Short abstract
Objective
This study was performed to determine whether the results of prevailing in vivo and in vitro studies offer a reliable model for investigation of medication-related osteonecrosis of the jaw (MRONJ).
Methods
Embase, Medline, and the Cochrane Library were searched for articles published from September 2003 to June 2017 involving experimental approaches to the pathogenesis of MRONJ. In vivo and in vitro trials were analyzed with respect to the scientific question, study design, methodology, and results.
Results
Of 139 studies, 87, 46, and 6 conducted in vivo, in vitro, and both in vivo and in vitro experiments, respectively. Rats, mice, dogs, minipigs, sheep, and rabbits were the preferred animal models used. Osteoblasts, osteoclasts, fibroblasts, keratinocytes, macrophages, and human umbilical vein endothelial cells were the preferred cell types. Zoledronate, alendronate, ibandronate, and risedronate were the most frequent bisphosphonates used. MRONJ was most reliably induced in minipigs because of the close relationship with human bone physiology. In vitro studies showed that reduced viability, growth, and migration of cells in the bone and soft tissues were causative for MRONJ. Other than exposed jawbone after tooth extraction, no reliable cofactors were found.
Conclusion
The minipig is the most suitable animal model for MRONJ.
Keywords: Osteonecrosis, medication-related, jaw, in vivo, in vitro, minipig
Introduction
Bisphosphonates (BPs) are pharmaceuticals commonly used for the treatment of resorptive bone diseases such as multiple myeloma,1 bone metastases,2 primary and secondary osteoporosis,3 and Paget’s disease of bone.4 BPs were introduced into medicine around 1960 as derivatives of endogenous inorganic pyrophosphate (PPi), a substance with a chemical P-O-P bond that is well known for its biological activity in interfering with the dissolution and precipitation of calcium phosphate, the main component of mineralized bone.5
Initial attempts to medicinally influence in vivo bone remodeling by administering PPi showed insufficient clinical effects. Despite its high affinity for skeletal calcium phosphate containing hydroxyapatite crystals, PPi was found to be extremely susceptible to rapid hydrolysis of the P-O-P bond perpetrated by pyrophosphatases.6 BPs resemble PPi in structure, but the P-O-P bond is substituted with a non-hydrolyzable P-C-P bond enabling BPs to bind to the bone surface before enzymatic breakdown.7 Once adherent to bone, BPs are incorporated by adjacent cells, predominantly osteoclasts, and induce antiresorptive effects by suppressing osteolytic cell activities.8 Besides BPs, several other medications also interfere with regular bone physiology, such as denosumab, an immunoglobulin G2 antibody to receptor activator of nuclear factor kappa B ligand (RANKL).9
Generally, two types of BPs can be distinguished: the less potent non-nitrogen-containing BPs (non-N-BPs) and the highly potent nitrogen-containing BPs (N-BPs). After uptake into the cell, the latter interfere with the mevalonate pathway by inhibiting its key enzyme farnesyl pyrophosphate synthase, thus blocking the biosynthesis of isoprenoids essential for cell signaling.10 In this group of BPs, the highly potent BP subtypes pamidronate, ibandronate, alendronate, risedronate, and zoledronate inhibit the enzymatic switch of dimethylallyl pyrophosphate to geranyl pyrophosphate.11 Their linear chemical formula exhibits steric likeness to the dimethylallyl pyrophosphate carbocation within the enzyme; hence, they are called linear BPs.11 In contrast, non-N-BPs are metabolized into nondegradable adenosine triphosphate compounds that disrupt regular energy gain from adenosine triphosphate hydrolysis.12 Recent studies suggest that besides their effect on osteoclasts, BPs have a decisive influence on other cells involved in bone metabolism, such as osteoblasts,13 fibroblasts,14 macrophages,15 and keratinocytes.16
Despite frequent medical prescription and good general tolerability of BPs, a rare yet severe adverse effect called medication-related osteonecrosis of the jaw (MRONJ) was first described in 2003.17 This adverse drug reaction presents as exposed necrotic maxillary or mandibular jaw bone often associated with mucosal swelling, erythema, ulceration, and pain.18 The American Association of Oral and Maxillofacial Surgeons describes MRONJ as the persistence of exposed alveolar bone for >8 weeks in patients who currently receive or have a history of receiving BPs in the absence of radiation to the head and neck area.19 The potency and route of application (oral versus intravenous) seem to be strong predisposing factors because patients with cancer undergoing intravenous N-BP treatment are more likely to show signs of MRONJ than patients receiving non-N-BPs orally.20 Furthermore, dental surgical procedures in patients on BP medication pose an additional precipitating factor for MRONJ development with an incidence ranging from 1% to 12% in intravenously treated patients and <1% in orally treated patients.21
Since first described in 2003, the etiology of MRONJ has not been clarified. Many case reports and various literature reviews have outlined the clinical appearance and causative agents of MRONJ, but they have failed to decipher the precise molecular pathway of its pathogenesis. However, several in vivo and in vitro studies have been performed in an attempt to determine the cellular mechanisms of MRONJ development. On the basis of these studies, two scenarios seem likely. In the first (and currently favored) scenario, primary bone infection is the origin of pathogenicity with secondary spread to the overlying soft tissues (inside-out theory). In the second (and less likely) scenario, MRONJ originates from soft tissue necrosis with secondary bone involvement (outside-in theory).
It has become obvious that scientific research in the field of MRONJ does not follow a common line. Therefore, the goal of this study was to compare all in vivo and in vitro studies associated with BP-induced MRONJ research regarding outcomes and methodology to gain a common consensus on current knowledge. In addition, we have paid special attention to what reliable animal models have been developed to date.
Material and methods
We scanned Medline, Embase, and the Cochrane Library for literature concerning MRONJ. We searched a timespan from September 2003 (first description of MRONJ) until June 2017. To retrieve literature solely associated with BP-induced MRONJ, we applied a search filter including the terms “jaw,” “osteonecrosis,” “bisphosphonate,” and “bisphosphonates” connected by the Boolean operator “AND.” The inclusion criteria were 1) literature with special emphasis on MRONJ, 2) availability of an abstract, 3) articles available in the English or German language, and 4) a listing in the three aforementioned databases. We retrieved a total of 1,781 articles. Of these, 404 articles had no available abstract and were discarded. Obtainable abstracts were examined, culminating in further exclusion of 89 articles not available in English or German and another 252 that bypassed the topic of MRONJ. Abstracts unavailable online were attained from Düsseldorf University and State Library. The remaining 1,036 articles were thoroughly reviewed and ultimately included in this study. A reference list is available upon request from the corresponding author. Among articles incorporated in our study, we focused on literature elucidating the pathogenesis of MRONJ using an experimental approach. Attention was paid exclusively to in vivo and in vitro trials. Accordingly, we identified 145 articles with an in vivo or in vitro study design. Data collection from these articles concentrated on the year of publication, authors, scientific question, study design, and final study results.
Next, we divided the in vivo and in vitro trials into two groups. For each group, we tested the intragroup conformity of the presented study results. In addition, we examined the intergroup agreement in understanding the pathogenesis of MRONJ.
This study was fully retrospective with no need for ethics approval. No experiments were conducted and no patient data collected that may have required approval.
Results
Trials with an experimental approach
Of all 1,036 articles included in this study, 145 (14.0%) used an experimental approach to investigate BP-induced MRONJ. Among these, 81 (55.9%) conducted in vivo trials, 57 (39.3%) conducted in vitro trials, and 7 (4.8%) conducted both in vivo and in vitro trials.
In vivo trials
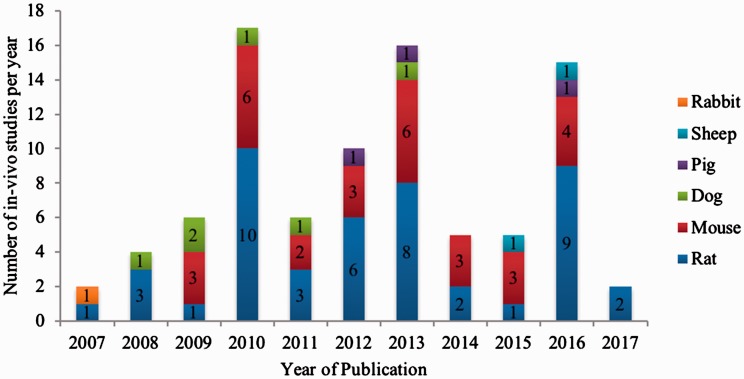
Within the timespan analyzed (2003 to June 2017), the experimental in vivo publications showed a steady increase from 2007 to 2010 and from 2011 to 2013. From 2011 to 2014, the number of in vivo trials reached an all-time low of merely six publications (Figure 1). In 2016, the number of animal studies conducted rose to 15. All studies in the in vivo subset used a mammalian test animal model. Itemized by animal type, 46 (52.3%) studies used rats, 30 (34.1%) used mice, 6 (6.8%) used dogs, 3 (3.4%) used pigs, 1 (1.1%) used rabbits, and 2 (2.3%) used sheep as test animal species. Table 1 summarizes all in vivo studies assorted by test animal species and year of publication.
Figure 1.
Number of published in vivo studies on medication-related osteonecrosis of the jaw published from 2003 to 2017 assorted by the test animal species used.
Table 1.
Summary of in vivo studies using different test animal models assorted by year of publication.
| Rat | Mouse | Rabbit | Dog | Pig | Sheep |
|---|---|---|---|---|---|
| 2007, Yang et al. | 2009, Huja et al. | 2007, Choi et al. | 2008, Allen and Burr | 2012, Pautke et al. | 2016, Voss et al. |
| 2009, Sonis et al. | 2009, Oizumi et al. | 2009, Burr and Allen | 2013, Li et al. | 2016, Voss et al. | |
| 2008, Cetinkaya et al. | 2009, Pozzi et al. | 2009, Allen | 2016, Mitsimponas et al. | ||
| 2008, Bauss et al. | 2010, Shikama et al. | 2011, Allam et al. | |||
| 2009, Hikita et al. | 2010, Kikuiri et al. | 2011, Huja et al. | |||
| 2010, Senel et al | 2010, Bi et al. | 2013, Allen et al. | |||
| 2011, Maahs et al. | 2010, Kobayashi et al. | ||||
| 2010, Lopez-Jornet et al. | 2010, Kubek et al. | ||||
| 2010, Hokugo et al. | 2010, Oizumi et al. | ||||
| 2011, Ali-Erdem et al. | 2012, Shimizu et al. | ||||
| 2010, Aguirre et al. | 2011, Mawardi et al. | ||||
| 2010, Biasotto et al. | 2012, Yu et al. | ||||
| 2011, Wen et al. | 2012, Zhao et al. | ||||
| 2010, Yamashita et al. | 2012, Kuiper et al. | ||||
| 2010, Kozloff et al. | 2013, Kang et al. | ||||
| 2011, Basi et al. | 2013, Bonnet et al. | ||||
| 2011, Aghaloo et al. | 2013, Kuroshima and Yamashita | ||||
| 2013, Abtahi et al. | 2013, Zhang et al. | ||||
| 2012, Vasconcelos et al. | 2014, Pabst et al. | ||||
| 2012, Marino et al. | 2014, Aghaloo et al. | ||||
| 2011, Conte-Neto et al. | 2014, Williams et al. | ||||
| 2012, Aguirre et al. | 2014, de Molon et al. | ||||
| 2012, Abtahi et al. | 2015, Su et al. | ||||
| 2013, Okamoto et al. | 2015, de Molon et al. | ||||
| 2013, Berti-Couto et al. | 2015, Park et al. | ||||
| 2013, Abtahi et al. | 2015, Tseng et al. | ||||
| 2013, Cankaya et al. | 2016, Song et al. | ||||
| 2014, Barba-Recreo et al. | 2017, Vermeer et al. | ||||
| 2013, Dayisoylu et al. | 2017, Kim et al. | ||||
| 2013, Tsurushima et al. | 2016, Cordova et al. | ||||
| 2014, Ersan et al. | |||||
| 2013, Hokugo et al. | |||||
| 2014, Dayisoylu et al. | |||||
| 2015, Janovszky et al. | |||||
| 2015, Ogata et al. | |||||
| 2016, Zandi et al. | |||||
| 2016, Silveira et al. | |||||
| 2017, Gong et al. | |||||
| 2016, Neto et al. | |||||
| 2016, Curra et al. | |||||
| 2016, De Ponte et al. | |||||
| 2016, Yanik et al. | |||||
| 2017, Zandi et al. | |||||
| 2015, Takaoka et al. | |||||
| 2017, Vilarinho et al. | |||||
| 2017, Vidal-Gutierrez et al. |
Rats
Used in 46 studies, the rat was the favored test animal among in vivo studies. In 2007, 4 years after the initial description of MRONJ, Yang et al.22 were the first to investigate the effects of BPs on mechanical bone properties in a femur rat model. The authors showed that high concentrations (0.5 mg/kg) of pamidronate, an N-BP, decreased mineral bone density and weakened mechanical bone strength. Subsequently, additional scientific questions from 2008 to 2017 addressed the effects of BP administration on MRONJ development with (30 studies) and without (15 studies) tooth extraction. Four trials studied periodontitis as a risk factor for jaw osteonecrosis under BP treatment.23–26 Eventually, oral wound healing (1 study) and bone site-specific BP uptake (4 studies) moved to the focus of scientific interest.27–31 The tissues investigated were bone, oral mucosa, periodontium, and blood vessel supply.23,24,32–43 The studies differed in the type of BP used as well as the route of application.25,44–49 Only 2 studies analyzed non-N-BPs (clodronate at 20 mg/kg and etidronate at 5 mg/kg); the remaining 44 trials used N-BPs with zoledronate (0.01–0.6 mg/kg) being the most commonly used (30 studies), followed by alendronate (0.01–1 mg/kg) (14 studies), pamidronate (0.01–3 mg/kg) (5 studies), and ibandronate (0.003–0.3 mg/kg) and risedronate (0.1–1 mg/kg) (1 study each).26,48,50–53 Twenty-two studies used an intraperitoneal route of application, 15 used subcutaneous, 10 used intravenous, 1 used intramuscular, and 1 used topical.54–63 The absolute number of studies does not equal 46 because some tested more than one BP or tried different application routes. Table 1 summarizes all conducted studies using rats as test animals.
Mice
To date, 30 studies have created a mouse MRONJ model. The first in vivo study using mice as test animals was conducted in 2009 by Huja et al.64 They injected zoledronate intraperitoneally at a concentration of 0.1 mg/kg and found that new bone formation was significantly decreased while osteoclast apoptosis could not be observed. Since then, many study groups have analyzed a broad variety of different scientific questions such as the effects of BPs on bone formation and remodeling (5 studies) and oral wound healing and angiogenesis (3 studies).65–76 Two studies also showed that non-N-BPs have protective effects on MRONJ development in animals undergoing N-BP treatment.65,77 One study concentrated on the BP effects at different bone sites.78 In 2013, RANKL inhibitors such as denosumab attained increasing interest, and their effects were compared with those of BPs on bone metabolism (3 studies).70,71,76 Similar to the rat trials, the influence of tooth extraction (13 studies) and periodontal disease (3 studies) on MRONJ development was also analyzed in studies using mice as test animals.77,79–84 Immunotherapeutics and chemotherapeutics such as dexamethasone and melphalan were applied in combination with BP treatment in three studies.68,69,80 The roles of dental infections, systemic and jaw osteopenia, osteoblast function, fracture repair, and technetium-99 conjugated with methylene diphosphonate were each assessed in two studies.81,85 Twenty-four studies used zoledronate (0.001–1 mg/kg), three studies used alendronate (0.1–1 mg/kg), two studies used etidronate (250 μg/kg), two studies used clodronate (21 μg/kg), one study used ibandronate (0.085 μg/kg), two studies used pamidronate (1.3 μg/kg), and one study used risedronate (0.1 mg/kg).85,86 The route of application was intraperitoneal in 14 studies, subcutaneous in 6 studies, and intravenous in 6 studies.87–89 Table 1 summarizes all studies conducted using mice as test animals.
Rabbits
In 2007, Choi et al.90 established a rabbit test animal model of MRONJ development. Locally applied pamidronate at concentrations of 2 and 3 mg inhibited bone healing in rabbit calvarial bony defects. Table 1 summarizes all conducted studies using rabbits as test animals.
Dogs
Six in vivo studies analyzed MRONJ in dogs.91–96 The preferred BP was zoledronate (0.006–0.1 mg/kg) in five studies and alendronate (0.2–1 mg/kg) in two studies (one study tested more than one BP). Zoledronate was always administered intravenously and alendronate orally. In 2008, Allen and Burr91 conducted a 3-year study of oral alendronate application in Beagle dogs at doses of 0.2 and 1 mg/kg. The authors observed a decreased intracortical bone turnover rate in the test group compared with the control group. Other trials tested the effects of BPs on osteocytic death (1 study),94 bone remodeling after tooth extraction (2 studies),91,92 and oral mucosal epithelial cells (1 study).95 One trial hypothesized that severe MRONJ develops when zoledronate is combined with the immunosuppressive agent dexamethasone.93 However, this hypothesis could not be proven. Table 1 summarizes all studies conducted using dogs as test animals.
Pigs
Three studies published in 2012, 2013, and 2016, respectively applied zoledronate (0.05 mg/kg and 4 mg) intravenously in a pig model of MRONJ.97–99 After tooth extraction, animals in the zoledronate group displayed improper bone remodeling compared with the controls. One study showed that bone marrow mesenchymal stem cell transplantation after BP treatment restored the number of Foxp3-positive regulatory T cells and the interleukin-17 serum level.98 Table 1 summarizes all studies conducted using pigs as test animals.
Sheep
In 2016, Voss et al.100,101 established a MRONJ sheep model. After intravenous injection of zoledronate (75 μg/kg), the sheep developed jaw osteonecrosis after tooth extraction. Table 1 summarizes all studies conducted using sheep as test animals.
In vitro trials
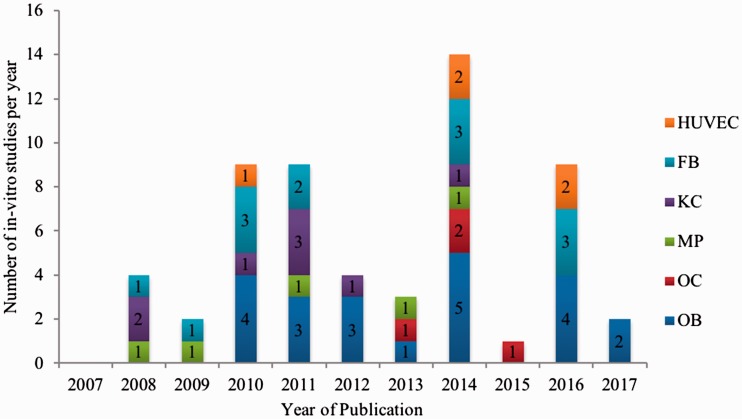
Within the in vitro subset, two groups were distinguished. Group I comprised 47 (82.5%) studies that investigated only one cell type, and Group II comprised 10 (17.5%) studies that investigated several cell types. The analyzed cell types in Group I were osteoblasts (15 studies), macrophages (4 studies), osteoclasts (4 studies), keratinocytes (8 studies), fibroblasts (11 studies), and human umbilical vein endothelial cells (HUVECs) (5 studies). In Group II, four studies (40.0%) investigated keratinocytes and fibroblasts; two studies (20.0%) investigated osteoblasts, fibroblasts, and HUVECs; one study (10.0%) investigated osteoblasts and fibroblasts; one study (10.0%) investigated osteoblasts and keratinocytes; one study (10.0%) investigated osteoblasts, keratinocytes, fibroblasts, and HUVECs; and one study (10.0%) investigated osteoblasts and osteoclasts. Figure 2 shows the in vitro studies that were conducted with respect to cell type and year of publication. The figure shows more than 57 in vitro studies because some studies tested more than one cell type.
Figure 2.
Number of in vitro studies on medication-related osteonecrosis of the jaw published from 2003 to 2017 assorted by the cell type analyzed. HUVEC = human umbilical vein endothelial cells; FB = fibroblasts; KC = keratinocytes; MP = macrophages; OC = osteoclasts; OB = osteoblasts.
Osteoblasts
Represented by 22 in vitro studies, osteoblasts were the most frequently analyzed cell type.13,102–108 Nineteen studies solely used osteoblasts, and three studies simultaneously experimented with other cell types.109–112 Osteoblast viability, differentiation, proliferation, growth, and migration under BP treatment were the main scientific focus in 19 studies. One study analyzed the gene expression of RANKL and osteoprotegerin,109 and four trials concentrated on the positive effects of low-level laser therapy and herbal extracts to counteract MRONJ development.107,113–115 Thirteen studies analyzed only one BP; of these, 10 studies used zoledronate (0.01–100 μM), 1 used pamidronate (10−5, 3 × 10−5, 6 × 10−5, and 10−4 M/L), 1 used clodronate (10−5 to 10−9 mM/L), and 1 used alendronate (0.01–100 μM).113,114,116–118 The remaining 11 studies applied a combination of zoledronate, ibandronate, pamidronate, clodronate, and alendronate at concentrations varying from 0.00001 to 500 μM.115,119–121 Table 2 summarizes all studies that evaluated osteoblasts in MRONJ.
Table 2.
Summary of in vitro studies using different cell culture models assorted by year of publication.
| Osteoblasts | HUVECs | Osteoclasts | Macrophages | Keratinocytes | Fibroblasts |
|---|---|---|---|---|---|
| 2010, Koch et al. | 2011, Walter et al. | 2013, Vermeer et al. | 2009, Deng et al. | 2008, Landesberg et al. | 2009, Scheper et al. |
| 2011, Koch et al. | 2015, Walter et al. | 2015, Nakagawa et al. | 2009, Masuda et al. | 2009, Scheper et al. | 2009, Agis et al. |
| 2011, Koch et al. | 2015, Hagelauer et al. | 2014, Mukudai et al. | 2011, Scheller et al | 2011, Ravosa et al. | 2011, Tipton et al. |
| 2011, Walter et al. | 2016, Lang et al. | 2015, Nagaoka et al. | 2013, Muratsu et al. | 2011, Kim et al. | 2011, Ravosa et al. |
| 2011, Cornish et al. | 2016, Lu et al. | 2015, Hoefert et al. | 2011, Scheller et al.2012, Pabst et al. | 2011, Walter et al.2012, Acil et al. | |
| 2012, Acil et al. | 2012, Saracino et al. | 2011, Kim et al. | |||
| 2012, Saracino et al. | 2015, Walter et al. | 2015, Walter et al. | |||
| 2012, Marolt et al. | 2015, Hagelauer et al. | ||||
| 2012, Patntirapong et al. | 2014, Zafar et al. | ||||
| 2013, Basso et al. | 2016, Basso et al. | ||||
| 2014, Basso et al. | 2016, Komatsu et al. | ||||
| 2015, Hagelauer et al. | 2016, Ohlrich et al. | ||||
| 2015, Walter et al. | |||||
| 2015, Manzano-Moreno et al. | |||||
| 2014, Mukudai et al. | |||||
| 2016, Zafar et al. | |||||
| 2016, Hu et al. | |||||
| 2016, Manzano-Moreno et al. | |||||
| 2016, Huang et al. | |||||
| 2017, Camacho-Alonso et al. | |||||
| 2017, Coates et al. |
HUVECs, human umbilical vein endothelial cells
HUVEC
HUVECs were analyzed in five studies.108,112,113,122,123 In all studies, the effects of zoledronate, ibandronate, pamidronate, and clodronate were tested at concentrations of 5 to 500 μM. After BP application, HUVEC viability and migration decreased and apoptosis was induced. Low-level laser therapy and the isoprenoid geranylgeraniol had positive effects on HUVEC survival. Table 2 summarizes all studies that evaluated HUVECs in MRONJ.
Osteoclasts
Four studies tested the effects of zoledronate (1–10 μM), alendronate (0.01–100 μM), pamidronate, and risedronate (1–100 μM) on osteoclasts.114,124–126 Differentiation of osteoclasts was found to be inhibited by BPs. Herbal extracts from Melia azedarach, Corydalis turtschaninovii, and Cerithium atratum induced apoptosis in osteoclasts. Isoprenoids restored BP-associated inhibition of osteoclast differentiation. Osteoclasts in the jaw showed increased uptake of BPs in the jaw region compared with long bones. Table 2 summarizes all studies that evaluated osteoclasts in MRONJ.
Macrophages
The effects of BPs on macrophages were assessed in five studies.15,127–130 The BPs of choice were zoledronate (0.5–50 μM), alendronate (5–100 μM), and clodronate (10−4 M). Three studies described increased proinflammatory cytokine production by macrophages after BP treatment.15,127,128 In 2009, Masuda et al.128 found decreased proinflammatory cytokine production. Further, BPs suppressed macrophage differentiation. Table 2 summarizes all studies that evaluated osteoclasts in MRONJ.
Keratinocytes
In 2008, oral keratinocytes gained the attention of many study groups in the pursuit of understanding the pathogenesis of MRONJ. In seven studies, the main objective was to analyze the effects of BPs on this cell type.14,16,104,131–134 One other trial tested the possible positive effects of low-level laser therapy on keratinocyte viability.113 The BPs used were zoledronate (0.01–500 μM), clodronate, ibandronate (5–500 μM), and pamidronate (0.003–500 μM). Table 2 summarizes all studies that evaluated keratinocytes in MRONJ.
Fibroblasts
As part of the oral connective tissue, oral fibroblasts were analyzed in 13 studies.14,16,103,112,113,135,136 In 2009, Scheper et al.132 studied the adverse effects of BPs on this cell type. Cell viability and migration deteriorated under BP treatment. Concentrations of 0.01 to 500 μM of zoledronate, 0.01 to 500 μM of pamidronate, 0.01 to 20 μM of alendronate, and 5 to 500 μM of clodronate and ibandronate were used.108,137–140 Table 2 summarizes all studies that evaluated fibroblasts in MRONJ.
Combined in vivo/in vitro trials
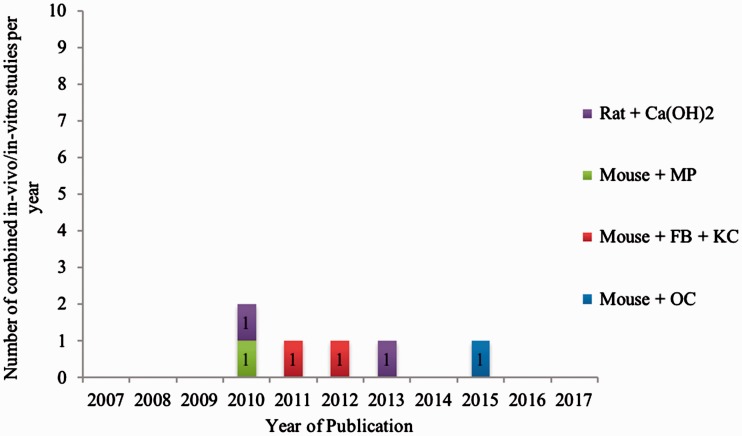
Seven studies performed both in vivo and in vitro MRONJ assessment.141–147 Four chose a mouse model141–144 and three chose a rat model.145–147 Figure 3 provides an overview of all combined in vivo/in vitro studies since the first description of MRONJ in 2003.
Figure 3.
Number of combined in vivo/in vitro studies on medication-related osteonecrosis of the jaw published from 2003 to 2017 assorted by the test animal species used and cell type analyzed. Ca(OH)2 = hydroxyapatite; MP = macrophages; FB = fibroblasts; KC = keratinocytes; OC = osteoclasts.
Mice
In 2010, Shikama et al.141 analyzed the effects of BPs on macrophages. They concluded that alendronate stimulates the production of the proinflammatory cytokine interleukin-1β in macrophages, while clodronate inhibits this effect. In macrophage-depleted mice, this production was almost absent. In 2012, Kuiper et al.142 also concentrated on immune cells, but focused on neutrophils. They showed in vitro and in vivo inhibition of chemotaxis and NADPH oxidase activity by zoledronate and pamidronate. In 2015, Tseng et al.143 found that N-BPs but not non-N-BPs triggered increased release of proinflammatory mediators from osteoclasts. Additionally, in 2011, Mawardi et al.144 showed diminished wound healing after pamidronate treatment in animals infected with Fusobacterium nucleatum, suggesting a bacterial role in the etiopathogenesis of MRONJ.
Rats
Hokugo et al.145 and Kozloff et al.146 concentrated on calcium hydroxide and the adhesion of BPs to the bone surface. Ogata et al.147 showed that serum-free conditioned media from human mesenchymal stem cells had potential therapeutic effects on MRONJ.
Discussion
Since the first description of MRONJ in patients undergoing BP therapy, many studies have been performed in an attempt to develop a suitable mammalian model for its etiopathogenesis. Among 46 studies, most of the in vivo rat studies concentrated on the influence that post-BP tooth extractions have on the occurrence of MRONJ. In this manner, only Vasconcelos et al.29 applied a non-N-BP (clodronate) in their study, accounting for only 2.0% of all studies. They found that clodronate was less likely to cause MRONJ than zoledronate. All other studies applied N-BPs (zoledronate, alendronate, or pamidronate). Trials in which only zoledronate was administered showed signs resembling jaw osteonecrosis.37 The effects of alendronate are more controversial. Conte-Neto et al.148 linked alendronate application to the occurrence of osteonecrosis of the jaw (ONJ)-like lesions, while Maahs et al.45 found no sufficient correlation. Conversely, Aguirre et al.46 only found a transient negative influence on wound healing in alendronate-only treated rats. A second subset of trials analyzed the effects of concomitant application of a BP (zoledronate, alendronate, or pamidronate) and a corticosteroid. Generally, BP + corticosteroid therapy resulted in the development of ONJ-like lesions.24 Furthermore, Sonis et al.32 found that BP + corticosteroid therapy was more relevant for MRONJ development than zoledronate treatment alone. However, Berti-Couto et al.34 stated that this combination therapy was not at all associated with the occurrence of MRONJ. Two studies showed that systemic antibiotic therapy and mucoperiosteal coverage of extraction sockets were reliable parameters for MRONJ prevention.149 Besides treatment with different BPs or immunosuppressants, Aghaloo et al.25 were the first to identify periodontitis as a precipitating factor for the occurrence of MRONJ under high-dose zoledronate treatment. Studies published in 2012 and 2016 support these findings.89
In another group of studies that used a rodent test animal model, the effects of tooth extraction under BP treatment were analyzed in mice. In contrast to the abundance of rat studies, only 13 tooth extraction studies were conducted in mice. More of these studies examined the effects of BP + corticosteroid combination therapy on MRONJ development than zoledronate-only treatment. In general, BP + corticosteroid therapy predisposes to the occurrence of ONJ-like lesions after tooth extraction. However, like Berti-Couto et al.,34 who studied rats, Kuroshima and Yamashita68 did not find these effects. Bi et al.81 induced MRONJ in mice under zoledronate treatment and tooth extraction. Again, a non-N-BP (etidronate) did not cause jaw osteonecrosis.82 Interestingly, the presence of periapical disease facilitated jaw osteonecrosis under treatment with zoledronate as well as treatment with antireceptor activator of nuclear factor kappa beta ligand antibody.87 This has not yet been tested in rats. However, rodent bone physiology and regeneration significantly differ from those of humans; therefore, whether the results from these studies can be transferred to MRONJ in humans remains unclear.
One of the first trials concerning avascular bone necrosis as observed in MRONJ was conducted in 2007 by Choi et al.90 In this study, however, the inhibitory effects of pamidronate on bone healing were only tested in the rabbit calvaria and not the jaw.
While rodent models generally induce jaw osteonecrosis under zoledronate-only treatment, the effects of which could even be intensified by addition of a corticosteroid, dog models do not show these effects. In the studies examined, no significant difference in the occurrence of MRONJ could be distinguished between zoledronate-only therapy and zoledronate + corticosteroid therapy.93 In two studies without tooth extraction, a reduced bone remodeling rate was observed under alendronate and zoledronate treatment in the absence of exposed jawbone.94 Among the vast variety of existing dog models of MRONJ, we still lack a uniformly applicable dog model in which BP administration is reliably followed by exposure of jawbone. This was discussed in 2007 by Allen.150
Li et al.,98 Pautke et al.,97 and Voss et al.100 showed induction of MRONJ after tooth extraction in a pig model (zoledronate) and sheep model (zoledronate + corticosteroid). Pig models are often used for bone studies because their bone regeneration physiology is similar to that of humans.97 Histomorphologic conditions found in human MRONJ samples can be reliably reproduced in the pig models. As already stated by Mitsimponas et al.,99 it is necessary to conduct further studies using pig models to understand the immunohistochemical and biomolecular structures leading to MRONJ. We currently regard studies using a minipig model as ideal for the analysis of the occurrence of MRONJ. This was also recently stated by Otto et al.151–153 The minipig model has shown the most promising effects with respect to the comparability of human bone physiology and morphology.
In vitro trials predominantly focused on the inhibitory effects of BPs, most often zoledronate, on the viability, migration, and growth of osteoblasts, osteoclasts, fibroblasts, keratinocytes, macrophages, and HUVECs. We found no direct correlation to an in vivo trial could. Several studies tested whether zoledronate causes apoptosis of bone and soft tissue cells; however, whether jaw osteonecrosis is preceded by soft tissue necrosis or if soft tissue necrosis follows development of necrotic bone remains unclear. A common consensus, as noted by Walter et al.112 in 2011, is the belief that MRONJ is a multifactorial disease involving many cell lines.
We conclude that in vivo and in vitro analyses of the etiology of MRONJ have not yet been able to present a universally accepted theorem. In line with what has already been observed in patients undergoing BP therapy, tooth extractions seem to profoundly increase the susceptibility to jaw osteonecrosis in rodents, markedly in rats. Whether rodent research can be transferred to humans is questionable; therefore, studies involving models with larger animals were conducted. Of these, minipig test animal models depict the most reliable relationship between BP treatment and jawbone necrosis. With all studies choosing a slightly different study design that varied in both the type and concentration of BPs and culminating in diverse outcomes, we hypothesize that the development of MRONJ might be metabolically dependent. Responsible cofactors, currently unidentifiable, in the pathogenesis of this disease need further exploration using the minipig test animal model in future trials.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Mhaskar R, Redzepovic J, Wheatley K, et al. Bisphosphonates in multiple myeloma. Cochrane Database Syst Rev 2010: CD003188. doi: 10.1002/14651858.CD003188.pub3. [DOI] [PubMed] [Google Scholar]
- 2.Aapro M, Saad F, Costa L. Optimizing clinical benefits of bisphosphonates in cancer patients with bone metastases. Oncologist 2010; 15: 1147–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen MR. Skeletal accumulation of bisphosphonates: implications for osteoporosis treatment. Expert Opin Drug Metab Toxicol 2008; 4: 1371–1378. [DOI] [PubMed] [Google Scholar]
- 4.Wat WZ. Current perspectives on bisphosphonate treatment in Paget’s disease of bone. Ther Clin Risk Manag 2014; 10: 977–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fleisch H, Russell RGG, Bisaz S, et al. The influence of pyrophosphate analogues (diphosphonates) on the precipitation and dissolution of calcium phosphate in vitro and in vivo. Calc Tis Res 1968; 2(Suppl 1): 10. [DOI] [PubMed] [Google Scholar]
- 6.Fleisch H, Russell RG, Francis MD. Diphosphonates inhibit hydroxyapatite dissolution in vitro and bone resorption in tissue culture and in vivo. Science 1969; 165: 1262–1264. [DOI] [PubMed] [Google Scholar]
- 7.Fleisch H. Diphosphonates: history and mechanisms of action. Metab Bone Dis Relat Res 1981; 3: 279–287. [DOI] [PubMed] [Google Scholar]
- 8.Fleisch H, Bisaz S. [Isolation from the plasma of pyrophosphate, an inhibitor of calcification]. Helv Physiol Pharmacol Acta 1962; 20: C52–C53 [in French, English abstract]. [PubMed] [Google Scholar]
- 9.Voss PJ, Steybe D, Poxleitner P, et al. Osteonecrosis of the jaw in patients transitioning from bisphosphonates to denosumab treatment for osteoporosis. Odontology 2018. [DOI] [PubMed]
- 10.Dominguez L, Di Bella G, Belvedere M, et al. Physiology of the aging bone and mechanisms of action of bisphosphonates. Biogerontology 2011; 12: 397–408. [DOI] [PubMed] [Google Scholar]
- 11.Bartl R, Frisch B, Tresckow E, et al. Bisphosphonates in medical practice: Actions-side effects-indications-strategies: Springer Science & Business Media, Berlin, 2007. [Google Scholar]
- 12.Russell RGG. Bisphosphonates: mode of action and pharmacology. Pediatrics 2007; 119(Suppl 2): S150–S62. [DOI] [PubMed] [Google Scholar]
- 13.Basso FG, Silveira Turrioni AP, Hebling J, et al. Zoledronic acid inhibits human osteoblast activities. Gerontology 2013; 59: 534–541. [DOI] [PubMed] [Google Scholar]
- 14.Ravosa MJ, Ning J, Liu Y, et al. Bisphosphonate effects on the behaviour of oral epithelial cells and oral fibroblasts. Arch Oral Biol 2011; 56: 491–498. [DOI] [PubMed] [Google Scholar]
- 15.Scheller EL, Hankenson KD, Reuben JS, et al. Zoledronic acid inhibits macrophage SOCS3 expression and enhances cytokine production. J Cell Biochem 2011; 112: 3364–3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim RH, Lee RS, Williams D, et al. Bisphosphonates induce senescence in normal human oral keratinocytes. J Dent Res 2011; 90: 810–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg 2003; 61: 1115–1117. [DOI] [PubMed] [Google Scholar]
- 18.Rasmusson L, Abtahi J. Bisphosphonate associated osteonecrosis of the jaw: an update on pathophysiology, risk factors, and treatment. Int J Dent 2014; 2014: 471035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruggiero SL, Dodson TB, Fantasia J, et al. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw–2014 update. J Oral Maxillofac Surg 2014; 72: 1938–1956. [DOI] [PubMed] [Google Scholar]
- 20.Ryan P, Saleh I, Stassen LF. Osteonecrosis of the jaw: a rare and devastating side effect of bisphosphonates. Postgrad Med J 2009; 85: 674–677. [DOI] [PubMed] [Google Scholar]
- 21.Sigua-Rodriguez EA, da Costa Ribeiro R, de Brito AC, et al. Bisphosphonate-related osteonecrosis of the jaw: a review of the literature. Int J Dent 2014; 2014: 192320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang KH, Won JH, Yoon HK, et al. High concentrations of pamidronate in bone weaken the mechanical properties of intact femora in a rat model. Yonsei Med J 2007; 48: 653–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okamoto Y, Hirota M, Monden Y, et al. High-dose zoledronic acid narrows the periodontal space in rats. Int J Oral Maxillofac Surg 2013; 42: 627–631. [DOI] [PubMed] [Google Scholar]
- 24.Abtahi J, Agholme F, Sandberg O, et al. Effect of local vs. systemic bisphosphonate delivery on dental implant fixation in a model of osteonecrosis of the jaw. J Dent Res 2013; 92: 279–283. [DOI] [PubMed] [Google Scholar]
- 25.Aghaloo TL, Kang B, Sung EC, et al. Periodontal disease and bisphosphonates induce osteonecrosis of the jaws in the rat. J Bone Miner Res 2011; 26: 1871–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aguirre JI, Akhter MP, Kimmel DB, et al. Oncologic doses of zoledronic acid induce osteonecrosis of the jaw-like lesions in rice rats (Oryzomys palustris) with periodontitis. J Bone Miner Res 2012; 27: 2130–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bauss F, Pfister T, Papapoulos S. Ibandronate uptake in the jaw is similar to long bones and vertebrae in the rat. J Bone Miner Metab 2008; 26: 406–408. [DOI] [PubMed] [Google Scholar]
- 28.Wen D, Qing L, Harrison G, et al. Anatomic site variability in rat skeletal uptake and desorption of fluorescently labeled bisphosphonate. Oral Dis 2011; 17: 427–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vasconcelos AC, Berti-Couto SA, Azambuja AA, et al. Comparison of effects of clodronate and zoledronic acid on the repair of maxilla surgical wounds - histomorphometric, receptor activator of nuclear factor-kB ligand, osteoprotegerin, von Willebrand factor, and caspase-3 evaluation. J Oral Pathol Med 2012; 41: 702–712. [DOI] [PubMed] [Google Scholar]
- 30.Cankaya M, Cizmeci Senel F, Kadioglu Duman M, et al. The effects of chronic zoledronate usage on the jaw and long bones evaluated using RANKL and osteoprotegerin levels in an animal model. Int J Oral Maxillofac Surg 2013; 42: 1134–1139. [DOI] [PubMed] [Google Scholar]
- 31.Tsurushima H, Kokuryo S, Sakaguchi O, et al. Bacterial promotion of bisphosphonate-induced osteonecrosis in Wistar rats. Int J Oral Maxillofac Surg 2013; 42: 1481–1487. [DOI] [PubMed] [Google Scholar]
- 32.Sonis ST, Watkins BA, Lyng GD, et al. Bony changes in the jaws of rats treated with zoledronic acid and dexamethasone before dental extractions mimic bisphosphonate-related osteonecrosis in cancer patients. Oral Oncol 2009; 45: 164–172. [DOI] [PubMed] [Google Scholar]
- 33.Ali-Erdem M, Burak-Cankaya A, Cemil-Isler S, et al. Extraction socket healing in rats treated with bisphosphonate: animal model for bisphosphonate related osteonecrosis of jaws in multiple myeloma patients. Med Oral Patol Oral Cir Bucal 2011; 16: e879–e883. [DOI] [PubMed] [Google Scholar]
- 34.Berti-Couto SA, Vasconcelos AC, Iglesias JE, et al. Diabetes mellitus and corticotherapy as risk factors for alendronate-related osteonecrosis of the jaws: a study in Wistar rats. Head Neck 2014; 36: 84–93. [DOI] [PubMed] [Google Scholar]
- 35.Dayisoylu EH, Senel FC, Ungor C, et al. The effects of adjunctive parathyroid hormone injection on bisphosphonate-related osteonecrosis of the jaws: an animal study. Int J Oral Maxillofac Surg 2013; 42: 1475–1480. [DOI] [PubMed] [Google Scholar]
- 36.Ersan N, van Ruijven LJ, Bronckers AL, et al. Teriparatide and the treatment of bisphosphonate-related osteonecrosis of the jaw: a rat model. Dentomaxillofac Radiol 2014; 43: 20130144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Janovszky A, Szabo A, Varga R, et al. Periosteal microcirculatory reactions in a zoledronate-induced osteonecrosis model of the jaw in rats. Clin Oral Investig 2015; 19: 1279–1288. [DOI] [PubMed] [Google Scholar]
- 38.Zandi M, Dehghan A, Malekzadeh H, et al. Introducing a protocol to create bisphosphonate-related osteonecrosis of the jaw in rat animal model. J Craniomaxillofac Surg 2016; 44: 271–278. [DOI] [PubMed] [Google Scholar]
- 39.Gong X, Yu W, Zhao H, et al. Skeletal site-specific effects of zoledronate on in vivo bone remodeling and in vitro BMSCs osteogenic activity. Sci Rep 2017; 7: 36129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Ponte FS, Catalfamo L, Micali G, et al. Effect of bisphosphonates on the mandibular bone and gingival epithelium of rats without tooth extraction. Exp Ther Med 2016; 11: 1678–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zandi M, Dehghan A, Janbaz P, et al. The starting point for bisphosphonate-related osteonecrosis of the jaw: alveolar bone or oral mucosa? A randomized, controlled experimental study. J Craniomaxillofac Surg 2017; 45: 157–161. [DOI] [PubMed] [Google Scholar]
- 42.Takaoka K, Yamamura M, Nishioka T, et al. Establishment of an animal model of bisphosphonate-related osteonecrosis of the jaws in spontaneously diabetic Torii rats. PloS One 2015; 10:e0144355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vidal-Gutierrez X, Gomez-Clavel JF, Gaitan-Cepeda LA. Dental extraction following zoledronate, induces osteonecrosis in rat's jaw. Med Oral Patol Oral Cir Bucal 2017; 22: e177–e184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hikita H, Miyazawa K, Tabuchi M, et al. Bisphosphonate administration prior to tooth extraction delays initial healing of the extraction socket in rats. J Bone Miner Metab 2009; 27: 663–672. [DOI] [PubMed] [Google Scholar]
- 45.Maahs MP, Azambuja AA, Campos MM, et al. Association between bisphosphonates and jaw osteonecrosis: a study in Wistar rats. Head Neck 2011; 33: 199–207. [DOI] [PubMed] [Google Scholar]
- 46.Aguirre JI, Altman MK, Vanegas SM, et al. Effects of alendronate on bone healing after tooth extraction in rats. Oral Dis 2010; 16: 674–685. [DOI] [PubMed] [Google Scholar]
- 47.Conte-Neto N, Bastos AS, Spolidorio LC, et al. Oral bisphosphonate-related osteonecrosis of the jaws in rheumatoid arthritis patients: a critical discussion and two case reports. Head Face Med 2011; 7: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abtahi J, Agholme F, Sandberg O, et al. Bisphosphonate-induced osteonecrosis of the jaw in a rat model arises first after the bone has become exposed. No primary necrosis in unexposed bone. J Oral Pathol Med 2012; 41: 494–499. [DOI] [PubMed] [Google Scholar]
- 49.Dayisoylu EH, Ungor C, Tosun E, et al. Does an alkaline environment prevent the development of bisphosphonate-related osteonecrosis of the jaw? An experimental study in rats. Oral Surg Oral Med Oral Pathol Oral Radiol 2014; 117: 329–334. [DOI] [PubMed] [Google Scholar]
- 50.Biasotto M, Chiandussi S, Zacchigna S, et al. A novel animal model to study non-spontaneous bisphosphonates osteonecrosis of jaw. J Oral Pathol Med 2010; 39: 390–396. [DOI] [PubMed] [Google Scholar]
- 51.Yamashita J, McCauley LK, Van Poznak C. Updates on osteonecrosis of the jaw. Curr Opin Support Palliat Care 2010; 4: 200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Basi DL, Hughes PJ, Thumbigere-Math V, et al. Matrix metalloproteinase-9 expression in alveolar extraction sockets of Zoledronic acid-treated rats. J Oral Maxillofac Surg 2011; 69: 2698–2707. [DOI] [PubMed] [Google Scholar]
- 53.Marino KL, Zakhary I, Abdelsayed RA, et al. Development of a rat model of bisphosphonate-related osteonecrosis of the jaw (BRONJ). J Oral Implantol 2012; 38 Spec No: 511–518. [DOI] [PubMed] [Google Scholar]
- 54.Cetinkaya BO, Keles GC, Ayas B, et al. Effects of risedronate on alveolar bone loss and angiogenesis: a stereologic study in rats. J Periodontol 2008; 79: 1950–1961. [DOI] [PubMed] [Google Scholar]
- 55.Senel FC, Kadioglu Duman M, Muci E, et al. Jaw bone changes in rats after treatment with zoledronate and pamidronate. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010; 109: 385–391. [DOI] [PubMed] [Google Scholar]
- 56.Lopez-Jornet P, Camacho-Alonso F, Molina-Minano F, et al. An experimental study of bisphosphonate-induced jaws osteonecrosis in Sprague-Dawley rats. J Oral Pathol Med 2010; 39: 697–702. [DOI] [PubMed] [Google Scholar]
- 57.Hokugo A, Christensen R, Chung EM, et al. Increased prevalence of bisphosphonate-related osteonecrosis of the jaw with vitamin D deficiency in rats. J Bone Miner Res 2010; 25: 1337–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barba-Recreo P, Del Castillo Pardo de Vera JL, Garcia-Arranz M, et al. Zoledronic acid - related osteonecrosis of the jaws. Experimental model with dental extractions in rats. J Craniomaxillofac Surg 2014; 42: 744–750. [DOI] [PubMed] [Google Scholar]
- 59.Silveira FM, Etges A, Correa MB, et al. Microscopic evaluation of the effect of oral microbiota on the development of bisphosphonate-related osteonecrosis of the Jaws in Rats. J Oral Maxillofac Res 2016; 7: e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vilarinho JLP, Ferrare N, Moreira AMR, et al. Early bony changes associated with bisphosphonate-related osteonecrosis of the jaws in rats: a longitudinal in vivo study. Arch Oral Biol 2017; 82: 79–85. [DOI] [PubMed] [Google Scholar]
- 61.Yanik S, Aras MH, Erkilic S, et al. Histopathological features of bisphosphonates related osteonecrosis of the jaw in rats with and without vitamin d supplementation. Arch Oral Biol 2016; 65: 59–65. [DOI] [PubMed] [Google Scholar]
- 62.Curra C, Cardoso CL, Ferreira O Júnior, et al. Medication-related osteonecrosis of the jaw. Introduction of a new modified experimental model. Acta Cir Bras 2016; 31: 308–313. [DOI] [PubMed] [Google Scholar]
- 63.Neto T, Horta R, Balhau R, et al. Resection and microvascular reconstruction of bisphosphonate-related osteonecrosis of the jaw: the role of microvascular reconstruction. Head Neck 2016; 38: 1278–1285. [DOI] [PubMed] [Google Scholar]
- 64.Huja SS, Fernandez SA, Phillips C, et al. Zoledronic acid decreases bone formation without causing osteocyte death in mice. Arch Oral Biol 2009; 54: 851–856. [DOI] [PubMed] [Google Scholar]
- 65.Oizumi T, Yamaguchi K, Funayama H, et al. Necrotic actions of nitrogen-containing bisphosphonates and their inhibition by clodronate, a non-nitrogen-containing bisphosphonate in mice: potential for utilization of clodronate as a combination drug with a nitrogen-containing bisphosphonate. Basic Clin Pharmacol Toxicol 2009; 104: 384–392. [DOI] [PubMed] [Google Scholar]
- 66.Kang B, Cheong S, Chaichanasakul T, et al. Periapical disease and bisphosphonates induce osteonecrosis of the jaws in mice. J Bone Miner Res 2013; 28: 1631–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bonnet N, Lesclous P, Saffar JL, et al. Zoledronate effects on systemic and jaw osteopenias in ovariectomized periostin-deficient mice. PloS One 2013; 8: e58726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kuroshima S, Yamashita J. Chemotherapeutic and antiresorptive combination therapy suppressed lymphangiogenesis and induced osteonecrosis of the jaw-like lesions in mice. Bone 2013; 56: 101–109. [DOI] [PubMed] [Google Scholar]
- 69.Su J, Feng M, Han W, et al. The effects of bisphosphonate on the remodeling of different irregular bones in mice. J Oral Pathol Med 2015; 44: 638–648. [DOI] [PubMed] [Google Scholar]
- 70.de Molon RS, Cheong S, Bezouglaia O, et al. Spontaneous osteonecrosis of the jaws in the maxilla of mice on antiresorptive treatment: a novel ONJ mouse model. Bone 2014; 68: 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.de Molon RS, Shimamoto H, Bezouglaia O, et al. OPG-Fc but not zoledronic acid discontinuation reverses osteonecrosis of the jaws (ONJ) in mice. J Bone Miner Res 2015; 30: 1627–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vermeer J, Renders G, van Duin MA, et al. Bone-site-specific responses to zoledronic acid. Oral Dis 2017; 23: 126–133. [DOI] [PubMed] [Google Scholar]
- 73.Cordova LA, Guilbaud F, Amiaud J, et al. Severe compromise of preosteoblasts in a surgical mouse model of bisphosphonate-associated osteonecrosis of the jaw. J Craniomaxillofac Surg 2016; 44: 1387–1394. [DOI] [PubMed] [Google Scholar]
- 74.Kim S, Williams DW, Lee C, et al. IL-36 induces bisphosphonate-related osteonecrosis of the Jaw-like lesions in mice by inhibiting TGF-beta-mediated collagen expression. J Bone Miner Res 2017; 32: 309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pabst AM, Ziebart T, Ackermann M, et al. Bisphosphonates’ antiangiogenic potency in the development of bisphosphonate-associated osteonecrosis of the jaws: influence on microvessel sprouting in an in vivo 3D Matrigel assay. Clin Oral Investig 2014; 18: 1015–1022. [DOI] [PubMed] [Google Scholar]
- 76.Williams DW, Lee C, Kim T, et al. Impaired bone resorption and woven bone formation are associated with development of osteonecrosis of the jaw-like lesions by bisphosphonate and anti-receptor activator of NF-kappaB ligand antibody in mice. Am J Pathol 2014; 184: 3084–3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Oizumi T, Funayama H, Yamaguchi K, et al. Inhibition of necrotic actions of nitrogen-containing bisphosphonates (NBPs) and their elimination from bone by etidronate (a non-NBP): a proposal for possible utilization of etidronate as a substitution drug for NBPs. J Oral Maxillofac Surg 2010; 68: 1043–1054. [DOI] [PubMed] [Google Scholar]
- 78.Yu YY, Lieu S, Hu D, et al. Site specific effects of zoledronic acid during tibial and mandibular fracture repair. PLoS One 2012; 7: e31771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pozzi S, Vallet S, Mukherjee S, et al. High-dose zoledronic acid impacts bone remodeling with effects on osteoblastic lineage and bone mechanical properties. Clin Cancer Res 2009; 15: 5829–5839. [DOI] [PubMed] [Google Scholar]
- 80.Kikuiri T, Kim I, Yamaza T, et al. Cell-based immunotherapy with mesenchymal stem cells cures bisphosphonate-related osteonecrosis of the jaw-like disease in mice. J Bone Miner Res 2010; 25: 1668–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bi Y, Gao Y, Ehirchiou D, et al. Bisphosphonates cause osteonecrosis of the jaw-like disease in mice. Am J Pathol 2010; 177: 280–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kobayashi Y, Hiraga T, Ueda A, et al. Zoledronic acid delays wound healing of the tooth extraction socket, inhibits oral epithelial cell migration, and promotes proliferation and adhesion to hydroxyapatite of oral bacteria, without causing osteonecrosis of the jaw, in mice. J Bone Miner Metab 2010; 28: 165–175. [DOI] [PubMed] [Google Scholar]
- 83.Kubek DJ, Burr DB, Allen MR. Ovariectomy stimulates and bisphosphonates inhibit intracortical remodeling in the mouse mandible. Orthod Craniofac Res 2010; 13: 214–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shimizu E, Tamasi J, Partridge NC. Alendronate affects osteoblast functions by crosstalk through EphrinB1-EphB. J Dent Res 2012; 91: 268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhao Y, Wang L, Liu Y, et al. Technetium-99 conjugated with methylene diphosphonate ameliorates ovariectomy-induced osteoporotic phenotype without causing osteonecrosis in the jaw. Calcif Tissue Int 2012; 91: 400–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang Q, Atsuta I, Liu S, et al. IL-17-mediated M1/M2 macrophage alteration contributes to pathogenesis of bisphosphonate-related osteonecrosis of the jaws. Clin Cancer Res 2013; 19: 3176–3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Aghaloo TL, Cheong S, Bezouglaia O, et al. RANKL inhibitors induce osteonecrosis of the jaw in mice with periapical disease. J Bone Miner Res 2014; 29: 843–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Park S, Kanayama K, Kaur K, et al. Osteonecrosis of the jaw developed in mice: disease variants regulated by γδ T cells in oral mucosal barrier immunity. J Biol Chem 2015; 290: 17349–17366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Song M, Alshaikh A, Kim T, et al. Preexisting periapical inflammatory condition exacerbates tooth extraction-induced bisphosphonate-related osteonecrosis of the jaw lesions in mice. J Endod 2016; 42: 1641–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Choi JY, Kim HJ, Lee YC, et al. Inhibition of bone healing by pamidronate in calvarial bony defects. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007; 103: 321–328. [DOI] [PubMed] [Google Scholar]
- 91.Allen MR, Burr DB. Mandible matrix necrosis in beagle dogs after 3 years of daily oral bisphosphonate treatment. J Oral Maxillofac Surg 2008; 66: 987–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Allen MR. Bisphosphonates and osteonecrosis of the jaw: moving from the bedside to the bench. Cells Tissues Organs 2009; 189: 289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Allen MR, Chu TM, Ruggiero SL. Absence of exposed bone following dental extraction in beagle dogs treated with 9 months of high-dose zoledronic acid combined with dexamethasone. J Oral Maxillofac Surg 2013; 71: 1017–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Burr DB, Allen MR. Mandibular necrosis in beagle dogs treated with bisphosphonates. Orthod Craniofac Res 2009; 12: 221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Allam E, Allen M, Chu TM, et al. In vivo effects of zoledronic acid on oral mucosal epithelial cells. Oral Dis 2011; 17: 291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Huja SS, Mason A, Fenell CE, et al. Effects of short-term zoledronic acid treatment on bone remodeling and healing at surgical sites in the maxilla and mandible of aged dogs. J Oral Maxillofac Surg 2011; 69: 418–427. [DOI] [PubMed] [Google Scholar]
- 97.Pautke C, Kreutzer K, Weitz J, et al. Bisphosphonate related osteonecrosis of the jaw: a minipig large animal model. Bone 2012; 51: 592–599. [DOI] [PubMed] [Google Scholar]
- 98.Li Y, Xu J, Mao L, et al. Allogeneic mesenchymal stem cell therapy for bisphosphonate-related jaw osteonecrosis in swine. Stem Cells Dev 2013; 22: 2047–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mitsimponas KT, Moest T, Iliopoulos C, et al. Search for a reliable model for bisphosphonate-related osteonecrosis of the jaw: establishment of a model in pigs and description of its histomorphometric characteristics. Br J Oral Maxillofac Surg 2016; 54: 883–888. [DOI] [PubMed] [Google Scholar]
- 100.Voss PJ, Stoddart MJ, Bernstein A, et al. Zoledronate induces bisphosphonate-related osteonecrosis of the jaw in osteopenic sheep. Clin Oral Investig 2016; 20: 31–38. [DOI] [PubMed] [Google Scholar]
- 101.Voss P, Ludwig U, Poxleitner P, et al. Evaluation of BP-ONJ in osteopenic and healthy sheep: comparing ZTE-MRI with µCT. Dentomaxillofac Radiol 2016; 45: 20150250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cornish J, Bava U, Callon KE, et al. Bone-bound bisphosphonate inhibits growth of adjacent non-bone cells. Bone 2011; 49: 710–716. [DOI] [PubMed] [Google Scholar]
- 103.Acil Y, Moller B, Niehoff P, et al. The cytotoxic effects of three different bisphosphonates in-vitro on human gingival fibroblasts, osteoblasts and osteogenic sarcoma cells. J Craniomaxillofac Surg 2012; 40: e229–e235. [DOI] [PubMed] [Google Scholar]
- 104.Saracino S, Canuto RA, Maggiora M, et al. Exposing human epithelial cells to zoledronic acid can mediate osteonecrosis of jaw: an in vitro model. J Oral Pathol Med 2012; 41: 788–792. [DOI] [PubMed] [Google Scholar]
- 105.Marolt D, Cozin M, Vunjak-Novakovic G, et al. Effects of pamidronate on human alveolar osteoblasts in vitro. J Oral Maxillofac Surg 2012; 70: 1081–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Patntirapong S, Singhatanadgit W, Chanruangvanit C, et al. Zoledronic acid suppresses mineralization through direct cytotoxicity and osteoblast differentiation inhibition. J Oral Pathol Med 2012; 41: 713–720. [DOI] [PubMed] [Google Scholar]
- 107.Basso FG, Turrioni AP, Soares DG, et al. Low-level laser therapy for osteonecrotic lesions: effects on osteoblasts treated with zoledronic acid. Support Care Cancer 2014; 22: 2741–2748. [DOI] [PubMed] [Google Scholar]
- 108.Hagelauer N, Ziebart T, Pabst AM, et al. Bisphosphonates inhibit cell functions of HUVECs, fibroblasts and osteogenic cells via inhibition of protein geranylgeranylation. Clin Oral Investig 2015; 19: 1079–1091. [DOI] [PubMed] [Google Scholar]
- 109.Koch FP, Yekta SS, Merkel C, et al. The impact of bisphosphonates on the osteoblast proliferation and collagen gene expression in vitro. Head Face Med 2010; 6: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Koch FP, Wunsch A, Merkel C, et al. The influence of bisphosphonates on human osteoblast migration and integrin aVb3/tenascin C gene expression in vitro. Head Face Med 2011; 7: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Koch FP, Merkel C, Al-Nawas B, et al. Zoledronate, ibandronate and clodronate enhance osteoblast differentiation in a dose dependent manner–a quantitative in vitro gene expression analysis of Dlx5, Runx2, OCN, MSX1 and MSX2. J Craniomaxillofac Surg 2011; 39: 562–569. [DOI] [PubMed] [Google Scholar]
- 112.Walter C, Pabst A, Ziebart T, et al. Bisphosphonates affect migration ability and cell viability of HUVEC, fibroblasts and osteoblasts in vitro. Oral Dis 2011; 17: 194–199. [DOI] [PubMed] [Google Scholar]
- 113.Walter C, Pabst AM, Ziebart T. Effects of a low-level diode laser on oral keratinocytes, oral fibroblasts, endothelial cells and osteoblasts incubated with bisphosphonates: an in vitro study. Biomed Rep 2015; 3: 14–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mukudai Y, Kondo S, Koyama T, et al. Potential anti-osteoporotic effects of herbal extracts on osteoclasts, osteoblasts and chondrocytes in vitro. BMC Complement Altern Med 2014; 14: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Camacho-Alonso F, Urrutia-Rodriguez I, Onate-Cabrerizo D, et al. Cytoprotective effects of melatonin on zoledronic acid-treated human osteoblasts. J Craniomaxillofac Surg 2017; 45: 1251–1257. [DOI] [PubMed] [Google Scholar]
- 116.Manzano-Moreno FJ, Ramos-Torrecillas J, De Luna-Bertos E, et al. High doses of bisphosphonates reduce osteoblast-like cell proliferation by arresting the cell cycle and inducing apoptosis. J Craniomaxillofac Surg 2015; 43: 396–401. [DOI] [PubMed] [Google Scholar]
- 117.Zafar S, Coates DE, Cullinan MP, et al. Effects of zoledronic acid and geranylgeraniol on the cellular behaviour and gene expression of primary human alveolar osteoblasts. Clin Oral Investig 2016; 20: 2023–2035. [DOI] [PubMed] [Google Scholar]
- 118.Hu L, Han J, Yang X, et al. Apoptosis repressor with caspase recruitment domain enhances survival and promotes osteogenic differentiation of human osteoblast cells under zoledronate treatment. Molecular medicine reports 2016; 14: 3535–3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Manzano-Moreno FJ, Ramos-Torrecillas J, De Luna-Bertos E, et al. Effect of clodronate on antigenic profile, growth, and differentiation of osteoblast-like cells. J Oral Maxillofac Surg 2016; 74: 1765–1770. [DOI] [PubMed] [Google Scholar]
- 120.Huang X, Huang S, Guo F, et al. Dose-dependent inhibitory effects of zoledronic acid on osteoblast viability and function in vitro. Mol Med Rep 2016; 13: 613–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Coates DE, Zafar S, Milne TJ. Quantitative real-time gene profiling of human alveolar osteoblasts. Methods Mol Biol 2017; 1537: 447–459. [DOI] [PubMed] [Google Scholar]
- 122.Lang M, Zhou Z, Shi L, et al. Influence of zoledronic acid on proliferation, migration, and apoptosis of vascular endothelial cells. Br J Oral Maxillofac Surg 2016; 54: 889–893. [DOI] [PubMed] [Google Scholar]
- 123.Lu Y, Wang Z, Han W, et al. Zoledronate induces autophagic cell death in human umbilical vein endothelial cells via Beclin-1 dependent pathway activation. Mol Med Rep 2016; 14: 4747–4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vermeer JA, Jansen ID, Marthi M, et al. Jaw bone marrow-derived osteoclast precursors internalize more bisphosphonate than long-bone marrow precursors. Bone 2013; 57: 242–251. [DOI] [PubMed] [Google Scholar]
- 125.Nakagawa T, Ohta K, Kubozono K, et al. Zoledronate inhibits receptor activator of nuclear factor kappa-B ligand-induced osteoclast differentiation via suppression of expression of nuclear factor of activated T-cell c1 and carbonic anhydrase 2. Arch Oral Biol 2015; 60: 557–565. [DOI] [PubMed] [Google Scholar]
- 126.Nagaoka Y, Kajiya H, Ozeki S, et al. Mevalonates restore zoledronic acid-induced osteoclastogenesis inhibition. J Dent Res 2015; 94: 594–601. [DOI] [PubMed] [Google Scholar]
- 127.Deng X, Tamai R, Endo Y, et al. Alendronate augments interleukin-1beta release from macrophages infected with periodontal pathogenic bacteria through activation of caspase-1. Toxicol Appl Pharmacol 2009; 235: 97–104. [DOI] [PubMed] [Google Scholar]
- 128.Masuda T, Deng X, Tamai R. Mouse macrophages primed with alendronate down-regulate monocyte chemoattractant protein-1 (MCP-1) and macrophage inflammatory protein-1alpha (MIP-1alpha) production in response to Toll-like receptor (TLR) 2 and TLR4 agonist via Smad3 activation. Int Immunopharmacol 2009; 9: 1115–1121. [DOI] [PubMed] [Google Scholar]
- 129.Muratsu D, Yoshiga D, Taketomi T, et al. Zoledronic acid enhances lipopolysaccharide-stimulated proinflammatory reactions through controlled expression of SOCS1 in macrophages. PloS One 2013; 8: e67906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hoefert S, Hoefert CS, Albert M, et al. Zoledronate but not denosumab suppresses macrophagic differentiation of THP-1 cells. An aetiologic model of bisphosphonate-related osteonecrosis of the jaw (BRONJ). Clin Oral Investig 2015; 19: 1307–1318. [DOI] [PubMed] [Google Scholar]
- 131.Landesberg R, Cozin M, Cremers S, et al. Inhibition of oral mucosal cell wound healing by bisphosphonates. J Oral Maxillofac Surg 2008; 66: 839–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Scheper MA, Badros A, Chaisuparat R, et al. Effect of zoledronic acid on oral fibroblasts and epithelial cells: a potential mechanism of bisphosphonate-associated osteonecrosis. Br J Haematol 2009; 144: 667–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Scheller EL, Baldwin CM, Kuo S, et al. Bisphosphonates inhibit expression of p63 by oral keratinocytes. J Dent Res 2011; 90: 894–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pabst AM, Ziebart T, Koch FP, et al. The influence of bisphosphonates on viability, migration, and apoptosis of human oral keratinocytes–in vitro study. Clin Oral Investig 2012; 16: 87–93. [DOI] [PubMed] [Google Scholar]
- 135.Agis H, Blei J, Watzek G, et al. Is zoledronate toxic to human periodontal fibroblasts? J Dent Res 2010; 89: 40–45. [DOI] [PubMed] [Google Scholar]
- 136.Tipton DA, Seshul BA, Dabbous M. Effect of bisphosphonates on human gingival fibroblast production of mediators of osteoclastogenesis: RANKL, osteoprotegerin and interleukin-6. J Periodontal Res 2011; 46: 39–47. [DOI] [PubMed] [Google Scholar]
- 137.Zafar S, Coates DE, Cullinan MP, et al. Zoledronic acid and geranylgeraniol regulate cellular behaviour and angiogenic gene expression in human gingival fibroblasts. J Oral Pathol Med 2014; 43: 711–721. [DOI] [PubMed] [Google Scholar]
- 138.Basso FG, Soares DG, Pansani TN, et al. Response of a co-culture model of epithelial cells and gingival fibroblasts to zoledronic acid. Braz Oral Res 2016; 30: e122. [DOI] [PubMed] [Google Scholar]
- 139.Komatsu Y, Ibi M, Chosa N, et al. Zoledronic acid suppresses transforming growth factor-beta-induced fibrogenesis by human gingival fibroblasts. Int J Mol Med 2016; 38: 139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ohlrich EJ, Coates DE, Cullinan MP, et al. The bisphosphonate zoledronic acid regulates key angiogenesis-related genes in primary human gingival fibroblasts. Arch Oral Biol 2016; 63: 7–14. [DOI] [PubMed] [Google Scholar]
- 141.Shikama Y, Nagai Y, Okada S, et al. Pro-IL-1beta accumulation in macrophages by alendronate and its prevention by clodronate. Toxicol Lett 2010; 199: 123–128. [DOI] [PubMed] [Google Scholar]
- 142.Kuiper JW, Forster C, Sun C, et al. Zoledronate and pamidronate depress neutrophil functions and survival in mice. Br J Pharmacol 2012; 165: 532–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tseng HC, Kanayama K, Kaur K, et al. Bisphosphonate-induced differential modulation of immune cell function in gingiva and bone marrow in vivo: role in osteoclast-mediated NK cell activation. Oncotarget 2015; 6: 20002–20025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mawardi H, Giro G, Kajiya M, et al. A role of oral bacteria in bisphosphonate-induced osteonecrosis of the jaw. J Dent Res 2011; 90: 1339–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hokugo A, Sun S, Park S, et al. Equilibrium-dependent bisphosphonate interaction with crystalline bone mineral explains anti-resorptive pharmacokinetics and prevalence of osteonecrosis of the jaw in rats. Bone 2013; 53: 59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kozloff KM, Volakis LI, Marini JC, et al. Near-infrared fluorescent probe traces bisphosphonate delivery and retention in vivo. J Bone Miner Res 2010; 25: 1748–1758. [DOI] [PubMed] [Google Scholar]
- 147.Ogata K, Katagiri W, Osugi M, et al. Evaluation of the therapeutic effects of conditioned media from mesenchymal stem cells in a rat bisphosphonate-related osteonecrosis of the jaw-like model. Bone 2015; 74: 95–105. [DOI] [PubMed] [Google Scholar]
- 148.Conte Neto N, Spolidorio LC, Andrade CR, et al. Experimental development of bisphosphonate-related osteonecrosis of the jaws in rodents. Int J Exp Pathol 2013; 94: 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Abtahi J, Agholme F, Aspenberg P. Prevention of osteonecrosis of the jaw by mucoperiosteal coverage in a rat model. Int J Oral Maxillofac Surg 2013; 42: 632–636. [DOI] [PubMed] [Google Scholar]
- 150.Allen MR. Animal models of osteonecrosis of the jaw. J Musculoskelet Neuronal Interact 2007; 7: 358–360. [PubMed] [Google Scholar]
- 151.Otto S, Troltzsch M, Jambrovic V, et al. Tooth extraction in patients receiving oral or intravenous bisphosphonate administration: a trigger for BRONJ development? J Craniomaxillofac Surg 2015; 43: 847–854. [DOI] [PubMed] [Google Scholar]
- 152.Otto S, Ristow O, Pache C, et al. Fluorescence-guided surgery for the treatment of medication-related osteonecrosis of the jaw: a prospective cohort study. J Craniomaxillofac Surg 2016; 44: 1073–1080. [DOI] [PubMed] [Google Scholar]
- 153.Otto S, Pautke C, Martin Jurado O, et al. Further development of the MRONJ minipig large animal model. J Craniomaxillofac Surg 2017; 45: 1503–1514. [DOI] [PubMed] [Google Scholar]