Abstract
Background:
To compare the long-term clinical outcomes of different antihypertensive drugs in stable patients after acute hemorrhagic stroke (HS).
Methods:
From January 2001 to December 2013, patients with first-ever primary HS were identified in the National Health Insurance Research Database, Taiwan. Patients with traumatic intracerebral hemorrhage and secondary HS were excluded. Those with first-ever HS were recruited and classified into three groups: (1) angiotensin-converting enzyme inhibitor/angiotensin receptor blocker (ACEI/ARB); (2) calcium channel blocker (CCB); and (3) other antihypertensive drugs (comparison) groups. Propensity score matching was used to balance the distribution of baseline characteristics, stroke severity, and medications between any two of the three groups. A validation study was performed using the databank of the Stroke Registry in Chang-Gung Healthcare System to reduce the bias. Primary outcomes were recurrent HS, ischemic stroke, any stroke, and all-cause mortality.
Results:
Compared to the comparison group, the ACEI/ARB group [35.4% versus 39.3%; hazard ratio (HR), 0.84; 95% confidence interval (CI), 0.74–0.95] and CCB group (33.0% versus 41.9%; HR, 0.72; 95% CI, 0.64–0.81) had a lower risk of all-cause mortality during long-term follow up. The CCB group had a similar risk of all-cause mortality to the ACEI/ARB group. Risks of recurrent HS, ischemic stroke, or any stroke were not different between the study groups.
Conclusions:
Antihypertensive drug class could be important to long-term outcomes in HS patients in addition to the target control of blood pressure. Both ACEIs/ARBs and CCBs are associated with lower risks of all-cause mortality. Our results may be applied to inform future research on hypertensive control in HS patients.
Keywords: angiotensin converting enzyme inhibitor, angiotensin receptor blocker, calcium channel blocker, hemorrhagic stroke, hypertension
Introduction
Stroke is the leading cause of death worldwide. Both ischemic stroke (IS) and hemorrhagic stroke (HS) survivors have high mortality rates during long-term follow up. Our previous study showed that in IS survivors, recurrent IS, cancer-related death, and cardiac disease are the main causes of death.1 However, in HS survivors, preventing recurrent HS is the most effective method to reduce mortality.1 To prevent recurrent HS, blood pressure (BP) should be well and intensively controlled.2–5 However, it remains uncertain whether there were pleiotropic effects in different classes of antihypertensive drugs on HS patients. Although angiotensin-converting enzyme inhibitors (ACEIs) with diuretics are reported to be the treatment of choice for secondary stroke prevention,5 the results were mainly derived from IS patients and may be less applicable to HS patients.
Asia has an ideal population to study HS due to the population’s characteristic of having a high proportion of small vessel disease (SVD), which accounts for the increased frequency of HS and lacunar infarction.6,7 ACEIs and angiotensin receptor blockers (ARBs) are usually used as the first-line drugs for hypertensive patients.5,8,9 Calcium channel blockers (CCBs) may have some roles in SVD through their effects on voltage-gated calcium channels, but this assumption lacks supporting sufficient clinical studies.10,11 Until now, few outcome studies focused on direct comparison among different classes of antihypertensive drugs in HS patients. Therefore, the latest guidelines cannot give conclusive recommendations on the choice of antihypertensive drug class in HS patients.5,12,13 The present study compared the long-term outcomes of CCBs and ACEIs/ARBs with other classes of antihypertensive drugs in stable patients after their first-ever HS. We hypothesized that in hypertension control, the class of antihypertensive drugs could be associated with additional benefits beyond the target control of BP in HS patients.
Methods
Patient enrollment and inclusion/exclusion criteria
This study was an open prospective nationwide cohort study including all patients admitted due to HS in the National Health Insurance Research Database (NHIRD) between January 1, 2001 and December 31, 2013. The NHIRD prospectively records the data submitted to the National Health Insurance (NHI) program, which covers more than 99% of the population in Taiwan. Diagnoses are registered using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes, and are routinely monitored by the NHI Bureau.14 The patients of interest were first-ever primary HS survivors. In total, 114,219 hospitalized patients with a primary diagnosis of HS in the NHIRD (ICD-9-CM code 431) were initially included for analysis. We excluded patients with traumatic intracerebral hemorrhage (ICD-9-CM code 853) or with a previous history of HS including intracerebral hemorrhage and subarachnoid hemorrhage. We also excluded patients assumed to be associated with secondary HS if they also had a concurrent diagnosis of venous sinus thrombosis, cerebral aneurysm or arteriovenous fistula, non-aneurysmal subarachnoid hemorrhage, or non-traumatic subdural hemorrhage. In order to validate the diagnostic accuracy of a first-ever HS in NHIRD, we compared the data of patients with the primary diagnosis of HS from both the NHIRD and the Stroke Registry in Chang Gung Healthcare System (SRICHS) from 2009 to 2013.15 The details are provided in Supplemental Figure 1.
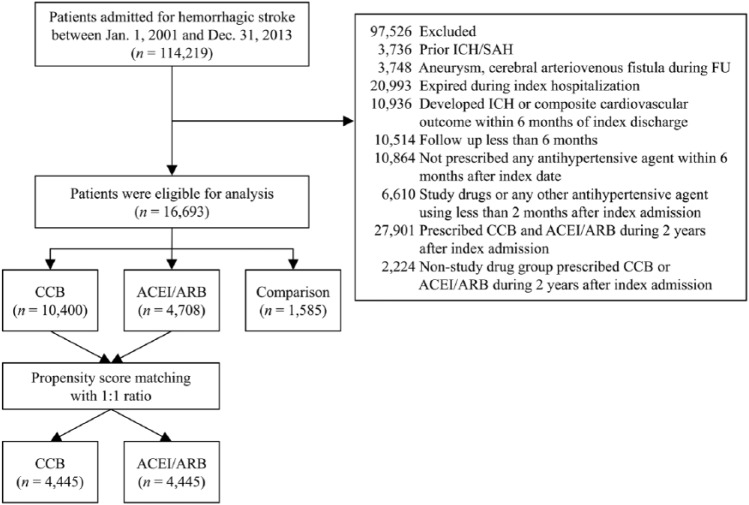
According to our previous study,1 more than half of the mortality in HS patients occurred within the first month after stroke onset. Drug switching or discontinuation occurs more commonly within the first 180 days after the start of medication.16 These factors may lead to misinterpretation of the correlations between antihypertensive drugs and clinical outcomes. To study HS patients in the stable phase, we excluded those patients who died during the index hospitalization and those who developed HS or had composite cardiovascular outcomes within 180 days after the index hospitalization. We also excluded those patients who had follow up of fewer than 180 days and who did not receive any antihypertensive agents within 180 days after the index hospitalization (Figure 1). The Ethics Institutional Review Board of Chang Gung Memorial Hospital approved this study (approval number: 201601164B0). Because the enrolled patients cannot be identified in this claims database study, informed consent was waived by our Ethics Institutional Review Board.
Figure 1.
Flow chart of the recruitment of the study patients. Patients with their first-ever hemorrhagic stroke are included after relevant exclusions and then further divided into three groups according to the prescribed antihypertensive therapy.
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CCB, calcium channel blocker; FU, follow up; ICH, intracerebral hemorrhage; SAH, subarachnoid hemorrhage.
Exposure to study drugs
The eligible patients were divided into three groups according to the main antihypertensive drugs prescribed during the follow-up period: (1) ACEI/ARB, (2) CCB, and (3) comparison (other antihypertensive medications) groups. To ensure the consistent use of study drugs in each group, patients were excluded if they took any CCB in the ACEI/ARB group, any ACEI/ARB in the CCB group, and any ACEI/ARB or CCB in the comparison group for even 1 day during a 2-year exposure period. Patients were also excluded if they received the study drug for fewer than 60 days. Since BP levels were not recorded in the NHIRD, the add-on antihypertensive drugs and the number of antihypertensive drug classes within a 2-year exposure period were adjusted to minimize the bias related to the different levels of BP. Also, with the linkage between SRICHS and NHIRD, the mean BPs at admission in the SRICHS were used to represent the baseline BP levels of the matched patients in the three study groups. The medication possession ratio was calculated to assess the adherence of the study drug in each group. The index hospitalization was defined as the first hospitalization due to HS throughout the study period. The follow-up period was calculated from the admission day of index hospitalization to the day of death or until December 31, 2013, whichever occurred first.
Outcomes and covariate measurements
Medications and ICD-9-CM diagnosis codes during the index hospitalization were used to represent the baseline medications and comorbidities. The diagnosis code in at least two consecutive outpatient follow-up visits or in one inpatient record in the previous year of the index hospitalization was used to confirm the comorbidity. In addition, the diagnoses of hemodialysis and cancer were further verified using catastrophic illness certificates (Supplemental Table 1). The prescribed medications were confirmed using Anatomical Therapeutic Chemical codes (Supplemental Table 2). Over 99% of the population and hospitals in Taiwan are enrolled in the NHI program. Therefore, almost all the major outcomes that occurred between January 1, 2001 and December 31, 2013 were recorded. The primary outcomes were defined as admission due to recurrent HS, IS, or any stroke, all-cause mortality, and composite end-points. The composite end-points included IS, HS, and all-cause mortality. Recurrent HS was identified by hospitalization with ICD-9-CM code 431 during the follow-up period. IS was identified by hospitalization with ICD-9-CM codes 433–435, except 433.00, 433.10, 433.20, 433.30, 433.80, 433.90, 434.90, 434.00, 434.10, and 434.90 during the follow-up period. The secondary end-points included cardiovascular death, hemodialysis, and myocardial infarction (MI). The definitions of MI, cardiovascular death, and all-cause mortality were the same as those used in the NHIRD study (Supplemental Table 1).17
Statistical analysis
We performed propensity score matching (PSM) to balance the distribution of baseline characteristics, the numbers of antihypertensive drug class used at baseline, and the use of non-antihypertensive medications between any two study groups. We also included the estimated National Institutes of Health Stroke Scale (NIHSS) score as a covariate when generating propensity score.18 We adopted the greedy nearest-neighbor matching algorithm and set the caliper as 0.2 times the standard deviation of the propensity score. To minimize bias of treatment effect estimation, we used a 1:1 matching ratio.19
The baseline characteristics were initially compared using one-way analysis of variance for continuous variable and the Chi-square test for categorical variable before PSM. These data were further compared between any two of the three groups using the two-sample t test for continuous variables and the Chi-square test for categorical variables after PSM. The risk of time to event between any two of the three groups after PSM was compared using a Cox proportional hazard model in which the study group was the independent variable and propensity score was treated as a covariate. The cumulative incidence comparing the time to all-cause mortality, recurrent HS, and IS between any two of the three groups was depicted using the adjusted survival curves in the multivariable Cox model. All data analyses were conducted using SAS software version 9.4 (SAS Institute, Cary, NC). Statistical significance was set at p < 0.05.
Results
Study patients
Between January 1, 2001 and December 31, 2013, a total of 699,291 patients admitted due to stroke (ICD-9-CM codes 430–437) were available in the NHIRD. In total, 114,219 patients who were admitted due to a HS (ICD-9-CM code 431) were initially included. Overall, 16,693 first-ever HS patients were confirmed eligible for analysis based on the inclusion/exclusion criteria. A total of 2343 and 234 from the 114,219 HS and 16,693 first-ever HS patients, respectively, were matched with the SRICHS for validation. The positive predictive values of HS and first-ever HS diagnoses in this study were 97.98% and 90.54%, respectively (Supplemental Figure 1). There were 10,400 patients in the CCB group, 4708 patients in the ACEI/ARB group, and 1585 patients in the comparison group (Figure 1).
Baseline characteristics (Table 1)
Table 1.
Baseline clinical characteristics of the study patients before propensity score matching.
| Characteristics | CCB (n = 10,400) |
ACEI/ARB (n = 4708) |
Comparison (n = 1585) |
p |
|---|---|---|---|---|
| Age (years) | 63.4 ± 13.2 | 63.2 ± 13.6 | 64.9 ± 14.7 | <0.001 |
| <40 | 342 (3.3) | 197 (4.2) | 71 (4.5) | <0.001 |
| 40–75 | 7813 (75.1) | 3421 (72.7) | 1045 (65.9) | |
| >75 | 2245 (21.6) | 1090 (23.2) | 469 (29.6) | |
| Gender | 0.002 | |||
| Male | 6372 (61.3) | 3026 (64.3) | 992 (62.6) | |
| Female | 4028 (38.7) | 1682 (35.7) | 593 (37.4) | |
| Previous myocardial infarction | 81 (0.8) | 90 (1.9) | 31 (2.0) | <0.001 |
| Previous ischemic stroke | 422 (4.1) | 210 (4.5) | 91 (5.7) | 0.008 |
| Previous antiplatelet use | 2010 (19.3) | 1195 (25.4) | 382 (24.1) | <0.001 |
| Previous anticoagulant use | 100 (1.0) | 126 (2.7) | 79 (5.0) | <0.001 |
| Comorbidity | ||||
| Coronary artery disease | 874 (8.4) | 653 (13.9) | 239 (15.1) | <0.001 |
| Chronic kidney disease | 278 (2.7) | 114 (2.4) | 39 (2.5) | 0.632 |
| Hemodialysis | 109 (1.0) | 40 (0.8) | 9 (0.6) | 0.133 |
| Chronic obstructive pulmonary disease | 541 (5.2) | 287 (6.1) | 172 (10.9) | <0.001 |
| Atrial fibrillation | 176 (1.7) | 202 (4.3) | 78 (4.9) | <0.001 |
| Diabetes mellitus | 1667 (16.0) | 1183 (25.1) | 333 (21.0) | <0.001 |
| Dyslipidemia | 1136 (10.9) | 773 (16.4) | 189 (11.9) | <0.001 |
| Malignancy | 335 (3.2) | 163 (3.5) | 75 (4.7) | 0.009 |
| NIHSS | 15.4 ± 6.9 | 14.1 ± 7.0 | 15.7 ± 7.3 | <0.001 |
| NIHSS group | <0.001 | |||
| ⩽5 | 920 (8.8) | 627 (13.3) | 175 (11.0) | |
| 6–13 | 3308 (31.8) | 1678 (35.6) | 436 (27.5) | |
| >13 | 6172 (59.3) | 2403 (51.0) | 974 (61.5) | |
| Follow-up years | 5.3 ± 3.4 | 4.4 ± 3.3 | 4.7 ± 3.4 | <0.001 |
| Baseline antihypertensive drugs | ||||
| ACEI/ARB | 0 (0) | 100 (100) | 0 (0) | <0.001 |
| CCB | 100 (0) | 0 (0) | 0 (0) | <0.001 |
| Alpha-blocker | 874 (8.4) | 417 (8.9) | 244 (15.4) | <0.001 |
| Beta-blocker | 3559 (34.2) | 1578 (33.5) | 1018 (64.2) | <0.001 |
| Thiazide | 693 (6.7) | 383 (8.1) | 158 (10.0) | <0.001 |
| Loop diuretics | 833 (8.0) | 501 (10.6) | 463 (29.2) | <0.001 |
| Spironolactone | 114 (1.1) | 97 (2.1) | 188 (11.9) | <0.001 |
| Others | 330 (3.2) | 126 (2.7) | 44 (2.8) | 0.218 |
| Numbers of antihypertensive drug types used at baseline | <0.001 | |||
| 1 | 5363 (51.6) | 2375 (50.4) | 1138 (71.8) | |
| 2 | 3879 (37.3) | 1706 (36.2) | 371 (23.4) | |
| ⩾3 | 1158 (11.1) | 627 (13.3) | 76 (4.8) | |
| Add-on antihypertensive drugs within two years | ||||
| Antihypertensive drug not of interest | ||||
| Beta-blocker | 546 (5.3) | 256 (5.4) | 29 (1.8) | <0.001 |
| Alpha-blocker | 209 (2.0) | 93 (2.0) | 14 (0.9) | 0.008 |
| Thiazide | 255 (2.5) | 116 (2.5) | 24 (1.5) | 0.064 |
| Loop diuretics | 409 (3.9) | 205 (4.4) | 84 (5.3) | 0.032 |
| Spironolactone | 84 (0.8) | 58 (1.2) | 33 (2.1) | <0.001 |
| Other | 99 (1.0) | 31 (0.7) | 8 (0.5) | 0.060 |
| Numbers of antihypertensive drug types used at two years | <0.001 | |||
| 0 | 837 (8.1) | 393 (8.4) | 264 (16.7) | |
| 1 | 5177 (49.8) | 2316 (49.2) | 944 (59.6) | |
| ⩾2 | 4386 (42.2) | 1999 (42.5) | 377 (23.8) | |
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CCB, calcium channel blocker; NIHSS, National Institutes of Health Stroke Scale.
Before PSM, the ACEI/ARB group had a higher frequency of male patients, diabetes mellitus (DM), and dyslipidemia than the other two groups. The CCB group had a lower prevalence of previous MI, previous IS, coronary artery disease, atrial fibrillation (AF), DM, dyslipidemia, and previous antiplatelet or anticoagulant therapy than the other two groups. The proportions of patients confined to a single class of antihypertensive drug within 2 years were 49.8%, 49.2%, and 59.6% in the CCB, ACEI/ARB, and comparison groups (p < 0.001), respectively, compared to 42.2%, 42.5%, and 23.8% in those using more than two classes of antihypertensive drugs. The proportions of patients receiving non-study medications before PSM were recorded (Supplemental Table 2). The medication possession ratios of CCBs, ACEIs/ARBs, and other antihypertensive drugs were 83.7%, 84.5%, and 87.1% in the CCB, ACEI/ARB, and comparison groups during the 2-year follow-up period. Comparisons of the baseline characteristics and medications after PSM between any two of the three groups are shown in Supplemental Tables 3–5.
Primary outcomes
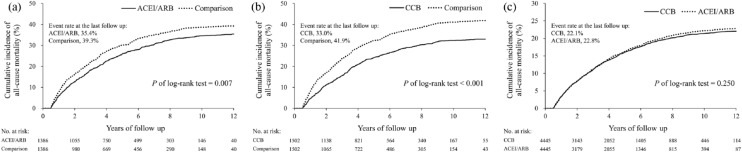
The primary outcomes were compared between any two of the three study groups after PSM. Compared to the comparison group, the ACEI/ARB group had a lower risk of all-cause mortality at 2-year [ACEI/ARB versus comparison: 12.6% versus 16.3%; hazard ratio (HR), 0.74; 95% confidence interval (CI), 0.61–0.91], 5-year (26.0% versus 30.1%; HR, 0.81; 95% CI, 0.70–0.93), and the last follow up (35.4% versus 39.3%; HR, 0.84; 95% CI, 0.74–0.95) (Table 2). The CCB group also had a lower risk of all-cause mortality at 2-year (CCB versus comparison: 11.1% versus 16.8%; HR, 0.63; 95% CI, 0.52–0.77), 5-year (24.2% versus 32.3%; HR, 0.69; 95% CI, 0.60–0.79), and the last follow up (33.0% versus 41.9%; HR, 0.72; 95% CI, 0.64–0.81) (Table 3). The incidence rates of HS and IS were similar among the ACEI/ARB, CCB, and comparison groups.
Table 2.
Primary outcomes in the ACEI/ARB and comparison groups after propensity score matching.
| Outcome | ACEI/ARB (n = 1386) |
Comparison (n = 1386) |
ACEI/ARB versus
comparison |
|
|---|---|---|---|---|
| HR (95% CI)† | p | |||
| 2-year follow up | ||||
| All-cause mortality | 175 (12.6) | 226 (16.3) | 0.74 (0.61, 0.91) | 0.003 |
| Any stroke# | 107 (7.7) | 108 (7.8) | 0.95 (0.73, 1.24) | 0.704 |
| Hemorrhagic stroke | 47 (3.4) | 49 (3.5) | 0.92 (0.62, 1.38) | 0.699 |
| Ischemic stroke | 66 (4.8) | 66 (4.8) | 0.96 (0.68, 1.35) | 0.813 |
| Primary composite end-point§ | 261 (18.8) | 308 (22.2) | 0.81 (0.69, 0.96) | 0.013 |
| 5-year follow up | ||||
| All-cause mortality | 360 (26.0) | 417 (30.1) | 0.81 (0.70, 0.93) | 0.003 |
| Any stroke# | 234 (16.9) | 217 (15.7) | 1.01 (0.84, 1.21) | 0.933 |
| Hemorrhagic stroke | 112 (8.1) | 103 (7.4) | 1.02 (0.78, 1.33) | 0.902 |
| Ischemic stroke | 147 (10.6) | 137 (9.9) | 1.01 (0.80, 1.27) | 0.949 |
| Primary composite end-point§ | 523 (37.7) | 554 (40.0) | 0.88 (0.78, 0.99) | 0.037 |
| At the last follow up | ||||
| All-cause mortality | 491 (35.4) | 545 (39.3) | 0.84 (0.74, 0.95) | 0.005 |
| Any stroke# | 295 (21.3) | 274 (19.8) | 1.02 (0.86, 1.20) | 0.849 |
| Hemorrhagic stroke | 143 (10.3) | 142 (10.2) | 0.95 (0.75, 1.19) | 0.642 |
| Ischemic stroke | 185 (13.3) | 175 (12.6) | 1.000 (0.81, 1.23) | 0.999 |
| Primary composite end-point§ | 666 (48.1) | 678 (48.9) | 0.92 (0.83, 1.03) | 0.133 |
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CI, confidence interval; HR, hazard ratio.
Any one of all-cause mortality, hemorrhagic stroke, and ischemic stroke.
Either one of hemorrhagic or ischemic stroke.
Propensity score was additionally treated as a covariate in the model.
Table 3.
Primary outcomes in the CCB and comparison groups after propensity score matching.
| Outcome | CCB (n = 1502) |
Comparison (n = 1502) |
CCB versus
comparison |
|
|---|---|---|---|---|
| HR (95% CI)† | p | |||
| 2-year follow up | ||||
| All-cause mortality | 167 (11.1) | 253 (16.8) | 0.63 (0.52, 0.77) | <0.001 |
| Any stroke# | 116 (7.7) | 118 (7.9) | 0.95 (0.73, 1.22) | 0.665 |
| Hemorrhagic stroke | 51 (3.4) | 53 (3.5) | 0.92 (0.63, 1.36) | 0.685 |
| Ischemic stroke | 73 (4.9) | 71 (4.7) | 0.99 (0.72, 1.38) | 0.959 |
| Primary composite end-point§ | 263 (17.5) | 342 (22.8) | 0.74 (0.63, 0.87) | <0.001 |
| 5-year follow up | ||||
| All-cause mortality | 363 (24.2) | 485 (32.3) | 0.69 (0.60, 0.79) | <0.001 |
| Any stroke# | 241 (16.0) | 235 (15.6) | 0.95 (0.79, 1.13) | 0.557 |
| Hemorrhagic stroke | 115 (7.7) | 111 (7.4) | 0.96 (0.74, 1.24) | 0.741 |
| Ischemic stroke | 158 (10.5) | 146 (9.7) | 1.001 (0.80, 1.25) | 0.993 |
| Primary composite end-point§ | 528 (35.2) | 627 (41.7) | 0.78 (0.69, 0.88) | <0.001 |
| At the last follow up | ||||
| All-cause mortality | 495 (33.0) | 629 (41.9) | 0.72 (0.64, 0.81) | <0.001 |
| Any stroke# | 323 (21.5) | 296 (19.7) | 1.002 (0.86, 1.17) | 0.979 |
| Hemorrhagic stroke | 159 (10.6) | 151 (10.1) | 0.96 (0.77, 1.20) | 0.743 |
| Ischemic stroke | 222 (14.8) | 187 (12.5) | 1.09 (0.90, 1.33) | 0.373 |
| Primary composite end-point§ | 681 (45.3) | 767 (51.1) | 0.82 (0.74, 0.91) | <0.001 |
CCB, calcium channel blocker; CI, confidence interval; HR, hazard ratio.
Any one of all-cause mortality, hemorrhagic stroke, and ischemic stroke.
Either one of hemorrhagic or ischemic stroke.
Propensity score was additionally treated as a covariate in the model.
The incidence rates of all-cause mortality, HS, IS, any stroke, and primary composite end-points were not significantly different between the ACEI/ARB and CCB groups throughout the follow-up period (Table 4). The mean follow-up period was 4.5 ± 3.3 and 4.6 ± 3.4 years in the ACEI/ARB and CCB groups (p = 0.77). The multivariate adjusted survival curves showed lower trends of all-cause mortality in the ACEI/ARB and CCB groups compared to the comparison group (p < 0.05). Compared to the ACEI/ARB group, the CCB group showed a similar trend of all-cause mortality throughout the follow-up period (Figure 2). The survival curves of IS and HS between the study groups are shown in Supplemental Figures 2 and 3.
Table 4.
Primary outcomes in the ACEI/ARB and CCB groups after propensity score matching.
| Outcome | ACEI/ARB (n = 4445) |
CCB (n = 4445) |
ACEI/ARB versus
CCB |
|
|---|---|---|---|---|
| HR (95% CI)† | p | |||
| 2-year follow up | ||||
| All-cause mortality | 347 (7.8) | 344 (7.7) | 1.01 (0.87, 1.17) | 0.940 |
| Any stroke# | 316 (7.1) | 295 (6.6) | 1.07 (0.91, 1.26) | 0.397 |
| Hemorrhagic stroke | 150 (3.4) | 134 (3.0) | 1.12 (0.89, 1.41) | 0.343 |
| Ischemic stroke | 186 (4.2) | 185 (4.2) | 1.002 (0.82, 1.23) | 0.987 |
| Primary composite end-point§ | 603 (13.6) | 579 (13.0) | 1.04 (0.93, 1.17) | 0.480 |
| 5-year follow up | ||||
| All-cause mortality | 726 (16.3) | 715 (16.1) | 1.01 (0.91, 1.12) | 0.818 |
| Any stroke# | 621 (14.0) | 595 (13.4) | 1.04 (0.93, 1.17) | 0.463 |
| Hemorrhagic stroke | 297 (6.7) | 276 (6.2) | 1.08 (0.91, 1.27) | 0.383 |
| Ischemic stroke | 382 (8.6) | 398 (9.0) | 0.95 (0.83, 1.10) | 0.508 |
| Primary composite end-point§ | 1179 (26.5) | 1145 (25.8) | 1.03 (0.95, 1.12) | 0.485 |
| At the last follow up | ||||
| All-cause mortality | 1015 (22.8) | 982 (22.1) | 1.05 (0.96, 1.15) | 0.265 |
| Any stroke# | 802 (18.0) | 803 (18.1) | 1.01 (0.92, 1.12) | 0.777 |
| Hemorrhagic stroke | 390 (8.8) | 358 (8.1) | 1.11 (0.96, 1.28) | 0.160 |
| Ischemic stroke | 502 (11.3) | 555 (12.5) | 0.91 (0.81, 1.03) | 0.140 |
| Primary composite end-point§ | 1533 (34.5) | 1518 (34.2) | 1.03 (0.96, 1.10) | 0.484 |
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CCB, calcium channel blocker; CI, confidence interval; HR, hazard ratio.
Any one of all-cause mortality, hemorrhagic stroke, and ischemic stroke.
Either one of hemorrhagic or ischemic stroke.
Propensity score was additionally treated as a covariate in the model.
Figure 2.
Comparisons of cumulative incidence of all-cause mortality between the study groups. The cumulative incidence comparing the time to all-cause mortality between the study groups. The multivariate adjusted survival curves of the ACEI/ARB (a) and CCB (b) groups show a lower trend of all-cause mortality compared to the comparison group. The CCB and ACEI/ARB groups have similar all-cause mortality throughout the follow-up period (c).
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CCB, calcium channel blocker.
Secondary outcomes
The ACEI/ARB group had a lower risk of new-onset hemodialysis compared to the CCB group at the last follow up (0.7% versus 1.5%; HR, 0.42; 95% CI, 0.27–0.65) (Supplemental Tables 6–8).
Discussion
In addition to the target control of BP, our results demonstrate that class selection of antihypertensive drugs may also be important in HS patients. Reduction of mortality is the major goal of antihypertensive treatment, and our results show both ACEI-/ARB- and CCB-based regimens were associated with lower risks of all-cause mortality compared to other drugs in HS patients. ACEIs/ARBs are usually the drug of choice in hypertensive patients,5,8,9 and combination therapy with ACEIs and CCBs is reported to have better protective effects than other regimens.8,20 Our finding that CCBs have an advantage in HS patients has rarely been studied before, and our results suggest that CCBs could also be used as a priority in HS survivors.
Renin-angiotensin system inhibitors (RASI), including ACEIs and ARBs, can effectively reduce cardiovascular mortality, especially in patients with heart diseases.21 Since coronary artery disease and IS share similar risk factors, IS patients may also be at risk of cardiovascular mortality, particularly the IS subtypes of cardioembolic stroke and large artery disease as seen in our previous study.1 It is reported that RASI could be beneficial to IS patients,8 but the benefits to HS patients are undetermined due to HS patients being infrequently enrolled in the previous studies.8,20,21 Although RASIs were found to have benefits in animal models with HS,22 the Scandinavian Candesartan Acute Stroke Trial, which used candesartan in 144 HS patients, only showed conflicting results with possibly harmful effects in the acute phase but neutral effects in the following 6 months.23,24 Moreover, risk factor controls with benefits on IS patients may not show identical effects on HS patients.25,26 Nevertheless, our study revealed that in HS patients the ACEI/ARB group had a 4% reduction of overall mortality compared to the comparison group, which was close to the previous studies focusing on all hypertensive patients.27,28 Similar to the report in a recent meta-analysis,29 our study also suggested that besides lowering BP, the ACEI/ARB group may have pleiotropic effects which result in a better clinical outcome compared to other antihypertensive drugs in HS patients. Our results could be of value because we demonstrated the potential benefits with regards to the long-term use of RASI in patients with HS.
The CCBs were noted to have better death-reduction results compared to beta-blockers in a systemic review.30 However, the advantages of CCBs are not identical in patients with different comorbidities and very few clinical studies have focused on the use of CCBs in stable HS patients.31 CCBs were noted to be superior to beta-blockers or diuretics in patients with metabolic syndrome or DM, but CCBs were reported only non-inferior to diuretics or beta-blockers in hypertensive patients with stable ischemic heart disease, AF, or chronic kidney disease.5 Our HS population had a high frequency of DM and dyslipidemia but a low frequency of previous MI, previous IS, chronic kidney disease, and AF. Beta-blockers were used in 64.2% of patients in our comparison group. These factors may explain why our CCB group had a lower mortality rate compared to the comparison group. Of note, our study demonstrated that the benefits of CCBs and ACEIs/ARBs could be equivalent on the reduction of all-cause mortality in HS patients. Previous studies focusing on uncomplicated hypertensive patients have shown a similar trend.32,33 Our results may further extend the potential benefits of CCBs when used in stable HS patients.
A recent meta-analysis reported that ACEIs, ARBs, or CCBs were better than beta-blockers in stroke prevention.34 Theoretically, ACEIs/ARBs may reduce recurrent strokes,35 since ACEIs may influence plasminogen activator inhibitor-1 antigen and endothelial function,35 and ARBs can mediate the protective effects against ischemic injury in brain tissue.36 ARBs also prevent the progression of diabetes and new-onset AF, both of which are major risk factors for IS.37 The risk of SVD may be associated with abnormal vascular tone,38 and CCBs can act on the voltage-gated calcium channels which are known to participate in the control of vascular tone and associate with the contraction of cerebral vessels in hypertensive patients.39 Therefore, CCBs are suggested to be effective in primary stroke prevention.31,40,41 In a recent meta-analysis,42 CCBs helped to reduce the risks of recurrent stroke. Nicardipine and labetalol are recommended for BP control during the acute stage of HS,43 but there is a lack of evidence with regards to the most appropriate antihypertensive drugs in the stationary phase after acute HS.13 Our HS patients taking ACEIs/ARBs or CCBs did not show better protective effects to the upcoming IS compared to other drugs. It is possible the frequency of upcoming IS might be low, or the follow-up period might not be long enough to show the clinical significance. Patients taking ACEIs/ARBs or CCBs were also not associated with significantly lower risks of recurrent HS in our study, which is similar to a recent meta-analysis focusing on the Asian population.44 We assume that the target of lowering BP rather than the class effectiveness of antihypertensive drugs remains the key effect for the prevention of recurrent HS.
There are limitations in this study. First, the characteristics and locations of the hematoma in HS were reported to be associated with risks of recurrent HS and long-term prognosis.45 However, the image reports were not available in the NHIRD. Nevertheless, we have excluded patients with ICD-9-CM codes of cerebral vascular abnormalities (e.g. aneurysm or arteriovenous malformation or fistula) to reduce the confounding effect from secondary HS. In the future, a nationwide cloud-based medical image-sharing platform with convolutional neural network analysis could be a potential solution.46,47 Second, BP levels are not recorded in the NHIRD for the examination of BP targets and variability, which may be a major confounder for the evaluation of clinical outcomes. To reduce the bias of BP levels after antihypertensive treatment, we have balanced the medication possession ratios and the frequency of patients taking more than two types of antihypertensive drugs at baseline and at 2 years. These factors have been reported as alternative parameters for the effectiveness evaluation of BP control.48–50 Also, since these patients were enrolled between 2000 and 2013, the clinicians usually followed the seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure to control BP.51 In Taiwan, all insurance claims will be scrutinized and peer-reviewed by medical reimbursement specialists. Physicians and their institutions will be accredited and penalized if they violate clinical guidelines. Third, stroke severity and outcome scores were not available in the NHIRD. However, a previous NHIRD study has established the “stroke severity index” to estimate “NIHSS scores” using treatment and intervention in the ICH admission.18 In the present study, we used the stroke severity index,18 alternative covariates, and medications to evaluate the disease severity of HS patients. The information on functional outcome, such as modified Rankin score, is also not available in the NHIRD. Since it has been reported that the modified Rankin score at discharge is correlated well with the NIHSS scores, we did not try to further adjust functional outcome.18,52 Fourth, drug switching, combinations, and adherence are important confounders. In this study, adherence to the study drugs was controlled, and only the patients who used the study drugs continuously were included. PSM was also used for statistical adjustments. Fifth, ICD-9-CM may be coded incorrectly in the claim database. Our validation study using SRICHS supported the coding accuracy of the HS and first-ever HS patients in this study. Lastly, conclusions with regards to the causal effects of the study drugs may be limited in this observational study, and the generalizability of our findings to other ethnicities is undefined. Despite these limitations, our study is valuable because of the paucity of outcome studies regarding hypertensive controls in stable HS patients. Our hypothesis-driven cohort study with a large nationwide HS population, strictly controlled variables, and long-term follow up may be applied to inform well-designed randomized clinical trials to determine the most effective regimen of antihypertensive drugs for HS in the future.
Conclusion
In our study, ACEI-/ARB- and CCB-based regimens are both associated with lower risk of all-cause mortality during long-term follow up compared to other antihypertensive drugs. Our results suggest both ACEIs/ARBs and CCBs may be used as a priority in BP control in stable HS patients, and also inform future researches.
Supplemental Material
Supplemental material, Supplemental_Table_20180618_Final for Choices for long-term hypertensive control in patients after first-ever hemorrhagic stroke: a nationwide cohort study by Chi-Hung Liu, Yu-Sheng Lin, Ching-Chi Chi, Chia-Wei Liou, Jiann-Der Lee, Tsung-I Peng and Tsong-Hai Lee in Therapeutic Advances in Neurological Disorders
Acknowledgments
The authors thank Alfred Hsing-Fen Lin and Zoe Ya-Jhu Syu for statistical assistance.
Footnotes
Funding: This study was supported by Chang Gung Memorial Hospital research project grant CMRPG3F2211 and BMRP 274 (TH Lee).
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Chi-Hung Liu  https://orcid.org/0000-0001-8965-2096
https://orcid.org/0000-0001-8965-2096
Contributor Information
Chi-Hung Liu, Stroke Center and Department of Neurology, Linkou Chang Gung Memorial Hospital, Taoyuan, Taiwan; College of Medicine, Chang Gung University, Taoyuan, Taiwan; Graduate Institute of Clinical Medical Sciences, Division of Medical Education, College of Medicine, Chang Gung University, Taoyuan, Taiwan.
Yu-Sheng Lin, Department of Medicine, College of Medicine, Chang Gung University, Taoyuan, Taiwan; Division of Cardiology, Department of Internal Medicine, Chiayi Chang Gung Memorial Hospital, Chiayi, Taiwan.
Ching-Chi Chi, Department of Dermatology, Linkou Chang Gung Memorial Hospital, Taoyuan, Taiwan College of Medicine, Chang Gung University, Taoyuan, Taiwan.
Chia-Wei Liou, Department of Neurology, Kaohsiung Chang Gung Memorial Hospital, Kaohsiung, Taiwan.
Jiann-Der Lee, Department of Neurology, Chiayi Chang Gung Memorial Hospital, Chiayi, Taiwan.
Tsung-I Peng, Department of Neurology, Keelung Chang Gung Memorial Hospital, Keelung, Taiwan.
Tsong-Hai Lee, Stroke Center and Department of Neurology, Chang Gung Memorial Hospital, Linkou Medical Center, and College of Medicine, Chang Gung University, No. 5, Fu-Hsing St., Kueishan, Taoyuan, 33333 Taiwan.
References
- 1. Liu CH, Lin JR, Liou CW, et al. Causes of death in different subtypes of ischemic and hemorrhagic stroke. Angiology 2018; 69: 582–590. [DOI] [PubMed] [Google Scholar]
- 2. Manning L, Hirakawa Y, Arima H, et al. Blood pressure variability and outcome after acute intracerebral haemorrhage: a post-hoc analysis of INTERACT2, a randomised controlled trial. Lancet Neurol 2014; 13: 364–373. [DOI] [PubMed] [Google Scholar]
- 3. Qureshi AI, Palesch YY, Barsan WG, et al. Intensive blood-pressure lowering in patients with acute cerebral hemorrhage. N Engl J Med 2016; 375: 1033–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lattanzi S, Silvestrini M. Optimal achieved blood pressure in acute intracerebral hemorrhage: INTERACT2. Neurology 2015; 85: 557–558. [DOI] [PubMed] [Google Scholar]
- 5. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APHA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol 2017; 71: 1269–1324. [DOI] [PubMed] [Google Scholar]
- 6. Liu X, Xu G, Wu W, et al. Subtypes and one-year survival of first-ever stroke in Chinese patients: the Nanjing stroke registry. Cerebrovasc Dis 2006; 22: 130–136. [DOI] [PubMed] [Google Scholar]
- 7. Tsai CF, Thomas B, Sudlow CL. Epidemiology of stroke and its subtypes in Chinese vs white populations: a systematic review. Neurology 2013; 81: 264–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jamerson K, Weber MA, Bakris GL, et al. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med 2008; 359: 2417–2428. [DOI] [PubMed] [Google Scholar]
- 9. Coleman JJ, Kendall MJ. The Anglo-Scandinavian cardiac outcomes trial: blood pressure lowering arm. J Clin Pharm Ther 2006; 31: 299–307. [DOI] [PubMed] [Google Scholar]
- 10. Inzitari D, Poggesi A. Calcium channel blockers and stroke. Aging Clin Exp Res 2005; 17:16–30. [PubMed] [Google Scholar]
- 11. Gurkoff G, Shahlaie K, Lyeth B, et al. Voltage-gated calcium channel antagonists and traumatic brain injury. Pharmaceuticals (Basel) 2013; 6: 788–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hemphill JC, III, Greenberg SM, Anderson CS, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2015; 46: 2032–2060. [DOI] [PubMed] [Google Scholar]
- 13. Steiner T, Al-Shahi Salman R, Beer R, et al. European Stroke Organisation (ESO) guidelines for the management of spontaneous intracerebral hemorrhage. Int J Stroke 2014; 9: 840–855. [DOI] [PubMed] [Google Scholar]
- 14. Hsieh CY, Chen CH, Li CY, et al. Validating the diagnosis of acute ischemic stroke in a National Health Insurance claims database. J Formos Med Assoc 2015; 114: 254–259. [DOI] [PubMed] [Google Scholar]
- 15. Lee TH, Chang CH, Chang YJ, et al. Establishment of electronic chart-based stroke registry system in a medical system in Taiwan. J Formos Med Assoc 2011; 110: 543–547. [DOI] [PubMed] [Google Scholar]
- 16. Wong MC, Tam WW, Cheung CS, et al. Initial antihypertensive prescription and switching: a 5 year cohort study from 250,851 patients. PLoS One 2013; 8: e53625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu CH, Chen TH, Lin MS, et al. Ezetimibe-simvastatin therapy reduce recurrent ischemic stroke risks in type 2 diabetic patients. J Clin Endocrinol Metab 2016; 101: 2994–3001. [DOI] [PubMed] [Google Scholar]
- 18. Hung LC, Sung SF, Hsieh CY, et al. Validation of a novel claims-based stroke severity index in patients with intracerebral hemorrhage. J Epidemiol 2017; 27: 24–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Austin PC. Statistical criteria for selecting the optimal number of untreated subjects matched to each treated subject when using many-to-one matching on the propensity score. Am J Epidemiol 2010; 172: 1092–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dahlof B, Sever PS, Poulter NR, et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet 2005; 366: 895–906. [DOI] [PubMed] [Google Scholar]
- 21. Van Vark LC, Bertrand M, Akkerhuis KM, et al. Angiotensin-converting enzyme inhibitors reduce mortality in hypertension: a meta-analysis of randomized clinical trials of renin–angiotensin–aldosterone system inhibitors involving 158,998 patients. Eur Heart J 2012; 33: 2088–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Smeda JS, Daneshtalab N. The effects of poststroke captopril and losartan treatment on cerebral blood flow autoregulation in SHRsp with hemorrhagic stroke. J Cereb Blood Flow Metab 2011; 31: 476–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jusufovic M, Sandset EC, Bath PM, et al. Blood pressure-lowering treatment with candesartan in patients with acute hemorrhagic stroke. Stroke 2014; 45: 3440–3442. [DOI] [PubMed] [Google Scholar]
- 24. Hornslien AG, Sandset EC, Igland J, et al. Effects of candesartan in acute stroke on vascular events during long-term follow-up: results from the Scandinavian Candesartan acute stroke trial (SCAST). Int J Stroke 2015; 10: 830–835. [DOI] [PubMed] [Google Scholar]
- 25. Anderson DC. ACP journal club. Review: statins do not increase risk for intracerebral hemorrhage. Ann Intern Med 2012; 156: JC3–JC6. [DOI] [PubMed] [Google Scholar]
- 26. Kroll ME, Green J, Beral V, et al. Adiposity and ischemic and hemorrhagic stroke: prospective study in women and meta-analysis. Neurology 2016; 87: 1473–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Group PC. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet 2001; 358: 1033–1041. [DOI] [PubMed] [Google Scholar]
- 28. Schrader J, Luders S, Kulschewski A, et al. Morbidity and mortality after stroke. Eprosartan compared with nitrendipine for secondary prevention: principal results of a prospective randomized controlled study (MOSES). Stroke 2005; 36: 1218–1226. [DOI] [PubMed] [Google Scholar]
- 29. Xie W, Zheng F, Evangelou E, et al. Blood pressure-lowering drugs and secondary prevention of cardiovascular disease: systematic review and meta-analysis. J Hypertens 2018; 36: 1256–1265. [DOI] [PubMed] [Google Scholar]
- 30. Wiysonge CS, Bradley HA, Volmink J, et al. Beta-blockers for hypertension. Cochrane Database Syst Rev 2017; 1: CD002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen GJ, Yang MS. The effects of calcium channel blockers in the prevention of stroke in adults with hypertension: a meta-analysis of data from 273,543 participants in 31 randomized controlled trials. PLoS One 2013; 8: e57854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xue H, Lu Z, Tang WL, et al. First-line drugs inhibiting the renin angiotensin system versus other first-line antihypertensive drug classes for hypertension. Cochrane Database Syst Rev 2015; 1: CD008170. [DOI] [PubMed] [Google Scholar]
- 33. Lee CJ, Hwang J, Oh J, et al. Treatment of uncomplicated hypertension is associated with a reduction in cardiovascular mortality: a Korean national cohort study. J Hypertens 2017; 35(Suppl. 1): S41–S49. [DOI] [PubMed] [Google Scholar]
- 34. Mukete BN, Cassidy M, Ferdinand KC, et al. Long-term anti-hypertensive therapy and stroke prevention: a meta-analysis. Am J Cardiovasc Drugs 2015; 15: 243–257. [DOI] [PubMed] [Google Scholar]
- 35. Pahor M, Franse LV, Deitcher SR, et al. Fosinopril versus amlodipine comparative treatments study: a randomized trial to assess effects on plasminogen activator inhibitor-1. Circulation 2002; 105: 457–461. [DOI] [PubMed] [Google Scholar]
- 36. Iadecola C, Gorelick PB. Hypertension, angiotensin, and stroke: beyond blood pressure. Stroke. 2004; 35: 348–350. [DOI] [PubMed] [Google Scholar]
- 37. Wachtell K, Lehto M, Gerdts E, et al. Angiotensin II receptor blockade reduces new-onset atrial fibrillation and subsequent stroke compared to atenolol: The losartan intervention for end point reduction in hypertension (LIFE) study. J Am Coll Cardiol 2005; 45: 712–719. [DOI] [PubMed] [Google Scholar]
- 38. Rajendran P, Rengarajan T, Thangavel J, et al. The vascular endothelium and human diseases. Int J Biol Sci 2013; 9: 1057–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Thuesen AD, Lyngso KS, Rasmussen L, et al. P/q-type and t-type voltage-gated calcium channels are involved in the contraction of mammary and brain blood vessels from hypertensive patients. Acta Physiol (Oxf) 2017; 219: 640–651. [DOI] [PubMed] [Google Scholar]
- 40. Neal B, MacMahon S, Chapman N, et al. Effects of ace inhibitors, calcium antagonists, and other blood-pressure-lowering drugs: results of prospectively designed overviews of randomised trials. Blood pressure lowering treatment trialists’ collaboration. Lancet 2000; 356: 1955–1964. [DOI] [PubMed] [Google Scholar]
- 41. Yamal JM, Oparil S, Davis BR, et al. Stroke outcomes among participants randomized to chlorthalidone, amlodipine or lisinopril in ALLHAT. J Am Soc Hypertens 2014; 8: 808–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jeffers BW, Robbins J, Bhambri R. Efficacy of calcium channel blockers versus other classes of antihypertensive medication in the treatment of hypertensive patients with previous stroke and/or coronary artery disease: a systematic review and meta-analysis. Am J Ther 2017; 24: e68–e80. [DOI] [PubMed] [Google Scholar]
- 43. Sato S, Carcel C, Anderson CS. Blood pressure management after intracerebral hemorrhage. Curr Treat Options Neurol 2015; 17: 49. [DOI] [PubMed] [Google Scholar]
- 44. Tran KC, Leung AA, Tang KL, et al. Efficacy of calcium channel blockers on major cardiovascular outcomes for the treatment of hypertension in Asian populations: a meta-analysis. Can J Cardiol 2017; 33: 635–643. [DOI] [PubMed] [Google Scholar]
- 45. Poon MT, Fonville AF, Al-Shahi Salman R. Long-term prognosis after intracerebral haemorrhage: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2014; 85: 660–667. [DOI] [PubMed] [Google Scholar]
- 46. Santos Simoes de Almeida LH, Costa Oliveira M. A medical image backup architecture based on a NoSQL database and cloud computing services. Stud Health Technol Inform 2015; 216: 929. [PubMed] [Google Scholar]
- 47. Pelt DM, Sethian JA. A mixed-scale dense convolutional neural network for image analysis. Proc Natl Acad Sci U S A 2018; 115: 254–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wu PH, Yang CY, Yao ZL, et al. Relationship of blood pressure control and hospitalization risk to medication adherence among patients with hypertension in Taiwan. Am J Hypertens 2010; 23: 155–160. [DOI] [PubMed] [Google Scholar]
- 49. Lee HJ, Jang SI, Park EC. Effect of adherence to antihypertensive medication on stroke incidence in patients with hypertension: a population-based retrospective cohort study. BMJ Open 2017; 7: e014486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kim S, Shin DW, Yun JM, et al. Medication adherence and the risk of cardiovascular mortality and hospitalization among patients with newly prescribed antihypertensive medications. Hypertension 2016; 67: 506–512. [DOI] [PubMed] [Google Scholar]
- 51. Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA 2003; 289: 2560–2572. [DOI] [PubMed] [Google Scholar]
- 52. Saver JL, Altman H. Relationship between neurologic deficit severity and final functional outcome shifts and strengthens during first hours after onset. Stroke 2012; 43: 1537–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental_Table_20180618_Final for Choices for long-term hypertensive control in patients after first-ever hemorrhagic stroke: a nationwide cohort study by Chi-Hung Liu, Yu-Sheng Lin, Ching-Chi Chi, Chia-Wei Liou, Jiann-Der Lee, Tsung-I Peng and Tsong-Hai Lee in Therapeutic Advances in Neurological Disorders