardiovascular Surgery. 2009;137:529–536. doi: 10.1016/j.jtcvs.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Franceschini MA, Thaker S, Themelis G, Krishnamoorthy KK, Bortfeld H, Diamond SG, Boas DA, Arvin K, Grant PE. Assessment of Infant Brain Development with Frequency-Domain Near-Infrared Spectroscopy. Pediatric Research. 2007;61:546–551. doi: 10.1203/pdr.0b013e318045be99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roche-Labarbe N, Fenoglio A, Aggarwal A, Dehaes M, Carp SA, Franceschini MA, Grant PE. Near-infrared spectroscopy assessment of cerebral oxygen metabolism in developing premature brain. Journal of Cerebral Blood Flow and Metabolism. 2012;32:481–488. doi: 10.1038/jcbfm.2011.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szwast A, Tian Z, McCann M, Soffer D, Rychik J. Comparitive analysis of cerebrovascular resistance in fetuses with single-ventricle congenital heart disease. Ultrasound Obstet Gynecol. 2012;40:62–67. doi: 10.1002/uog.11147. [DOI] [PubMed] [Google Scholar]
- 37.Kaltman JR, Di H, Tian Z, Rychik J. Impact of congenital heart disease on cerebrovascular blood flow dynamics inthe fetus. Ultrasound Obstet Gynecol. 2005;25:32–36. doi: 10.1002/uog.1785. [DOI] [PubMed] [Google Scholar]
- 38.Sun L, Macgown C, Sled J, Yoo S, Manlhoit C, Porayette P, Grosse-Wortmann L, Jaeggi E, McCrindle B, Kingdom J, Hickey E, Miller S, Seed M. Reduced fetal cerebral oxygen consumption is associated with smaller brain size in fetuses with congenital heart disease. Circulation. 2015;131:1313–1323. doi: 10.1161/CIRCULATIONAHA.114.013051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rudolph AM. The changes in the circulation after birth. Their importance in congenital heart disease. Circulation. 1970;41:343–359. doi: 10.1161/01.cir.41.2.343. [DOI] [PubMed] [Google Scholar]
- 40.Lynch JM, Licht DJ. First things first: The importance of the preoperative period for neurolic outcomes in hypoplastic left heart syndrome. The Journal of Thoracic and Cardiovascular Surgery. 2016;151:1367–1368. doi: 10.1016/j.jtcvs.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


