Short abstract
Objective
To assess the efficacy of cerebrally monitoring the depth of anaesthesia in reducing postoperative cognitive dysfunction and postoperative delirium (POD).
Methods
MEDLINE, EMBASE, and Cochrane Library databases were searched following PRISMA statement guidelines. We included randomized clinical trials (RCTs) comparing electroencephalogram-based and routine care-guided titration of anaesthesia in a systematic review. The risk estimate from each RCT was pooled in a meta-analysis. The primary outcome was POD and long-term cognitive dysfunction. Subgroup analyses were conducted for the subtypes of intervention group and surgery. We identified five RCTs with a total sample size of 2,868 and with bispectral index (BIS) or auditory evoked potential (AEP) as interventions.
Results
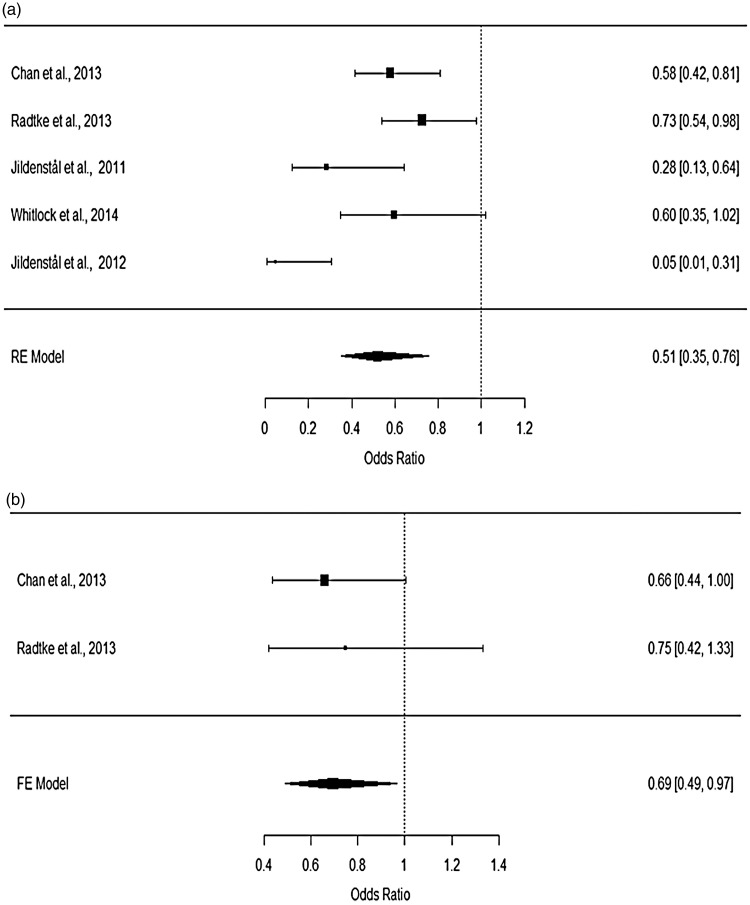
The odds ratio (OR) for POD and long-term cognitive decline was 0.51 (95%CI: 0.35–0.76) and 0.69 (95%CI: 0.49–0.97), respectively. Significant heterogeneity was identified in the POD data. There was no significant difference between BIS- and AEP-based titration of anaesthesia in reducing the risk of POD. Extensive heterogeneity for cardiac and thoracic surgery was identified in the study population, and significant publication bias was found among the POD results.
Conclusions
BIS- and AEP-guided anaesthesia are associated with significantly reduced risk of POD and long-term cognitive dysfunction.
Keywords: Cerebral monitoring, anaesthesia, cognitive dysfunction, postoperative delirium, systematic review, meta-analysis
Introduction
Postoperative delirium (POD) is a common perioperative complication among older adults, imposing a significant financial burden on society. Although the incidence of POD is high and more than half of clinical cases are undiagnosed, up to 40% of cases are preventable.1 Strategies such as intraoperative cerebral monitoring by processed electroencephalogram (EEG) have been shown to facilitate the tracking of depth of anaesthesia and to improve anaesthetic management by avoiding light and deep anaesthesia2,3 Intraoperative monitoring with the electroencephalogram bispectral index (BIS) or auditory evoked potential (AEP) devices are two commonly-used methods for guiding the titration of anaesthetic drugs. BIS is a newly established EEG parameter specifically developed to measure the hypnotic effects of anaesthesia, and is displayed as a dimensionless number ranging from 0 to 100 (completely conscious). The use of BIS monitoring alongside the bispectral analysis of raw EEG signals in anaesthesia titration appears to confer sufficient clinical and economic benefits to justify its routine use.4
Previous studies have shown that chronic exposure to neurotoxicity induced by anaesthesia may lead to POD and cognitive dysfunction.5–7 Specific brain regions (e.g. the hippocampus, insula, amygdala and prefrontal cortex) involved in cognitive function processing are influenced by anaesthesia. The inhibition of neural activity in distributed cortical and thalamocortical networks by sedative concentration of anaesthetic drugs produces amnesia and attention deficit.8 To avoid cognitive deficit caused by long-term neurotoxicity after anaesthesia, several clinical studies have demonstrated that AEP and BIS-guided anaesthetic titration reduced anaesthetic exposure and decreased the risk of postoperative cognitive dysfunction.3,9,10
The purpose of this systematic review was to summarize the effects of AEP or BIS-guided anaesthetic titration on POD and cognitive function in older adults from the results of available randomized clinical trials (RCTs) and to perform subgroup analysis by type of major surgery and intervention.
Methods
Search strategy
We conducted a literature search of the Medline, EMBASE, and Cochrane Library databases for relevant RCTs. Search terms used were “Bispectral Index”, “Auditory Evoked Potential”, “Anaesthesia”, “Postoperative Delirium”, “RCT”, “Cognitive Dysfunction” and their combinations. RCTs with extractable data were used in a meta-analysis. The detailed search strategy was as follows: 1) delirium; 2) deliri*; 3) cognitive; 4) cognitive dysfunction; 5) BIS or AEP; 6) postoperative; 7) operation*; 8) surgery; 9) surgical; 10) anaesthesia; 11) anaesthesia; 12) 1 OR 2 OR 3 OR 4; 13) 6 OR 7 OR 8 OR 9 OR 10 OR 11; 14) 5 and 12 and 13; 15) ‘English' (Language); 16) 14 AND 15; 17) Randomized Clinical Trial; 18) 16 NOT 17; 19) ‘Elderly' (Mesh); 20) 18 AND 19. Additional studies were identified by reviewing the reference lists of searched articles.
Inclusion criteria
The search and selection of eligible studies by title and abstract were conducted by two independent reviewers. Discrepancies were resolved by discussion and review of the original paper. The included studies met the following criteria: 1) RCTs in adults, 2) publications written in English, 3) studies aimed at evaluating the effectiveness of BIS- or AEP-guided anaesthesia on reducing postoperative cognitive decline or POD, and 4) risk ratio and its 95% confidence interval (CI) reported for dichotomous outcome parameters. Conference abstracts, reviews, and non-human studies were excluded.
Data extraction
We extracted the following information for each eligible study: name of the first author, year of publication, journal, and study population characteristics. For the primary analyses, crude or adjusted risk ratio for POD or cognitive decline with corresponding 95% CI and the adjusted covariates were determined. For the subgroup analysis, event rates of other relevant outcomes, type of BIS-guided anaesthesia, and major surgery details were extracted.
Statistical analysis
All statistical analyses were performed independently by two researchers using R version 3.4.1 (http://www.R-project.org/) with relevant packages. Meta-analysis was performed when two or more clinical studies were identified. Heterogeneity testing was performed using the chi-square or I-square (I2) test. A random-effects model was used when a large heterogeneity was observed (I2 > 50%); otherwise, a fixed-effects model was used for the meta-analysis. The incidence of POD or cognitive decline was extracted from each eligible study. Odds ratios with 95% CIs were calculated. Egger’s test and inverted funnel plots with Trim-and-Hill correction were used to evaluate potential publication bias and funnel asymmetry. Fail-safe N was used to determine the number of NULL studies to be added to reduce the significance of the meta-analysis to 0.05. A sensitivity analysis was performed by omitting each study and recalculating the pooled OR in turn to identify potential outliers. We intended to conduct a subgroup analysis, where possible, on the effects of BIS-guided anaesthesia in patients with different surgeries. For all analyses, a P value of less than 0.05 (two-tailed) was considered statistically significant.
Results
Study selection
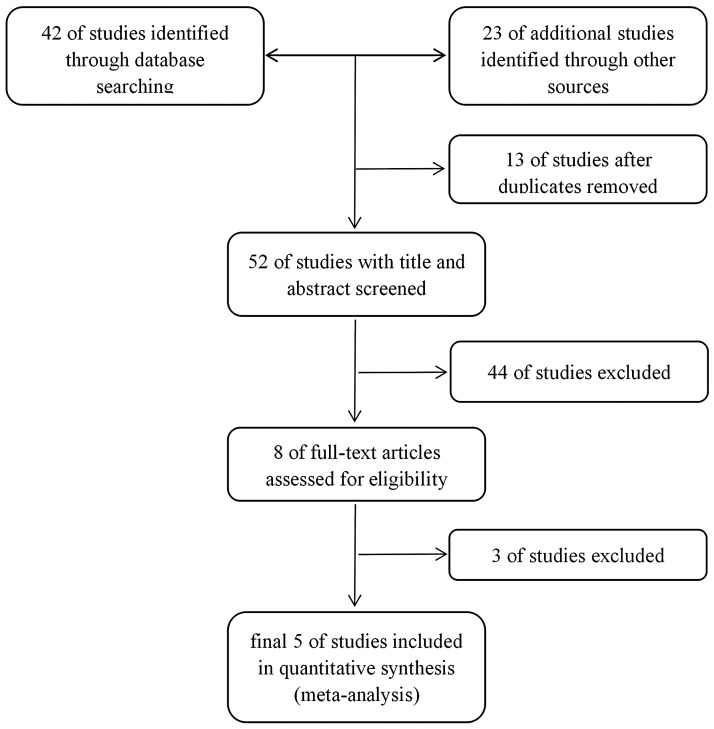
The process of searching, screening, and study selection is summarized in Figure 1. Our primary search yielded 65 articles (Medline = 23; Cochrane Library = 7; EMBASE = 12; other = 23). After removing duplicates (n = 13) and articles not satisfying the inclusion criteria (n = 47), 5 studies were included in our final analysis: 14 articles were removed because they were not RCTs; 3 articles were removed because they did not include cerebral monitoring and routine clinical sign-guided titration of anaesthesia as treatment arms; and 30 articles were removed because the relevant clinical question was not addressed or the data reported were incomplete.9–13
Figure 1.
Consort diagram showing study selection.
Study characteristics
The characteristics of selected studies are summarized in Table 1. A total of 2868 patients were randomized into either an intraoperative cerebral monitoring-guided group (n = 1426) or an endtidal anaesthetic concentration (EACT)-guided depth of anaesthesia protocols or routine care control group (n = 1442). The earliest date for patient enrolment was in January 2007. Three RCTs employed BIS and two employed AEP index as guidance for treatment groups. Patients from two studies underwent non-cardiac surgery, such as hip fracture repair, and others underwent cardiac and thoracic surgery. The majority of RCTs included older adults (>60 years), except for one RCT conducted in Sweden that included elective patients aged 18 to 92. Cognitive function and incidence of POD were evaluated in each study using the Cognitive Failures Questionnaire (CFQ), Reliable Change Index (RCI) and neuropsychological test, the Diagnostic and Statistical Manual of Mental Disorders (DSM IV), the Mini-Mental Test (MMT), Confusion Assessment Method (CAM)-ICU, or the Mini-Mental State Examination (MMSE).
Table 1.
Characteristics of the included studies.
| Study | Patient recruitment | Country | Sample size | Treatment arms | Age (years) | Study design | Cognitive measurement | Surgery |
|---|---|---|---|---|---|---|---|---|
| Chan et al., 2013 | Jan 2007–Dec 2009 | China | 921 | BIS-guided (462) and control (459) | >60 | RCT | Cognitive Failure Questionnaire (CFQ) | Non-cardiac |
| Radtke et al., 2013 | March 2009–May 2010 | Germany | 1155 | BIS-guided (575) and control (580) | >60 | RCT | Reliable Change Index and neuropsychological test | Non-cardiac |
| Jildenstål et al., 2011 | Jan 2005–April 2008 | Sweden | 450 | AEP-guided (224) and control (226) | 18–92 | RCT | The Mini-Mental Test (MMT) and CFQ | Cardiac or thoracic |
| Whitlock et al., 2014 | Aug 2009-April 2010 | US | 310 | BIS-guided (149) and control (161) | >60 | RCT | Confusion Assessment Method (CAM)-ICU | Cardiac or thoracic |
| Jildenstål et al., 2012 | Sept 2010–Feb 2011 | Sweden | 32 | AEP-guided (16) and control (16) | ∼ 60 | RCT | Mini-Mental State Examination (MMSE), CAM and CFQ | Cardiac or thoracic |
Abbreviations: BIS, bispectral index; AEP, auditory evoked potential; RCT, randomized controlled trial.
Primary analysis
Our primary analyses aimed to compare the effect of cerebral monitoring-guided anaesthesia with a control group using routine care or ECTA on POD and cognitive decline. Meta-analysis using a random-effects model revealed that BIS- or AEP-guided anaesthesia was associated with reduced incidence of POD (Figure 2a). The pooled OR for POD from 5 studies (treatment group vs control group) was 0.51 (95%CI: 0.35–0.76). Significant heterogeneity for POD among the selected studies was detected by I2 test (I2 = 62.34%). A fixed-effects model was used to summarize the evidence for long-term cognitive decline (>3 months) from 2 studies. The pooled OR for cognitive decline was 0.69 (95%CI: 0.49–0.97), suggesting a significant reduction in the incidence of cognitive decline in the group using BIS-guided anaesthesia compared with the control group. No significant heterogeneity for cognitive decline was observed among the included studies (Q test: p = 0.7357). These data were visualized using forest plots (Figure 2a, b).
Figure 2.
Forest plots for the incidence of (a) POD and (b) cognitive decline.
Subgroup analysis
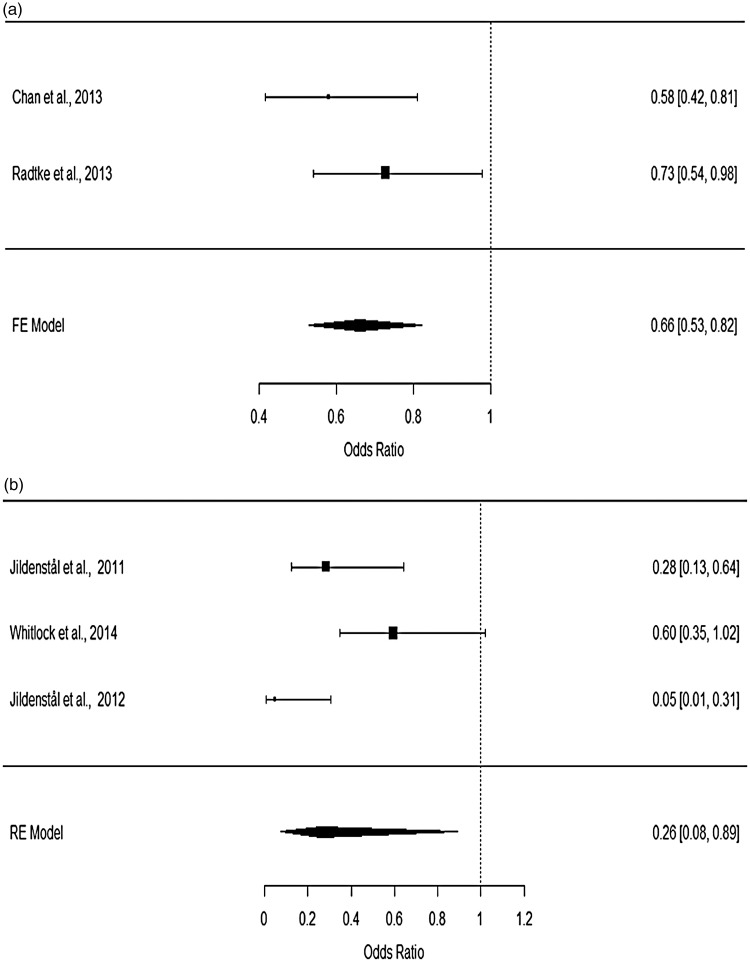
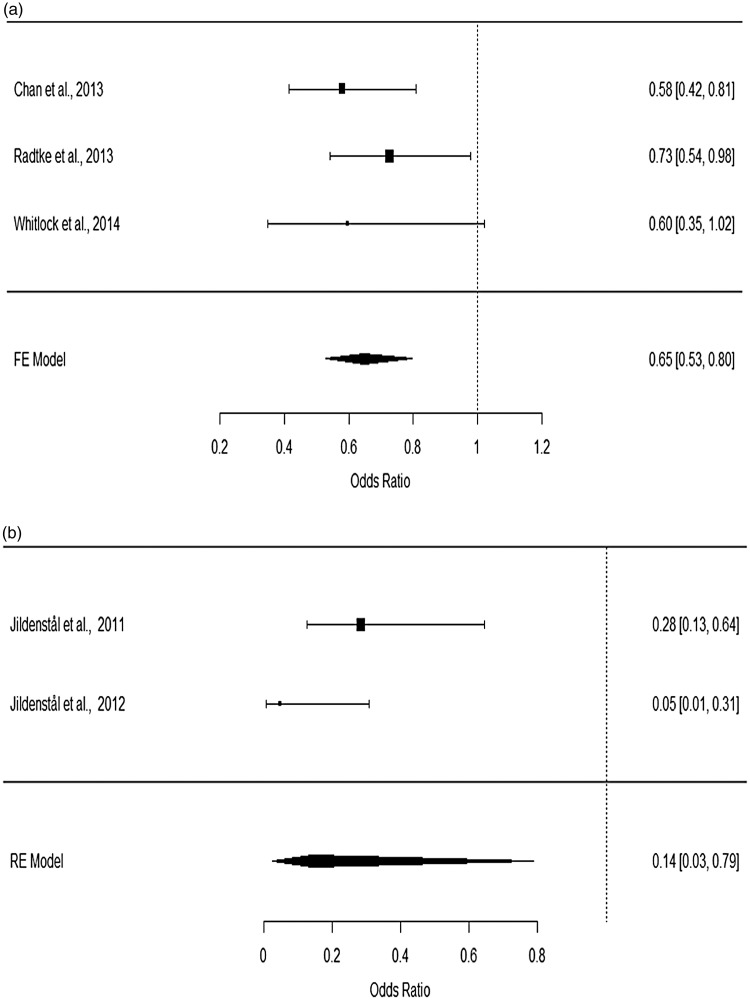
Subgroup analyses were performed based on the specific methods used to monitor the depth of anaesthesia or on the subtypes of major surgery. For patients undergoing non-cardiac surgery, heterogeneity testing showed that the data were consistent (p = 0.3177), while for patients undergoing cardiac and thoracic surgery, significant heterogeneity was detected (p < 0.05; I2 = 81.28). Using a fixed-effects model for patients undergoing non-cardiac surgery and a random-effects model on those undergoing cardiac and thoracic surgery, the pooled ORs for non-cardiac surgery (OR: 0.66; 95%CI: 0.53–0.82) and cardiac surgery (OR: 0.26; 95%CI: 0.08–0.89) were found to be significantly associated with a decreased risk of developing POD (Figure 3). In addition, after dichotomizing the treatment groups into BIS- and AEP-specific groups, no significant difference in reducing the risk of POD was observed between BIS- (OR: 0.65; 95%CI: 0.53–0.80) and AEP-guided anaesthesia (OR: 0.14; 95%CI: 0.03–0.79, Figure 4). Significant heterogeneity was observed in studies using AEP to index the depth of anaesthesia (p = 0.0849; I2 = 66.32%).
Figure 3.
Forest plots for (a) non-cardiac surgery and (b) cardiac and thoracic surgery.
Figure 4.
Forest plots for (a) BIS-guided and (b) AEP-guided titration of anaesthesia.
Publication bias
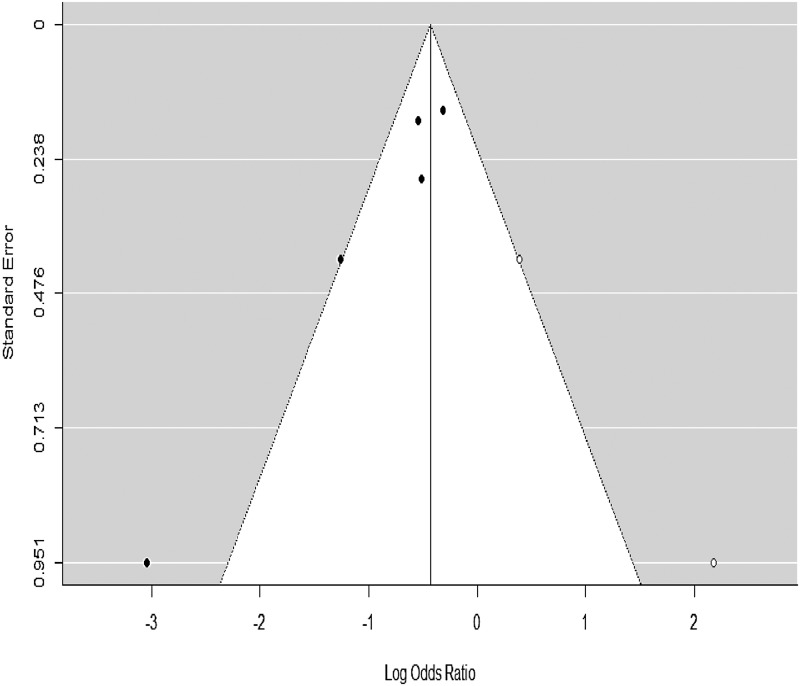
To evaluate the potential for publication bias in our primary analysis on the incident of POD, a funnel plot with trim-fill correction was developed (Figure 5). Using a fail-safe N calculation with the Rosenthal approach, we identified that 62 null studies were required to be added to reduce the significance of the meta-analysis to 0.05. Using a regression test for funnel plot asymmetry, we identified a significant publication bias among the eligible studies (z = 2.13; p < 0.05).
Figure 5.
Funnel plot for assessing publication bias.
Sensitivity analysis
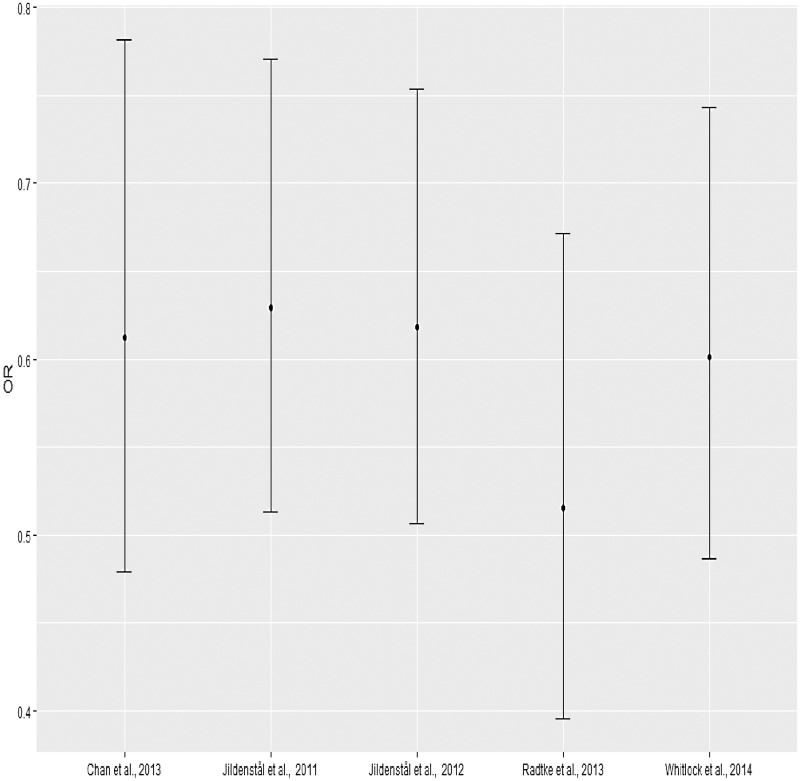
To identify potential outlier trials contributing to the observed heterogeneity, a leave-one-out method was used whereby we repeated the meta-analysis, iteratively removing studies. We found that the removal of any trial in our review did not affect the efficacy of cerebral monitoring-guided anaesthesia observed (Figure 6).
Figure 6.
Sensitivity analysis.
Discussion
Few studies have evaluated whether cognitive side-effects such as POD and cognitive decline in older and fragile patients can be reduced by the use of EEG-based depth-of-anaesthesia monitoring. Furthermore, several of these studies are limited by their single-centre design, small sample size, and ambiguous results. Therefore, the meta-analysis in the present study was performed to summarize the evidence on using cerebral monitoring during anaesthesia to reduce the incidence of postoperative cognitive dysfunction.
In our systematic review, we included 5 RCTs with a total sample size of 2868 patients to evaluate the efficacy of cerebral monitoring-guided anaesthesia in reducing the risk of POD or long-term cognitive decline. The main findings in our systematic review include that 1) BIS-guided or AEP-guided anaesthesia is associated with a significant decrease in the risk of POD and long-term cognitive decline, 2) BIS-guided and AEP-guided anaesthesia did not differ significantly in their ability to reduce the risk of POD, 3) significant heterogeneity was identified among studies in patients undergoing cardiac and thoracic surgery but not in patients undergoing non-cardiac surgery, 4) the removal of any trial from the analysis did not alter the outcome of the meta-analysis on POD, and 5) significant publication bias was identified among the POD data from the 5 clinical trials.
These conclusions are based on a limited number of studies, and there was no evidence of the comparative effectiveness of various monitoring approaches. We included 3 RCTs9,10,12 using BIS-based devices where cerebral monitoring appeared to reduce the risk of POD by excluding extremely low BIS values, which was highly correlated with the incidence of POD. In addition, the absolute and relative numbers of the average burst suppression ratio and BIS value < 20 were found to be increased in the routine care (control) group while the mean BIS value across the two groups did not differ significantly.
The cause of postoperative cognitive dysfunction has not been fully determined. Possible risk factors include hypoxemia; hypotension; abnormal preoperative serum sodium, potassium, or glucose levels; alcohol abuse; diminished cerebral oxygenation extraction; and decreased regional cerebrovenous oxygenation. Surgery may confound these risk factors and the outcome of postoperative cognitive dysfunction. A previous study has shown that the incidence of POD depends on the type of surgery.14
This systematic review and meta-analysis had several limitations: 1) we included different types of EEG-based devices for a single intervention, and this may have affected heterogeneity; 2) multiple methods were used to measure POD and cognitive decline; 3) for the same intervention, we disregarded the dose and duration of titration of anaesthesia; 4) included trials did not specify the subtype of POD; and 5) we only compared the efficacy of reducing postoperative cognitive dysfunction and POD between cerebral monitoring-guided and EACT-guided depth of anaesthesia protocols or routine care, while the effects of different anaesthetic depths under cerebral monitoring on reducing postoperative cognitive dysfunction and POD efficacy were not evaluated because of insufficient data, an important consideration for future research. These limitations may have contributed to significant publication bias and introduced uncertainty to the study findings.
In summary, we identified significant heterogeneity in the data for cardiac and thoracic surgery, but the overall OR did not differ significantly for the two types of surgery. Future studies using screening tools to classify the subtypes of delirium and evaluate their relationship with different types of intervention are required, as well as studies to validate the findings related to the use of EEG-based devices in older patients. Moreover, given that low-risk and high-risk patients may show different sensitivities to anaesthesia, clinical trials should dichotomize the study population into different levels of risk in their study design.
Declaration of conflicting interests
The authors declare that there is no conflict of interest.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults. American Geriatrics Society abstracted clinical practice guideline for postoperative delirium in older adults. J Am Geriatr Soc 2015; 63: 142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palanca BJ, Mashour GA, Avidan MS. Processed electroencephalogram in depth of anesthesia monitoring. Curr Opin Anaesthesiol 2009; 22: 553–559. [DOI] [PubMed] [Google Scholar]
- 3.Recart A, Gasanova I, White PF, et al. The effect of cerebral monitoring on recovery after general anesthesia: a comparison of the auditory evoked potential and bispectral index devices with standard clinical practice. Anesth Analg 2003; 97: 1667–1674. [DOI] [PubMed] [Google Scholar]
- 4.Punjasawadwong Y, Boonjeungmonkol N, Phongchiewboon A. Bispectral index for improving anaesthetic delivery and postoperative recovery. Cochrane Database Syst Rev. 2007; 17: CD003843. [DOI] [PubMed] [Google Scholar]
- 5.Sprung J, Roberts RO, Knopman DS, et al. Mild cognitive impairment and exposure to general anesthesia for surgeries and procedures: a population-based case-control study. Anesth Analg 2017; 124: 1277–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang C, Zhang Y, Shen Y, et al. Anesthesia/surgery induces cognitive impairment in female Alzheimer's disease transgenic mice. J Alzheimers Dis 2017; 57: 505–518. [DOI] [PubMed] [Google Scholar]
- 7.Sprung J, Roberts RO, Knopman DS, et al. Association of mild cognitive impairment with exposure to general anesthesia for surgical and nonsurgical procedures: a population-based study. Mayo Clin Proc 2016; 91: 208–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heinke W, Koelsch S. The effects of anesthetics on brain activity and cognitive function. Curr Opin Anaesthesiol 2005; 18: 625–631. [DOI] [PubMed] [Google Scholar]
- 9.Radtke FM, Franck M, Lendner J, et al. Monitoring depth of anaesthesia in a randomized trial decreases the rate of postoperative delirium but not postoperative cognitive dysfunction. Br J Anaesth 2013; 110(Suppl 1): i98–i105. [DOI] [PubMed] [Google Scholar]
- 10.Chan MT, Cheng BC, Lee TM, et al. BIS-guided anesthesia decreases postoperative delirium and cognitive decline. J Neurosurg Anesthesiol 2013; 25: 33–42. [DOI] [PubMed] [Google Scholar]
- 11.Jildenstal PK, Hallen JL, Rawal N, et al. Effect of auditory evoked potential-guided anaesthesia on consumption of anaesthetics and early postoperative cognitive dysfunction: a randomised controlled trial. Eur J Anaesthesiol 2011; 28: 213–219. [DOI] [PubMed] [Google Scholar]
- 12.Whitlock EL, Torres BA, Lin N, et al. Postoperative delirium in a substudy of cardiothoracic surgical patients in the BAG-RECALL clinical trial. Anesth Analg 2014; 118: 809–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jildenstål PK, Hallén JL, Rawal N, et al. Does depth of anesthesia influence postoperative cognitive dysfunction or inflammatory response following major ENT surgery? J Anesth Clin Res 2014; 3: 6. [Google Scholar]
- 14.Marcantonio ER, Goldman L, Mangione CM, et al. A clinical prediction rule for delirium after elective noncardiac surgery. JAMA 1994; 271: 134–139. [PubMed] [Google Scholar]