Short abstract
Objective
To investigate the prognostic significance of and risk factors for solitary lymph node metastasis (SLNM) of patients with cervical carcinoma.
Methods
Clinical data from patients with International Federation of Gynecology and Obstetrics (FIGO) stages IA2 to IIA cervical carcinoma who underwent radical hysterectomy and pelvic lymphadenectomy between January 2003 and December 2010 were analysed retrospectively. Histopathological analysis was used to identify SLNM. Long-term survival and risk factors associated with SLNM were analysed.
Results
The study enrolled 302 patients with cervical cancer: 48 with SLNM (SLNM group) and 254 patients with no lymph node metastases (nLNM group). FIGO stage, tumour grade, depth of tumour invasion, uterine body involvement, parametrial involvement and lymphovascular invasion differed significantly between the two groups. Logistic regression analysis revealed that FIGO stage, depth of tumour invasion and lymphovascular invasion were independent factors associated with SLNM. The 5-year survival rates of the SLNM and nLNM groups were 54.2% and 87.8%, respectively. Multivariate analysis identified SLNM as an independent factor affecting survival.
Conclusions
The occurrence of just one solitary lymph node metastasis significantly worsened the prognosis in patients with cervical carcinoma compared with patients without lymph node metastases.
Keywords: Cervical carcinoma, lymph node metastasis, survival
Introduction
Cervical cancer is a major cause of cancer-related deaths of women worldwide.1 Although lymph node metastasis is not included in the International Federation of Gynecology and Obstetrics (FIGO) stages, it is still one of the most important prognostic factors for cervical carcinoma.2–4 Pelvic lymph node dissection can increase the long-term survival of patients with cervical carcinoma with lymph node metastasis, although it may be unnecessary for patients without lymph node metastasis.5–7 To decrease the perioperative morbidity and mortality and to improve the quality of life, patients with node-negative cervical carcinoma may undergo less invasive surgery. However, it is difficult to precisely diagnose lymph node metastasis using preoperative examinations such as endoscopic ultrasonography and computed tomography.8,9 Numerous studies have focused on patients with or without lymph node metastasis to explore the influence of lymph node metastasis on prognosis.5,6,9 Although this represents a critical step in the process of lymph node metastasis, the effect of solitary lymph node metastasis (SLNM) on prognosis is unknown.
To determine the value of using the presence of SLNM to assess the progression of cervical carcinoma, this present study retrospectively analysed the clinicopathological characteristics and long-term outcomes of patients with cervical carcinoma with proven SLNM who underwent primary radical hysterectomy and pelvic lymphadenectomy.
Patients and methods
Patient population
This retrospective study reviewed the medical records and pathological materials obtained from patients with invasive carcinoma of the cervix with FIGO stages IA2 to IIA. The patients were treated at the Department of Gynaecology and Obstetrics, Fujian Provincial Maternity and Children’s Hospital, Fuzhou, Fujian Province, China between January 2003 and December 2010. Patients were included who met the inclusion criteria as follows: (i) FIGO stages IA2 to IIA and primary radical hysterectomy with pelvic lymphadenectomy; (ii) the histological examination of all resected lymph nodes revealed only one involved lymph node; (iii) preoperative examination such as sternum, abdominal ultrasound revealed no lung, liver, abdominal or more distant metastases. Patients who received preoperative chemotherapy or radiotherapy were excluded from the study. FIGO staging was performed according to the findings of a clinical examination, preoperative computed tomography and magnetic resonance imaging.
The study was approved by the Ethics Committee of Fujian Provincial Maternity and Children’s Hospital. Written informed consent was given by the patients for their information to be stored in the hospital database and used for research.
Surgical retrieval of lymph node metastases
Radical hysterectomy is performed in our institute as a standard treatment option for patients with FIGO stage IA2 to IIA disease according to the National Comprehensive Cancer Network (NCCN) guidelines.10 The operation was carried out with initial pelvic lymphadenectomy followed by radical hysterectomy. The systematic pelvic lymphadenectomy was carried out by removing all fatty tissue along both sides of the common iliac, external iliac, and internal iliac vessels, and also the lymphatic tissue in the obturator fossa. Para-aortic lymphadenectomy was performed only when gross metastasis to the common iliac nodes or para-aortic nodes was suspected. Parametrial involvement and positive pelvic lymph node metastasis were proven by pathological examination after surgery. Most nodal materials were separately dissected by the surgeons from the en bloc specimens at the end of the procedure. The remaining nodes were identified and retrieved by specialized pathologists from formalin-fixed surgical specimens.
Immunohistochemical analysis
Paraffin-embedded nodes were serially sectioned at intervals of 20–40 µm. The thickness of the slices was approximately 4 µm. Two different representative sections were selected for haematoxylin and eosin staining to detect tumour metastases. Additional cytokeratin 20 immunohistochemical staining was usually used to detect any micro-metastases by gynaecological pathologists in patients presenting with negative lymph node metastasis. Postoperative pathological classification was performed according to the Union for International Cancer Control TNM classification of malignant tumours. Patients with any high-risk pathological factors such as lymph node metastasis, parametrial invasion or positive surgical margins for cancer and patients who had two intermediate-risk pathological factors including deep stromal invasion and lymphovascular space invasion were suggested to receive an adjuvant concurrent chemoradiation with platinum-based chemotherapeutic regimen. Radiation alone was administered in patients who refused chemotherapy or in those with a poor performance status.
The follow-up was performed by trained investigators through mailings, telephone calls, visitations or recording the patients’ consultations at the outpatient service. All surviving patients were followed for more than 5 years. The survival time was from surgery until the date that the survival information was collected or the date of death.
Statistical Analyses
All statistical analyses were performed using the SPSS® statistical package, version 18.0 (SPSS Inc., Chicago, IL, USA) for Windows®. Data were analysed using Student’s t-test, χ2-test and Fisher’s exact test. The survival rate was analysed using the Kaplan–Meier method and the difference between the curves was assessed using the log-rank test. Multivariate analysis was performed using a logistic regression model for the analysis of solitary of lymph node metastasis. A Cox proportional hazards model was used to analyse survival. A P-value < 0.05 was considered statistically significant.
Results
This retrospective study reviewed the medical records and pathological materials obtained from 563 patients with invasive carcinoma of the cervix with FIGO stages IA2 to IIA. Of these, 261 patients were excluded because 249 had multiple lymph nodes metastases and 12 patients had proven distant metastases. A total of 302 patients were enrolled in the study: 254 patients were node-negative (nLNM group) and 48 patients were diagnosed with SLNM (SLNM group). Among the 48 patients with SLNM, 46 patients had pelvic lymph node involvement and two had para-aortic lymph node involvement. Overall, there were 60 patients with lymphovascular involvement. The clinicopathological characteristics of the patients with SLNM compared with the node-negative patients are shown in Table 1. Overall, the mean ± SD number of dissected lymph nodes was 21.3 ± 7.9 (range, 10–49); the mean ± SD number of dissected lymph nodes in the SLNM and nLNM groups were 21.7 ± 6.8 and 21.2 ± 8.1, respectively. The differences between the two groups in terms of FIGO stage, tumour grade, depth of tumour invasion, uterine body involvement, parametrial involvement and lymphovascular invasion were statistically significant (P < 0.05 for all comparisons). There were no significant differences between the two groups in terms of age, tumour size, pathological cell type and ovarian shift.
Table 1.
Comparison of the clinicopathologic characteristics between patients with solitary lymph node metastasis (SLNM group) and patients without lymph node metastasis (nLNM group).
| Characteristic | SLNM groupn = 48 | nLNM groupn = 254 | Statistical significancea |
|---|---|---|---|
| Age, years | 46.2 ± 8.7 | 44.9 ± 7.4 | NS |
| FIGO stage | P = 0.001 | ||
| IA2 | 0(0.0) | 34 (13.4) | |
| IB | 14 (29.2) | 111 (43.7) | |
| IIA | 34 (70.8) | 109 (42.9) | |
| Tumour size, cm | NS | ||
| ≥4 | 24 (50.0) | 132 (52.0) | |
| <4 | 24 (50.0) | 122 (48.0) | |
| Tumour grade | P = 0.035 | ||
| Well differentiated | 2 (4.2) | 32 (12.6) | |
| Moderately differentiated | 6 (12.5) | 58 (22.8) | |
| Poorly differentiated | 40 (83.3) | 164 (64.6) | |
| Depth of tumour invasion | P < 0.001 | ||
| T1 | 8 (16.7) | 134 (52.8) | |
| T2 | 30 (62.5) | 110 (43.3) | |
| T3 | 10 (20.8) | 10 (3.9) | |
| Pathological cell type | NS | ||
| Squamous cell carcinoma | 40 (83.3) | 234 (92.1) | |
| Adenocarcinoma | 6 (12.5) | 16 (6.3) | |
| Adenosquamous | 2 (4.2) | 4 (1.6) | |
| Uterine body involvement | P = 0.001 | ||
| Yes | 8 (16.7) | 10 (3.9) | |
| No | 40 (83.3) | 244 (96.1) | |
| Parametrial involvement | P < 0.001 | ||
| Yes | 10 (20.8) | 12 (4.7) | |
| No | 38 (79.2) | 242 (95.3) | |
| Number of dissected lymph nodes | 21.7 ± 6.8 | 21.2 ± 8.1 | NS |
| Lymphovascular invasion | P < 0.001 | ||
| Positive | 24 (50.0) | 36 (14.2) | |
| Negative | 24 (50.0) | 218 (85.8) | |
| Ovarian shift | NS | ||
| Yes | 6 (12.5) | 56 (22.0) | |
| No | 42 (87.5) | 198 (78.0) |
Data presented as mean ± SD or n of patients (%).
aThe two groups were compared using Student’s t-test for continuous variables and χ2-test for categorical variables.
FIGO, International Federation of Gynecology and Obstetrics; T1, invasive <1/2 muscle layer; T2, invasive ≥1/2 muscle layer; T3, invasive cervical internal surface; NS, no significant between-group difference (P ≥0.05).
Multivariate analysis was performed using a logistic regression model for the analysis of the characteristics associated with a risk of SLNM. The logistic regression analysis revealed that the FIGO stage (P = 0.041), depth of tumour invasion (P = 0.002) and lymphovascular invasion (P = 0.038) were independent factors for SLNM (Table 2).
Table 2.
Multivariate logistic regression analysis of the risk for solitary lymph node metastasis.
| Parameter | β | SE | Wald | Statistical significance | Risk ratio | 95% CI |
|---|---|---|---|---|---|---|
| FIGO stage | 0.722 | 0.352 | 4.194 | P = 0.041 | 2.058 | 1.032, 4.106 |
| Tumour grade | 0.386 | 0.343 | 1.092 | NS | 0.260 | 0.751, 2.879 |
| Depth of tumour invasion | 0.979 | 0.323 | 9.182 | P = 0.002 | 2.662 | 1.413, 5.014 |
| Uterine body involvement | –0.841 | 0.689 | 1.490 | NS | 0.431 | 0.112, 1.665 |
| Parametrial involvement | –0.266 | 0.646 | 0.170 | NS | 0.766 | 0.216, 2.716 |
| Lymphovascular invasion | –0.863 | 0.415 | 4.323 | P = 0.038 | 0.422 | 0.187, 0.952 |
CI, confidence interval; FIGO, International Federation of Gynecology and Obstetrics; NS, no significant association (P ≥ 0.05).
The follow-up rate was 92.1% (278 of 302 patients) and the follow-up duration ranged from 4 to 103 months. The SLNM and nLNM groups lost three and 21 patients to follow-up, respectively. The 5-year survival rates of patients in the SLNM and nLNM groups differed significantly (54.2% [26 of 48 patients] and 87.8% [223 of 254 patients], respectively; P < 0.001) (Figure 1).
Figure 1.
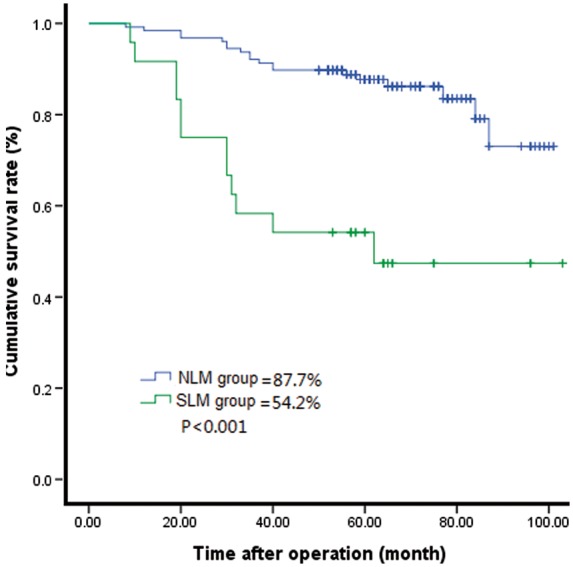
Comparison of the Kaplan-Meier survival curves between patients with solitary lymph node metastasis (SLNM group) and patients without lymph node metastasis (nLNM group) (P < 0.001; log-rank test).
The clinicopathological variables tested using univariate analyses are shown in Table 3. Factors that significantly influenced the 5-year survival rate were as follows: FIGO stage (P < 0.001), tumour size (P = 0.002), tumour grade (P < 0.001), depth of tumour invasion (P < 0.001), uterine body involvement (P < 0.001), lymphovascular invasion (P < 0.001), parametrial involvement (P < 0.001), SLNM (P < 0.001), postoperative treatment (P = 0.018) and ovarian shift (P = 0.023). The covariates age and pathological cell type had no significant influence on survival.
Table 3.
Univariate analysis of prognostic factors for survival in patients with and without solitary lymph node metastasis (SLNM).
| Parameter | n | 5-year overall survival rate, % | χ2 | Statistical significance |
|---|---|---|---|---|
| Age | 0.938 | NS | ||
| <40 | 68 | 74.5 | ||
| ≥40 | 234 | 78.9 | ||
| FIGO stage | 17.397 | P < 0.001 | ||
| IA2 | 34 | 94.1 | ||
| IB | 125 | 88.5 | ||
| IIA | 143 | 74.3 | ||
| Tumour size (cm) | 9.189 | P = 0.002 | ||
| <4 | 156 | 87.0 | ||
| ≥4 | 146 | 77.4 | ||
| Tumour grade | 33.018 | P < 0.001 | ||
| Well differentiated | 274 | 84.9 | ||
| Moderately differentiated | 22 | 45.5 | ||
| Poorly differentiated | 6 | 100.0 | ||
| Depth of tumour invasion | 67.827 | P < 0.001 | ||
| T1 | 142 | 93.0 | ||
| T2 | 140 | 81.4 | ||
| T3 | 20 | 40.0 | ||
| Pathological cell type | 0.428 | NS | ||
| Squamous cell carcinoma | 34 | 88.2 | ||
| Adenocarcinoma | 64 | 80.4 | ||
| Adenosquamous | 204 | 82.1 | ||
| Uterine body metastasis | 19.554 | P < 0.001 | ||
| Yes | 18 | 50.0 | ||
| No | 284 | 84.8 | ||
| Lymphovascular invasion | 66.991 | P < 0.001 | ||
| Positive | 60 | 52.6 | ||
| Negative | 242 | 89.8 | ||
| Parametrial involvement | 36.712 | P < 0.001 | ||
| Yes | 22 | 13.6 | ||
| No | 280 | 87.6 | ||
| Postoperative treatment | 7.237 | P = 0.018 | ||
| None | 153 | 87.6 | ||
| Radiation therapy | 28 | 77.3 | ||
| Chemoradiation | 121 | 71.5 | ||
| SLNM | 41.487 | P < 0.001 | ||
| Yes | 48 | 87.7 | ||
| No | 254 | 54.2 | ||
| Ovarian shift | 5.187 | P = 0.023 | ||
| Yes | 62 | 93.5 | ||
| No | 240 | 75.4 |
FIGO, International Federation of Gynecology and Obstetrics; T1, invasive <1/2 muscle layer; T2, invasive ≥1/2 muscle layer; T3, invasive cervical internal surface; NS, no significant association (P ≥0.05).
The prognostic factors identified using univariate analyses were subjected to stepwise regression, which revealed significant differences between the variables as follows: tumour grade (P = 0.003), depth of tumour invasion (p = 0.035), parametrial involvement (P < 0.001) and SLNM (P = 0.002). The risk ratios and their 95% confident intervals are presented in Table 4.
Table 4.
Multivariate analysis of prognostic factors for survival in patients with and without solitary lymph node metastasis (SLNM).
| Parameter | β | SE | Wald | Statistical significance | Risk ratio | 95% CI |
|---|---|---|---|---|---|---|
| FIGO stage | 0.416 | NS | ||||
| IB versus IA2 | –0.022 | 0.832 | 0.001 | NS | 0.978 | 0.191, 5.002 |
| IIA versus IA2 | –0.215 | 0.347 | 0.385 | NS | 0.807 | 0.409, 1.591 |
| Tumour size | –0.360 | 0.337 | 1.135 | NS | 0.698 | 0.360, 1.352 |
| Tumour grade | 0.746 | 0.252 | 8.720 | P = 0.003 | 2.108 | 1.285, 3.457 |
| Depth of tumour invasion | 6.719 | P = 0.035 | ||||
| T2 versus T1 | –1.272 | 0.537 | 5.604 | P = 0.018 | 0.280 | 0.098, 0.803 |
| T3 versus T1 | –1.025 | 0.421 | 5.914 | P = 0.015 | 0.359 | 0.157, 0.820 |
| Uterine body metastasis | 0.555 | 0.515 | 1.157 | NS | 1.741 | 0.634, 4.782 |
| Lymphovascular invasion | –0.725 | 0.390 | 3.463 | NS | 0.484 | 0.226, 1.039 |
| Parametrial involvement | –0.725 | 0.437 | 17.310 | P < 0.001 | 0.162 | 0.069, 0.382 |
| Postoperative treatment | 0.703 | 0.564 | 1.665 | NS | 1.734 | 0.769, 5.463 |
| SLNM | 0.955 | 0.302 | 9.973 | P = 0.002 | 2.598 | 1.437, 4.700 |
| Ovarian shift | 0.514 | 0.474 | 1.177 | NS | 1.672 | 0.661, 4.229 |
CI, confidence interval; FIGO, International Federation of Gynecology and Obstetrics; T1, invasive <1/2 muscle layer; T2, invasive ≥ 1/2 muscle layer; T3, invasive cervical internal surface; NS, no significant association (P ≥ 0.05).
Discussion
There are ongoing controversies regarding the surgical treatment of stage IA2 cervical cancer.11,12 FIGO stage IA2 and IIA cervical cancers are suggested to be treated with radical hysterectomy and bilateral lymph node dissection according to the NCCN guidelines.10 Lymph node metastasis is an important biological characteristic of cervical cancer that influences treatment and prognosis.13 The incidence of lymph node metastasis in cervical carcinoma was reported to range from 21.4% to 46%.14–16 There was a higher incidence of lymph node metastasis in this present study (297 of 563 patients, 52.8%), which might be partly explained by the fact that many Chinese patients with cancer, especially those living in rural areas, do not see a physician until they experience severe symptoms. As a result, they present with more advanced disease and lymph node metastasis. In addition, in various tumours, there is a phenomenon known as ‘skipping lymph node metastasis’, in which no lymph node metastases are found in the area of the primary tumour, but they occur in more distant lymph nodes.17–19 In the present study, two patients in the SLNM group had para-aortic lymph node metastasis, which could be skipping lymph node metastasis. The present study included these two patients in order to more comprehensively evaluate the status of lymph node metastasis. The 5-year survival rate of patients with node-negative cervical carcinoma ranges from 80% to 98%, in contrast to 50% of patients with node-positive cancer.3,9 FIGO reported that the 5-year survival rate of the patients with node-positive cervical carcinoma diagnosed with stages IA–V (n = 953) was 64.1% compared with 94.1% for node-negative patients (n = 3364).20 A previous study suggested that the 5-year survival rate differs significantly between patients with SLNM and node-negative patients (69.3% versus 95.4%, respectively).21
The present study found that the long-term oncological survival was worse for the SLNM group compared with the nLNM group. Once patients with cervical carcinoma were diagnosed with lymph node metastasis, their postoperative 5-year survival rate was reduced. Although lymph node metastasis is a recognized independent prognostic factor for cervical cancer after radical surgery, most investigations conducted in China or other countries present data that only focuses on the effects of the presence or absence of lymph node metastasis on the prognosis.13–15 Few studies address the question of whether SLNM is an independent prognostic factor for cervical cancer.8 It is a limitation of the present study that it did not compare the SLNM group with patients with more lymph node metastases. However, the present study focused on SLNM, which means the status of the lymph node changes from having no metastasis present to the presence of a solitary metastasis. This present study compared the SLNM group with the nLNM group in order to identify risk factors associated with SLNM; and SLNM was identified by multivariate analysis to be an independent factor affecting prognosis. SLNM was associated with advanced tumour stage and malignant tumour phenotype, which usually leads to more postoperative recurrence and metastasis and a decreased survival rate, even though patients have undergone radical resection. Pelvic lymphadenectomy may not be omitted for patients with stage IA2 to IIA cervical cancer, particularly those with a more advanced stage and tumour invasion deeper than one-half of the muscle layer. In order to reduce the incidence of SLNM and improve the efficacy of treatment, early detection and diagnosis should be performed.
The present study demonstrated that the 5-year survival rate of patients with SLNM decreased significantly compared with the nLNM group, which suggests that SLNM represents a pivotal step in the progression of lymph node metastasis from nLNM. Therefore, exploring clinicopathological factors that serve to predict lymph node metastasis in patients with cervical cancer has significance for their timely diagnosis and treatment. For example, the depth of tumour invasion, lymphovascular invasion and FIGO stage were the main risk factors for SLNM, which was consistent with previous findings.22 A previous study suggested that the risk of lymph node metastasis was associated with the depth of tumour invasion, being 3.8-times higher in patients with deeper myometrial invasion.21 Another study found that vascular invasion was an independent risk factor for lymph node metastasis; the mean number of lymph node metastases was 2.47 in the vascular invasion group and 0.33 in patients without vascular invasion (P = 0.001).23 Moreover, the rate of lymph node metastasis was higher for advanced stage disease.23 A higher rate of lymph node metastasis was reported with increasing clinical stage; stage IB for 12–22%, stage IIA for 10–27%, and stage IIB for 34–43%.24 Therefore, the present study demonstrated that invasion of one-half of the myometrial layer, vascular invasion and advanced clinical stage increased the risk of lymph node metastasis.
This present study had several limitations. First, its retrospective and non-randomized nature made it subject to selection bias. Secondly, the sample size of the SLNM group was small, therefore, the results need to be validated in a larger study. Thirdly, the present study did not compare the SLNM group with patients with more lymph node metastases.
In conclusion, this present study demonstrated that the occurrence of just one solitary lymph node metastasis significantly worsened the prognosis in patients with cervical carcinoma compared with patients without lymph node metastases. Therefore, identifying risk factors that predict lymph node metastasis is important. Patients with an advanced FIGO stage, invasion of one-half of the myometrial layer and lymphovascular invasion appear to be at greater risk of pelvic lymph node metastasis.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65: 87–108. [DOI] [PubMed] [Google Scholar]
- 2.Je HU, Han S, Kim YS, et al. A nomogram predicting the risks of distant metastasis following postoperative radiotherapy for uterine cervical carcinoma: a Korean radiation oncology group study (KROG 12-08). Radiother Oncol 2014; 111: 437–441. [DOI] [PubMed] [Google Scholar]
- 3.Tada H, Teramukai S, Fukushima M, et al. Risk factors for lower limb lymphedema after lymph node dissection in patients with ovarian and uterine carcinoma. BMC Cancer 2009; 9: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richard SD, Krivak TC, Castleberry A, et al. Survival for stage IB cervical cancer with positive lymph node involvement: a comparison of completed vs. abandoned radical hysterectomy. Gynecol Oncol 2008; 109: 43–48. [DOI] [PubMed] [Google Scholar]
- 5.Khatun S, Huda AQ, Begum SK, et al. Evaluation of Pelvic Lymphadenectomy during Radical Hysterectomy for Cervical Cancer. Mymensingh Med J 2017; 26: 287–292. [PubMed] [Google Scholar]
- 6.Obrzut B, Semczuk A, Naróg M, et al. Prognostic Parameters for Patients with Cervical Cancer FIGO Stages IA2-IIB: A Long-Term Follow-Up. Oncology 2017; 93: 106–114. [DOI] [PubMed] [Google Scholar]
- 7.Barquet-Muñoz SA, Rendón-Pereira GJ, Acuña-González D, et al. Role of pelvic and para-aortic lymphadenectomy in abandoned radical hysterectomy in cervical cancer. World J Surg Oncol 2017; 15: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martínez A, Mery E, Filleron T, et al. Accuracy of intraoperative pathological examination of SLN in cervical cancer. Gynecol Oncol 2013; 130: 525–529. [DOI] [PubMed] [Google Scholar]
- 9.Togami S, Kamio M, Yanazume S, et al. Can pelvic lymphadenectomy be omitted in stage IA2 to IIB uterine cervical cancer? Int J Gynecol Cancer 2014; 24: 1072–1076. [DOI] [PubMed] [Google Scholar]
- 10.Koh WJ, Greer BE, Abu-Rustum NR, et al. Cervical Cancer, Version 2.2015. J Natl Compr Canc Netw 2015; 13: 395–404. [DOI] [PubMed] [Google Scholar]
- 11.Billingsley CC, Kohler MF, Creasman WT, et al. A case of parametrial lymph node involvement in stage IA2 squamous cell carcinoma of the cervix treated with radical hysterectomy and a review of the literature: a case report. J Low Genit Tract Dis 2012; 16: 145–148. [DOI] [PubMed] [Google Scholar]
- 12.Buchanan T, Pierce JY, Graybill W, et al. Why do we continue to overtreat stage Ia carcinoma of the cervix? Am J Obstet Gynecol 2017; 217: 413–417. [DOI] [PubMed] [Google Scholar]
- 13.Polterauer S, Grimm C, Hofstetter G, et al. Nomogram prediction for overall survival of patients diagnosed with cervical cancer. Br J Cancer 2012; 107: 918–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lai JC, Chou YJ, Huang N, et al. Survival analysis of Stage IIA1 and IIA2 cervical cancer patients. Taiwan J Obstet Gynecol 2013; 52: 33–38. [DOI] [PubMed] [Google Scholar]
- 15.Garg G, Shah JP, Toy EP, et al. Stage IIA1 versus stage IIA2 cervical cancer: does the new staging criteria predict survival? Int J Gynecol Cancer 2011; 21: 711–716. [DOI] [PubMed] [Google Scholar]
- 16.Hongladaromp W, Tantipalakorn C, Charoenkwan K, et al. Locoregional spread and survival of stage IIA1 versus stage IIA2 cervical cancer. Asian Pac J Cancer Prev 2014; 15: 887–890. [DOI] [PubMed] [Google Scholar]
- 17.Riquet M, Assouad J, Bagan P, et al. Skip mediastinal lymph node metastasis and lung cancer: a particular N2 subgroup with a better prognosis. Ann Thorac Surg 2005; 79: 225–233. [DOI] [PubMed] [Google Scholar]
- 18.Prenzel KL, Bollschweiler E, Schröder W, et al. Prognostic relevance of skip metastases in esophageal cancer. Ann Thorac Surg 2010; 90: 1662–1667. [DOI] [PubMed] [Google Scholar]
- 19.Choi YY, An JY, Guner A, et al. Skip lymph node metastasis in gastric cancer: is it skipping or skipped? Gastric Cancer 2016; 19: 206–215. [DOI] [PubMed] [Google Scholar]
- 20.Quinn MA, Benedet JL, Odicino F, et al. Carcinoma of the cervix uteri. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer . Int J Gynaecol Obstet 2006; 95(Suppl 1): S43–S103. [DOI] [PubMed] [Google Scholar]
- 21.Feng SY, Zhang YN, Liu JG. Risk factors and prognosis of node-positive cervical carcinoma. Ai Zheng 2005, 24: 1261–1266 [Article in Chinese, English abstract]. [PubMed] [Google Scholar]
- 22.Sakuragi N, Satoh C, Takeda N, et al. Incidence and distribution pattern of pelvic and paraaortic lymph node metastasis in patients with Stages IB, IIA, and IIB cervical carcinoma treated with radical hysterectomy. Cancer 1999; 85: 1547–1554. [DOI] [PubMed] [Google Scholar]
- 23.Silva-Filho AL, Reis FM, Traiman P, et al. Clinicopathological features influencing pelvic lymph node metastasis and vaginal and parametrial involvement in patients with carcinoma of the cervix. Gynecol Obstet Invest 2005; 59: 92–96. [DOI] [PubMed] [Google Scholar]
- 24.Sakuragi N. Up-to-date management of lymph node metastasis and the role of tailored lymphadenectomy in cervical cancer. Int J Clin Oncol 2007; 12: 165–175. [DOI] [PubMed] [Google Scholar]


