Short abstract
Objective
This study was performed to determine the cut-off point of the Functional Independence Measure (FIM) to discriminate patients with acute stroke who develop adverse events during their stay in a stroke care unit (SCU).
Methods
All consecutive patients with stroke admitted to a single institute from January to March 2015 were enrolled. They were divided into two groups according to their average daily energy intake in the SCU: ≥66% or <66% of the target (high- and low-energy group, respectively). A receiver operating characteristic curve was used to determine the cut-off point of the FIM to predict adverse events in patients with acute stroke.
Results
The length of stay in the SCU was significantly longer and the serum C-reactive protein level (CRP) was significantly higher in the low- than high-energy group (7 vs. 4 days and 2.15 vs. 0.20 mg/dL, respectively). The total FIM score cut-off value was 63 points.
Conclusions
An energy intake of <66% of the target was associated with a significantly longer stay in the SCU and a higher CRP level. A total FIM score cut-off value of 63 points is useful to discriminate patients with adverse events among those with acute stroke.
Keywords: Stroke, energy intake, outcome, Functional Independence Measure, cut-off point, adverse events, receiver operating characteristic curve
Introduction
Stroke is a leading cause of death and functional disability.1 Each year, more than 700,000 strokes occur in the United States and 300,000 occur in Japan.1,2 Based on recent data regarding these two countries’ populations (325,886,000 and 126,748,000, respectively),3 the annual occurrence rate of stroke is 215 per 100,000 population in the United States and 237 per 100,000 population in Japan. These rates are very similar despite differences in genetics and lifestyle. Thus, the relatively common occurrence of stroke makes it worthwhile to study how to improve activities of daily living (ADL) following stroke not only in the Unites States but also in other regions such as Asia. After acute stroke, physical function is a key indicator of quality of life and should be appropriately assessed. In these patients, swallowing disability may lead to malnutrition associated with adverse events such as pneumonia, longer hospitalization, and poor rehabilitation outcomes.4 During the early period of a patient’s stay in a neuro-intensive care unit (neuro-ICU) or stroke care unit (SCU) after acute stroke onset, nutritional management is reportedly critical to prevent malnutrition and poor functional recovery.5,6 Another study also showed that proper energy intake is independently associated with improvement in ADL6 and reduction in the mortality rate.7 However, the proper amount of energy to prevent adverse events during the early period after stroke has not been studied.8–12
Upon admission to the SCU, the physical dependence of patients with acute stroke is often measured using several scales, mainly the following four: the Barthel Index (BI), developed in 1955; the Rankin Scale (RS), developed in 1957; the modified RS (mRS), revised in 1988; and the Functional Independence Measure (FIM), developed in 1989. Each of these scales involves summing all items to obtain a numerical score. Among them, the BI and FIM include the eating function, whereas the RS and mRS do not. Additionally, compared with the BI, the FIM includes only the cognitive functions of communication and memory. Because the eating function requires not only swallowing but communication, the FIM was developed even after the BI had become widespread among rehabilitation areas. The total FIM score is reportedly effective and reliable for assessment of the severity of physical functional disability and the burden of care in inpatient rehabilitation settings.13 Research on the total FIM score and achievements in nutritional therapies after acute stroke has also shown that the eating function in the FIM is associated with physical function recovery.14 Another study revealed that nutritional improvement was strongly associated with functional recovery.15
In this context, we performed the present study to examine the hypothesis that nutritional management with an energy intake of <66% of the energy target is associated with a poor outcome during a stay in the SCU. This cut-off point of 66% of the energy target was set according to a guideline of nutrition support for critically ill adult patients16 and observational studies.17,18 Whether the cut-off point on the total FIM score at admission to the SCU is associated with poor outcomes was also determined.
Methods
Patients
This study was designed as a retrospective observational chart review. All consecutive patients enrolled in this study had been admitted to the SCU in a single institution from January 2015 to March 2015 with a diagnosis of stroke. The exclusion criteria were a <7-day length of stay in the SCU and missing data for the FIM scores, height, or weight. Approval for the study was obtained from the ethics committee of the study institution (approval number: 120038). Given the nature of this study, the requirement for informed patient consent was considered unnecessary.
Data collection
The following data were collected from the medical records on the day of SCU admission:
Demographic parameters, including age, sex, height, body weight, body mass index, primary diagnosis (cerebral infarction or cerebral hemorrhage), and Charlson Comorbidity Index score (a comorbidities index including hypertension, diabetes, dyslipidemia, cerebrovascular disease, and 15 other diseases)
National Institutes of Health Stroke Scale (NIHSS) score19
FIM score (both the NIHSS and FIM total scores were evaluated on admission by a licensed physical therapist or occupational therapist, and the details of the FIM structure are described later)
Laboratory data during the SCU stay, including the serum albumin and C-reactive protein (CRP) levels
Nutritional parameters, including energy intake during the first 7 days after admission to the SCU (expressed as the average daily energy intake during the first 7 days after admission to the SCU in kcal/kg of actual body weight/day) and whether oral intake was available or not
Outcome parameters. The primary outcome was the length of stay in the SCU, and the secondary outcomes were the highest CRP level during the first 7 days in the SCU and the presence of a CRP level of ≥6.0 mg/dL20 during the stay in the SCU. The length of stay in the SCU was determined by reference to the guide to resource allocation of intensive monitoring and care proposed by the American College of Critical Care Medicine.21 Patients were able to be discharged from the SCU when their hemodynamic and life-threatening conditions had stabilized to allow movement to a lower-acuity area or step-down unit.
Analysis 1
All patients were divided into two groups according to their average daily energy intake during the first 7 days in the SCU: ≥66% (E-H group) or <66% (E-L group) of the target energy. All collected data were compared between the two groups. The target energy intake was set at 25 kcal/kg of actual body weight/day as proposed by the guidelines of the American Society for Parenteral and Enteral Nutrition16 and European Society for Parenteral and Enteral Nutrition.22
Analysis 2
Assuming that one group defined in Analysis 1 showed a significantly lower FIM score associated with adverse events during the stay in the SCU, we used a receiver operating characteristic (ROC) curve for the total FIM score at admission along with its sensitivity and specificity to determine the cut-off point of the FIM score at admission to predict adverse events in patients with acute stroke.
Statistical analysis
The outcome parameters in the two groups were divided into different categories and compared using the Mann–Whitney U test for continuous variables and the chi-square test or Fisher’s exact test for categorical variables. In the ROC curve analysis, the area under the curve (AUC) and the 95% confidence interval (CI) were also determined. The AUC with a 95% CI was considered if the AUC was 1.0, and an AUC of 0.5 was not considered to be confident. The point with the larger Youden index, equal to sensitivity + specificity − 1, was defined as the superior cut-off point. Two-sided p values of <0.05 were considered statistically significant. All analyses were performed using SPSS Statistics version 24 (IBM Corp., Armonk, NY, USA), and significance was examined at P < 0.05.
Results
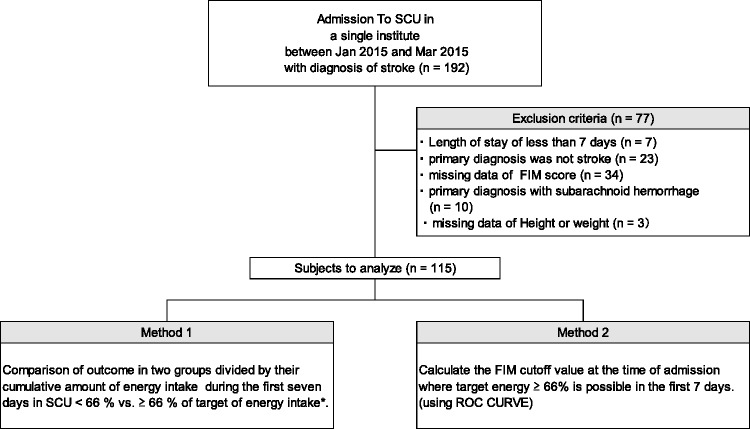
In total, 192 patients were enrolled. After 77 patients were excluded according to the above-described exclusion criteria, the remaining 115 patients underwent Analyses 1 and 2 (Figure 1). The patients’ demographics are shown in Table 1. The total and motor and cognitive subscale scores in the low- and high-energy groups are shown in Table 2. All except three motor items showed significant differences.
Figure 1.
Flowchart of the study. SCU, stroke care unit; FIM, Functional Independence Measure; ROC, receiver operating characteristic curve.
Table 1.
Demographic and anthropometric characteristics, stroke types, NIHSS scores, and comorbidity indexes in the low- and high-energy intake groups
| Total (n = 115) | Low (n = 40) | High (n = 75) | P value | |
|---|---|---|---|---|
| Demographic | ||||
| Age, years | 71 (62, 78) | 72 (59, 79) | 70 (63, 78) | 0.567 |
| Male sex | 78 (68) | 30 (75) | 48 (64) | 0.296 |
| Anthropometric parameters | ||||
| Height, cm | 163 (154, 169) | 165 (156, 170) | 163 (154, 168) | 0.320 |
| Weight, kg | 61 (51, 70) | 64 (55, 74) | 60 (51, 69) | 0.182 |
| BMI, kg/m2 | 23 (21, 26) | 24 (21, 27) | 23 (21, 25) | 0.320 |
| Type of stroke | <0.001 | |||
| Cerebral infarction | 75 (65) | 14 (35) | 61 (81) | |
| Cerebral hemorrhage | 40 (35) | 26 (65) | 14 (19) | |
| NIHSS score | 5 (2, 12) | 15 (7, 17) | 3 (1, 6) | <0.001 |
| Comorbidity | ||||
| CCI | 2 (1, 4) | 3 (2, 4) | 2 (1,4) | 0.169 |
| Old cerebral infarction | 32 (28) | 8 (2) | 24 (32) | 0.196 |
Data are expressed as median (25%, 75% quartile) or n (%).
Target energy intake of <66% (low-energy group) vs. ≥66% (high-energy group): Mann–Whitney U test and chi-square test or Fisher’s exact test for categorical variables. Target energy was set at 25 kcal/kg of actual body weight/day.
Low, low-energy intake group; High, high-energy intake group; BMI, body mass index; CCI, Charlson comorbidity index; NIHSS, National Institutes of Health Stroke Scale; SCU, stroke care unit.
Table 2.
FIM scores at admission between the low- and high-energy intake groups
| Total (n = 115) | Low (n = 40) | High (n = 75) | P value | ||
|---|---|---|---|---|---|
| Nutrition domain | |||||
| % of target energy intake | 80 (50, 97) | 30 (14, 53) | 93 (84, 104) | <0.001 | |
| Oral intake without EN/PN, n (%) | 77 (67) | 22 (55) | 55 (73) | 0.061 | |
| Admission FIM score | |||||
| Total-FIM | 75 (33, 91) | 21 (18, 51) | 88 (70, 99) | <0.001 | |
| Motor-FIM | 44 (16, 57) | 13 (13, 28) | 57 (44, 64)* | <0.001 | |
| Cognitive-FIM | 31 (16, 35) | 7 (5, 25) | 34 (30, 35) | <0.001 | |
| FIM scores | Subcategories | ||||
| Motor 1. Eating | Self-care | 5 (1, 5) | 1 (1, 4) | 5 (3, 5) | <0.001 |
| Motor 2. Grooming | Self-care | 1 (1, 1) | 1 (1, 1) | 5 (3, 7) | <0.001 |
| Motor 3. Bathing | Self-care | 1 (1, 1) | 1 (1, 1) | 1 (1, 1) | 0.848 |
| Motor 4. Dress upper body | Self-care | 3 (1, 5) | 1 (1, 1) | 4 (3, 4) | <0.001 |
| Motor 5. Dress lower body | Self-care | 4 (1, 4) | 1 (1, 1) | 4 (3, 5) | <0.001 |
| Motor 6. Toileting | Self-care | 4 (1, 5) | 1 (1, 1) | 5 (4, 7) | <0.001 |
| Motor 7. Bladder management | Sphincter control | 7 (1, 7) | 1 (1, 1) | 7 (7, 7) | <0.001 |
| Motor 8. Bowel management | Sphincter control | 7 (1, 7) | 1 (1, 1) | 7 (7, 7) | <0.001 |
| Motor 9. Bed/chair | Transfers (mobility) | 5 (1, 5) | 1 (1, 1) | 5 (5, 7) | <0.001 |
| Motor 10. Toilet | Transfers (mobility) | 5 (1, 5) | 1 (1, 1) | 5 (4, 7) | <0.001 |
| Motor 11. Tub/shower | Transfers (mobility) | 1 (1, 1) | 1 (1, 1) | 1 (1, 1) | 0.848 |
| Motor 12. Walk/wheelchair | Locomotion | 1 (1, 5) | 1 (1, 1) | 5 (1, 6) | <0.001 |
| Motor 13. Stairs | Locomotion | 1 (1, 1) | 1 (1, 1) | 1 (1, 1) | 0.177 |
| Cognitive 14. Comprehension | Communication | 7 (4, 7) | 3 (1, 6) | 7 (6, 7) | <0.001 |
| Cognitive 15. Expression | Communication | 7 (3, 7) | 2 (1, 5) | 7 (7, 7) | <0.001 |
| Cognitive 16. Social interaction | Social cognition | 7 (3, 7) | 1 (1, 6) | 7 (7, 7) | <0.001 |
| Cognitive 17. Problem-solving | Social cognition | 5 (3, 7) | 1 (1, 4) | 7 (5, 7) | <0.001 |
| Cognitive 18. Memory | Social cognition | 6 (2, 7) | 1 (1, 5) | 7 (5, 7) | <0.001 |
All data except oral intake are expressed as median (25%, 75% quartile).
Low, energy intake of <66% of target; High, energy intake of ≥66% of target (target energy was set at 25 kcal/kg of actual body weight); FIM, Functional Independence Measure.
Results of Analysis 1
The comparison of energy intake during the first 7 days after admission to the SCU showed that the length of stay in the SCU was significantly longer and the CRP level was significantly higher in the low- than high-energy group (7 vs. 4 days and 2.15 vs. 0.20 mg/dL, respectively; both P < 0.001) (Table 3).
Table 3.
Comparison of clinical outcomes in the low- and high-energy intake groups
| Total (n = 115) | Low (n = 40) | High (n = 75) | P value | |
|---|---|---|---|---|
| Length of stay in SCU, days | 5 (3, 8) | 7 (5, 10) | 4 (3, 6) | <0.001 |
| Serum CRP, mg/dL | 0.60 (0.12, 2.60) | 2.15 (1.00, 5.41) | 0.20 (0.10, 0.85) | <0.001 |
| Serum CRP of ≥6.0 mg/dL | 14 (12) | 10 (25) | 4 (5) | 0.005 |
Data are expressed as median (25%, 75% quartile) or n (%).
CRP, C-reactive protein; Low, energy intake of <66% of target; High, energy intake of ≥66% of target; SCU, stroke care unit.
Percent of target energy intake of <66% vs. ≥66%: Mann–Whitney U test and the chi-square test or Fisher’s exact test for categorical variables; target energy was set at 25 kcal/kg of actual body weight.
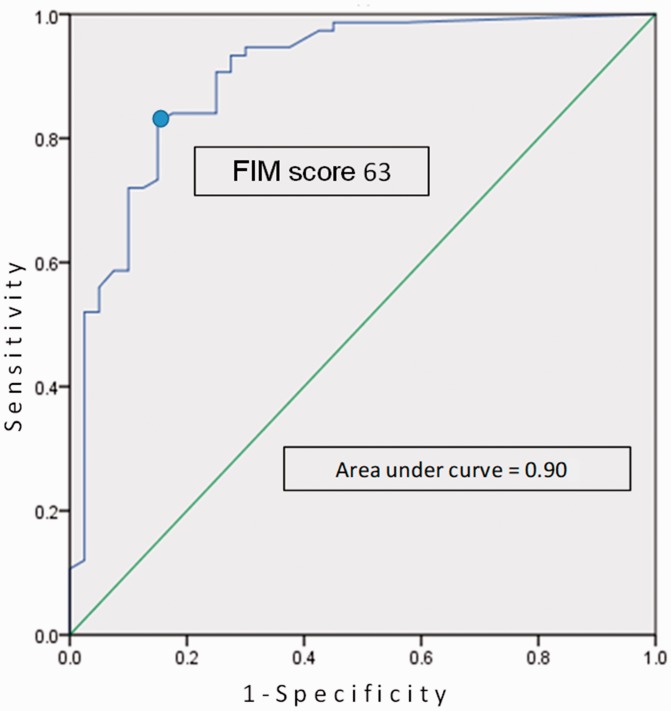
Results of Analysis 2
The ROC curve analysis showed that the cut-off point of the total FIM score at admission was 63 points. The AUC, sensitivity, and specificity were 0.90, 82.7%, and 85.0%, respectively (Figure 2).
Figure 2.
Determination of the cut-off point of the Functional Independence Measure (FIM) using a receiver operating characteristic curve. The cut-off point of the total FIM scale to discriminate patients with poor outcomes was calculated as 63 points, with an area under the curve, sensitivity, and specificity of 0.90, 82.7%, and 85.0%, respectively.
Discussion
FIM score as a predictor of functional recovery following stroke
The NIHSS is the most widely used deficit rating scale in modern neurology and is one of several stroke severity evaluation scales. Since its effectiveness was reported by Brott et al.19 in 1989, it has been used frequently in clinical practice and clinical research. The higher the score of each item, the greater the severity, and the maximum score is 42 points. The influence of the nutritional status on the clinical outcome after acute stroke has also been evaluated, and one study showed that the serum albumin level and mRS score were strong, independent predictors of mortality at 3 months after acute stroke [hazard ratio, 0.91 (95% CI, 0.84–0.99) and 1.63 (95% CI, 1.20–2.22), respectively].23 However, a systematic review comparing multiple functional evaluation assessment tools, such as the mRS, BI, NIHSS, and others, proved that the FIM is the most clinically accurate predictor of functional outcomes in post-stroke populations.1 This was one of the reasons that we chose the FIM in the present study, setting a cut-off point to identify post-stroke patients who showed adverse events.
FIM as a disability scale of ADL
Multiple scales have been developed to measure ADL after acute stroke. The BI, RS, mRS, FIM, and NIHSS use numbers to represent the sum of all items involved. All but the NIHSS are assessment tools to measure functional disabilities; the NIHSS was developed to assess the severity of stroke. The BI score was developed more than 30 years ago and is calculated from the amount of time and assistance required by a patient.24 Since its development, the FIM has become increasingly more widespread because it includes items to assess cognitive functions.25 The FIM comprises 18 items: 13 define disabilities in motor functions, and 5 define disabilities in cognitive functions (Table 2). Each item is rated on a 7-point scale [7 = complete independence (performed in a timely and safe manner), 6 = modified independence (using a device), 5 = supervision, 4 = minimal assistance (subject 75%+), 3 = moderate assistance (subject 50%+), 2 = maximal assistance (subject 25%+), and 0 = total assistance (subject 0%+)].24 A recent systematic review of 3,260 articles reported that the FIM score measured at admission has been well validated to predict functional ability during rehabilitation periods.26
Determination of the cut-off FIM score to predict adverse events in patients with acute stroke
In general, a cut-off point is used to identify subjects with a binary opposite status, such as “presence” and “absence” of disease, morbidity, or mortality. In the present study, when the total FIM score of post-stroke patients at admission was <63 points (as the cut-off point drawn from the study), he or she might have a high likelihood of adverse events, such as a significantly longer stay in the SCU or a higher CRP level. To identify a relevant cut-off point in the clinical setting, an ROC curve is applied in biomedical research to evaluate the effectiveness and accuracy of the measurement method used.27,28 The accuracy of determining a cut-off point using an ROC curve seems high enough to validate.30 In contrast, however, a previously reported study of 106 patients with acute stroke determined an FIM cut-off point of 38.29 (95% CI, 34.07–42.25) and 70.62 (95% CI, 66.65–75.22) for discriminating severe and moderate disability, respectively.4 The difference in cut-off points between the previously reported study and ours (shown in Figure 2) might have occurred because of the difference in the study aims. We aimed to distinguish patients with adverse events, whereas the authors of the previous study aimed to classify patients by severity of functional disability. In this context, our study might be the first to show a cut-off FIM score with which to predict the possibilities of adverse events, such as a longer stay in the ICU and a higher CRP level with infectious events after acute stroke.
Association between lower energy intake during early period in ICU and adverse events
The reason why patients in the low-energy group had a significantly lower energy intake might be related to several factors associated with their functional and metabolic conditions. One reason might involve the physical functional impairments as shown by significantly lower total FIM scores at admission (Table 2). Additionally, a significantly lower eating function score, which was included in the FIM motor score (Table 2), might have been associated with significantly poorer eating function in the low-energy group [expressed in oral intake without enteral or parenteral support (%)] than in the high-energy group (Table 2). The association of a lower energy intake with significantly poorer eating function suggests that patients in the low-energy group with low oral energy intake might have easily developed aspiration pneumonia. In a previously reported study that examined factors influencing aspiration pneumonia in older adults, the odds ratio of aspiration pneumonia in older adults with deteriorated swallowing function was 3.584 (95% CI, 1.948–6.502).29 Moreover, dysphagia has also been reported as an independent risk factor for mortality.30 The latter study also showed that the odds ratios of mortality at 30 days and 1 year after admission were 3.43 (95% CI, 1.34–8.79) and 7.99 (95% CI, 3.43–18.6), respectively. In the present study, the significantly lower energy intake associated with a longer stay in the SCU and higher CRP level in the low-energy group might have been related to the significantly lower total FIM score and eating score. In the other words, the low-energy group might have been unable to tolerate a higher amount of energy compared with the high-energy group. As a result, a significantly higher CRP level and malnutrition might occur in patients with low energy intake during the acute period after stroke. In our study, malnutrition at discharge could not be estimated because the study institute was an advanced acute stroke hospital and the length of hospital stay was too short. Malnourished patients with stroke reportedly have a significantly higher incidence of pneumonia (P = 0.048).31 Motor disability, aspiration pneumonia, and malnutrition seem to form a vicious circle in patients with stroke. This might be interpreted that dysphagia as estimated by the FIM motor score and malnutrition following stroke seem to be risk factors and discharge outcome indicators in the acute period.32–35 However, because swallowing dysfunction and the incidence of aspiration pneumonia were not investigated in our study, further studies must be conducted to examine these relationships.
Limitations of the study
This study has several limitations. First, it was conducted as a retrospective observational study. Therefore, we did not examine whether patients with an FIM point of <63 points and who were nutritionally managed with an energy intake of ≥66% of the target showed a decrease in the incidences of adverse events. As a result, we also did not assess whether the relationship between the total FIM point at admission and the average energy intake during the acute period was due to causality or association. Further prospective validation studies for the cut-off FIM points must be conducted to examine whether the predicted poor clinical outcomes can be changed or improved by intensive energy management of ≥66% of the target. Second, patients with a diagnosis of subarachnoid hemorrhage were excluded because these patients have less direct motor neuronal damage than the patients with cerebral infarction or hemorrhage who were included in this study. The main purpose of this study was to determine the cut-off FIM points at admission; therefore, only patients with pathological entities that directly invade cerebral neurons were included. To confirm the effectiveness of the FIM and its cut-off point with which to identify patients who might have a poor outcome in the neuro-ICU, further studies including patients with subarachnoid hemorrhage must also be conducted. Third, the types of strokes in the low- and high-energy groups were significantly different. The high-energy group, in which the SCU stay was shorter and the CRP value was lower, consisted of 81% of patients with cerebral infarction; in contrast, the low-energy group consisted of 35% of patients with cerebral infarction. A previous study showed that the FIM score at admission in patients with cerebral infarction was not significantly different from that in patients with cerebral hemorrhage;32 this was also observed also in our study (Table 1). Further prospective studies must be designed to equally allocate the types of strokes into two groups. Fourth, the difference in outcomes of this study might be explained by the difference in the FIM scores at admission between the two groups. The total FIM score in the low-energy group was significantly lower than that in the high-energy group (21 vs. 88, respectively; P < 0.001) (Table 1). This lower FIM score in the low-energy group might have been associated with the patients’ poor outcomes. To test the possibility of an association between the FIM score and energy intake in patients with acute stroke, prospective random allocation of patients into low- and high-energy intake groups is necessary. Finally, the sample size was too small to draw definitive conclusions. Studies with a larger sample size are necessary.
Conclusion
For patients with acute stroke, an energy intake of <66% of the target (equal to 16.5 kcal/kg of actual body weight/day) during the first 7 days after admission may be associated with a significantly longer stay in the SCU and a higher CRP level. Additionally, a cut-off point of 63 points on the FIM scale at admission might be a predictor of adverse events, such as a longer stay in the SCU and a higher CRP level, as a result of infectious comorbidities (mainly aspiration pneumonia) among patients with acute stroke.
Authors’ contributions
Study design: Kurokawa N, Kai C, Amagai T
Data collection: Kurokawa N.
Data analysis: Kurokawa N, Hokotachi Y, Hasegawa M, Amagai T.
Writing of the manuscript: Kurokawa N, Amagai T.
Declaration of Conflicting Interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Chumney D, Nollinger K, Shesko K, et al. Ability of functional independence measure to accurately predict functional outcome of stroke-specific population: systematic review. J Rehabil Res Dev 2010; 47: 17–29. [DOI] [PubMed] [Google Scholar]
- 2.Takashima N, Arima H, Kita Y, et al. Incidence, management and short-term outcome of stroke in a general population of 1.4 million Japanese - Shiga stroke registry. Circ J 2017; 81: 1636–1646. [DOI] [PubMed] [Google Scholar]
- 3.Economics/Macroeconomics/country macro data. https://countryeconomy.com/countries/compare/japan/usa?sc=XE23. Accessed on 29 Jun 2018.
- 4.Balasch i Bernat M, Balasch i Parisi S, Sebastián EN, et al. Determining cut-off points in functional assessment scales in stroke. NeuroRehabilitation 2015; 37: 165–172. [DOI] [PubMed] [Google Scholar]
- 5.Nishida Y, Wakabayashi H, Maeda K, et al. Nutritional status is associated with the return home in a long-term care health facility. J Gen Fam Med 2018; 19: 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nishioka S, Wakabayashi H, Momosaki R. Nutritional status changes and activities of daily living after hip fracture in convalescent rehabilitation units: a retrospective observational cohort study from the Japan rehabilitation nutrition database. J Acad Nutr Diet 2018; 118: 1270–1276. pii: S2212-2672(18)30252-1. [DOI] [PubMed] [Google Scholar]
- 7.Wakita M, Wakayama A, Omori Y, et al. Impact of energy intake on the survival rate of patients with severely ill stroke. Asia Pac J Clin Nutr 2013; 22: 474–481. [PubMed] [Google Scholar]
- 8.Franzosi OS, von Frankenberg AD, Loss SH, et al. Underfeeding versus full enteral feeding in critically ill patients with acute respiratory failure: a systematic review with meta-analysis of randomized controlled trials. Nutr Hosp, 2017; 34: 19–29. [DOI] [PubMed] [Google Scholar]
- 9.Arabi YM, Tamim HM, Dhar GS, et al. Permissive underfeeding and intensive insulin therapy in critically ill patients: a randomized controlled trial. Am J Clin Nutr 2011; 93: 569–577. [DOI] [PubMed] [Google Scholar]
- 10.Rice TW, Mogan S, Hays MA, et al. Randomized trial of initial trophic versus full-energy enteral nutrition in mechanically ventilated patients with acute respiratory failure. Crit Care Med 2011; 39: 967–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network, Rice TW, Wheeler AP, et al. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA 2012; 307: 795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casaer MP, Mesotten D, Hermans G, et al. Early versus late parenteral nutrition in critically Ill adults. N Engl J Med 2011; 365: 506–517. [DOI] [PubMed] [Google Scholar]
- 13.Granger CV, Hamilton BB, Linacre JM, et al. Performance profiles of the functional independence measure. Am J Phys Med Rehabil 1993; 72: 84–89. [DOI] [PubMed] [Google Scholar]
- 14.Mizrahi EH, Arad M, Weiss A, et al. Eating management and functional outcome of elderly patients with symptomatic ischemic stroke undergoing inpatient rehabilitation. Geriatr Gerontol Int 2013; 13: 701–705. [DOI] [PubMed] [Google Scholar]
- 15.Nii M, Maeda K, Wakabayashi H, et al. Nutritional improvement and energy intake are associated with functional recovery in patients after cerebrovascular disorders. J Stroke Cerebrovasc Dis 2016; 25: 57–62. [DOI] [PubMed] [Google Scholar]
- 16.Taylor BE, McClave SA., Martindale RG, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) . Crit Care Med 2016; 44: 390–438. doi: 10.1097/CCM.0000000000001525. [DOI] [PubMed] [Google Scholar]
- 17.Stapleton RD, Jones N, Heyland DK. Feeding critically ill patients: what is the optimal amount of energy? Crit Care Med 2007; 35: S535–S540. [DOI] [PubMed] [Google Scholar]
- 18.Levy MM, Artigas A, Phillips GS, et al. Outcomes of the surviving sepsis campaign in intensive care in the USA and Europe: a prospective cohort study. Lancet Infect Dis 2012; 12: 919–924. [DOI] [PubMed] [Google Scholar]
- 19.Brott T, Adams HP, Jr, Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke 1989; 20: 864–870. doi: 10. 1161/01. STR. 20. 7. 864. [DOI] [PubMed] [Google Scholar]
- 20.Liu A, Van Nguyen H, Ong B, et al. Serum C-reactive protein as a biomarker for early detection of bacterial infection in the older patient. Age Ageing 2010; 39: 559–565 [DOI] [PubMed] [Google Scholar]
- 21.Nates JL, Nunnally M, Kleinpell R, et al. ICU admission, discharge, and triage guidelines: a framework of enhance clinical operations, development of institutional policies, and future research. Crit Care Med 2016; 44: 1553–1602. [DOI] [PubMed] [Google Scholar]
- 22.Kreymann KG, Berger MM, Deutz NE, et al. ESPEN guidelines on enteral nutrition: intensive care. Clin Nutr 2006; 5: 210–223. [DOI] [PubMed] [Google Scholar]
- 23.Cariballa SE, Parker SG, Taub N, et al. Influence of nutritional status on clinical outcome after acute stroke. Am J Clin Nutr 1998; 68: 275–281. [DOI] [PubMed] [Google Scholar]
- 24.Langhammer B, Sunnerhagen KS, Lundgren-Nilsson Å, et al. Factors enhancing activities of daily living after stroke in specialized rehabilitation: an observational multicenter study within the Sunnaas International Network. Eur J Phys Rehabil Med 2017; 53: 725–734. [DOI] [PubMed] [Google Scholar]
- 25.Kidd D, Stewart G, Baldry J, et al. The functional independence measure: a comparative validity and reliability study. Disabil Rehabil 1995; 17: 10–14. [DOI] [PubMed] [Google Scholar]
- 26.Meyer MJ, Pereira S, McClure A. A systematic review of studies reporting multivariable models to predict functional outcomes after post-stroke inpatient rehabilitation. Disabil Rehabil 2015; 37: 1316–1323. [DOI] [PubMed] [Google Scholar]
- 27.Schisterman EF, Perkins NJ, Liu A, et al. Optimal cut-point and its corresponding Youden Index to discriminate individuals using pooled blood samples. Epidemiol 2005; 16: 73–81. [DOI] [PubMed] [Google Scholar]
- 28.Swets JA. Measuring the accuracy of diagnostic systems. Science 1988; 240: 1285–1293. [DOI] [PubMed] [Google Scholar]
- 29.Manabe T, Teramoto S, Tamiya N, et al. Risk factors for aspiration pneumonia in older adults. PLoS One 2015; 10: e0140060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cabre M, Serra-Prat M, Palomera E, et al. Prevalence and prognostic implications of dysphagia in elderly patients with pneumonia. Age Ageing 2010; 39: 39–45. [DOI] [PubMed] [Google Scholar]
- 31.Nishioka S, Okamoto T, Takayama M, et al. Malnutrition risk predicts recovery of full oral intake among older adult stroke patients undergoing enteral nutrition: secondary analysis of a multicentre survey (the APPLE study). Clin Nutr 2017; 36: 1089–1096. [DOI] [PubMed] [Google Scholar]
- 32.Smithard DG, O’Neill PA, Parks C, et al. Complication and outcome after acute stroke. Does dysphagia matter? Stroke 1996; 27: 1200–1204. [DOI] [PubMed] [Google Scholar]
- 33.Foley NC, Martin RE, Salter KL, Teasell RW. A review of the relationship between dysphagia and malnutrition following stroke. J Rehabil Med 2009; 41: 707–713. [DOI] [PubMed] [Google Scholar]
- 34.Nishioka S, Wakabayashi H, Nishioka E, et al. Nutritional improvement correlated with recovery of activities of daily living among malnourished elderly stroke patients in the convalescent stage: a cross-sectional study. J Acad Nutr Diet 2016; 116: 837–843. [DOI] [PubMed] [Google Scholar]
- 35.Brown AW, Therneau TM, Schultz BA, et al. Measure of functional independence dominates discharge outcome prediction after inpatient rehabilitation for stroke. Stroke 2015; 46: 1038–1044. doi: 10.1161/STROKEAHA.114.007. [DOI] [PubMed] [Google Scholar]