Abstract
Background:
Cirrhosis-related complications are associated with poor prognosis. With our analyses, we examined the potential benefit of rifaximin in reducing the risk of developing cirrhosis-related complications.
Methods:
Adults with cirrhosis and hepatic encephalopathy (HE) in remission were randomly assigned to receive rifaximin 550 mg twice daily or placebo for 6 months with concomitant lactulose permitted. Post hoc analyses examined time to cirrhosis-related complications (HE, spontaneous bacterial peritonitis (SBP), variceal bleeding, acute kidney injury/hepatorenal syndrome). Subgroup analyses evaluated efficacy for select baseline disease characteristics.
Results:
Of patients receiving rifaximin (n = 140) and placebo (n = 159), 53.6% and 49.1%, respectively, had baseline Model for End-Stage Liver Disease (MELD) score ⩾ 12 and international normalized ratio (INR) ⩾ 1.2. Baseline ascites was observed in 36.4% (rifaximin) and 34.6% (placebo) of patients. In patients with MELD score ⩾ 12 and INR ⩾ 1.2, rifaximin reduced the relative risk (RR) of any first complication experienced during trial by 59% [hazard ratio (HR) = 0.41, 95% confidence interval (CI): 0.25–0.67; p < 0.001] versus placebo. For patients with baseline ascites, rifaximin reduced the RR of any first complication experienced during trial by 42% versus placebo (HR = 0.58, 95% CI: 0.34–1.0; p = 0.045). For some subgroups, there was a decrease in RR of complications of SBP, variceal bleeding, and acute kidney injury/hepatorenal syndrome with rifaximin versus placebo, although there were few events reported in the study.
Conclusion:
Rifaximin may reduce the incidence of cirrhosis-related complications and the recurrence of overt HE.
[ClinicalTrials.gov identifier: NCT00298038.]
Keywords: antibiotic, cirrhosis, decompensation, hepatic encephalopathy, rifaximin
Introduction
Cirrhosis has two phases: a compensated phase with favorable prognosis and a decompensated phase with poor prognosis (median survival > 12 years versus ~2 years, respectively).1,2 The shift from compensated to decompensated cirrhosis is characterized by the onset of complications, including ascites, hepatic encephalopathy (HE), varices and variceal bleeding, and spontaneous bacterial peritonitis (SBP),2–4 which are associated with substantial morbidity and mortality.5–12 For patients with cirrhosis, the 30-day rehospitalization rate has been shown to increase with the number of cirrhosis-related complications, from 11.1% with no complications to 24.3% with at least three complications.13 Ascites is the most common complication of cirrhosis.1 Occurrence of ascites is associated with mortality in 38% and 78% of patients with cirrhosis after 2 and 5 years, respectively.14 Ascites is also associated with increased risk of developing other complications of cirrhosis, including hepatorenal syndrome, SBP, varices, and variceal bleeding.15–18
Hepatic encephalopathy is a neurologic complication of cirrhosis that affects 30–70% of patients.19,20 Patients may present with symptoms that are not clinically apparent (minimal or covert HE; West Haven grade 1) or with more severe symptoms (overt HE; West Haven grades 2–4).20 For patients with cirrhosis who visit the emergency department, those with HE are at significantly greater risk of hospitalization compared with patients without HE [odds ratio (OR) = 4.4, 95% confidence interval (CI): 4.3–4.5; p < 0.01].21
The gut microbiota are thought to play a role in cirrhosis and development of cirrhosis-related complications.22,23 Intestinal permeability plays a role in the development of bacterial translocation and may be involved in the development of complications of cirrhosis; indeed, translocation of endotoxins is increased in patients with cirrhosis.24–27 In a prospective study, patients with more severe cirrhosis (i.e. patients with decompensated cirrhosis, hospitalized patients) had significantly greater serum endotoxin concentrations compared with patients with compensated cirrhosis (p < 0.0001), and a positive correlation between endotoxin concentration and the Model for End-Stage Liver Disease [MELD; calculated using three laboratory parameters: the international normalized ratio (INR), serum creatinine, and serum bilirubin]28 score was observed.29 Further, serum endotoxin concentrations were positively correlated with the severity of HE in patients with cirrhosis in a single-center prospective study.30 Thus, it is apparent that endotoxins may mediate complications of cirrhosis, including HE.
Rifaximin is a nonsystemic antibiotic administered as secondary prophylaxis for adults with cirrhosis and a history of overt HE.31 Rifaximin significantly decreased endotoxin concentrations from baseline in patients with cirrhosis after 8 weeks (p = 0.02).32 In patients with cirrhosis and previous episodes of overt HE, rifaximin significantly decreased the risk of recurrence of overt HE and overt HE-related hospitalization compared with placebo after 6 months of therapy (p < 0.001 and p = 0.01, respectively).33 In a retrospective study of patients with cirrhosis, rifaximin in combination with lactulose was associated with a significantly decreased risk of mortality compared with lactulose alone [adjusted hazard ratio (HR) = 0.70; 95% CI: 0.51–0.95; p = 0.02].34 Further, during a median 18 months of follow-up, rifaximin plus lactulose significantly decreased the risk of recurrence of overt HE and SBP compared with lactulose alone (overt HE: HR = 0.45, 95% CI: 0.28–0.71; p < 0.001; SBP: HR = 0.21, 95% CI: 0.11–0.41; p < 0.001).34
Cirrhosis and its complications (e.g. HE) have a substantial economic, social, and personal impact on affected patients, as well as their families and caregivers.35 Given that the number of primary prophylaxis treatments that prevent complications of cirrhosis is limited, it is important to examine whether rifaximin has the potential to reduce the risk of developing several complications of cirrhosis, including HE, SBP, variceal bleeding, and hepatorenal syndrome.36,37 Thus, the objective of the current analysis was to evaluate the potential impact of rifaximin on the reduction of the risk of developing complications of cirrhosis (i.e. HE, SBP, variceal bleeding, acute kidney injury/hepatorenal syndrome) in patients with a history of overt HE. As the MELD score is often used to determine prognosis in patients with cirrhosis and help prioritize patients for liver transplantation,38,39 and the occurrence of ascites in patients with cirrhosis is associated with the subsequent development of other complications of cirrhosis (e.g. SBP, variceal bleeding, hepatorenal syndrome),15–18 this post hoc analysis evaluated the efficacy of rifaximin as prophylaxis for complications in patients with cirrhosis, based on baseline MELD/INR scores and the presence of ascites.
Methods
Patients and study design
Details of the patient population, inclusion and exclusion criteria, and study design have been published elsewhere.33 Briefly, adults with cirrhosis, history of ⩾ 2 episodes of overt HE (Conn score ⩾ 2) within 6 months of screening, but currently in remission from HE (i.e. Conn score ⩽ 1), with a MELD score ⩽ 25 at study entry, were eligible for inclusion in the study. Exclusion criteria included active SBP or requirement of daily prophylactic therapy for SBP, renal insufficiency (serum creatinine > 2.0 mg/dl), anemia (hemoglobin < 8 g/dl), and hypovolemia or any electrolyte abnormality with the potential to affect mental function. The trial design was a randomized, phase III, placebo-controlled, multicenter trial of rifaximin 550 mg (Xifaxan®, Salix Pharmaceuticals, Bridgewater, NJ, USA) or placebo administered twice daily (BID) for 6 months. Concomitant use of lactulose was permitted during the study. As previously reported, the study protocol received approval by the institutional review board or ethics committee at each center, and the study was conducted in adherence to guidelines from the International Conference on Harmonisation.33 All patients provided written informed consent.
Assessments
Clinic visits occurred on days 7 and 14, and every 2 weeks thereafter through day 168 (end of treatment), with optional visits on days 42, 70, 98, 126, and 154. The primary endpoint was time to a breakthrough episode of overt HE (i.e. an increase in Conn score from ⩽1 at baseline to ⩾2, or an increase in Conn score and asterixis score of 1 grade for patients with a baseline Conn score of 0). Other common complications of cirrhosis (i.e. SBP, variceal bleeding, and acute kidney injury/hepatorenal syndrome) were further analyzed post hoc by baseline disease severity characteristics of MELD score, INR value, and presence of ascites. All complication events, except for HE, were collected from adverse event (AE) reporting (which included any discontinuation because of complications of cirrhosis). The investigator collected information on these AEs based on standard guidelines for diagnosing these events [i.e. abdominal ultrasound for ascites, endoscopy for detection of esophageal varices or variceal bleeding, and assessment of ascetic fluid (obtained by paracentesis)] for detection of SBP. The risk of complications of cirrhosis was compared in subgroups of patients with MELD scores ⩾ 12 and INR ⩾ 1.2 versus MELD scores < 12 and INR < 1.2, or in the presence or absence of ascites at baseline. For the subgroup analysis based on MELD/INR scores, a cutoff MELD score > 12 was used because the original study only included patients with MELD score of ⩽ 25 and the median MELD score was 13.1 for rifaximin and 12.4 for placebo. In addition, an INR of ⩾ 1.2 threshold was used as an internal barometer to ensure that the MELD scores reflected liver function changes and to potentially avoid inclusion of patients with pre-existing primary renal dysfunction unrelated to cirrhosis.
Statistical analyses
The intent-to-treat (ITT) population included all patients randomly assigned to treatment who received at least one dose of study drug. Demographic and baseline characteristics were summarized using descriptive statistics. Time to the first episode, experienced during the trial, of breakthrough overt HE (primary efficacy endpoint) or other complications of cirrhosis (i.e. SBP, variceal bleeding, and acute kidney injury/hepatorenal syndrome) for patients receiving rifaximin or placebo was analyzed using the Cox proportional hazards model, specifying a two-sided test at a significance level of 0.05 under the proportional hazards assumption. For each treatment group, Kaplan–Meier time-to-event methods were used to estimate the percentage of patients who experienced a breakthrough episode of overt HE on days 28, 56, 84, 140, and 168. Patients who completed the study without experiencing a breakthrough episode of overt HE were censored at 6 months. Patients who discontinued the study for other reasons (e.g. AE, patient request, liver transplantation) were contacted 6 months after randomization to ascertain whether a breakthrough episode of overt HE or other outcome (e.g. mortality) had occurred.
Results
A total of 299 patients [rifaximin (n = 140), placebo (n = 159)] were included in the ITT population.33 Demographic and baseline characteristics were generally comparable between groups (Table 1). Concomitant lactulose use during the study was reported by 91.4% and 91.2% of patients receiving rifaximin or placebo, respectively. A comparable percentage of patients receiving rifaximin or placebo had a baseline MELD score ⩾ 12 and INR ⩾ 1.2 [53.6% (n = 75) versus 49.1% (n = 78)]. Approximately one third of patients in each treatment group had ascites present at baseline.
Table 1.
Baseline demographics and disease characteristics.
| Parameter | Rifaximin (n = 140) |
Placebo (n = 159) |
||
|---|---|---|---|---|
| Age, years, mean (SD) | 55.5 (9.6) | 56.8 (9.2) | ||
| Male sex, n (%) | 75 (53.6) | 107 (67.3) | ||
| Race, White, n (%) | 118 (84.3) | 139 (87.4) | ||
| Duration of current HE remission, days, mean (SD) | 68.8 (47.7) | 73.1 (51.3) | ||
| Time since advanced liver disease diagnosis, months, mean (SD) | 51.2 (49.2) | 60.5 (64.9) | ||
| MELD score, mean (SD) | 13.1 (3.6) | 12.7 (3.9)* | ||
| INR, mean (SD) | 1.4 (0.3)$ | 1.4 (0.4)‡ | ||
| Ascites present, n (%) | 51 (36.4) | 55 (34.6) | ||
| MELD ⩾12 and INR ⩾1.2 |
MELD <12 and INR <1.2 |
MELD ⩾12 and INR ⩾1.2 |
MELD <12 and INR <1.2 |
|
| MELD score, mean (SD)* | 15.4 (2.5) | 8.0 (1.5) | 15.7 (2.9) | 7.9 (1.6) |
| INR, mean (SD)$‡ | 1.6 (0.2) | 1.1 (0.1) | 1.6 (0.3) | 1.1 (0.1) |
| Ascites, yes | Ascites, no | Ascites, yes | Ascites, no | |
| MELD score, mean (SD)* | 14.1 (3.7) | 12.5 (3.5) | 14.0 (4.2) | 12.0 (3.6) |
| INR, mean (SD)$‡ | 1.5 (0.3) | 1.4 (0.3) | 1.5 (0.3) | 1.4 (0.4) |
n = 158 in placebo group.
n = 130 in rifaximin group.
n = 147 in placebo group.
HE, hepatic encephalopathy; INR, international normalized ratio; MELD, Model for End-Stage Liver Disease; SD, standard deviation.
Table created from study data, with additional data from Bass et al.33
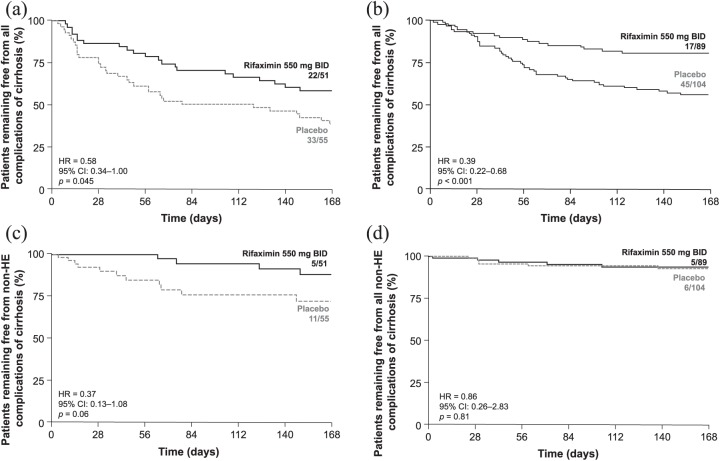
For patients with MELD scores ⩾ 12 and INR scores ⩾ 1.2, the HR for the time to any first complication of cirrhosis experienced during the trial for rifaximin (n = 75) versus placebo (n = 78) was 0.41, indicating a relative risk (RR) reduction of 59% with rifaximin during 6 months of treatment; p < 0.001 [Figure 1(a)].
Figure 1.
Time to a first complication of cirrhosis experienced during the trial by treatment group and baseline disease severity.
(a) All complications of cirrhosis in patients with MELD score ⩾ 12 and INR ⩾1.2; (b) non-HE complications of cirrhosis in patients with MELD score ⩾ 12 and INR ⩾ 1.2; and (c) all complications of cirrhosis in patients with MELD score <12 and INR <1.2.
BID, twice daily; CI, confidence interval; HE, hepatic encephalopathy; HR, hazard ratio; INR, international normalized ratio; MELD, Model for End-Stage Liver Disease.
Rifaximin was associated with a 68% reduction in the RR of developing overt HE during 6 months of treatment in patients with MELD scores ⩾12 and INR scores ⩾1.2 (Table 2). There was a trend for reduction in the RR for overall non-HE complications when event data were pooled (i.e. SBP, variceal bleeding, and acute kidney injury/hepatorenal syndrome) in these patients (MELD scores ⩾ 12 and INR scores ⩾ 1.2); however, this reduction did not reach statistical significance [HR = 0.46, 95% CI: 0.18–1.17; p = 0.10; Figure 1(b)].
Table 2.
Risk of complications of cirrhosis.
| Complication | MELD ⩾ 12 and INR ⩾ 1.2 |
MELD < 12 and INR < 1.2 |
||||||
|---|---|---|---|---|---|---|---|---|
| Events, n |
Hazard ratio (95% CI) |
p value | Events, n |
Hazard ratio (95% CI) |
p value | |||
| Rifaximin (n = 75) | Placebo (n = 78) |
Rifaximin (n = 24) |
Placebo (n = 30) |
|||||
| Hepatic encephalopathy | 18 | 44 | 0.32 (0.19–0.56) | <0.0001 | 1 | 9 | 0.13 (0.02–1.03) | 0.02 |
| Acute kidney injury/hepatorenal syndrome | 2 | 6 | 0.26 (0.05–1.31) | 0.08 | 0 | 1 | 0 (NC) | 0.37 |
| Variceal bleeding | 4 | 3 | 0.99 (0.22–4.49) | 0.99 | 2 | 0 | >1000 (NC) | 0.13 |
| Spontaneous bacterial peritonitis | 1 | 4 | 0.21 (0.02–1.88) | 0.12 | 0 | 0 | NC | NC |
| Complication | Baseline ascites present |
No baseline ascites present |
||||||
| Events, n |
Hazard ratio (95% CI) |
p value | Events, n |
Hazard ratiobreak/>(95% CI) | p value | |||
| Rifaximin (n = 51) | Placebo (n = 55) | Rifaximin (n = 89) | Placebo (n = 104) | |||||
| Hepatic encephalopathy | 18 | 31 | 0.52 (0.29–0.93) | 0.03 | 13 | 42 | 0.32 (0.17–0.59) | 0.0001 |
| Acute kidney injury/hepatorenal syndrome | 2 | 4 | 0.42 (0.08–2.30) | 0.30 | 0 | 4 | 0 (NC) | 0.07 |
| Variceal bleeding | 3 | 4 | 0.62 (0.14–2.79) | 0.53 | 3 | 3 | 1.0 (0.20–5.00) | 0.99 |
| Spontaneous bacterial peritonitis | 0 | 4 | 0 (NC) | 0.03 | 2 | 1 | 2.15 (0.19– 23.8) | 0.52 |
CI, confidence interval; INR, international normalized ratio; MELD, Model for End-Stage Liver Disease; NC, not calculated.
For patients with MELD scores <12 and INR scores <1.2, rifaximin reduced the RR of their first cirrhosis-related complication experienced during the trial by 74% during 6 months of treatment [Figure 1(c)], although significance was not achieved (p = 0.06).
Patients with MELD scores <12 and INR scores <1.2 experienced low numbers of events, which made RR determination difficult for several individual complications [HE (n = 10), acute kidney injury/hepatorenal syndrome (n = 1), varices, variceal bleeding, and gastrointestinal bleeding (n = 2), SBP (n = 0)]. However, rifaximin reduced the RR of overt HE for these patients during 6 months of treatment (87%) compared with placebo (Table 2).
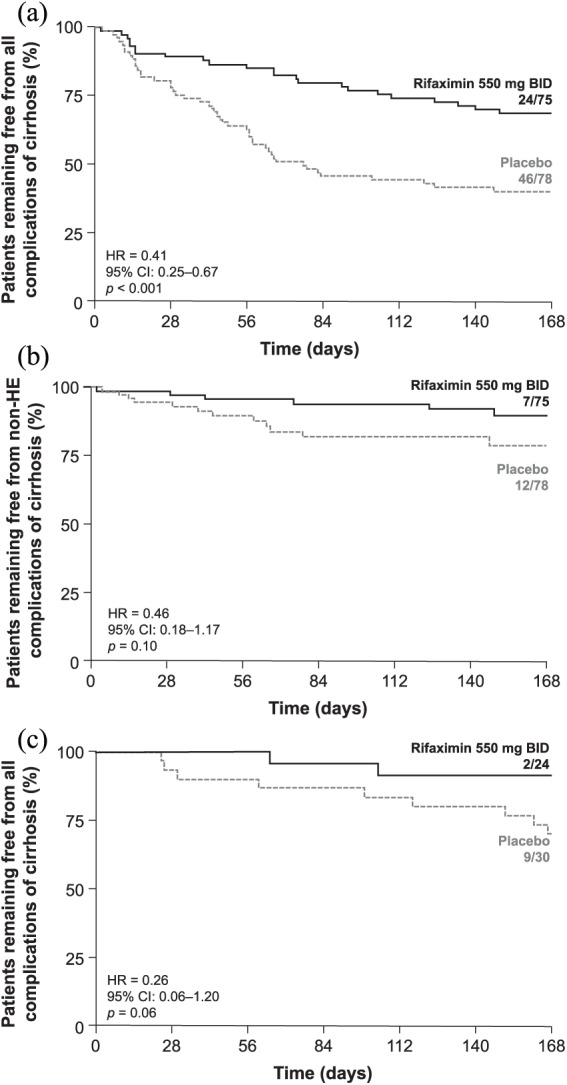
For patients with ascites at baseline, rifaximin treatment during the trial was associated with a 42% decrease in the RR of experiencing their first cirrhosis complication during 6 months of treatment versus placebo [Figure 2(a)].
Figure 2.
Time to a first complication of cirrhosis experienced during the trial by treatment group.
All complications of cirrhosis according to (a) presence or (b) absence of ascites at baseline; and non-HE complications of cirrhosis according to (c) presence or (d) absence of ascites at baseline.
BID, twice daily; CI, confidence interval; HE, hepatic encephalopathy; HR, hazard ratio.
For patients without ascites at baseline, rifaximin treatment during the trial decreased the RR of their first cirrhosis complication by 61% versus placebo during 6 months of treatment [Figure 2(b)].
Further, rifaximin treatment for 6 months decreased the RR of overt HE by 48% and 68% in patients with and without baseline ascites, respectively (Table 2). Other complications of cirrhosis occurred in only a small number of patients, either with or without ascites at baseline (i.e. SBP, variceal bleeding, and acute kidney injury/hepatorenal syndrome; Table 2). While the reductions in complications appeared to be driven by the reduction in HE events, given the small number of non-HE events reported, a pooled analysis showed a 63% reduction in the RR of non-HE complications in patients with ascites at baseline, although significance was not achieved [HR = 0.37, 95% CI: 0.13–1.08, p = 0.06; Figure 2(c)].
No significant difference in the RR of non-HE complications was observed in patients without ascites at baseline [HR = 0.86, 95% CI: 0.26–2.83; p = 0.81; Figure 2(d)].
In the subgroup of patients with ascites at baseline, four patients experienced SBP events (all in the placebo group; p = 0.03), and in the subgroup of patients without ascites at baseline, three patients experienced SBP events (rifaximin, n = 2; placebo, n = 1). The onset of SBP AEs (n = 7 overall) in this study ranged from 13 to 152 days, and only one patient (placebo group) had a history of SBP.
Discussion
In the treatment of patients with cirrhosis, prophylactic agents are needed to reduce the overall risk of complications. Given the role of the INR-driven MELD score in determining prognosis in patients with cirrhosis38 and that the presence of ascites is associated with the subsequent development of other complications of cirrhosis (e.g. SBP, variceal bleeding, hepatorenal syndrome),15–18 this post hoc analysis evaluated the efficacy of rifaximin 550 mg BID for 6 months as prophylaxis of complications in patients with cirrhosis, based on baseline INR, MELD score, and presence of ascites.
During 6 months of treatment, rifaximin decreased the RR of any first complication of cirrhosis experienced during the trial for patients with MELD score ⩾ 12 and INR ⩾ 1.2, compared with placebo. Similarly, rifaximin decreased the RR of any first complication of cirrhosis in patients with and without baseline ascites. Findings in these subgroups were driven by the inclusion of HE in the analysis. A separate pooled analysis that excluded HE found a trend in RR reduction with rifaximin in either MELD subgroup that did not reach statistical significance, potentially because non-HE complications were experienced by only a few patients. Not unexpectedly for the complication of HE, rifaximin significantly reduced the RR of its recurrence in both MELD subgroups and both ascites subgroups during 6 months of treatment.
In this current study, the number of patients in all subgroups who experienced individual complications of cirrhosis other than HE (i.e. SBP, variceal bleeding, acute kidney injury/hepatorenal syndrome) was small. Although the data from this study do not unequivocally show that rifaximin reduces the rate of non-HE complications of cirrhosis, a trend in RR reduction was observed that did not reach statistical significance, conceivably related to the small number of events. Thus, this warrants examining the occurrence of non-HE-related events in future studies, with larger patient populations, that are appropriately powered. However, results from another study have shown that patients receiving rifaximin had significantly decreased incidence of acute kidney injury and hepatorenal syndrome compared with patients not receiving rifaximin (p = 0.02 and p = 0.01, respectively).40 Likewise, in the current study, rifaximin was associated with a RR reduction for incidence of SBP compared with placebo in the subgroup of patients with ascites at baseline; however, the number of patients experiencing SBP in this study was small (n = 7 overall). Consistent with this finding, a separate retrospective medical records review reported that patients receiving rifaximin for the primary prevention of SBP had a 72% decrease in the risk of occurrence of SBP compared with patients not receiving rifaximin (HR = 0.28, 95% CI: 0.11–0.71; p = 0.007).36 A second retrospective review of 421 patients with cirrhosis found that 6.8% and 42.7% of patients receiving rifaximin 600 mg BID plus lactulose or lactulose alone, respectively, for a median 18 months experienced SBP; rifaximin plus lactulose decreased the risk of developing SBP by 79% compared with lactulose alone.34 In the current study, although the number of events was low overall, fewer patients with ascites and fewer patients with MELD ⩾ 12 and INR ⩾ 1.2 receiving rifaximin experienced SBP compared with those receiving placebo. Another randomized study, which compared rifaximin and norfloxacin for the prevention of SBP in patients with cirrhosis and ascites, reported that significantly fewer patients who received rifaximin 400 mg three times daily for 6 months experienced SBP compared with those who received norfloxacin (3.9% versus 14.1%, respectively; p = 0.04).41 However, another study of patients with cirrhosis-related ascites reported that rifaximin did not exhibit greater efficacy for the prevention of SBP compared with systemic antibiotics (incidence of 30% versus 0%, respectively).42 Future larger prospective studies are warranted to examine whether rifaximin has the potential to decrease the risk of SBP, given the association of SBP with HE, hospitalization, and mortality.17,43,44
The post hoc nature of the current analysis is a limitation, as the trial was powered for prevention of complications of overt HE and was not powered to examine the efficacy of rifaximin for prevention of all complications or other individual complications of cirrhosis (e.g. SBP). In addition, only patients with a baseline MELD score ⩽ 25 were included in this study, hence, excluding patients with more severe disease. Further, the study was designed to assess rifaximin as secondary prophylaxis for overt HE and included patients who experienced at least two episodes of HE in the previous 6 months. Whether the number of baseline episodes of HE may have impacted the development of future episodes or other complications of cirrhosis remains unclear. However, a post hoc analysis of a 24-month, open-label study examining rifaximin efficacy in the prevention of recurrence of overt HE that included both patients from the current study and newly enrolled patients indicated that there was an association between the number of previous episodes of HE reported at baseline and an increased risk of additional episodes, particularly for patients with baseline MELD scores ⩽ 10.45 In addition, non-HE complications of cirrhosis events were collected as part of the AE reporting in this analysis by the investigating physician, based on standard guidelines for diagnosing these events. Lastly, the current analysis was restricted to 6 months of treatment, and thus the potential long-term impact of rifaximin on preventing the complications of cirrhosis remains to be elucidated.
Conclusions
Rifaximin 550 mg BID for 6 months reduced the incidence of complications of cirrhosis and the recurrence of overt HE in patients with more severe cirrhosis (i.e. MELD score ⩾ 12 and INR ⩾ 1.2 versus MELD score < 12 and INR < 1.2) and in patients with baseline ascites. Further research on the potential role of rifaximin as prophylaxis against the complications of cirrhosis and HE in larger, prospective studies is warranted.
Acknowledgments
Technical editorial assistance was provided, under the direction of the authors, by Mary Beth Moncrief, PhD, and Sophie Bolick, PhD, Synchrony Medical Communications, LLC, West Chester, Pennsylvania, USA.
SL Flamm was involved with the study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, and critically revising the manuscript for important intellectual content.
KD Mullen was involved with the study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, and critically revising the manuscript for important intellectual content.
Z Heimanson was involved with drafting of the manuscript and critically revising the manuscript for important intellectual content.
AJ Sanyal was involved with the study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, and critically revising the manuscript for important intellectual content.
This research was, in part, presented at the American Association for the Study of Liver Diseases Annual Meeting, held from November 1–5, 2013, in Washington, DC, USA.
The trial and current analyses were supported by Salix Pharmaceuticals, Bridgewater, NJ, USA.
Footnotes
Funding: This study was supported by Salix Pharma-ceuticals, Bridgewater, NJ, USA.
The technical editorial assistance was financially supported by Salix Pharmaceuticals, Bridgewater, NJ, USA.
Conflict of interest statement: SL Flamm is a consultant and has served on the speakers’ bureau for AbbVie, Gilead Sciences, Inc., Intercept Pharmaceuticals, Inc., Merck & Co., Inc., and Salix Pharmaceuticals; and has received research support from AbbVie, Gilead Sciences, Inc., and Intercept Pharmaceuticals, Inc. KD Mullen has served as a consultant for Salix Pharmaceuticals and Norgine. Z Heimanson is an employee of Salix Pharmaceuticals. AJ Sanyal has stock options in Genfit and is the president of Sanyal Biotechnology. He has served as a consultant to AbbVie, AstraZeneca, Bristol-Myers Squibb Company, DURECT Corporation, Enanta Pharmaceuticals, Inc., Galmed Pharmaceuticals Ltd., Genfit, Gilead Sciences, Inc., Ikaria, Immuron, Intercept Pharmaceuticals, Inc., Eli Lilly and Company, Merck & Co., Inc., Nitto Denko Corporation, Novartis, Salix Pharmaceuticals, and Zafgen.
Contributor Information
Steven L. Flamm, Northwestern University Feinberg School of Medicine, 676 North Saint Clair, Arkes 19-041, Chicago, IL 60611, USA.
Kevin D. Mullen, West Virginia University, Morgantown, WV, USA
Zeev Heimanson, Salix Pharmaceuticals, Bridgewater, NJ, USA.
Arun J. Sanyal, Virginia Commonwealth University, Richmond, VA, USA
References
- 1. Ginés P, Quintero E, Arroyo V, et al. Compensated cirrhosis: natural history and prognostic factors. Hepatology 1987; 7: 122–128. [DOI] [PubMed] [Google Scholar]
- 2. D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol 2006; 44: 217–231. [DOI] [PubMed] [Google Scholar]
- 3. Starr SP, Raines D. Cirrhosis: diagnosis, management, and prevention. Am Fam Physician 2011; 84: 1353–1359. [PubMed] [Google Scholar]
- 4. Garcia-Tsao G, Lim JK; Members of the Veterans Affairs Hepatitis C Resource Center Program. Management and treatment of patients with cirrhosis and portal hypertension: recommendations from the Department of Veterans Affairs Hepatitis C Resource Center Program and the National Hepatitis C Program. Am J Gastroenterol 2009; 104: 1802–1829. [DOI] [PubMed] [Google Scholar]
- 5. Landis CS, Ghabril M, Rustgi V, et al. Prospective multicenter observational study of overt hepatic encephalopathy. Dig Dis Sci 2016; 61: 1728–1734. [DOI] [PubMed] [Google Scholar]
- 6. Bajaj JS, O’Leary JG, Tandon P, et al. Hepatic encephalopathy is associated with mortality in patients with cirrhosis independent of other extrahepatic organ failures. Clin Gastroenterol Hepatol 2017; 15: 565–574. [DOI] [PubMed] [Google Scholar]
- 7. Fernández J, Acevedo J, Prado V, et al. Clinical course and short-term mortality of cirrhotic patients with infections other than spontaneous bacterial peritonitis. Liver Int 2016; 37: 385–395. [DOI] [PubMed] [Google Scholar]
- 8. Bal CK, Daman R, Bhatia V. Predictors of fifty days in-hospital mortality in decompensated cirrhosis patients with spontaneous bacterial peritonitis. World J Hepatol 2016; 8: 566–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim HY, Kim CW, Choi JY, et al. Complications requiring hospital admission and causes of in-hospital death over time in alcoholic and nonalcoholic cirrhosis patients. Gut Liver 2016; 10: 95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brunner F, Berzigotti A, Bosch J. Prevention and treatment of variceal haemorrhage in 2017. Liver Int 2017; 37: 104–115. [DOI] [PubMed] [Google Scholar]
- 11. Schmidt ML, Barritt AS, Orman ES, et al. Decreasing mortality among patients hospitalized with cirrhosis in the United States from 2002 through 2010. Gastroenterology 2015; 148: 967–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mohammed SEA, AE Abdo, HMY. Mudawi. Mortality and rebleeding following variceal haemorrhage in liver cirrhosis and periportal fibrosis. World J Hepatol 2016; 8: 1336–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tapper EB, Halbert B, Mellinger J. Rates of and reasons for hospital readmissions in patients with cirrhosis: a multistate population-based cohort study. Clin Gastroenterol Hepatol 2016; 14: 1181–1188. [DOI] [PubMed] [Google Scholar]
- 14. D’Amico G, Pasta L, Morabito A, et al. Competing risks and prognostic stages of cirrhosis: a 25-year inception cohort study of 494 patients. Aliment Pharmacol Ther 2014; 39: 1180–1193. [DOI] [PubMed] [Google Scholar]
- 15. Ginès A, Escorsell A, Ginès P, et al. Incidence, predictive factors, and prognosis of the hepatorenal syndrome in cirrhosis with ascites. Gastroenterology 1993; 105: 229–236. [DOI] [PubMed] [Google Scholar]
- 16. Planas R, Montoliu S, Ballesté B, et al. Natural history of patients hospitalized for management of cirrhotic ascites. Clin Gastroenterol Hepatol 2006; 4: 1385–1394. [DOI] [PubMed] [Google Scholar]
- 17. Schwabl P, Bucsics T, Soucek K, et al. Risk factors for development of spontaneous bacterial peritonitis and subsequent mortality in cirrhotic patients with ascites. Liver Int 2015; 35: 2121–2128. [DOI] [PubMed] [Google Scholar]
- 18. Garcia-Tsao G, Bosch J. Varices and variceal hemorrhage in cirrhosis: a new view of an old problem. Clin Gastroenterol Hepatol 2015; 13: 2109–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Romero-Gómez M, Boza F, García-Valdecasas MS, et al. Subclinical hepatic encephalopathy predicts the development of overt hepatic encephalopathy. Am J Gastroenterol 2001; 96: 2718–2723. [DOI] [PubMed] [Google Scholar]
- 20. Patidar KR, Bajaj JS. Covert and overt hepatic encephalopathy: diagnosis and management. Clin Gastroenterol Hepatol 2015; 13: 2048–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pant C, Olyaee M, Gilroy R, et al. Emergency department visits related to cirrhosis: a retrospective study of the nationwide emergency department sample 2006 to 2011. Medicine (Baltimore). 2015; 94: e308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Quigley EM, Stanton C, Murphy EF. The gut microbiota and the liver. Pathophysiological and clinical implications. J Hepatol 2013; 58: 1020–1027. [DOI] [PubMed] [Google Scholar]
- 23. Bajaj JS. The role of microbiota in hepatic encephalopathy. Gut Microbes 2014; 5: 397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Benjamin J, Singla V, Arora I, et al. Intestinal permeability and complications in liver cirrhosis: a prospective cohort study. Hepatol Res 2013; 43: 200–207. [DOI] [PubMed] [Google Scholar]
- 25. Fukui H. Gut-liver axis in liver cirrhosis: how to manage leaky gut and endotoxemia. World J Hepatol 2015; 7: 425–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Koutsounas I, Kaltsa G, Siakavellas SI, et al. Markers of bacterial translocation in end-stage liver disease. World J Hepatol 2015; 7: 2264–2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tumgor G. Cirrhosis and hepatopulmonary syndrome. World J Gastroenterol 2014; 20: 2586–2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kamath PS, Kim WR. The Model for End-Stage Liver Disease (MELD). Hepatology 2007; 45: 797–805. [DOI] [PubMed] [Google Scholar]
- 29. Bajaj JS, Heuman DM, Hylemon PB, et al. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol 2014; 60: 940–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jain L, Sharma BC, Sharma P, et al. Serum endotoxin and inflammatory mediators in patients with cirrhosis and hepatic encephalopathy. Dig Liver Dis 2012; 44: 1027–1031. [DOI] [PubMed] [Google Scholar]
- 31. Salix Pharmaceuticals. Xifaxan® (rifaximin) tablets, for oral use [package insert]. Bridgewater, NJ: Salix Pharmaceuticals, 2018. [Google Scholar]
- 32. Bajaj JS, Heuman DM, Sanyal AJ, et al. Modulation of the metabiome by rifaximin in patients with cirrhosis and minimal hepatic encephalopathy. PLoS One. 2013; 8: e60042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bass NM, Mullen KD, Sanyal A, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010; 362: 1071–1081. [DOI] [PubMed] [Google Scholar]
- 34. Kang SH, Lee YB, Lee JH, et al. Rifaximin treatment is associated with reduced risk of cirrhotic complications and prolonged overall survival in patients experiencing hepatic encephalopathy. Aliment Pharmacol Ther 2017; 46: 845–855. [DOI] [PubMed] [Google Scholar]
- 35. Bajaj JS, Wade JB, Gibson DP, et al. The multi-dimensional burden of cirrhosis and hepatic encephalopathy on patients and caregivers. Am J Gastroenterol. 2011; 106: 1646–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hanouneh MA, Hanouneh IA, Hashash JG, et al. The role of rifaximin in the primary prophylaxis of spontaneous bacterial peritonitis in patients with liver cirrhosis. J Clin Gastroenterol 2012; 46: 709–715. [DOI] [PubMed] [Google Scholar]
- 37. Vlachogiannakos J, Viazis N, Vasianopoulou P, et al. Long-term administration of rifaximin improves the prognosis of patients with decompensated alcoholic cirrhosis. J Gastroenterol Hepatol 2013; 28: 450–455. [DOI] [PubMed] [Google Scholar]
- 38. Peng Y, Qi X, Guo X. Child–Pugh versus MELD score for the assessment of prognosis in liver cirrhosis: a systematic review and meta-analysis of observational studies. Medicine (Baltimore). 2016; 95: e2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Berg CL. Liver transplantation in 2016: an update. N C Med J 2016; 77: 194–197. [DOI] [PubMed] [Google Scholar]
- 40. Dong T, Aronsohn A, Gautham Reddy K, et al. Rifaximin decreases the incidence and severity of acute kidney injury and hepatorenal syndrome in cirrhosis. Dig Dis Sci. 2016; 61: 3621–3626. [DOI] [PubMed] [Google Scholar]
- 41. Elfert A, Abo Ali L, Soliman S, et al. Randomized-controlled trial of rifaximin versus norfloxacin for secondary prophylaxis of spontaneous bacterial peritonitis. Eur J Gastroenterol Hepatol 2016; 28: 1450–1454. [DOI] [PubMed] [Google Scholar]
- 42. Lutz P, Parcina M, Bekeredjian-Ding I, et al. Impact of rifaximin on the frequency and characteristics of spontaneous bacterial peritonitis in patients with liver cirrhosis and ascites. PLoS One 2014; 9: e93909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tsung PC, Ryu SH, Cha IH, et al. Predictive factors that influence the survival rates in liver cirrhosis patients with spontaneous bacterial peritonitis. Clin Mol Hepatol 2013; 19: 131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Singal AK, Salameh H, Kamath PS. Prevalence and in-hospital mortality trends of infections among patients with cirrhosis: a nationwide study of hospitalised patients in the United States. Aliment Pharmacol Ther 2014; 40: 105–112. [DOI] [PubMed] [Google Scholar]
- 45. Bannister CA, Orr JG, Reynolds AV, et al. Natural history of patients taking rifaximin-α for recurrent hepatic encephalopathy and risk of future overt episodes and mortality: a post-hoc analysis of clinical trials data. Clin Ther 2016; 38: 1081–1089. [DOI] [PubMed] [Google Scholar]