ABSTRACT
Introduction
Hepatitis C virus (HCV) infection is a major cause of chronic liver disease. Six different genotypes (GT) of HCV (genotypes 1-6) have been identified. The genotype is clinically relevant since the majority of current direct antiviral agents (DAA´s) do not have pangenotypic efficacy. The purpose of this study was to describe the clinical characteristics of real world patients and evaluate the effectiveness of different treatment regimens.
Material and methods
Retrospective and observational study carried out in a third level hospital. Study period: January 2015-January 2016. Inclusion criteria: HCV patients of any genotype treated with either DAAs ± rivabirin (RBV) or DAAs + RBV + pegilated interferon (Peg-IFN) regimens for 12 weeks. Exclusion criteria: patients without adequate clinical or analytical information available for further analysis. Patients treated for 24 weeks were excluded. The main endpoint was sustained virologic response 12 weeks after the end of treatment (SVR12), and secondary endpoint was SVR24.
Results
A total of 515 patients were included (aged 55.52±8.97 years). GT1: patients treated with simeprevir + sofosbuvir (SIM + SOF), ledipavir (LDV)/SOF and paritaprevir/ritonavir/ombitasvir + dasabuvir (PTV/r/OBV + DSV) ± RBV had a SVR12 of 93.59% (190/203), 98.82% (N=84/85), 94.28% (66/70), respectively. Regarding daclatasvir (DCV) + SOF and SIM + DCV, everybody (19/19) and 87.5% (7/8) got SVR12, respectively. GT2: 71.42% (N=10/14) of patients achieved SVR12, concretely, SOF + RBV had a SVR12 75% (N=6/8). GT3: 43.75% (N=7/16), 90% (N=9/10) and 95% (N=19/20) of patients treated with LDV/SOF, LDV/SOF + RBV and SOF + DCV obtained SVR12, respectively. GT4: patients treated with LDV/SOF, SIM + SOF and PTV/r/OBV ± RBV had a SVR12 rate of 100% (21/21), 91.67% (22/24) and 92% (23/25), respectively. All patients that got SVR12 achieved SVR24.
Conclusion
Our study confirmed the efficacy data reported in clinical trials in a cohort of patients with GT1-4 and a wide range of basal characteristics.
Keywords: Hepatitis C virus, direct antiviral agents, genotypes 1-4, effectiveness
RESUMEN
Introducción
La infección por el virus de la hepatitis C (VHC) es una causa importante de enfermedad hepática crónica. Se han identificado seis genotipos (GT) diferentes de VHC (genotipos 1-6). El genotipo es relevante dado que la mayoría de los antivirales de acción directa (AAD) actuales no tienen eficacia pangenotípica. El objetivo del presente estudio fue describir las características clínicas de los pacientes y evaluar la efectividad de los diferentes tratamientos en condiciones de uso real.
Material y métodos
Estudio observacional, retrospectivo realizado en un hospital de tercer nivel. Período de estudio: enero-2015 a enero-2016. Criterios de inclusión: pacientes con VHC de cualquier genotipo tratados con AAD ± ribavirina (RBV) o AAD + RBV + interferón-α pegilado (Peg-IFN) durante 12 semanas. Criterios de exclusión: pacientes de quienes no se dispuso de información clínica/analítica adecuada para análisis posterior. Los pacientes tratados durante 24 semanas fueron excluidos. La variable principal fue la respuesta viral sostenida 12 semanas después de terminar el tratamiento (RVS12) y la secundaria la RVS24.
Resultados
Se incluyeron 515 pacientes (55,52 ± 8,97 años). GT1: pacientes tratados con simeprevir + sofosbuvir (SIM + SOF), ledipavir (LDV)/SOF y paritaprevir/ritonavir/ombitasvir + dasabuvir (PTV/r/OBV + DSV) ± RBV, tuvieron una RVS12 de 93,59% (190/203), 98,82% (84/85), 94,28% (66/70). En cuanto a daclatasvir (DCV) + SOF y SIM + DCV, todos (19/19) y 87,5% (7/8) obtuvieron RVS12, respectivamente. GT2: 71,42% (10/14) de los pacientes lograron RVS12, concretamente, los tratados con SOF+RBV tuvieron una RVS12 75% (6/8). GT3: 43,75% (7/16), 90% (9/10) y 95% (19/20) de los pacientes tratados con LDV/SOF, LDV/SOF + RBV y SOF + DCV alcanzaron RVS12, correspondientemente. GT4: pacientes tratados con LDV/SOF, SIM + SOF y PTV/r/OBV ± RBV tuvieron RVS12 del 100% (21/21), 91,67% (22/24) y 92% (23/25), respectivamente. Todos los pacientes que obtuvieron RVS12 lograron RVS24.
Conclusión
Nuestro estudio confirmó los datos de eficacia publicados en los ensayos clínicos en una cohorte de pacientes con GT1-4 y con una amplia gama de características basales.
Keywords: Virus de la hepatitis c, agentes antivirales directos, genotipos 1-4, efectividad
INTRODUCTION
Hepatitis C virus (HCV) infection is a major cause of chronic liver disease. Chronic hepatitis C (CHC) is a worldwide cause of liver-related morbidity and mortality and its long-term impacts range from minimal changes to extensive fibrosis and cirrhosis with or without hepatocellular carcinoma [1]. It affects over 185 million people, approximately 2–3% of the world’s population [2].
The objective of chronic HCV infection treatment is to achieve a sustained virological response (SVR). A SVR is stable over time, reduces morbidity and mortality, and is equivalent in most cases to curing the HCV infection [1].
Historically, HCV drug therapy was based on interferon-α (administered by injection) and ribavirin orally over many months, which is associated with severe side effects [3].
In 2011, the association of pegylated-interferons (Peg-INFs) and ribavirin (RBV) with the first direct antiviral agents (DAAs), telaprevir and boceprevir, increased the rate of SVRs in HCV genotype 1 from 30%-40% to 65%-75%. However, all these treatments had limited efficacy and low tolerability [1].
However, over the last several years, the management of CHC has been revolutionized by the development of cell-mediated targeted therapies, DAAs, against HCV. New regimens are simple, safe, effective, with short duration and minimal side effects [4].
Six different genotypes (GT) of HCV (genotypes 1, 2, 3, 4, 5, 6) have been identified [3]. GT1, specifically 1-b, is the most typical subtype worldwide, affecting 42% of HCV-infected patients [3]. This is followed by GT3 (26%), most typically found in Pakistan and India, and GT4 (14%), which is most typical in North Africa and the Middle East [3]. The GT is clinically relevant since the majority of current DAAs not have pangenotypic efficacy. In addition, each GT is associated with a different SVR rate [4]. Moreover, information derived from HCV anti-viral clinical trials have limited applicability in clinical practice. Understanding the effectiveness of anti-viral regimens in real-world settings is essential to providing practical information and adopting better HCV treatment decisions.
The purpose of this study is to describe the clinical characteristics of real world patients and evaluate the effectiveness of different treatment regimens with different HCV GTs according to real clinical practise.
MATERIAL AND METHODS
This retrospective and observational study analyzed different antiviral treatments for HCV-infected patients in routine clinical practice in a third level hospital. Inclusion criteria: HCV patients of any genotype treated with either DAAs ± RBV or DAAs plus RBV plus Peg-IFN treatment regimens from January 2015 to January 2016 for 12 weeks. Treatment regimens studied were: simeprevir + sofosbuvir (SIM+SOF), ledipavir/sofosbuvir (LDV/SOF), paritaprevir/ritonavir/ombitasvir + dasabuvir ± ribavirin (PTV/r/OBV+DSV ± RBV), daclatasvir + sofosbuvir (DCV + SOF), simeprevir + daclatasvir (SIM + DCV), SOF + RBV, SOF + RBV + Peg-IFN, LDV/SOF + RBV and PTV/r/OBV ± RBV.
DAAs reflects the evolution of HCV therapy in clinical practice. The treatment of choice was entirely at the discretion of the treating physician. It was made in accordance, of the majority of the cases, with the product label [5], the European Association for the Study of the Liver clinical practice guidelines [6] and the National Hepatitis C Plan developed by the Spanish Ministry of Health [7], giving priority for treatment to patients with significant liver fibrosis (F2-F4).
Exclusion Criteria: patients without adequate clinical and/or analytical information for further analysis. Patients treated for 24 weeks were also excluded.
The information was obtained from the electronic medical records and dispensing records from outpatient software (Cafydim® and Athos-Prisma®) Pharmacy Service
Outcomes collected:
•Demographic variables: age and sex.
•Clinical data: basal viral load (VL), sustained virologic response (SVR12), defined as HCV RNA titres lower than 15 IU/mL 12 weeks after end of treatment, and SVR24, defined as HCV RNA titres lower than 15 IU/mL 24 weeks after end of treatment. HCV-RNA levels were measured by the COBAS TaqMan HCV Test v2.0 (RCTM) (Roche Molecular Diagnostics) with lower limit quantification (LLOQ) of 15 IU/mL. Regarding fibrosis grade, patients were categorized according to METAVIR scale (F0-F4). F4 patients were considered as cirrhotic. Fibrosis stage was determined by non-invasive device: Fibroscan®. Other variables analysed were: platelet levels (cel/µL), albumin concentration (g/dL), transaminases hepatic levels (IU/L): aspartate transaminase (AST) and alanine transaminase (ALT) (IU/L), and bilirubin concentration (mg/dL). We also have assessed whether patients had had liver transplant, HIV co-infection or had been treated previously for HCV.
The main endpoint measured was the SVR12 and the secondary endpoint was SVR24.
Adherence variable: The calculation was made with the following formula:
Percentage of adherence = number of units of total DAAs agents medication dispensed/number of units of planned DAAs agents medication.
RESULTS
In the study period, in our hospital, were treated 603 HCV patients. We have genotype data of 97.84% (N=590). The genotypic distribution of all patients is summarised in table 1.
Table 1.
Genotypic distribution of different patients treated from January 2015 to January 2016.
| Genotypic distribution | Number of patients (N=590) |
|---|---|
| GT 1 | 431 (73.05%) |
| GT 2 | 14 (2.37%) |
| GT 3 | 71 (12.03%) |
| GT 4 | 74 (12.54%) |
The patients included were 515: 385 GT1-infected patients, 14 GT2-infected patients, 46 GT3-infected patients and 70 GT4-infected patients. All of them were treated for 12 weeks. We lost 75 patients due to insufficient clinical or analytical information.
Baseline characteristics. Of the 515 patients included in the study, 332 (64.46%) were male, with mean age of 55.52±8.97 years. Cirrhosis (F4) was present at baseline in 46.40% (N=239) of the cohort, 62 patients (12.04%) had received liver transplant, and 113 patients (21.95%) were pre-treated patients, but they did not achieve SVR12. Also, 102 patients (19.80%) were HIV co-infected and 290 (56.31%) had VL upper than 800,000 IU/mL. We have measured other serum biomarkers (mean ± standard deviation) related to stage of liver fibrosis and liver function such as platelet, albumin, AST, ALT and also bilirubin [8]. Finally, treatment adherence was analysed and it was 100% in all patients, except in two patients, which was 57.14% and 87.50%.
The most frequent treatment prescribed was SIM + SOF (44.46%), which was followed by LDV/SOF (24.07%) and PTV/r/OBV + DSV ± RBV (13.59%). In concrete, 385 GT1-infected patients [1] were studied (203 treated with SIM + SOF, 85 with LDV/SOF, 70 with PTV/r/OBV + DSV ± RBV, 19 with DCV + SOF, and 8 with SIM + DCV). 14 GT2-infected patients (2 of them treated with LDV/SOF, 8 with SOF + RBV, 2 with SIM + SOF, 1 with DCV + SOF and 1 with SOF + RBV + Peg-IFN). 46 GT3-infected patients (20 treated with SOF + DCV, 16 with LDV/SOF and 10 with LDV/SOF + RBV). 70 GT4-infected patients (24 treated with SIM + SOF, 21 treated with LDV/SOF, 25 treated with PTV/r/OBV ± RBV). The baseline characteristics of the patients are summarized in table 2.
Table 2.
Summary of the characteristics of all analysed patients (n=515).
| GT1 (n=385) |
GT2 (n=14) | GT3 (n=46) | GT4 (n=70) | ||||
|---|---|---|---|---|---|---|---|
| GT1 (undefined) (N=51) | GT1a (N=105) | GT1b (N=229) | |||||
| Age (years) | 55.52±8.97 | 58.83±9.44 | 52.68±8.04 | 60.81±10.42 | 54.78±12.36 | 54.17±8.54 | 51.87±5.05 |
| Sex | |||||||
| Male | 332 | 33 | 81 | 122 | 11 | 33 | 52 |
| Female | 183 | 18 | 24 | 107 | 3 | 13 | 18 |
| Stage of fibrosis | |||||||
| F4 | 239 | 25 | 52 | 107 | 4 | 22 | 29 |
| F3 | 152 | 15 | 22 | 67 | 8 | 14 | 26 |
| F2 | 100 | 9 | 24 | 50 | 1 | 7 | 9 |
| F1 | 18 | - | 7 | 2 | - | 3 | 6 |
| F0 | 6 | 2 | - | 3 | 1 | - | - |
| Liver transplant | 62 | 7 | 12 | 36 | 1 | 2 | 4 |
| Previously treated | 113 | 8 | 16 | 50 | 2 | 11 | 26 |
| VIH co-infected | 102 | 6 | 36 | 14 | 4 | 7 | 35 |
| Basal viral load > 800,000 IU/mL | 290 | 33 | 68 | 139 | 3 | 16 | 31 |
| Platelet (cel/µL) | 163.54±65.89 | 157.23±61.03 | 161.05±77.74 | 162.36±73.62 | 192.71±47.42 | 138.68±60.70 | 169.26±74.85 |
| Albumin (g/dL) | 4.01±0.51 | 3.99±0.44 | 3.93±0.54 | 4.00±0.51 | 4.10±0.61 | 3.97±0.48 | 4.09±0.46 |
| AST (IU/L) | 78.00±49.64 | 70.41±36.47 | 74.63±52.18 | 74.52±58.62 | 71.86±50.46 | 114.5±64.36 | 62.09±35.78 |
| ALT (IU/L) | 93.09±65.19 | 79.62±48.90 | 87.99±66.06 | 77.59±64.61 | 105.86±90.43 | 133.68±72.19 | 73.82±48.99 |
| Bilirrubin (mg/dL) | 0.84±0.54 | 0.92±0.68 | 0.84±0.55 | 0.86±0.52 | 0.90±0.70 | 0.77±0.34 | 0.77±0.47 |
| Treatment prescribed | |||||||
| SIM + SOF | 229 | 32 | 55 | 116 | 2 | - | 24 |
| LDV/SOF | 124 | 10 | 29 | 46 | 2 | 16 | 21 |
| PTV/r/OBV +DSV±RBV | 70 | 5 | 15 | 50 | - | - | - |
| DCV + SOF | 40 | 4 | 5 | 10 | 1 | 20 | - |
| SIM + DCV | 8 | - | 1 | 7 | - | - | |
| SOF + RBV | 8 | - | - | - | 8 | - | - |
| SOF + RBV + Peg-IFN | 1 | - | - | - | 1 | - | - |
| LDV/SOF + RBV | 10 | - | - | - | - | 10 | - |
| PTV/r/OBV ± RBV | 25 | - | - | - | - | - | 25 |
AST aspartate aminotransaminase, ALT alanine aminotransaminase, SIM simeprevir, SOF sofosbuvir, LDV ledipasvir, PTV paritaprevir, r ritonavir, OBV ombitasvir, DSV dasabuvir, RBV ribavirin, DCV daclatasvir, Peg-IFN peginterferon.
Sustained virologic response (SVR). Of the 515 patients included in the study 92.62% (477/515) achieved SVR12. However, there were not differences in response depending on VL because 38 patients in total did not reach SVR, 19 had VL <800,000 IU/mL and another 19 VL> 800,000 IU/mL. Also it is important to note that of the 38 patients who did not achieve SVR12, only 7 of them were HIV coinfected.
Genotype 1
The 95.06% (366/385) of all patients with chronic HCV GT1 achieved SVR12, likewise those patients that obtained SVR12 had SVR24, as well.
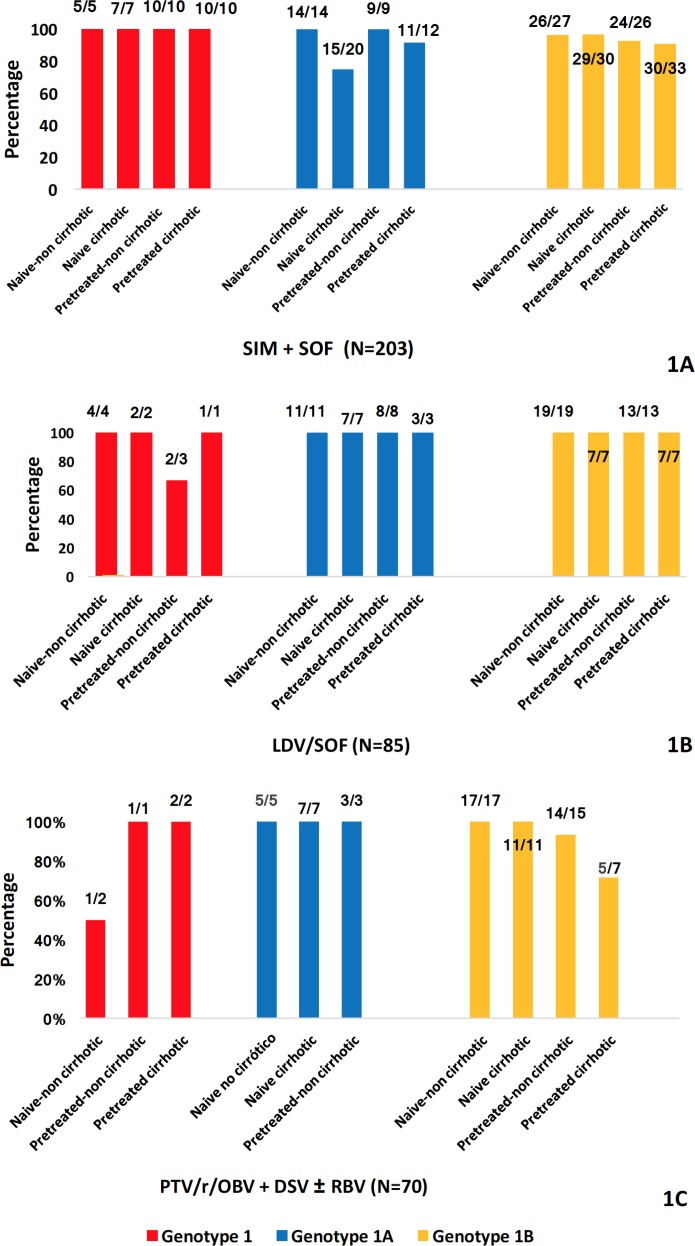
The 93.59% (190/203) of the patients treated with SIM + SOF got SVR12. If we analyse the different subgroups of patients, we observe that: 100% (N=32) of all patients with chronic HCV GT1 treated with SIM + SOF achieved SVR12.
The 89.09% (N=49/55) of the patients with GT1a achieved SVR12. It is remarkable that 5 naive cirrhotic patients (15/20) and one pre-treated cirrhotic patient (11/12) did not got SVR12. Regarding GT1b, the 93.96% (N=109/116) of the patients achieved SVR12. (figure 1A).
Figure 1.
SVR12 rates of HCV genotype 1-infected patients.
SVR12 of all patients with chronic HCV genotype 1 infection treated with SIM+SOF (figure 1A), LDV/SOF (figure 1B), PTV/r/OBV+DSV±RBV (figure 1C), DCV+SOF (figure 1D) and SIM+DCV (figure 1E). SIM simeprevir, SOF sofosbuvir, LDV ledipasvir, PTV paritaprevir, r ritonavir, OBV ombitasvir, DSV dasabuvir, RBV ribavirin, DCV daclatasvir.
As to LDV/SOF, 98.82% (N=84/85) of the patients treated got SVR12 (figure 1B), about PTV/r/OBV+DSV ± RBV (66/70), 94.28% of them reached SVR12 (figure 1C), and regarding DCV + SOF, everybody (19/19) achieved SVR12 (figure 1D). Finally, of the patients treated with SIM + DCV, 87.5% (7/8) got SVR12 (figure 1E).
Genotype 2 (n=14)
The 71.42% (N=10/14) of all patients with chronic HCV GT2 achieved SVR12. All patients that achieved SVR12 had SVR24, as well. Two naive-patients were treated with LDV/SOF achieving SVR12 only one of them (naive non-cirrhotic patient). Other two non-cirrhotic patients were treated with SIM + SOF, achieving SVR12 one of them (naive non-cirrhotic patient). 8 patients were treated with SOF+RBV, getting SVR12 a 75% of them (N=6/8). These two patients who did not achieve SVR12 were naive non-cirrhotic patients. Lastly, one patient was treated with SOF + RBV + Peg-IFN and another one with DCV+ SOF achieving both SVR12 (table 3).
Table 3.
SVR12 rates of HCV genotype 2-infected patients (N=14).
| Treatment regimens | SVR12 rate |
|---|---|
| LDV/SOF | 50% (1/2) |
| SIM + SOF | 50% (1/2) |
| SOF + RBV | 75% (6/8) |
| SOF + RBV + Peg-IFN | 100% (1/1) |
| DCV + SOF | 100% (1/1) |
| Total | 71.42% (10/14) |
LDV: ledipasvir, SOF: sofosbuvir, SIM: simeprevir, RBV: ribavirin, Peg-IFN: peginterferon, DCV: daclatasvir.
Genotype 3 (n=46)
The 76.08% (35/46) of all patients with chronic HCV GT3 achieved SVR12. Concretely, 43.75% (N=7/16) of patients treated with LDV/SOF obtained SVR12, 90% (N=9/10) of the patients treated with LDV/SOF + RBV got SVR12 and 95% (N=19/20) of the patients treated with SOF + DCV achieved SVR12.
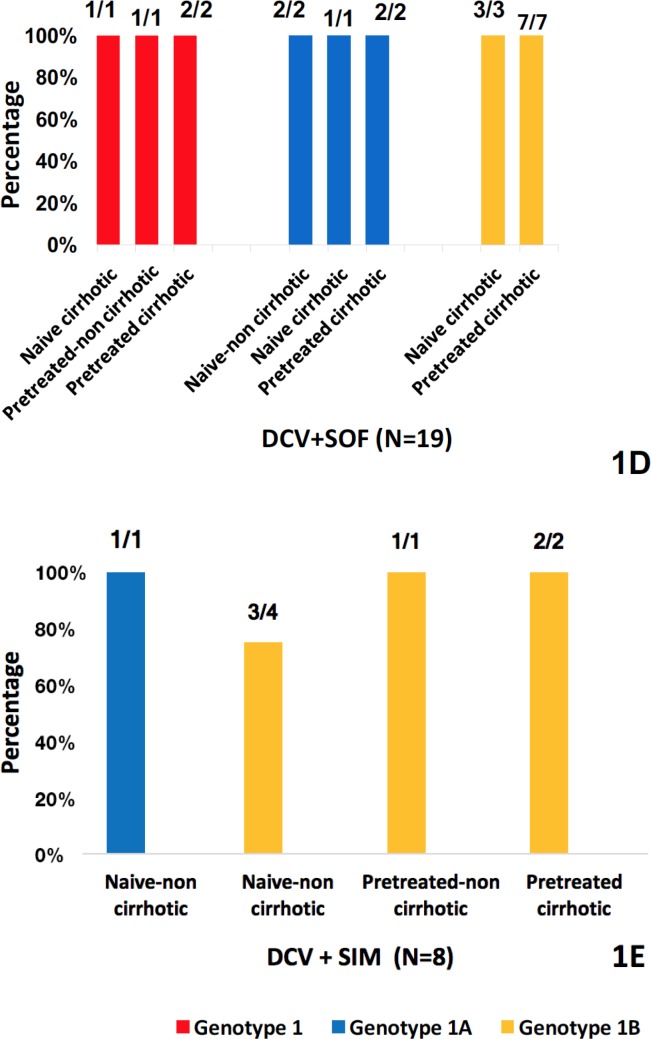
Considering patients treated with LDV/SOF (N=16), if we analyse the different subgroups of patients we observe that: 50% (N=3/6) naive-non cirrhotic patients achieved SVR12; 66.66% (N=2/3) of pre-treated-non cirrhotic patients achieved SVR12; 33.33% (N=2/6) of naive cirrhotic achieved SVR12; and no one of pre-treated cirrhotic patients (N=0/1) achieved SVR12 (figure 2).
Figure 2.
SVR12 rates of HCV genotype 3-infected patients.
It is represented the percentage of different subgroups of patients with HCV genotype 3 infection who achieved SVR12, treated with either LDV/SOF, or LDV/SOF + RBV, or DCV + SOF (LDV = ledipasvir, SOF = sofosbuvir, RBV = ribavirin, DCV =daclatasvir).
Figure 3.
SVR12 rates of HCV genotype 4-infected patients
It is represented the percentage of different subgroups of patients with HCV genotype 4 infection who achieved SVR12 treated with either SIM + SOF or LDV/SOF or PTV/r/OBV ± RBV (SIM = simeprevir, SOF = sofosbuvir, LDV = ledipasvir, PTV = paritaprevir, r = ritonavir, OBV = ombitasvir, RBV = ribavirin).\
As to LDV/SOF + RBV, all naive patients; non-cirrhotic (N=5) and cirrhotic (N=3) achieved SVR12. However, respect to pre-treated cirrhotic patients only 50% (N=1/2) achieved SVR12 (figure 2).
As regards DCV + SOF, all patients: naive non-cirrhotic (N=9), pre-treated non-cirrhotic patients (N=1), naive cirrhotic (N=6) achieved SVR12. Only 75% (N=3/4) of pre-treated cirrhotic patients achieved SVR12 (figure 2).
Genotype 4 (n=70)
The 94.28% (66/70) of all patients with chronic HCV GT4 achieved SVR12. In concrete, only one naive non-cirrhotic and one pre-treated cirrhotic (both treated with SIM + SOF), and one pre-treated non cirrhotic, and one naive cirrhotic (both treated with PTV/r/OBV ± RBV), did not get SVR12. Like GT1, 2 and 3, everybody that got SVR12 achieved SVR24.
DISCUSSION
In this study, we have investigated the real world effectiveness of various regimens of DAAs: SIM + SOF, LDV/SOF, PTV/r/OBV + DSV ± RBV, DCV + SOF, SIM + DCV, SOF + RBV, SOF + Peg-IFN + RBV, LDV/SOF + RBV, PTV/r/OBV ± RBV. Every regimen has been administered for 12 weeks in patients infected with HCV GT1, 2, 3 and 4 who met inclusion criteria, explained previously.
Our starting population was 603, but we only had GT data of 590 (only 515 met inclusion criteria), whose genotypic distribution was similar to that one published by Ramos H., et al. 2017 [1]. Concretely, the percentage of GT1 of our patients was 73.05% vs. 78.4%, GT2 of our patients was 2.37% vs. 2.4%, GT3 was 12.03% vs. 9.7% and GT4 was 12.54% vs. 9.5%.
In the general cohort, the global effectiveness was high, 92.23% of patients (475/515) got SVR12 and similar to those achieved in the study performed by Ramos H., et al. 2017 (94.6% SVR12) [1]. However, in this study 12 out of 462 patients were treated for 8 weeks and 43 out of 462 patients for 24 weeks, unlike ours, where every patient was treated for 12 weeks.
Until now, few real world setting studies have included results that consider the most frequent GTs (1 to 4). The most significant study is the US retrospective analysis of data from 17,487 patients with GTs 1 to 4 from the Veterans Affairs (VA) National Healthcare System, which had a global SVR12 of 90.7% lower than that in our study. This difference may be linked to early discontinuation of treatment in 4.4% of patients with available SVR12 data [1].
We have found that every subject who achieved SVR12 subsequently got SVR24, however in other studies obtained that between 0.4%-2% of the subjects who achieved a SVR12 subsequently relapsed at week 24 (did not achieve SVR24) [1,9,10]. These studies demonstrated that in DAAs regimens, with or without interferon, SVR12 and SVR24 are closely correlated.
Genotype 1
Most real world studies reported results in HCV GT1 patients [11,12,13]. The SVR12 rate in our study, which included 385 GT1 patients, was 95.06% (364/385) of the overall GT1 patients, which was practically the same than previously reported rates by Ramos et al. 2017 (94.5%) [1].
a) SIM + SOF. The SIM + SOF regimen was the most used in our GT1 patients, which was likely because it was the best combination available at the beginning of the study. This treatment was used in 203 of the total GT1 patients achieving a SVR12 of 93.59% (190/203). Results were similar to the study published by Ramos et al. 2017 (93.3%). In other study [14] with 802 HCV GT1-infected patients treated with this regimen, the SVR12 rates were lower, accurately 84%. The main cause of the difference was likely due to the lower rate of subtype 1a in our study (27.09%). Q80K polymorphism is found in up to 50% of patients with GT1a infection in the United States and the presence of Q80K has been associated with a decreased response in HCV GT1a-infected patients with cirrhosis treated with SIM+SOF but had not impact on non-cirrhotic patients treated for 12 weeks [14]. This variant was not analysed in the current study because it appears in only 2.7% of Spanish GT1 patients [1]. The COSMOS study, a phase II trial [15], reported high SVR12 rates in treatment-naive and prior null-responder HCV GT1-infected patients receiving SIM + SOF ± RBV for 12 or 24 weeks, concretely, 92.21% (154/167) of the patients had SVR12. The OPTIMIST-I study [16] was a multicenter, randomized, open-label study assessing the efficacy and safety of 12 and 8 weeks of SIM + SOF in HCV GT1-infected treatment-naive and pre-treated patients without cirrhosis. In the study OPTIMIST-I [16], was obtained a SVR12 of 97% in the 12-week SIM+SOF arm which similar to our study, 96.7% (88/91).
If we analysed the different subtypes, it is remarkable that HCV GT1a-infected non-cirrhotic patients achieved a SVR12 of 100% (23/23) and GT1b infected patients got a SVR12 of 94.33% (50/53), as well. In the OPTIMIST-I study [16], the SVR12 was 97% (112/116) for GT1a-infected non-cirrhotic patients and 97% (38/39) for GT1b.
Regarding to HCV GT1a-infected cirrhotic patients, our cohort got a SVR12 of 81.25% (26/32), similar to the OPTIMIST-II [17], [83%, (60/72)]. About GT1b, in our study the SVR12 achieved was of 93.65% (59/63), this response rate is higher than obtained in the OPTIMIST-II study [17], 84% (26/31). This difference could be related to the sample size or better to the basal conditions of the patients. Thus, it could be related to the percentage of patients with basal viral load upper than 800,000 IU/ml in each group, treatment-experienced patients, transplanted patients and other comorbidities.
b) LDV/SOF. LDV/SOF, a pangenotypic treatment combination, also showed high rates of SVR12 in our study: 98.82% (N=84/85). This rate was similar to 95% SVR12 rate derived from the study Ramos et al. (2017). We analyzed different subgroups of patients treated with LDV/SOF and we observed that all naive patients achieved SVR12 (100%, N=50/50), same result as ION-1 study (99%, 211/213) [18]. As to pre-treated patients, 97.17% (34/35) obtained SVR12, it is similar to ION-2 study [19] where SVR12 rate was of 94% (202/215). However, it is important to underline that the sample size in the ION-1 and ION-2 studies was bigger than our study and basal conditions of the patients could differ.
c) PTV/r/OBV + DSV ± RBV. With respect to PTV/r/OBV + DSV ± RBV, in our study, 94.28% of patients (66/70) got SVR12, which matches results published by Ramos H. et al. 2017, SVR12 rate of 94.5% [1]. By other side, we analysed different subgroups and 95.83% (23/24) of the naive non-cirrhotic patients got SVR12. In SAPPHIRE-I study [20], same results were obtained in naive non-cirrhotic patients (RVS12 was of 96.2%) (455/473). However, in SAPPHIRE-I study [20] all patients were treated with PTV/r/OBV + DSV + RBV while in our cohort, only 3 out of 24 received RBV.
If we break down our results according to the viral subtype, in our cohort, naive non-cirrhotic patients with GT1a infection got SVR12 100% of them (5/5). It is important to remark that every patient received PTV/r/OBV + DSV + RBV except two who did not take RBV. In SAPPHIRE-I study, all patients were treated with PTV/r/OBV + DSV + RBV and 95.3% (307/322) of them with HCV GT1a infection had SVR12. In PEARL-IV study [21], patients with GT1a infection and treated with PTV/r/OBV+DSV+RBV achieved a SVR12 rate of 97% (97/100) and who took PTV/r/OBV + DSV got a SVR12 rate of 90.2% (185/205).
About naive non-cirrhotic patients with GT1b infection, in our study, all of them had SVR12 (17/17), which is similar to the global response got in PEARL-III study [21], 99.28% (416/419). On the other hand, pre-treated non-cirrhotic patients with GT1b infection obtained a SVR12 of 93.33% (14/15), response that matches the PEARL-II study [22] (98.3%, N=176/179).
Therefore, both patients with GT1a and GT1b HCV infection achieved SVR12 similar to previous studies, although the sample size are not comparable.
d) DCV+SOF. Another treatment used in our study was DCV + SOF, concretely 19 subjects were treated achieving everyone SVR12, regardless of viral subtype. This response rate agrees with the results published by Ramos et al. 2017 [1] where all patients (N=15) with HCV GT1 infection reached SVR12. Likewise, also, these results are aligned with the response rate obtained in the AI444040 Study [23], where every patient (N=41) treated for 12 weeks had SVR12.
Finally, we analysed also SIM+DCV regimen, 85.71% (6/7) of patients with GT1b infection had SVR12, which matches SVR12 (81.57%, N=62/76) obtained in the study developed by Zeuman S. et al. 2016 [24] after 12 weeks of treatment.
Genotype 2
In our cohort, only fourteen patients with GT2 were treated with the following regimens: SOF + RBV; SOF + RBV + Peg-IFN; LDV/SOF; DCV + SOF and SIM + SOF and 71.42% (10/14) of them achieved SVR12.
The SVR12 rate (75%, N=6/8) with the SOF+RBV combination was similar to the SVR12 rate of 79.0% achieved in clinical practice in the VA study [11]. However, the low number of patients with GT2 in our study indicates that the combinations recommended in the clinical guidelines, such as DCV+SOF [25] should have been favoured due to the high rates of SVR12. Although SOF + RBV and SOF + RBV + Peg were the therapies of choice at that time according to the Ministry of Health, social services and equality in Spain. Currently, sofosbuvir/velpatasvir (VEL) [26] is also considered an effective therapy to treat HCV GT2 infection with high rates of SVR12.
Genotype 3
Patients with HCV GT3 are at a higher risk of liver disease progression and hepatocellular carcinoma development [27,28]. However, compared with other HCV GTs, DAAs combinations have lower efficacy against GT3 in patients with liver cirrhosis. In our study, the global SVR12 in patients with HCV GT3 infection was 76.08% (35/46). In Ramos H. et al. 2017 the global SVR12 in patients with HCV GT3 infection was 93.3% (42/45) [1]. This difference may be due in part to the fact that in the Ramos et al study (2017), most patients were treated with SOF + DCV (82.2%) vs 43.47% in our study, 6.7% with LDV/SOF (34.78% in our study) and 11.1% with SOF only and in our study 21.73% of total patients were treated with LDV/SOF + RBV. If we analyzed every regimen used in our study to treat HCV GT3 infection, it is essential to remark that 95% of the patients treated with SOF + DCV achieved SVR12, 90% of the patients treated with LDV/SOF + RBV achieved SVR12 and 43.75% of the patients treated with LDV/SOF achieved SVR12.
ALLY-3 clinical trial [29] supports the use of SOF + DCV for 12 weeks in patients infected with GT3. Patients were either treatment naive (n=101) or pretreated (n=51). SVR12 rates were 90% (91/101) and 86% (44/51) in treatment-naive and pre-treated patients, respectively. Concretely, in patients without cirrhosis SOF + DCV for 12 weeks achieved SVR12 rates of 97% (73/75) in treatment-naive patients and 94% (32/34) in pre-treated patients with GT3 infection, while in our study we got SVR12 rates of 100% in both cases, but our sample (n=10) was smaller than in the ALLY-3 study. However, lower rates were obtained for cirrhotic patients in the ALLY-3 study, exactly 63% (20/32) achieved SVR12, while in our cohort 90% (9/10).
ELECTRON-2 [30] study evaluated LDV/SOF for GT3. Of the 51 naive patients included, 100% (26/26) achieved RVS12 in the treatment arm with LDV/SOF + RVB 12 weeks [30]. These results are in line with those obtained in our study: 100% (8/8). In the treatment arm with LDV/SOF 12 weeks, only 64% (16/25) of them achieved SVR12 vs. 41.66% (5/12) in our cohort. These results are aligned with the treatment regimens as valuable options for GT3 recommended by European Association for the Study of the Liver (EASL) (guideline 2016). EASL establishes that in patients infected with HCV GT3, the combination of LDV/SOF is not recommended because LDV is considerably less potent against GT3 than VEL or DCV [25].
Genotype 4
Patients with HCV GT4 [25] infection are poorly represented in pivotal clinical trials of second-generation DAAs and in most real world studies. In our cohort, 94.28% (66/70) of all patients with HCV GT4 infection achieved SVR12, that is to say, a similar SVR12 rate to other real world studies such as Ramos et al. 2017 [1] and Ioannou G.N. et al. 2016 [31] where 95% (N=44) and 89.6% (N=135) of the patients got SVR12, respectively.
If we analysed the different treatment regimens used in our study, we underlined that LDV/SOF, SIM + SOF and PTV/r/OBV ± RBV had a SVR12 rate of 100% (21/21), 91.67% (22/24) and 92% (23/25), respectively. The same rates of SVR12 were obtained in the study of Ramos et al. 2017: 100% with LDV/SOF, 94.7% with SIM + SOF and 92.3% with PTV/r/OBV ± RBV [1].
Likewise, the SVR12 rates achieved in this study with the treatments SOF/LDV and PTV/r/OBV ± RBV match the results obtained in published clinical trials, ION-4 [32] with SVR12=96% (N=322/335) and PEARL-I [33] with SVR12=97%, (N=131/135).
In the SIM + SOF group, 91.67% (22/24) had SVR12, which is slightly lower than the value obtained in the PLUTO study [34], where all patients treated for 12 weeks got SVR12. This difference could be explained because in our cohort 45% (11/24) of the patients treated with SIM + SOF were cirrhotic and in the PLUTO study only 17.5% (7/40).
In conclusion, our study confirmed the efficacy data reported in clinical trials in a cohort of patients with GTs 1-4 and a wide range of basal characteristics, including a high proportion of pre-treated patients and with advanced fibrosis. Indeed, these new drugs show a high rate of response, which has revolutionized the management of chronic hepatitis C.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest
FUNDING
None to declare
REFERENCES
- 1.Ramos H, Linares P, Badia E, Martin I, Gomez J, Almohalla C, et al. Interferon-free treatments in patients with hepatitis C genotype 1-4 infections in a real-world setting. World J Gastrointest Pharmacol Ther. 2017. May;8(2):137–46. DOI: 10.4292/wjgpt.v8.i2.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013. April;57(4):1333–42. DOI: 10.1002/hep.26141 [DOI] [PubMed] [Google Scholar]
- 3.Messina JP, Humphreys I, Flaxman A, Brown A, Cooke GS, Pybus OG, et al. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology. 2015. January;61(1):77–87. DOI: 10.1002/hep.27259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lam BP,Jeffers T, Younoszai Z, Fazel Y, Younossi ZM. The changing landscape of hepatitis C virus therapy: focus on interferon-free treatment. Therap Adv Gastroenterol. 2015. September;8(5):298–312. DOI: 10.1177/1756283X15587481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centro de información online de medicamentos de la AEMPS [Internet].. Madrid: Agencia Española del Medicamento y Productos Sanitarios; 1997- [Cited 2018 January 07]. Available from: https://www.aemps.gob.es/cima/publico/home.html [Google Scholar]
- 6.European Association for Study of Liver.. EASL Recommendations on Treatment of Hepatitis C 2015. J Hepatol. 2015. July;63(1):199-236. DOI: 10.1016/j.jhep.2015.03.025 [DOI] [PubMed] [Google Scholar]
- 7.Ministerio de Sanidad, Servicios Sociales e Igualdad. Plan Estratégico para el abordaje de la Hepatitis C en el Sistema Nacional de Salud [Internet].. Madrid: Ministerio de Sanidad, Servicios Sociales e Igualdad; 2015. [Cited 2017 December 10]. Available from: http://www.plataformadeafectadosporhepatitisc.org/sites/default/files/plan_estrategico_nacional_definitivo.pdf [Google Scholar]
- 8.Bang CS, Kang HY, Choi GH, Kim SB, Lee W, Song IH. The Performance of Serum Biomarkers for Predicting Fibrosis in Patients with Chronic Viral Hepatitis. Korean J Gastroenterol. 2017. May;69(5):298–307. DOI: 10.4166/kjg.2017.69.5.298 [DOI] [PubMed] [Google Scholar]
- 9.Chen J, Florian J, Carter W, Fleischer RD, Hammerstrom TS, Jadhav PR, et al. Earlier sustained virologic response end points for regulatory approval and dose selection of hepatitis C therapies. Gastroenterology. 2013. June;144(7):1450–5.e2. DOI: 10.1053/j.gastro.2013.02.039 [DOI] [PubMed] [Google Scholar]
- 10.Yoshida EM, Sulkowski MS, Gane EJ, Herring RWJ, Ratziu V, Ding X, et al. Concordance of sustained virological response 4, 12, and 24 weeks post-treatment with sofosbuvir-containing regimens for hepatitis C virus. Hepatology. 2015. January;61(1):41–5. DOI: 10.1002/hep.27366 [DOI] [PubMed] [Google Scholar]
- 11.Backus LI, Belperio PS, Shahoumian TA, Loomis TP, Mole LA. Effectiveness of sofosbuvir-based regimens in genotype 1 and 2 hepatitis C virus infection in 4026 U.S. Veterans. Aliment Pharmacol Ther. 2015. September;42(5):559–73. DOI: 10.1111/apt.13300 [DOI] [PubMed] [Google Scholar]
- 12.Backus LI, Belperio PS, Shahoumian TA, Loomis TP, Mole LA. Real-world effectiveness and predictors of sustained virological response with all-oral therapy in 21,242 hepatitis C genotype-1 patients. Antivir Ther. 2017;22(6):481–93. DOI: 10.3851/IMP3117 [DOI] [PubMed] [Google Scholar]
- 13.Backus LI, Belperio PS, Shahoumian TA, Loomis TP, Mole LA. Comparative effectiveness of ledipasvir/sofosbuvir +/- ribavirin vs. ombitasvir/paritaprevir/ritonavir + dasabuvir +/- ribavirin in 6961 genotype 1 patients treated in routine medical practice. Aliment Pharmacol Ther. 2016. August;44(4):400–10. DOI: 10.1111/apt.13696 [DOI] [PubMed] [Google Scholar]
- 14.Lawitz E, Sulkowski MS, Ghalib R, Rodriguez-Torres M, Younossi ZM, Corregidor A, et al. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomised study. Lancet (London, England). 2014. November;384(9956):1756–65. DOI: 10.1016/S0140-6736(14)61036-9 [DOI] [PubMed] [Google Scholar]
- 15.Sulkowski MS, Vargas HE, Di Bisceglie AM, Kuo A, Reddy KR, Lim JK, et al. Effectiveness of Simeprevir Plus Sofosbuvir, With or Without Ribavirin, in Real-World Patients With HCV Genotype 1 Infection. Gastroenterology. 2016. February;150(2):419–29. DOI: 10.1053/j.gastro.2015.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwo P, Gitlin N, Nahass R, Bernstein D, Etzkorn K, Rojter S, et al. Simeprevir plus sofosbuvir (12 and 8 weeks) in hepatitis C virus genotype 1-infected patients without cirrhosis: OPTIMIST-1, a phase 3, randomized study. Hepatology. 2016. August;64(2):370–80. DOI: 10.1002/hep.28467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lawitz E, Matusow G, DeJesus E, Yoshida EM, Felizarta F, Ghalib R, et al. Simeprevir plus sofosbuvir in patients with chronic hepatitis C virus genotype 1 infection and cirrhosis: A phase 3 study (OPTIMIST-2). Hepatology. 2016. August;64(2):360–9. DOI: 10.1002/hep.28422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014. May;370(20):1889–98. DOI: 10.1056/NEJMoa1402454 [DOI] [PubMed] [Google Scholar]
- 19.Afdhal N, Reddy KR, Nelson DR, Lawitz E, Gordon SC, Schiff E, et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370(16):1483–93. DOI: 10.1056/NEJMoa1316366 [DOI] [PubMed] [Google Scholar]
- 20.Feld JJ, Kowdley K V, Coakley E, Sigal S, Nelson DR, Crawford D, et al. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014. April;370(17):1594–603. DOI: 10.1056/NEJMoa1315722 [DOI] [PubMed] [Google Scholar]
- 21.Ferenci P, Bernstein D, Lalezari J, Cohen D, Luo Y, Cooper C, et al. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med. 2014. May;370(21):1983–92. DOI: 10.1056/NEJMoa1402338 [DOI] [PubMed] [Google Scholar]
- 22.Andreone P, Colombo MG, Enejosa J V, Koksal I, Ferenci P, Maieron A, et al. ABT-450, ritonavir, ombitasvir, and dasabuvir achieves 97% and 100% sustained virologic response with or without ribavirin in treatment-experienced patients with HCV genotype 1b infection. Gastroenterology. 2014. August;147(2):359–65.e1. DOI: 10.1053/j.gastro.2014.04.045 [DOI] [PubMed] [Google Scholar]
- 23.Sulkowski MS, Gardiner DF, Rodriguez-Torres M, Reddy KR, Hassanein T, Jacobson I, et al. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med. 2014. January;370(3):211–21. DOI: 10.1056/NEJMoa1306218 [DOI] [PubMed] [Google Scholar]
- 24.Zeuzem S, Hezode C, Bronowicki J-P, Loustaud-Ratti V, Gea F, Buti M, et al. Daclatasvir plus simeprevir with or without ribavirin for the treatment of chronic hepatitis C virus genotype 1 infection. J Hepatol. 2016. February;64(2):292–300. DOI: 10.1016/j.jhep.2015.09.024 [DOI] [PubMed] [Google Scholar]
- 25.European Association for the Study of the Liver.. EASL Recommendations on Treatment of Hepatitis C 2016. J Hepatol. 2017. January;66(1):153-94. DOI: 10.1016/j.jhep.2016.09.001 [DOI] [PubMed] [Google Scholar]
- 26.Asociación Española para el Estudio del Hígado, Sociedad Española de Enfermedades Infecciosas y Microbiología.. Guías AEEH/SEIMC de manejo de la Hepatitis C [Internet]. Madrid; 2017. [Cited 2017 december 17]. Available from http://aeeh.es/wp-content/uploads/2017/06/consenso.pdf [Google Scholar]
- 27.Bochud P-Y, Cai T, Overbeck K, Bochud M, Dufour J-F, Mullhaupt B, et al. Genotype 3 is associated with accelerated fibrosis progression in chronic hepatitis C. J Hepatol. 2009. October;51(4):655–66. DOI: 10.1016/j.jhep.2009.05.016 [DOI] [PubMed] [Google Scholar]
- 28.Nkontchou G, Ziol M, Aout M, Lhabadie M, Baazia Y, Mahmoudi A, et al. HCV genotype 3 is associated with a higher hepatocellular carcinoma incidence in patients with ongoing viral C cirrhosis. J Viral Hepat. 2011. October;18(10):e516-22. DOI: 10.1111/j.1365-2893.2011.01441 [DOI] [PubMed] [Google Scholar]
- 29.Nelson DR, Cooper JN, Lalezari JP, Lawitz E, Pockros PJ, Gitlin N, et al. All-oral 12-week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study. Hepatology. 2015. April;61(4):1127–35. DOI: 10.1002/hep.27726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gane EJ, Hyland RH, An D, Svarovskaia E, Pang PS, Brainard D, et al. Efficacy of ledipasvir and sofosbuvir, with or without ribavirin, for 12 weeks in patients with HCV genotype 3 or 6 infection. Gastroenterology. 2015. November;149(6):1454–61.e1. DOI: 10.1053/j.gastro.2015.07.063 [DOI] [PubMed] [Google Scholar]
- 31.Ioannou GN, Beste LA, Chang MF, Green PK, Lowy E, Tsui JI, et al. Effectiveness of Sofosbuvir, Ledipasvir/Sofosbuvir, or Paritaprevir/Ritonavir/Ombitasvir and Dasabuvir Regimens for Treatment of Patients With Hepatitis C in the Veterans Affairs National Health Care System. Gastroenterology. 2016. September;151(3):457–71.e5. DOI: 10.1053/j.gastro.2016.05.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naggie S, Cooper C, Saag M, Workowski K, Ruane P, Towner WJ, et al. Ledipasvir and Sofosbuvir for HCV in Patients Coinfected with HIV-1. N Engl J Med. 2015. August;373(8):705–13. DOI: 10.1056/NEJMoa1501315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hezode C, Asselah T, Reddy KR, Hassanein T, Berenguer M, Fleischer-Stepniewska K, et al. Ombitasvir plus paritaprevir plus ritonavir with or without ribavirin in treatment-naive and treatment-experienced patients with genotype 4 chronic hepatitis C virus infection (PEARL-I): a randomised, open-label trial. Lancet (London, England). 2015. June;385(9986):2502–9. DOI: 10.1053/j.gastro.2016.05.049 [DOI] [PubMed] [Google Scholar]
- 34.Buti M, Calleja JL, Lens S, Diago M, Ortega E, Crespo J, et al. Simeprevir in combination with sofosbuvir in treatment-naive and -experienced patients with hepatitis C virus genotype 4 infection: a Phase III, open-label, single-arm study (PLUTO). Aliment Pharmacol Ther. 2017. February;45(3):468–75. DOI: 10.1111/apt.13883 [DOI] [PubMed] [Google Scholar]