Abstract
Background
The six-food elimination diet (SFED) is an effective treatment approach for eosinophilic esophagitis (EoE), but it can be challenging and affect patients’ quality of life.
Aim
Assess patients’ long-term adherence to SFED and potential factors influencing adherence.
Methods
EoE patients were recruited online via multiple platforms. Patients were classified as reaching the maintenance stage if they responded to SFED and identified specific trigger foods by reintroduction. Maintenance stage patients were categorized into those actively following the elimination diet (ACTIVE) and those no longer on their prescribed diet (FORMER). Participants completed a study-specific questionnaire assessing patient experiences related to SFED use.
Results
Forty-two participants were identified as having reached the SFED’s maintenance stage. 57% (24/42) of the maintenance stage patients were ACTIVE users. FORMER users rated the SFED’s effectiveness at treating symptoms (5.45 ± 3.96, 10 max.) lower than ACTIVE users (8.29 ± 2.76, p = .02). A greater percentage of FORMER users (100%) agreed social situations create challenges in following the diet compared to ACTIVE users (67%, p < .05). Anxiety related to SFED was also higher among FORMER users (64%) compared to ACTIVE users (21%, p < .01). Both ACTIVE (95.8%) and FORMER (81.8%, NSS) users would recommend the elimination diet to other EoE patients.
Conclusions
Understanding SFED adherence is multifactorial and complex. Factors influencing SFED adherence during long-term maintenance with diet therapy include diet effectiveness, social situations, and diet-related anxiety. Despite a lower than expected long-term adherence to maintenance of an elimination diet, the majority would recommend diet therapy as a treatment to other EoE patients.
Keywords: Eosinophilic esophagitis, Gastroesophageal reflux disease, Dysphagia, Food allergy, Esophageal stricture, Esophagitis
Introduction
Eosinophilic esophagitis (EoE) is an immune-mediated chronic inflammatory disorder of the esophagus [1–5]. Treatment modalities for EoE consist of esophageal dilation [6], corticosteroids [7–11], and elimination or elemental diets [12–14]. One of the most popular diet approaches to treat EoE is an empirical elimination diet known as the six-food elimination diet (SFED) [12, 13]. Patients exclude cow’s milk protein, wheat, soy, egg, peanuts/tree nuts, and fish/shellfish from their diet. Prospective but uncontrolled studies have demonstrated that about two-thirds of patients achieve a histologic response [15, 16]. Through systematic reintroduction, the goal of SFED is the identification of one or a limited number of trigger foods, rather than prolonged exclusion of all six food groups. Elimination of even a small number of commonly ingested foods for long-term management of the disease can be challenging and affect the patient’s quality of life (QOL) [17–19]. One study demonstrated that EoE by itself has substantial impact on several psychosocial domains. Some of the patients’ concerns included EoE’s impact on eating and social relationships [17]. By intentionally restricting patients’ diets, SFED could further adversely affect their QOL.
The literature pertaining to the long-term follow-up on EoE patients who have been put on an elimination diet is limited. An important step in treating EoE patients with diet restriction is coming to a better understanding of the barriers that confront patients as they attempt to adhere to their diet. The focus of our study was to identify EoE patients in the “maintenance” stage of the SFED, a period in which patients were intended to continue long-term avoidance of trigger foods. The aim of our study was to assess patients’ adherence to the diet and identify their attitudes, beliefs, and potential barriers to SFED that patients may face with sustained diet therapy. Ensuring that the patient–provider interaction can lead to improved diet adherence requires a better understanding of the patient perspective. A better understanding of the patient’s perspective will help physicians tailor a personalized treatment plan for EoE to the specific needs and concerns of an individual patient.
Methods
Adult participants (aged 18–70) diagnosed with EoE were recruited online via Northwestern, a patient advocacy group (CURED Foundation) social media, the online research-dedicated Web site (ResearchMatch.org), and social media (Facebook, Twitter). All participants filled out the survey via an online link hosted by the third party, secure Web site Qualtrics (www.qualtrics.com). Screening questions were used to identify patients between 18 and 70 years of age with a diagnosis of EoE and no past history of serious mental illness (bipolar disorder, schizophrenia, and personality disorder). However, EoE diagnosis could not be confirmed in some participants due to lack of access to the participants’ medical records. Another screening question was used to identify patients who had completed the six-food elimination diet (SFED) and reaching the maintenance stage of the diet. Maintenance stage patients were defined as having removed the six foods from their regular diet, reintroducing the foods, and determining which food, if any, were a trigger for their EoE. This could not be confirmed for those participants recruited via social media and the research-dedicated Web site due to lack of access to these participants’ medical records; however, patients were asked to specify a date in which they began the maintenance stage. Maintenance stage patients were further categorized into those who actively followed an elimination diet (ACTIVE) and those no longer on their prescribed diet (FORMER). This study was approved by the institutional review board of Northwestern University. All participants completed informed consent prior to the beginning of the study.
Measures
All participants completed a series of questionnaires with an estimated 30-min completion time that collected the following information:
Demographic information such as gender, race/ethnicity, age, marital status, employment status, and education level.
Clinical information specifically diagnosis duration, symptom duration prior to diagnosis, symptom severity, whether they meet with a dietician or psychologist, and location of physician’s practice.
NIH-PROMIS short form[20] Emotional distress–depression and anxiety (16 items) assess self-reported negative mood (sadness, guilt), views of self (self-criticism, worthlessness), and social cognition (loneliness, interpersonal alienation), as well as decreased positive affect and engagement (loss of interest, meaning, and purpose). Anxiety items assess fear (fearfulness, panic), anxious misery (worry, dread), hyperarousal (tension, nervousness, restlessness), and somatic symptoms related to arousal (racing heart, dizziness). Each question has five response options ranging in value from one to five.
Eosinophilic Esophagitis Quality of Life Scale-Adult[21] The EoE-QOL-A is a self-report questionnaire designed to assess disease-specific health-related quality of life in EoE patients. Questions are designed to evaluate established domains of HRQOL such as social functioning, emotional functioning, and eating and symptom anxiety. Items were created based on EoE patient interview data. The EoE-QOLA includes 30 questions on a 5-point Likert scale. Higher scores indicate better HRQOL.
Study-specific questionnaire 17 statements assessing potential difficulties and barriers that SFED patients may face were given. This assessed patient experiences related to SFED use on a 5-point Likert scale. The questionnaire also assessed the patients’ trigger foods, how often patients were exposed to their trigger food, and a self-rated scale of the diet’s effectiveness at treating their symptoms.
Statistical Analysis
Data were exported from the online survey system directly into SPSS v. 23 for analyses. Participants with missing data were identified and removed from the sample. Preliminary descriptive statistics (percentage, mean ± standard deviation) analyzed the demographic and clinical characteristics of the sample, symptom severity, and psychological distress. Participants were dichotomized into ACTIVE and FORMER groups based on screening questions about SFED diet use. Independent sample t-Tests determined significant differences between the ACTIVE and FORMER users for continuous demographic and clinical variables and psychological distress, while z-tests for two population proportions evaluated differences between these groups for percentage variables. An additional series of z-tests for two population proportions determined significant differences for each question on the study-specific diet questionnaire (% agree or strongly agree summed for first percentage/N, % disagree or strongly disagree for second percentage/N). Statistical significance was set to p < .05 for all analyses.
Results
Sixty-seven participants identified themselves as over the age of 18 and with a diagnosis of EoE. Among these 67 patients, some had attempted the SFED, but did not reach the maintenance stage. Forty-two participants were identified as having reached the SFED’s maintenance stage, with at least 19 of these participants being Northwestern patients who had SFED data verified. 57% (24/42) of the maintenance stage patients were ACTIVE users. Demographic data for ACTIVE users and FORMER users are provided in Table 1. No significant differences were found for demographics, symptom duration, diagnosis duration, or duration between the start of the maintenance stage and completion of the survey. FORMER users rated the SFED’s effectiveness at treating symptoms (5.45 ± 3.96, 10 max.) lower than ACTIVE users (8.29 ± 2.76, p = .02). 96% of ACTIVE users agreed that the benefits of SFED for improving EoE symptoms outweigh the inconveniences that come with a restricted diet, compared to 36% of FORMER users (p < .01). 100% of ACTIVE users agreed that the SFED does a good job in keeping their EoE symptoms under control compared to 54.5% of FORMER users (p < .01). The majority of both ACTIVE (95.8%) and FORMER (81.8%) users would recommend the elimination diet to other patients with EoE (Table 2).
Table 1.
Demographics and clinical presentation by disease group
| Active SFED (N= 24) | Former SFED (N= 11) | P | |
|---|---|---|---|
| Age in years (mean±SD) | 45.3 ±9.0 | 43.6±13.5 | .72 |
| Male GENDER | 57.9% | 37.5% | .23 |
| Caucasian race | 95.8% | 100% | .49 |
| Non-hispanic ethnicity | 95.8% | 72.7% | .05 |
| Married | 70.8% | 81.8% | .49 |
| Employed (part or full time) | 83.3% | 90.9% | .56 |
| College educated | 87.5% | 81.9% | .65 |
| Primary EoE treatment provider | |||
| Gastroenterologist | 87.5% | 90.9% | .77 |
| Allergist | 8.3% | 9.1% | .94 |
| University-based practice | 83.3% | 81.8% | .91 |
| Worked with dietitian | 87.5% | 90.9% | .77 |
| Symptom duration prior to diagnosis (years) | 15.22±9.2 | 16.10±14.5 | .86 |
| Diagnosis duration (years) | 5.27±3.12 | 6.36 ±6.5 | .61 |
| Duration from start of maintenance stage to completion of survey (months) | 39 ±27.4 | 25.7 ±33.8 | .26 |
| Closely follow SFED most or all of time | 91.7% | 45.5% | .003 |
| Food trigger exposure | |||
| Accidental | 54.2% | 18.2% | .05 |
| Intentional | 20.8% | 18.2% | .86 |
| Both | 25.0% | 45.5% | .23 |
| Daily food trigger exposure | 0% | 54.5% | |
| Self-rated EoE symptom severity (out of 10) | 1.5 ±1.86 | 3.27 ±2.24 | .020 |
| Self-rated SFED effectiveness (out of 10) | 8.3 ±2.76 | 5.45 ±3.96 | .019 |
| PROM1S anxiety (out of 35) | 10.7±4.31 | 15.7 ±6.30 | .014 |
| PROM1S depression (out of 40) | 10.4 ±3.23 | 15.0±6.18 | .007 |
Table 2.
Agreement on SFED diet survey items by adherence group (Knowledge and Efficacy)
| Statement | ACTIVE SFED (N= 24) | FORMER SFED (N= 11) | Z |
|---|---|---|---|
| I have been educated about situations that put me at risk for cross-contamination of foods | 95.8% (23) | 72.7% (8) | 2.00* |
| My EoE healthcare providers have been helpful in providing dietary advice and options for my individual needs | 87.5% (21) | 72.7% (8) | 1.08 |
| I feel that I have been well-informed and educated about ways in which I can be most successful with the elimination diet | 91.7% (22) | 81.8% (9) | 0.85 |
| Following the elimination diet has improved my eating habits in general | 66.7% (16) | 63.6% (7) | 0.18 |
| In general, my nutrition knowledge has been improved since being on the elimination diet | 79.2% (19) | 90.9% (10) | −0.86 |
| I find the benefits of the elimination diet for improving my EoE symptoms outweigh the inconveniences that come with having a restricted diet | 95.8% (23) | 36.4% (4) | 3.89** |
| I would recommend the elimination diet to other patients with EoE | 95.8% (23) | 81.8% (9) | 1.38 |
| The elimination diet does a good job in keeping my EoE symptoms under control | 100% (24) | 54.5% (6) | 3.57** |
p < .05;
p < .01
However, 100% of FORMER users agreed that social situations make it harder to follow the elimination diet compared to 67% of ACTIVE users (p < .05). Anxiety related to SFED was higher among FORMER users (67%) compared to ACTIVE users (21%, p < .01). Finding the elimination diet to be much more difficult than expected was also higher among FORMER users (64%) compared to ACTIVE users (8%, p < .01). See Table 3.
Table 3.
Social and emotional
| Statement | ACTIVE SFED (N= 24) | FORMER SFED (N= 11) | Z |
|---|---|---|---|
| Social situations, like going out with friends or family, make it harder for me to follow the elimination diet | 66.7% (16) | 100% (11) | −2.18* |
| Following the elimination diet makes me anxious | 20.8% (5) | 63.6% (7) | −2.48** |
| When my symptoms are in control, I sometimes stop following the diet more than usual | 33.3% (8) | 36.4% (4) | −0.18 |
| Following the elimination diet makes me feel down or depressed | 29.2% (7) | 36.4% (4) | −0.43 |
| When I am stressed or feeling ill, I stop following the EoE diet more than usual | 16.7% (4) | 18.2% (2) | −0.11 |
| I find the elimination diet to be much more difficult than I expected it to be | 8.3% (2) | 63.6% (7) | 3.14** |
| Travel for pleasure/work has introduced a challenging hurdle in following my EoE diet | 75.0% (18) | 100% (11) | −1.82 |
p < .05;
p < .01
Questions concerning patients’ views on meal planning and costs identified that ACTIVE users were more concerned about finding safe foods to ingest and were also spending greater time planning meals (Table 4). Financial burden for SFED diet-related foods did not discriminate between ACTIVE and FORMER users.
Table 4.
EoEQOL, cost, and inconvenience
| Statement | ACTIVE SFED (N=24) | FORMER SFED (N=11) | Z |
|---|---|---|---|
| I worry when I’m out that I won’t find something to eat | 54.2% (13) | 18.2% (2) | 2.00* |
| I worry about eating out for fear of contamination | 70.8% (17) | 63.6% (7) | 0.43 |
| I spend a lot of time planning my meals | 58.3% (14) | 18.2% (2) | 2.24* |
| I find it troublesome to read food labels and shop at special stores | 87.5% (21) | 72.7% (8) | 1.08 |
| I find myself spending more money on food because of EoE | 45.8% (11) | 45.4% (5) | 0.02 |
| I have difficulty finding foods I can eat because of my EoE | 75.0% (18) | 54.5% (6) | 1.21 |
| I find the elimination diet difficult to follow because it’s expensive | 12.5% (3) | 27.3% (3) | −1.08 |
| I find the elimination diet difficult to follow because it’s restrictive | 58.3% (14) | 81.9% (9) | −1.36 |
p < .05;
p < .01
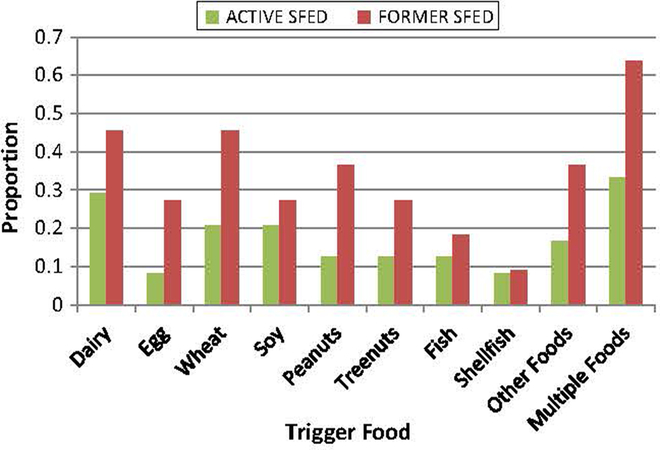
Specific food triggers identified were compared between ACTIVE and FORMER users. A greater proportion of FORMER users identified milk, wheat and/or multiple foods as triggers compared to ACTIVE users (Fig. 1). These differences, however, were not significant.
Fig. 1.
Proportion of patients with specific trigger foods among the two adherence groups. N = 24 for ACTIVE SFED, and N = 11 for FORMER SFED. There were no statistically significant differences among the two groups for trigger foods
Discussion
The present study is the first to evaluate factors associated with patient adherence to the long-term diet treatment with SFED for eosinophilic esophagitis. Among patients who reached the maintenance stage of the SFED, 43% were no longer following the elimination diet. Multiple factors appear to influence whether a patient remains adherent to the SFED including diet effectiveness, social situations, and diet-related anxiety. A significantly lower proportion of FORMER users reported that the diet was less effective in controlling their EoE symptoms, and a significantly lower proportion reported the symptom benefits outweighed the inconveniences. FORMER users had a greater number of identified food triggers than ACTIVE users but this was not statistically significant. If the SFED is not as effectively controlling symptoms, motivation to remain on the diet may, logically, degrade. The restrictiveness of the SFED likely outweighs the moderate benefits that come with it in such patients. Despite a lower than expected adherence to maintenance of an elimination diet, the majority (> 80%) of patients would recommend diet therapy as a treatment to other patients with EoE.
Few studies have assessed the long-term effects and patient adherence to the SFED. Lucendo et al. performed a three-year prospective study among EoE patients on the SFED demonstrating that at one-year follow-up, 25 of 42 patients continued to avoid offending foods, 15 patients at two-years follow-up, and four patients at three-years follow-up. All who were adherent with the food trigger avoidance diet were asymptomatic and were in histological remission [15]. Reed et al. [22] demonstrated that among 21 EoE patients who responded histologically to a food elimination diet, 10 remained adherent and in histologic remission at a follow-up period of 24.9 months. Gonsalves et al. [23] demonstrated that among 20 EoE patients who completed reintroduction and food trigger identification, nine had completed 1 year on the maintenance diet avoiding food triggers while also maintaining histological and symptomatic remission. Our study demonstrated that for ACTIVE users the average length of time from the start of the maintenance stage to completion of the survey was 39 months. Prior studies have assessed patient adherence to a gluten-free diet (GFD) in celiac disease. Similar to the SFED, eliminating gluten can be restrictive, difficult to follow, and affect quality of life. A systematic study demonstrated adherence to gluten-free diet by self-report that ranged widely, from about 42–91% [24]. Although evidence is limited, some factors most associated with GFD adherence are cognitive, emotional and sociocultural influences, membership of advocacy group, and regular dietary follow-up [24]. One study demonstrated that reported ability to follow the diet in social situations such as during travel, dining out, or at work is significantly related to adherence [25]. Our study has similar findings for diet adherence in relation to these sociocultural influences. Inflammatory bowel disease (IBD) is another chronic digestive illness population with significant treatment adherence data, and rates range from about 60–70% [26–28]. Factors associated with non-adherence include greater disease duration, weaker beliefs in the longevity of IBD, and being ambivalent, skeptical, or indifferent about maintenance therapy [26]. However, in our study, EoE disease duration appears unrelated to treatment adherence. Obstacles to adherence in IBD include cost of medication, side effects, unpleasant association with side effects, and uncertainty of effectiveness, suggesting that low adherence is a response to dealing with many day-to-day life matters [27].
There are several considerations physicians should weigh when recommending SFED as treatment. A large burden of EoE comes with eating as a social activity [17]. A significantly greater proportion of FORMER users also reports social situations create challenges to following SFED. It is possible that for these individuals, their social network inherently revolves around eating out more frequently. Thus, it is also important for physicians to consider the individual’s occupation. Certain individuals may have a professional travel schedule that requires them to eat out not only for themselves, but also with colleagues.
Anxiety may be a barrier to overall adherence to long-term diet therapy. All patients utilizing the SFED encounter similar difficulties (restrictiveness, social situations, reading food labels, time planning meals, etc.). A greater proportion of ACTIVE users report worrying about being able to find something to eat and spending time planning meals compared to FORMER users. However, with a greater proportion of FORMER users reporting diet-related anxiety, this could make social situations, diet restrictiveness, and food shopping more difficult to tolerate, and the cost–benefit ratio of following the SFED too high, thus reducing adherence. FORMER users also scored significantly higher on the PROMIS scale for depression and anxiety compared to ACTIVE users. However, given the methodology of the study, it is difficult to determine the temporal relationship between psychological distress and SFED adherence. Higher anxiety or depression could lead to reduced SFED adherence, or perhaps, because the SFED is not as effective at treating symptoms, this makes the FORMER users more distressed.
Truly understanding patient adherence to a prescribed treatment, whether it be medications, diet, or lifestyle, is multifactorial and complex. The negative feedback one receives from their symptoms may be a key driver in adherence. If an individual experiences severe anaphylaxis after exposure to a food allergen, it is probable that that individual would be much more adherent in avoiding that allergen. Unable to see the need for medication during periods of symptom quiescence was a reason given for non-adherence to 5-aminosalicylate therapy in patients with ulcerative colitis [29–31]. In EoE, the dysphagia and risk of having a food impaction may act as a negative feedback for these patients, but a difference is that exposure to food allergens typically does not immediately manifest as symptoms.
This study has some limitations that should be taken into consideration when interpreting its results. The current sample represents participants recruited from an outpatient gastroenterology practice, a patient advocacy group, and a research-dedicated Web site. We were unable to confirm an EoE diagnosis for all patients, specifically those recruited via online methods. It is unlikely that other patients without EoE completed our survey. We also were unable to confirm whether all these patients had truly completed all stages of the SFED; however, 19 of 42 participants were internal Northwestern patients with a confirmed SFED diagnosis and confirmed histologic response to certain trigger foods. External patients whom we could not confirm completion of all SFED stages provided a specific date on the questionnaire indicating when they had started the maintenance stage of the SFED. Not having confirmed these factors present a potential issue with validity. Future studies that assess diet adherence and use newer methods of recruitment such as social media and patient advocacy groups might request access to participant’s medical records to confirm EoE diagnosis and SFED status.
Additional limitations to our study include the generaliz-ability of the findings. The participants in our sample were mostly Caucasian, college educated, and at a university-based practice. Patients with lower educational levels may face additional challenges and barriers to dietary adherence. Caution must be taken before extrapolating the results to minority and other racial ethnic groups underrepresented in our cohort. The sample size of this study was also small. This could lead to inadequate power and potential Type 1 error. Although the prevalence of EoE is increasing, it is still uncommon and meets criteria for an “orphan disease.” Elimination diets are recognized as an effective first-line therapeutic option in EoE, but reaching an adequate sample size was difficult due to the small proportion of EoE patients who both reached the maintenance stage of the SFED and were available to complete our survey. There is a need for future larger scale studies addressing this topic to validate our observations and identify additional factors that might increase long-term adherence. Another limitation in this study is response bias. As with any questionnaire or self-reported survey, response bias can influence the validity of the study.
In conclusion, we have identified that more than half of patients who completed the SFED for EoE with identification of food triggers maintained their restricted diet. Despite a lower than expected lon-term adherence to maintenance of an elimination diet, the majority would recommend diet therapy as a treatment for other patients with EoE. Factors associated with adherence during long-term maintenance with diet therapy for EoE include perceived diet effectiveness, social situations, and diet-related anxiety. Understanding the adherence rate and barriers patients faces, when an elimination diet is central to optimizing this important treatment modality. Such knowledge should help tailor decisions regarding specific EoE medical or diet treatment options to the individualized needs and concerns of the patient.
Acknowledgments
Dr. Hirano would like to acknowledge grant support from the NIH Consortium of Eosinophilic Gastrointestinal disease Researchers (NIH U54AI117804), which is part of the Rare Disease Clinical Research Network, an initiative of the Office of Rare Disease Research funded through a collaboration between NIAID, NIDDK and NCATS.
Abbreviations
- EoE
Eosinophilic esophagitis
- eos/hpf
Eosinophils/high-power field
- GERD
Gastroesophageal reflux disease
- PPI
Proton pump inhibitor
Footnotes
Compliance with ethical standards
Conflict of interest None of the authors have potential conflicts related to this manuscript. Ikuo Hirano is a consultant and has received research funding from Adare, Shire, Regeneron, and Receptos. Tiffany Taft has served as a speaker for Janssen and Abbvie and Bethany Doer-fler for Nutricia North America and Allergan’s IBS counsel.
References
- 1.Liacouras CA, Furuta GT, Hirano I, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128:3–20. e6; quiz 1–2. [DOI] [PubMed] [Google Scholar]
- 2.Furuta GT, Liacouras CA, Collins MH, et al. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology. 2007;133:1342–1363. [DOI] [PubMed] [Google Scholar]
- 3.Dellon ES, Gonsalves N, Hirano I, et al. ACG clinical guideline: Evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE). Am J Gastroenterol. 2013;108:679–692. quiz 93. [DOI] [PubMed] [Google Scholar]
- 4.Prasad GA, Talley NJ, Romero Y, et al. Prevalence and predictive factors of eosinophilic esophagitis in patients presenting with dysphagia: a prospective study. Am J Gastroenterol. 2007;102:2627–2632. [DOI] [PubMed] [Google Scholar]
- 5.Dellon ES. Diagnosis and management of eosinophilic esophagitis. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2012;10:1066–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schoepfer AM, Gonsalves N, Bussmann C, et al. Esophageal dilation in eosinophilic esophagitis: effectiveness, safety, and impact on the underlying inflammation. Am J Gastroenterol. 2010;105:1062–1070. [DOI] [PubMed] [Google Scholar]
- 7.Liacouras CA, Spergel JM, Ruchelli E, et al. Eosinophilic esophagitis: a 10-year experience in 381 children. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2005;3:1198–1206. [DOI] [PubMed] [Google Scholar]
- 8.Schaefer ET, Fitzgerald JF, Molleston JP, et al. Comparison of oral prednisone and topical fluticasone in the treatment of eosinophilic esophagitis: a randomized trial in children. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2008;6:165–173. [DOI] [PubMed] [Google Scholar]
- 9.Konikoff MR, Noel RJ, Blanchard C, et al. A randomized, double-blind, placebo-controlled trial of fluticasone propionate for pediatric eosinophilic esophagitis. Gastroenterology. 2006;131:1381–1391. [DOI] [PubMed] [Google Scholar]
- 10.Dohil R, Newbury R, Fox L, Bastian J, Aceves S. Oral viscous budesonide is effective in children with eosinophilic esophagitis in a randomized, placebo-controlled trial. Gastroenterology. 2010;139:418–429. [DOI] [PubMed] [Google Scholar]
- 11.Straumann A, Conus S, Degen L, et al. Budesonide is effective in adolescent and adult patients with active eosinophilic esophagitis. Gastroenterology. 2010;139:1526–1537. 37 e1. [DOI] [PubMed] [Google Scholar]
- 12.Kagalwalla AF, Sentongo TA, Ritz S, et al. Effect of six-food elimination diet on clinical and histologic outcomes in eosinophilic esophagitis. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2006;4:1097–1102. [DOI] [PubMed] [Google Scholar]
- 13.Gonsalves N, Yang GY, Doerfler B, Ritz S, Ditto AM, Hirano I. Elimination diet effectively treats eosinophilic esophagitis in adults; food reintroduction identifies causative factors. Gastroenterology. 2012;142:1451–1459. e1; quiz e14–5. [DOI] [PubMed] [Google Scholar]
- 14.Kelly KJ, Lazenby AJ, Rowe PC, Yardley JH, Perman JA, Sampson HA. Eosinophilic esophagitis attributed to gastroesophageal reflux: improvement with an amino acid-based formula. Gastroenterology. 1995;109:1503–1512. [DOI] [PubMed] [Google Scholar]
- 15.Lucendo AJ, Arias A, Gonzalez-Cervera J, et al. Empiric 6-food elimination diet induced and maintained prolonged remission in patients with adult eosinophilic esophagitis: a prospective study on the food cause of the disease. J Allergy Clin Immunol. 2013;131:797–804. [DOI] [PubMed] [Google Scholar]
- 16.Arias A, Gonzalez-Cervera J, Tenias JM, Lucendo AJ. Efficacy of dietary interventions for inducing histologic remission in patients with eosinophilic esophagitis: a systematic review and meta-analysis. Gastroenterology. 2014;146:1639–1648. [DOI] [PubMed] [Google Scholar]
- 17.Taft TH, Kern E, Keefer L, Burstein D, Hirano I. Qualitative assessment of patient-reported outcomes in adults with eosinophilic esophagitis. J Clin Gastroenterol. 2011;45:769–774. [DOI] [PubMed] [Google Scholar]
- 18.Klinnert MD. Psychological impact of eosinophilic esophagitis on children and families. Immunol Allergy Clin North Am. 2009;29:99–107. x. [DOI] [PubMed] [Google Scholar]
- 19.Klinnert MD, Silveira L, Harris R, et al. Health-related quality of life over time in children with eosinophilic esophagitis and their families. J Pediatr Gastroenterol Nutr. 2014;59:308–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pilkonis PA, Choi SW, Reise SP, et al. Item banks for measuring emotional distress from the Patient-Reported Outcomes Measurement Information System (PROMIS(R)): depression, anxiety, and anger. Assessment. 2011;18:263–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taft TH, Kern E, Kwiatek MA, Hirano I, Gonsalves N, Keefer L. The adult eosinophilic oesophagitis quality of life questionnaire: a new measure of health-related quality of life. Aliment Pharmacol Ther. 2011;34:790–798. [DOI] [PubMed] [Google Scholar]
- 22.Reed CC, Fan C, Koutlas NT, Shaheen NJ, Dellon ES. Food elimination diets are effective for long-term treatment of adults with eosinophilic oesophagitis. Aliment Pharmacol Ther. 2017;46:836–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonsalves N, Doerfler B, Hirano I. Long term maintenance therapy with dietary restriction in adults with eosinophilic esophagitis. Gastroenterology. 2011;140:180 S-1.20955707 [Google Scholar]
- 24.Hall NJ, Rubin G, Charnock A. Systematic review: adherence to a gluten-free diet in adult patients with coeliac disease. Aliment Pharmacol Ther. 2009;30:315–330. [DOI] [PubMed] [Google Scholar]
- 25.Leffler DA, Edwards-George J, Dennis M, et al. Factors that influence adherence to a gluten-free diet in adults with celiac disease. Dig Dis Sci. 2008;53:1573–1581. https ://doi.org/10.1007/s1062 0-007-0055-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horne R, Parham R, Driscoll R, Robinson A. Patients’ attitudes to medicines and adherence to maintenance treatment in inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:837–844. [DOI] [PubMed] [Google Scholar]
- 27.Ediger JP, Walker JR, Graff L, et al. Predictors of medication adherence in inflammatory bowel disease. Am J Gastroenterol. 2007;102:1417–1426. [DOI] [PubMed] [Google Scholar]
- 28.Sewitch MJ, Abrahamowicz M, Barkun A, et al. Patient nonadherence to medication in inflammatory bowel disease. Am J Gastroenterol. 2003;98:1535–1544. [DOI] [PubMed] [Google Scholar]
- 29.Levy RL, Feld AD. Increasing patient adherence to gastroenterology treatment and prevention regimens. Am J Gastroenterol. 1999;94:1733–1742. [DOI] [PubMed] [Google Scholar]
- 30.Kane S, Huo D, Aikens J, Hanauer S. Medication nonadherence and the outcomes of patients with quiescent ulcerative colitis. Am J Med. 2003;114:39–43. [DOI] [PubMed] [Google Scholar]
- 31.Kane SV. Systematic review: adherence issues in the treatment of ulcerative colitis. Aliment Pharmacol Ther. 2006;23:577–585. [DOI] [PubMed] [Google Scholar]