Abstract
Dyspnoea, a common and multifactorial symptom in patients with acute coronary syndrome, has been associated with lower quality of life and hospital readmission. Prescriber preference for antiplatelet therapy, the standard of care in this patient group, is shifting to ticagrelor due to mortality benefits demonstrated in trials compared with clopidogrel. In these trials, dyspnoea was more commonly reported in patients prescribed ticagrelor but the aetiology is still debated.
An observational cohort study was conducted to quantify the rates and severity of dyspnoea reported in patients with acute coronary syndrome and newly prescribed ticagrelor compared with those prescribed clopidogrel. Dyspnoea was more commonly reported in patients prescribed ticagrelor at each follow up post-discharge (p = 0.016). Rates were higher than previously reported in clinical trials. In some patients, dyspnoea necessitated drug therapy change and was associated with readmission to hospital (p = 0.046). As ticagrelor is widely prescribed as a first-line antiplatelet agent for a range of patients with acute coronary syndrome, the incidence of dyspnoea in a generalized patient cohort may result in higher rates of drug discontinuation. This in turn could lead to higher rates of rehospitalisation and potential treatment failure than that reported from the controlled setting of a clinical trial.
Keywords: acute coronary syndromes, antiplatelet, ticagrelor, dyspnoea, adverse effects, cardiology
Introduction
Dyspnoea is common in patients with acute coronary syndrome (ACS) and may develop during admission or post-discharge, due to new-onset or worsening of pre-existing heart failure, treatment with beta blockers or recurrent ischaemia.1 ACS patients with dyspnoea report lower quality of life and have a higher risk of hospital readmission or mortality.2
Dual antiplatelet therapy with clopidogrel and aspirin became the standard therapy for secondary prevention in ACS after a 20% reduction in the composite endpoint of cardiovascular death, myocardial infarction or stroke was demonstrated, without a significant increase in life-threatening bleeding.3 Ticagrelor, a structurally novel P2Y12 antagonist administered twice daily, does not need enzymatic activation and has a more rapid onset of action compared with clopidogrel.4 In the Phase-III PLATelet inhibition and patient Outcomes (PLATO) trial, ticagrelor use was associated with lower all-cause mortality when compared with clopidogrel.5 Ticagrelor however, was also associated with higher rates of dyspnoea compared with clopidogrel (13.8% versus 7.8%).4–8
Dose-related dyspnoea associated with ticagrelor was first identified in the phase II DISPERSE trial,6,7 and subsequently in the PLATO trial.5,7 Drug discontinuation due to dyspnoea occurred in 0.9% of patients prescribed ticagrelor in PLATO compared with 0.1% of patients prescribed clopidogrel [odds ratio (OR) = 6.21, p = <0.001].7,9 Rates of ticagrelor discontinuation in the highly regulated environment of randomized controlled trials may be lower than in clinical practice.10
Despite the prevalence of dyspnoea in the ACS patient population, the burden of ticagrelor-related dyspnoea in patients with comorbid respiratory disease has not been explored in depth. In a sub-study of pulmonary function during PLATO, patients with chronic obstructive pulmonary disease (COPD) or asthma were excluded from analysis.11 Furthermore, the prevalence of patients with comorbid respiratory disease was considerably low in the PLATO patient population, and may not reflect a generalized patient cohort.6
The present study aims to investigate the incidence of ‘real-world’ ticagrelor-related dyspnoea in a cohort of ACS patients newly commenced on ticagrelor or clopidogrel.
Methods
This was a multicentre prospective observational cohort study conducted from March 2015 until October 2016. Patients were eligible for inclusion if they were admitted for ACS and newly prescribed ticagrelor or clopidogrel on discharge. Exclusion criteria were being unavailable for follow up or being discharged to a nursing home facility. Ethical approval was granted from the Southern Adelaide Clinical Human Research Ethics Committee, Australia (approval 473.14). Patients admitted to Flinders Medical Centre, The Royal Adelaide and The Queen Elizabeth Hospitals in South Australia were approached for voluntary enrolment during their hospital admission and followed up with a telephone interview (16 questions) at 3 weeks, 3 months, 6 months and 12 months post-discharge. All interviews were conducted by AP to ensure fidelity to questioning and documenting the patient’s experience of dyspnoea since the last follow up, the severity (mild, moderate, severe), pattern and impact on daily activities.
Analysis was performed using Stata version 14.2 (StataCorp, USA). Differences in patient characteristics between the two drug groups were compared using independent Student’s t-tests or Mann–Whitney tests for normally and non-normally distributed variables and using either Chi-square tests of association or Fisher’s exact test for categorical variables. The risk of obtaining a falsely significant p-value can increase when multiple analysis is performed. To mitigate this risk, we utilized and reported a p-value for linear trends in addition to Chi-square.
We assessed between-group differences in the occurrence of dyspnoea over time using multilevel binary logistic regression with drug, week (categorical) and a drug X week interaction term as fixed effects and the subject identity number as a random effect. Overall differences were assessed globally and where p < 0.05, were then assessed at the individual time points using a Wald test for the separate drug X week interaction terms with Bonferroni adjustment for multiple comparisons (p < 0.0125 considered as significant). Patient characteristics were assessed as predictors of dyspnoea, using both univariate and multivariate mixed effects regression with variables entered into the multivariate model when p < 0.20. The final multivariate model contained only those predictors significant at p < 0.05. The impact of dyspnoea on readmission to hospital was assessed using multivariate binary logistic regression with adjustment for age and gender. The log rank test, univariate Cox regression and Kaplan–Meier survival curves were used to assess the impact of dyspnoea on readmission to hospital. Variables significant at p < 0.05 in two-tailed hypothesis tests were considered statistically significant.
Results
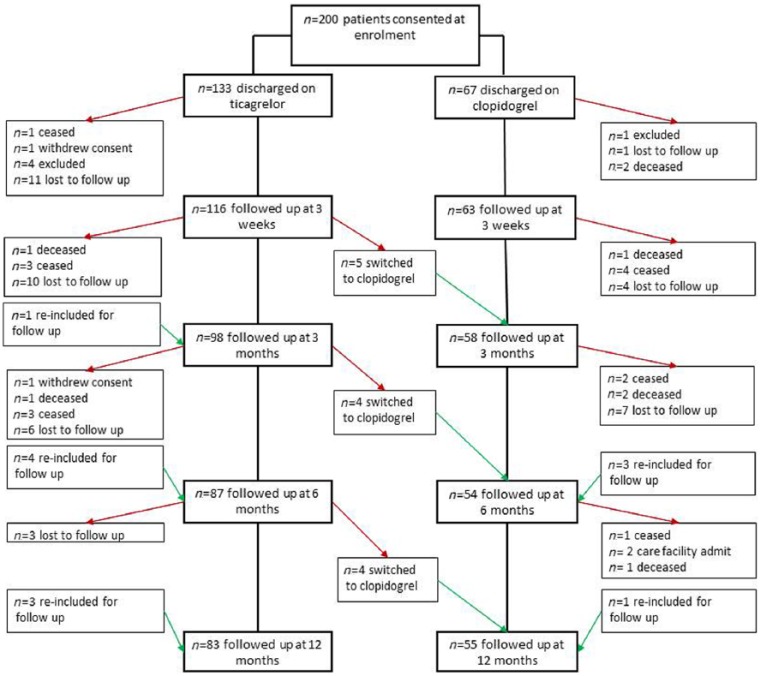
After 32 recruited patients were excluded, baseline patient demographics for participants (n = 200) were analysed (Figure 1). Sex, level of health cover, marital status and household income did not differ between groups (Table 1). Patients prescribed clopidogrel were older, had more comorbidities and scored higher for their medication regimen complexity index preadmission. Those taking ticagrelor were more likely to be admitted with a diagnosis of ST-elevation myocardial infarction (p = 0.002), managed with a drug-eluting stent (p = 0.022) and employed full time (p = 0.042).
Figure 1.
Study flow diagram of patients.
Table 1.
Baseline patient characteristics.
| Ticagrelor (n = 133) | Clopidogrel (n = 67) | p-value | |
|---|---|---|---|
| Age (years), mean SD | 62.9 ± 11.6 | 70.4 ± 11.6 | <0.001 |
| Female sex (%) | 27.0 | 31.3 | 0.616 |
| Health care card holder (%) | 54.4 | 64.2 | 0.221 |
| Annual household income (AUD) (%) | 0.229 | ||
| <40,000 | 56.8 | 71.2 | |
| 40,001–60,000 | 16.8 | 12.1 | |
| 60,001–80,000 | 6.4 | 4.5 | |
| 80,001–100,000 | 4.0 | 6.1 | |
| >100,000 | 12.0 | 6.1 | |
| Marital status (%) | 0.082 | ||
| Single | 14.5 | 3.0 | |
| Married/defacto | 54.8 | 67.2 | |
| Divorced/widowed | 30.6 | 29.9 | |
| Employed full time (%) | 28.6 | 16.4 | 0.042 |
| Body mass index (kg/m2 ), mean ± SD | 29.5 ± 6.1 | 28.7 ± 4.8 | 0.560 |
| Smoker (%) | 35.4 | 22.4 | 0.073 |
| Pre-existing ischaemic heart disease (%) | 27.6 | 40.3 | 0.077 |
| Atrial fibrillation (%) | 5.5 | 20.9 | 0.003 |
| Heart failure (%) | 11.0 | 14.9 | 0.493 |
| Asthma (%) | 11.0 | 16.4 | 0.367 |
| Chronic obstructive pulmonary disease (%) | 6.8 | 7.4 | 1.00 |
| Anaemia (%) | 1.6 | 9.0 | 0.021 |
| Number of preadmission medications, mean ± SD | 4.0 ± 3.9 | 5.5 ± 4.0 | 0.012 |
| Charlson comorbidity index | 3.5 ± 2.7 | 4.9 ± 3.0 | 0.001 |
| Diagnosis at admission (%) | 0.022 | ||
| STEMI | 45.7 | 34.3 | |
| NSTEMI | 40.9 | 34.3 | |
| Other | 13.4 | 31.4 | |
| Mean length of stay ± SD (days) | 4.3 ± 3.7 | 4.9 ± 3.4 | 0.290 |
| Medications prescribed at discharge (%) | |||
| ACE inhibitor | 68.5 | 65.7 | 0.748 |
| AT 2 receptor inhibitor | 20.5 | 16.4 | 0.567 |
| Beta blocker | 87.4 | 83.6 | 0.515 |
| Statin | 100 | 92.5 | 0.004 |
| Aspirin | 95.8 | 84.9 | 0.042 |
| Glyceryl trinitrate buccal spray/tablet | 78.7 | 68.2 | 0.117 |
| Diuretics | 15.6 | 9.0 | 0.280 |
| Mean MRCI preadmission ± SD | 12.7 ± 13.3 | 16.7 ± 13.1 | 0.049 |
| Mean MRCI post-admission ± SD | 25.2 ± 12.0 | 25.5 ± 10.3 | 0.860 |
| Mean change in MRCI ± SD | 12.6 ± 5.3 | 8.8 ± 7.2 | <0.001 |
ACE, angiotensin converting enzyme; AT, angiotensin; MRCI, medication regimen complexity index; NSTEMI, non-ST-elevation myocardial infarction; SD, standard deviation; STEMI, ST-elevation myocardial infarction.
observations missing for some patients.
Overall, patients prescribed ticagrelor were more likely to report dyspnoea as an adverse effect across all follow-up time points [OR = 2.54, 95% confidence interval (CI) 1.19–5.45, p = 0.016]. The prevalence of patient-reported dyspnoea with ticagrelor was higher than reported in patients prescribed clopidogrel at 3 weeks (p = 0.04) (Table 2). This trend, albeit not significant, persisted at 3, 6 and 12 months. There was a nonsignificant trend towards increased severity at each time point. In 4.3% of patients receiving ticagrelor, the persistence of dyspnoea necessitated a change to clopidogrel. The incidence of dyspnoea was also associated with hospital readmission (OR = 1.65, 95% CI 0.99–2.75, p = 0.051; Supplementary Figure) and was significant after adjusting for patients age and sex (OR = 1.68, 95% CI 1.01–2.80, p = 0.046).
Table 2.
Dyspnoea: patient-reported incidence and severity.
| Time interval |
Antiplatelet agent |
All dyspnoea n (%) |
Mild n (%) |
Moderate n (%) |
Severe n (%) |
p-value for χ2 |
p-value for linear trend |
|---|---|---|---|---|---|---|---|
| 3 weeks | Ticagrelor n = 116 | 65 (56.0) | 40 (34.5) | 19 (16.4) | 6 (5.2) | 0.040 | 0.11 |
| Clopidogrel n = 63 | 25 (39.7) | 15 (23.8) | 5 (7.9) | 5 (7.9) | |||
| 3 months | Ticagrelor n = 98 | 45 (45.9) | 25 (25.5) | 17 (17.3) | 3 (3.1) | 0.355 | 0.07 |
| Clopidogrel n = 58 | 20 (34.5) | 13 (22.4) | 6 (10.3) | 1 (1.6) | |||
| 6 months | Ticagrelor n = 87 | 36 (41.4) | 21 (24.1) | 11 (12.6) | 4 (4.6) | 0.306 | 0.17 |
| Clopidogrel n = 54 | 16 (29.6) | 8 (14.8) | 7 (13.0) | 1 (1.9) | |||
| 12 months | Ticagrelor n = 83 | 27 (32.5) | 14 (16.9) | 11 (13.3) | 2 (2.4) | 0.846 | 0.39 |
| Clopidogrel n = 55 | 15 (27.3) | 9 (16.4) | 5 (9.1) | 1 (1.8) |
In univariate analysis, significant baseline patient characteristics predictive of patient-reported dyspnoea at the p < 0.2 level included female sex (OR = 2.44, 95% CI 1.20–4.98, p = 0.014), COPD (OR = 4.21, 95% CI 1.19–14.8, p = 0.025), Charlson comorbidity index (OR = 2.22, 95% CI 0.90–5.47, p = 0.082), and anaemia (OR = 0.23, 95% CI 0.31–1.74, p = 0.156). In multivariate analysis, significant predictors were age, female sex (OR = 2.46, 95% CI 1.15–5.25, p = 0.19) and COPD (OR = 3.84, 95% CI 1.03–14.41, p = 0.046; Table 3). Other factors included for analysis that were not predictive of patient-reported dyspnoea included level of education, smoking status, asthma, heart failure, chronic kidney disease and treatment with diuretics or beta blockers. Ticagrelor was a significant predictor of dyspnoea in multivariate analysis (OR = 2.43, 95% CI 1.12–5.23, p = 0.024) as was follow-up time post-discharge (p < 0.0001).
Table 3.
Dyspnoea: baseline predictors.
| Univariate logistic
regression |
Global p-value | |||
|---|---|---|---|---|
| Odds ratio | 95% confidence interval | p-value | ||
| Age (years) | 0.99 | (0.96–1.02) | 0.510 | |
| Female sex | 2.44 | (1.20–4.98) | 0.014 | |
| Body mass index (kg/m2) | 1.02 | (0.91–1.31) | 0.736 | |
| Smoker | 0.94 | (0.45–1.94) | 0.858 | |
| Charlson comorbidity index | 0.167 | |||
| 0 | 1.00 | |||
| <2 | 1.03 | (0.17–6.24) | 0.978 | |
| >2 | 2.22 | (0.90–5.47) | 0.082 | |
| Diabetes | 1.68 | (0.80–3.55) | 0.174 | |
| Depression | 1.92 | (0.69–5.31) | 0.210 | |
| Chronic obstructive pulmonary disease | 4.21 | (1.19–14.8) | 0.025 | |
| Asthma | 1.57 | (0.58–4.23) | 0.373 | |
| Heart failure | 1.33 | (0.46–3.86) | 0.597 | |
| Atrial fibrillation | 0.78 | (0.27–2.29) | 0.658 | |
| Anaemia | 0.23 | (0.31–1.74) | 0.156 | |
| Chronic kidney disease | 1.44 | (0.35–5.95) | 0.616 | |
| Multivariate logistic regression | ||||
| Odds ratio | 95% confidence interval | p-value | ||
| Age (years) | 0.99 | (0.96–1.03) | 0.746 | |
| Female sex | 2.46 | (1.15–5.25) | 0.019 | |
| Ticagrelor | 2.43 | (1.12–5.23) | 0.024 | |
| Chronic obstructive pulmonary disease | 3.84 | (1.02–14.4) | 0.046 | |
| Time post-discharge | <0.0001 | |||
| 3 weeks | 3.90 | (2.07–7.33) | <0.001 | |
| 12 weeks | 1.97 | (1.05–3.68) | 0.033 | |
| 26 weeks | 1.41 | (0.75–2.67) | 1.06 | |
| 52 weeks | 1.00 | |||
Discussion
In the current study, we found ACS patients that were prescribed ticagrelor for secondary prevention were more likely to report dyspnoea post-discharge compared with patients prescribed clopidogrel. Our findings show rates of patient-reported dyspnoea with ticagrelor were much higher than those previously reported in the literature6 and this was consistently observed at all follow-up time points. Furthermore, patients reporting dyspnoea were more likely to discontinue drug therapy or be readmitted to hospital.
A number of comorbidities and drug treatments which are known to cause dyspnoea were analysed to assess their contribution to the reported dyspnoea. Baseline factors that significantly associated with dyspnoea were comorbid COPD, female sex and age. Recent guidelines for the management of ACS heed practitioners’ warnings regarding prescribing ticagrelor in patients with COPD due to the higher incidence of dyspnoea in trials.12 Such recommendations seem prudent given a sub-analysis of PLATO found that the mortality benefit of ticagrelor persisted for patients who reported dyspnoea on treatment, compared with those who reported dyspnoea on clopidogrel.13
Our study is the first to prospectively assess ticagrelor-induced dyspnoea at multiple follow-up times post-discharge in a real-world patient population. With our cohort size and recruitment from multiple sites, the results can be more easily applied to a general patient population to assess the severity and pattern of dyspnoea with ticagrelor after hospital discharge. Using this real-world patient population, we can also observe prescribing patterns of antiplatelet agents for patients with ACS. As our study did not exclude patients with comorbid respiratory disease, we were able to assess the incidence and severity of ticagrelor-induced dyspnoea in this patient group.
There are some limitations with our study, as the design was observational, differences in baseline patient characteristics were observed. Older patients were more likely to be prescribed clopidogrel which is consistent with other observational studies,14 and we cannot exclude that lower reported rates of dyspnoea were not influenced by their daily level of activity. A number of patients were lost to follow up which limited our sample size as the study progressed. Patients were asked to self-report whether they experienced dyspnoea and how they rated the severity. The current study sought principally to investigate the incidence of dyspnoea. At the time of study design, the severity of dyspnoea was underestimated and whilst the investigators developed probing questions to distinguish the nature and severity of patient self-reported dyspnoea, a validated tool was not employed. Furthermore, there was no assessment of dyspnoea at baseline to distinguish whether it was new onset. Although patients with comorbid asthma and COPD were included in the study, no respiratory function testing was performed to confirm diagnosis or assess disease severity.
As rates of dyspnoea were higher than expected at the onset of this study, further work is warranted to investigate validated dyspnoea assessment tools and to determine the nature of subsequent hospital readmissions.
Conclusion
Dyspnoea was more frequently reported in patients with ACS prescribed ticagrelor compared with clopidogrel. However, the observed rates in a naturalistic setting were significantly higher than those reported in clinical trials, and predicted the rate of subsequent hospital readmission. Further studies in larger cohorts are warranted to confirm these findings, to identify other predisposing factors, and to test the hypothesis that ticagrelor-associated dyspnoea predicts short and long-term adverse outcomes.
Supplemental Material
Supplemental material, Supp_figure for Real-world incidence of patient-reported dyspnoea with ticagrelor by Adaire E. Prosser, Jessica L. Dawson, KethLyn Koo, Karen M. O’Kane, Michael B. Ward, Richard J. Woodman, Arduino A. Mangoni and Cameron J. Phillips in Therapeutic Advances in Drug Safety
Acknowledgments
We thank Professor Derek Chew, Regional Director of Cardiology, Southern Region Adelaide Health Service; Dr Peter Steele, Director of Cardiology, the Royal Adelaide Hospital and Professor John Horowitz, Director of Cardiology and Clinical Pharmacology, The Queen Elizabeth Hospital for allowing the study to be conducted. Dr Luke Grzeskowiak and Mr Greg Roberts, SA Pharmacy Flinders Medical Centre for their assistance with data management and statistical advice; Professor Sepehr Shakib, Director of Clinical Pharmacology, Royal Adelaide Hospital for his advice and support. Sincere thanks to Ms Cassandra Potts SA Pharmacy, Flinders Medical Centre, Dr Adam Nelson, Advanced Trainee, Department of Cardiology, Royal Adelaide Hospital and Ms Amber Bendyk for their assistance with patient identification.
We acknowledge the following contributors. Study concepts and design: AEP, JLD, KK, KOK, MBW, CJP and AAM. Acquisition, analysis or interpretation of data: all authors. Statistical analysis: RJW. Drafting or revising manuscript for critically important content: all authors. Final approval: all authors approved the final version of the manuscript and agree to be accountable for all aspects of the work.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: AE Prosser  https://orcid.org/0000-0001-7500-8494
https://orcid.org/0000-0001-7500-8494
Supplemental Material: Supplemental material is available for this article online.
Contributor Information
Adaire E. Prosser, SA Pharmacy, Flinders Medical Centre, E-Block Room 288, Bedford Park, 5042, Australia.
Jessica L. Dawson, SA Pharmacy, Flinders Medical Centre, Bedford Park, Australia
KethLyn Koo, SA Pharmacy, The Royal Adelaide Hospital, Adelaide, Australia.
Karen M. O’Kane, SA Pharmacy, The Queen Elizabeth Hospital, Woodville South, Australia
Michael B. Ward, School of Pharmacy and Medical Sciences, University of South Australia, Adelaide, Australia
Richard J. Woodman, Flinders Centre for Epidemiology and Biostatistics, Flinders University, Adelaide, Australia College of Medicine and Public Health, Flinders University, Adelaide, Australia.
Arduino A. Mangoni, College of Medicine and Public Health, Flinders University, Adelaide, Australia Division of Medicine, Flinders Medical Centre, Bedford Park, Australia; Department of Clinical Pharmacology, Flinders University, Adelaide, Australia.
Cameron J. Phillips, SA Pharmacy, Flinders Medical Centre, Bedford Park, Australia School of Pharmacy and Medical Sciences, University of South Australia, Adelaide, Australia; College of Medicine and Public Health, Flinders University, Adelaide, Australia.
References
- 1. Parodi G, Storey RF. Dyspnoea management in acute coronary syndrome patients treated with ticagrelor. Eur Heart J Acute Cardiovasc Care 2015; 4: 555–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arnold SV, Spertus JA, Jones PG, et al. The impact of dyspnea on health-related quality of life in patients with coronary artery disease: results from the PREMIER registry. Am Heart J 2009; 157: 1042–1049. [DOI] [PubMed] [Google Scholar]
- 3. Mehta SR, Yusuf S, Peters RJ, et al. Clopidogrel in Unstable angina to prevent Recurrent Events trial (CURE) Investigators. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet 2001; 358: 527–533. [DOI] [PubMed] [Google Scholar]
- 4. Cannon CP, Husted S, Harrington RA, et al. Safety, tolerability, and initial efficacy of AZD6140, the first reversible oral adenosine diphosphate receptor antagonist, compared with clopidogrel, in patients with non-ST-segment elevation acute coronary syndrome: primary results of the DISPERSE-2 trial. J Am Coll Cardiol 2007; 50: 1844–1851. [DOI] [PubMed] [Google Scholar]
- 5. Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009; 361: 1045–1057. [DOI] [PubMed] [Google Scholar]
- 6. Husted S, Emanuelsson H, Heptinstall S, et al. Pharmacodynamics, pharmacokinetics, and safety of the oral reversible P2Y12 antagonist AZD6140 with aspirin in patients with atherosclerosis: a double-blind comparison to clopidogrel with aspirin. Eur Heart J 2006; 27: 1038–1047. [DOI] [PubMed] [Google Scholar]
- 7. Serebruany VL, Sibbing D, DiNicolantonio JJ. Dyspnea and reversibility of antiplatelet agents: Ticagrelor, Elinogrel, Cangrelor, and beyond. Cardiology 2014; 127: 20–24. [DOI] [PubMed] [Google Scholar]
- 8. Guerbaai RA, Mahata I, Marechaux S, et al. Is ticagrelor worth its high cost and side-effects? Acta Cardiol 2018; 7: 1–6. [DOI] [PubMed] [Google Scholar]
- 9. Serebruany VL, Steinhubl SR, Berger PB, et al. Variability in platelet responsiveness to clopidogrel among 544 individuals. J Am Coll Cardiol 2005; 45: 246–251. [DOI] [PubMed] [Google Scholar]
- 10. Gaubert M, Laine M, Richard T, et al. Effect of ticagrelor-related dyspnea on compliance with therapy in acute coronary syndrome patients. Int J Cardiol 2014; 173: 120. [DOI] [PubMed] [Google Scholar]
- 11. Storey RF, Becker RC, Harrington RA, et al. Pulmonary function in patients with acute coronary syndrome treated with ticagrelor or clopidogrel (from the Platelet Inhibition and Patient Outcomes [PLATO] pulmonary function substudy). Am J Cardiol 2011; 108: 1542–1546. [DOI] [PubMed] [Google Scholar]
- 12. Chew DP, Scott IA, Cullen L, et al. National Heart Foundation of Australia & Cardiac Society of Australia and New Zealand: Australian Clinical Guidelines for the Management of Acute Coronary Syndromes 2016. Heart Lung Circ 2016; 25: 895–951. [DOI] [PubMed] [Google Scholar]
- 13. Storey RF, Becker RC, Harrington RA, et al. Characterization of dyspnoea in PLATO study patients treated with ticagrelor or clopidogrel and its association with clinical outcomes. Eur Heart J 2011; 32: 2945–2953. [DOI] [PubMed] [Google Scholar]
- 14. Rezaei SS, Geroldinger A, Heinze G, et al. Clopidogrel, prasugrel, or ticagrelor use and clinical outcome in patients with acute coronary syndrome: A nationwide long-term registry analysis from 2009 to 2014. Int J Cardiol 2017; 235: 61–66. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supp_figure for Real-world incidence of patient-reported dyspnoea with ticagrelor by Adaire E. Prosser, Jessica L. Dawson, KethLyn Koo, Karen M. O’Kane, Michael B. Ward, Richard J. Woodman, Arduino A. Mangoni and Cameron J. Phillips in Therapeutic Advances in Drug Safety