Short abstract
Objective
To investigate the association between time from hospital admission to intensive care unit (ICU) admission (door to ICU time) and hospital mortality in patients with sepsis.
Methods
This retrospective observational study included routinely collected healthcare data from patients with sepsis. The primary endpoint was hospital mortality, defined as the survival status at hospital discharge. Door to ICU time was calculated and included in a multivariable model to investigate its association with mortality.
Results
Data from 13 115 patients were included for analyses, comprising 10 309 survivors and 2 806 non-survivors. Door to ICU time was significantly longer for non-survivors than survivors (median, 43.0 h [interquartile range, 12.4, 91.3] versus 26.7 h [7.0, 74.2]). In the multivariable regression model, door to ICU time remained significantly associated with mortality (odds ratio [OR] 1.11, 95% confidence interval [CI] 1.006, 1.017) and there was a significant interaction between age and door to ICU time (OR 0.99, 95% CI 0.99, 1.00).
Conclusion
A shorter time from hospital door to ICU admission was shown to be independently associated with reduced hospital mortality in patients with severe sepsis and/or septic shock.
Keywords: Sepsis, septic shock, mortality
Introduction
Severe sepsis and/or septic shock are important contributors to hospital mortality and morbidity.1–3 Investigators and clinicians continue to make every effort to combat these syndromes and patients with such disorders typically require multidisciplinary interventions.3–5 Management of patients with severe sepsis and/or septic shock includes, but is not limited to, early initiation of antibiotics, early goal-directed therapy, infection source control, protective mechanical ventilation and use of renal replacement therapy.6–8 Although the effectiveness of some of these interventions remains under debate, the multidisciplinary approach mandates close monitoring and intensive treatment.
The timing of intensive care unit (ICU) admission is associated with mortality outcome for critically ill patients,9–11 and delayed ICU admission has been related to increased risk of death for critically ill patients who require close monitoring and intensive treatment.12 In a mixed ICU cohort, patients who stayed in the emergency department (ED) for > 5 h before ICU admission were 2.5 times more likely to die than those with ED length of stay (LOS) < 5 h.12 The Surviving Sepsis Campaign guidelines recommend early goal-directed therapy for septic shock, aiming to provide early control of infection and adequate peripheral perfusion,13 however, several large trials investigating the effectiveness of early goal-directed therapy have reported neutral results.14,15 While neutral results may be due to an ineffective intervention, other explanations may include low quality monitoring and intensive care in the ED. This may be why early goal-directed therapy trials conducted in the ED have reported neutral results,14 whereas those performed in the ICU have shown beneficial effects of early goal-directed therapy.16,17 For patients with severe sepsis, it is important to transfer them into the ICU to receive intensive care.
The present authors hypothesized that prompt admission to the ICU will provide additional benefits for patients with severe sepsis and septic shock. In analogy to the concept of door to balloon time for myocardial infarction, the aim of the present study was to investigate the concept of time from hospital door to ICU admission (door to ICU time), and its association with patient outcome, in patients with severe sepsis and/or septic shock.
Patients and methods
Critical care database
This retrospective, observational study included de-identified patient data from the critical care big data Medical Information Mart for Intensive Care (MIMIC)-III database, a large, single-centre database comprising information relating to patients admitted to critical care units at Beth Israel Deaconess Medical Centre in Boston, MA, USA, a large tertiary care hospital.18,19 The database comprises 57 328 distinct admissions for adult patients (aged ≥16 years) admitted to ICUs between June 2001 and October 2012.18
The study employed the publicly available dataset from MIMC-III, for which ethical approval has been provided previously by the Massachusetts Institute of Technology (Cambridge, MA, USA) and the Institutional Review Boards of Beth Israel Deaconess Medical Centre (Boston, MA, USA). Requirement for individual patient consent was waived because the project did not impact clinical care and all protected health information was deidentified.
Study population
According to the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3), sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection,20 and corresponds to earlier definitions of severe sepsis and septic shock. The present study population was defined as those patients with documented or suspected infection, plus organ dysfunction.21,22 A patient was defined as having organ dysfunction if he or she had any of the following International Classification of Diseases, Ninth Revision (ICD-9) codes: unspecified thrombocytopenia (2875), hypotension (458), acute and subacute necrosis of liver (570), acute kidney failure (584), anoxic brain damage (3481), shock without mention of trauma (7855), encephalopathy (3483), transient mental disorders due to conditions classified elsewhere (293), secondary thrombocytopenia (2874), other and unspecified coagulation defects (2869), defibrination syndrome (2866), and hepatic infarction (5734). Requirement of mechanical ventilation (procedures ICD code: 9670, 9671, or 9672) was also defined as organ dysfunction. The method was adapted from Angus et al., 2001,23 and the SQL code used for data retrieval is available at https://github.com/MIT-LCP.
Demographic and laboratory variables
The following variables were extracted from the MIMIC- III database: age at hospital admission, sex, admission type, sequential organ failure assessment (SOFA) score (calculated on the first 24 h following ICU admission), SOFA score components, use of vasopressors and renal replacement therapy. Laboratory variables included platelet count, activated partial thromboplastin time (aPTT), international normalized ratio (INR), creatinine level, urine output (UO) for the first 24 h, Glasgow coma scale (GCS), mean blood pressure (BP), vasopressors, and arterial partial oxygen pressure (PaO2). If a variable was measured more than once in the first 24 h, the value associated with the greatest severity of illness was used. For example, the lowest mean BP and GCS were used in the study.
The primary endpoint was hospital mortality, which was defined as the status of survival at hospital discharge.
Missing values
Variables with >30% of values missing were excluded from the analysis. Single imputation was performed for missing values to account for mean and variance of the measured values.24 Specifically, missing values were replaced by values randomly drawn from the non-missing values. A set.seed function was used for reproducibility of the procedure.
Statistical analyses
Patients were divided into survivor and non-survivor subgroups based on hospital mortality. Demographic and clinical data are presented as n (%) prevalence for categorical data or median (interquartile range [IQR]) for continuous data. Categorical variables were compared using Pearson's χ2-test with Yates' continuity correction and continuous variables were compared using Student’s t-test.25 The Compare Baseline Characteristics Between Groups (CBCgrps) software package, version 2.1 (https://cran.r-project.org/web/packages/CBCgrps/index.html) was employed for statistical description and bivariate inference.26
Conservative variable selection strategy was used for multivariable analysis due to the large sample size. Variables with a P value <0.02 in bivariate statistical inference were candidates for inclusion into the logistic regression model. Timing of ICU admission may have different effects on mortality outcome for different subgroups of patients, thus, it was hypothesized that door to ICU time and SOFA would interact, assuming that patients with different severities of illness respond differently to door to ICU time. Age has long been recognized as an important predictor of mortality for ICU patients, and patients in different age groups often respond differently to a given therapeutic intervention or risk factor.27,28 Thus, an interaction term between door to ICU time and age was also included in the multivariable model. After derivation of the final model, model validation and calibration were performed. Bootstrapping was used to obtain bias-corrected estimates of predicted probability of death, which was then compared to the observed values based on a non-parametric smoother. Model diagnostics were performed using influence, leverage and outliers (Cook’s distance).29
All statistical analyses were performed using R software, version 3.2.3 (https://cran.r-project.org/). The Regression Modelling Strategies (rms) software package, version 5.1-2 was used for modelling.30 A P-value < 0.05 was considered to be statistically significant.
Results
Patient selection and baseline characteristics
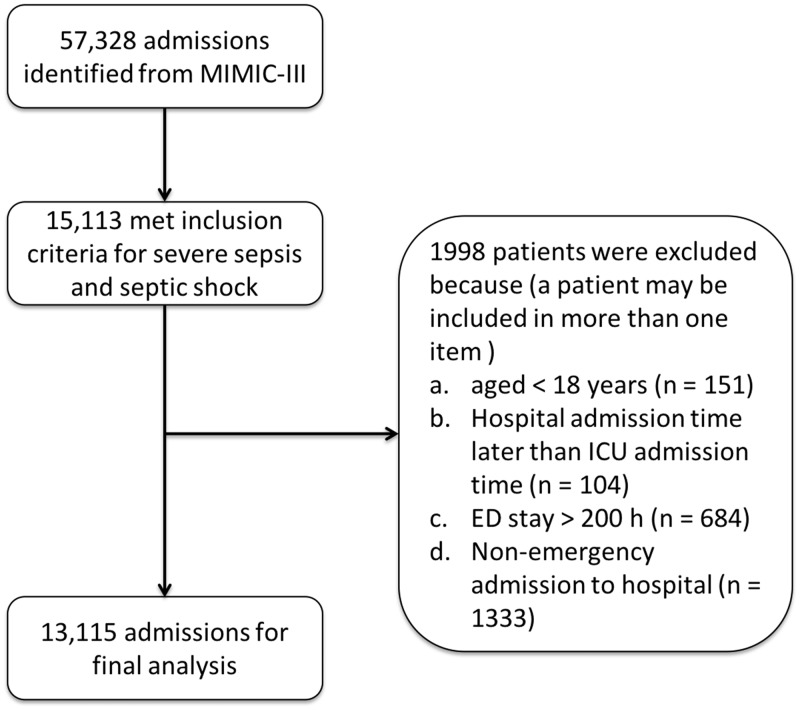
An initial search of the MIMIC-III database revealed 57 328 admissions, in which those with more than one ICU entry had already been excluded. A total of 15 113 patients met the inclusion criteria for severe sepsis and/or septic shock, among whom, 1 998 were then excluded due to the following: (1) aged <18 years, (2) hospital admission time later than ICU transfer time, (3) ED stay > 200 h or (4) non-emergency admission; resulting in 13 115 patients for final analyses (Figure 1).
Figure 1.
Flowchart of patient selection from the Medical Information Mart for Intensive Care (MIMIC)-III database. Of 57 328 admissions initially identified, hospital admissions with more than one intensive care unit (ICU) entry had already been excluded; ED, emergency department.
Baseline characteristics and comparisons between survivors and non-survivors are summarized in Table 1. There were slightly more male patients in the non-survivor versus survivor group (54.5% versus 52.5%, respectively) but the difference was not statistically significant. Non-survivors were significantly older than survivors (median, 74.0 versus 67.9 years; P <0.001) and had higher SOFA scores (median score, 7 versus 5; P <0.001), and every SOFA component score (respiration, coagulation, liver, cardiovascular, central nervous system and renal system) was higher in non-survivors. More patients in the non-survivor group required renal replacement therapy (16.4% versus 8.5%; P < 0.001) and mechanical ventilation (69.3% versus 48.4%; P < 0.001) than in the survivor group. Non-survivors had more comorbidity burdens such as metastatic cancer (10.4% versus 3.7%; P < 0.001) and congestive heart failure (33.2% versus 28.0%; P < 0.001) than survivors, however, obesity and chronic pulmonary disease appeared to be protective factors (4.1% versus 6.2% and 22.4 versus 24.3, respectively; P ≤ 0.037). Non-survivors spent more time in the ICU than survivors (median [IQR], 5 [2, 10] versus 3 [2, 8] days; P <0.001), and door to ICU time was significantly longer (43.0 [12.4, 91.3] versus 26.7 [7.0, 74.2] h; P <0.001). Furthermore, a higher proportion of patients in the survivor group were transferred to the ICU within 1 h (door to ICU time < 1 h) than those in the non-survivor group (69.8% versus 67%; P = 0.005), and out of 9 074 patients transferred within 1 h, 79.3% (7 194) survived and 20.7% (1 880) did not.
Table 1.
Comparison of demographic and clinical characteristics between survivors and nonsurvivors in a cohort of 13 115 adult patients admitted to the ICU and diagnosed with sever sepsis/septic shock
| Study group |
||||
|---|---|---|---|---|
| Clinical variable | Overall(n = 13 115) | Survivor(n = 10 309) | Nonsurvivor(n = 2 806) | Statistical significance* |
| Sex, male | 6942 (52.9) | 5414 (52.5) | 1528 (54.5) | NS |
| Age, years | 69.1 (55.9, 80.4) | 67.9 (54.7, 79.5) | 74.0 (61.3, 82.8) | P < 0.001 |
| SOFA score | 5 (3, 7) | 5 (3, 7) | 7 (4, 10) | P < 0.001 |
| Respiration | 2 (0, 3) | 2 (0, 3) | 3 (0, 3) | P < 0.001 |
| Coagulation | 0 (0, 1) | 0 (0, 1) | 0 (0, 2) | P < 0.001 |
| Liver | 0 (0, 1) | 0 (0, 1) | 0 (0, 2) | P < 0.001 |
| Cardiovascular system | 1 (1, 3) | 1 (1, 1) | 1 (1, 4) | P < 0.001 |
| Central nervous system | 0 (0, 1) | 0 (0, 1) | 0 (0, 1) | P < 0.001 |
| Renal system | 1 (0, 2) | 1 (0, 2) | 2 (0, 3) | P < 0.001 |
| Renal replacement therapy | 1335 (10.2) | 875 (8.5) | 460 (16.4) | P < 0.001 |
| Mechanical ventilation | 6931 (52.8) | 4986 (48.4) | 1945 (69.3) | P < 0.001 |
| Comorbidities | ||||
| Congestive heart failure | 3820 (29.1) | 2889 (28.0) | 931 (33.2) | P < 0.001 |
| Chronic pulmonary disease | 3133 (23.9) | 2505 (24.3) | 628 (22.4) | P = 0.037 |
| Obesity | 754 (5.7) | 638 (6.2) | 116 (4.1) | P < 0.001 |
| Weight loss | 841 (6.4) | 679 (6.6) | 162 (5.8) | NS |
| AIDS | 76 (0.6) | 65 (0.6) | 11 (0.4) | NS |
| Metastatic cancer | 671 (5.1) | 380 (3.7) | 291 (10.4) | P < 0.001 |
| ICU length of stay, days | 4 (2, 8) | 3 (2, 8) | 5 (2, 10) | P < 0.001 |
| ICU admission within 1 h (direct admission) | 9074 (69.2) | 7194 (69.8) | 1880 (67.0) | P = 0.005 |
| $Door to ICU time, h | 30.4 (8.0, 79.5) | 26.7 (7.0, 74.2) | 43.0 (12.4, 91.3) | P < 0.001 |
Data presented as n (%) prevalence, or median (interquartile range).
*Categorical variables were compared using Pearson's χ2-test with Yates' continuity correction; continuous variables were compared using Student’s t-test.
$Compared only in patients who were not directly admitted to ICU.
ICU, intensive care unit; Door to ICU time, time between admission to hospital and ICU admission; SOFA, sequential organ failure assessment.
NS, no statistically significant between-group difference (P > 0.05).
Multivariable Logistic regression model
All variables with a P-value <0.02 were included in the multivariable model. Interactions between door to ICU time and SOFA were not statistically significant (data not shown), however, there was significant interaction between age and door to ICU time (odds ratio [OR] 0.99; P = 0.006). ORs of other variables are presented in Table 2. After multivariable adjustment, door to ICU time remained significantly associated with mortality (OR 1.11; P <0.001). Other risk factors included metastatic cancer, age (for each one-year increase), SOFA (for each one-point increase), mechanical ventilation and renal replacement therapy (all P <0.001). Obesity was a protective factor against death (OR 0.69; P = 0.001) (Table 2).
Table 2.
Multivariable logistic regression to assess the impact of door to ICU time on mortality risk, controlling for confounding factors, in a cohort of 13 115 adult patients admitted to the ICU and diagnosed with sever sepsis/septic shock
| Clinical variable | Odds ratio (95% CI) | Statistical significance |
|---|---|---|
| Door to ICU time (each hour delay) | 1.11 (1.006, 1.017) | P < 0.001 |
| Metastatic cancer | 4.31 (3.59, 5.17) | P < 0.001 |
| Age (each year increase) | 1.031 (1.028, 1.034) | P < 0.001 |
| SOFA (each one-point increase) | 1.22 (1.21, 1.24) | P < 0.001 |
| Obesity | 0.69 (0.55, 0.86) | P = 0.001 |
| Mechanical ventilation | 2.45 (2.21, 2.71) | P < 0.001 |
| Renal replacement therapy | 1.40 (1.21, 1.62) | P < 0.001 |
| Door to ICU time*age | 0.99 (0.99, 1.00) | P = 0.006 |
Door to ICU time, time between admission to hospital and ICU admission; ICU, intensive care unit; OR, odds ratio; CI, confidence interval; SOFA, sequential organ failure assessment.
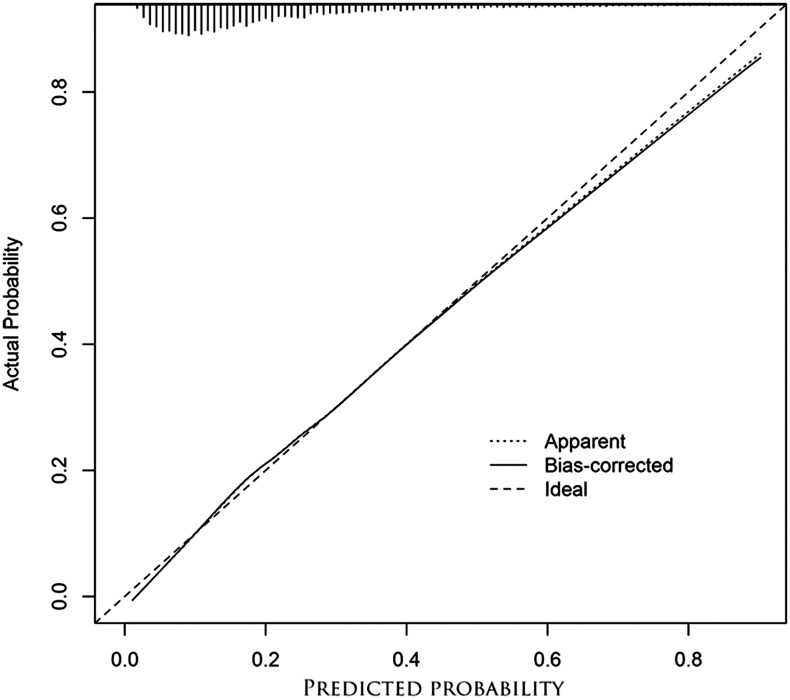
Model calibration was performed by plotting actual probability of death against predicted probability (Figure 2), and predicted probability was shown to fit well with observed probability. Model diagnostics were used to examine outliers and influential observations. Sensitivity analyses revealed that exclusion of two observations with the largest Cook’s distances (considered to be influential observations) did not alter the results (data not shown).
Figure 2.
Model calibration curves showing actual probability of death against predicted probability: Bias-corrected probability was estimated using the bootstrap method, and the ideal line (observed values exactly matched predicted values) was the reference line. Predicted probability fitted well with the observed probability, however, Hosmer–Lemeshow goodness of fit (GOF) test resulted in P = 0.008. Because the sample size was large, even a small difference between observed and predicted values would give a significant P value. Thus, Hosmer–Lemeshow GOF test was not suitable in this situation
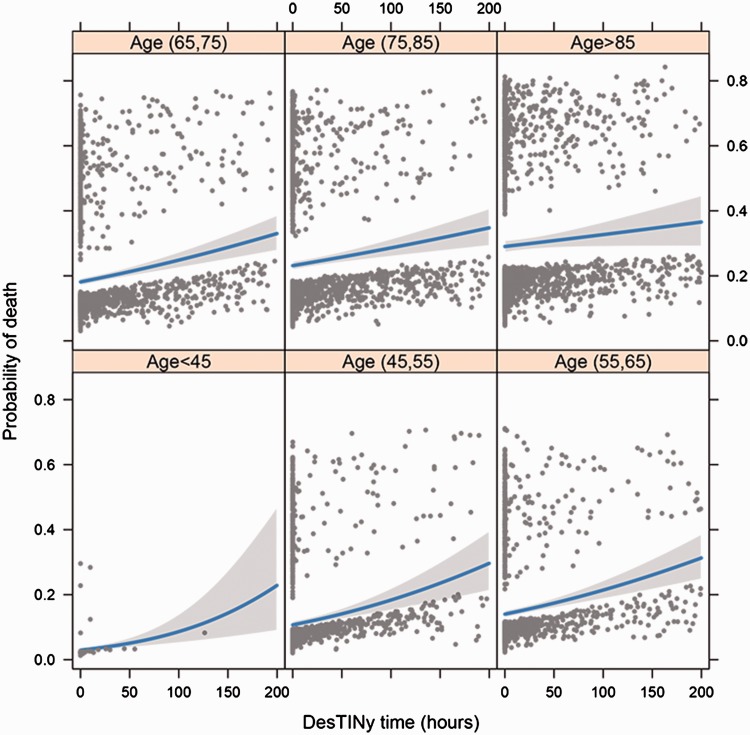
Probability of death was plotted against door to ICU time, stratified by age groups (Figure 3). Younger patients with severe sepsis and/or septic shock were shown to be more sensitive to door to ICU time, i.e., younger patients gained more benefits from urgent admission to ICU. For example, patients aged > 85 years had a probability of survival of 70% and 71% for door to ICU times of 50 h and 1 h, respectively. For patients aged between 45 and 55 years, probability of survival would be 90% and 85% for door to ICU times of 1 h and 50 h, respectively.
Figure 3.
Regression model showing probability of death against door to ICU time, stratified by age groups. Door to ICU time, time between admission to hospital and intensive care unit admission
Discussion
The study showed that door to ICU time was significantly associated with hospital mortality in patients with severe sepsis and/or septic shock. Shorter door to ICU time was associated with reduced risk of hospital death and the effect remained unchanged after multivariable adjustment. Furthermore, there was an interaction between door to ICU time and age. The effect size was larger in younger age groups, suggesting that younger patients with severe sepsis and/or septic shock would benefit more from shorter door to ICU time than the elderly.
The timing of ICU admission for critically ill patients has been investigated in several studies. In patients with community-acquired pneumonia, outcomes of patients who were directly admitted to the ICU were compared with those who were admitted to a ward prior to ICU transfer.9 Those directly managed in the ICU were found to be less likely to die and delayed ICU admission was an important risk factor (OR 9.61).9 Delayed transfer to the ICU with an ED-LOS >5 h is also thought to be an important risk factor of poor outcome (OR 3.8, 95% CI 1.6, 8.8).10 In a large clinical database study involving over 50 000 patients, delayed admission to ICU of > 6 h was found to be associated with lower hospital survival (OR 0.709, 95% CI 0.561, 0.895).10 To the best of the present authors’ knowledge, there is no study investigating the association between timing of ICU admission and mortality outcome in patients with severe sepsis and/or septic shock. Clinical practice guidelines recommended that patients with severe sepsis and/or septic shock should be managed and treated intensively.31 For example, in the first 4 or 6 hours, serum lactate should be closely monitored and predefined lactate clearance should be achieved.32 Other treatment goals include urine output, central venous pressure and central venous (or mixed) oxygen saturation, which may be met with interventions such as fluid bolus, vasopressors, inotropes and red blood cell transfusion.13 A prerequisite of this monitoring and intervention, however, is the adequacy of medical personnel and facilities, such as in the ICU, where close monitoring and intensive treatments can be provided. The present findings support the notion that early initiation of intensive care improves clinical outcomes for patients with severe sepsis and/or septic shock.
The strength of the present study was that it restricted the analyses to patients with severe sepsis and/or septic shock. Critically ill patients with different diagnoses may respond differently to timeliness of ICU admission, and treatment strategies during the early time window vary for different types of diseases. An interaction term between age and door to ICU time was included and showed that the importance of door to ICU time varied for different age groups. Also, the study employed a large clinical database and was pragmatic in design, with the understanding that a recognized gap exists between well-designed trials and pragmatic trials.33 Some interventions showing beneficial biological efficacy in well-designed trials may have no clinical effectiveness in real world settings.34,35 The present study employed critical care big data that reflected conditions in daily clinical practice, and thus, the results may be more applicable to real world settings. Nonetheless, the study was subject to the inherent limitations of observational, retrospective design. Although an attempt was made to control for confounding factors that may impact both the timing of ICU admission and hospital mortality, such as severity of illness, presence of comorbidity and demographics, there may have been unmeasured confounders. Furthermore, all the SOFA scores were calculated after ICU admission and as a result the initial presenting physiological status in the ED (i.e. at first presentation) remained unknown. Those patients transferred to ICU early may have had clinical syndromes that were easier to recognize compared with those who were transferred later.
In conclusion, the study provided evidence that the time between hospital door to ICU admission (door to ICU time) was independently associated with hospital mortality for patients with severe sepsis and/or septic shock. The study supports early recognition of sepsis.
Supplemental Material
Supplemental material for Prompt admission to intensive care is associated with improved survival in patients with severe sepsis and/or septic shock by Qiang Li, Jiajiong Wang, Guomin Liu, Meng Xu, Yanguo Qin, Qin Han, He Liu, Xiaonan Wang, Zonghan Wang, Kerong Yang, Chaohua Gao, Jin-cheng Wang and Zhongheng Zhang in Journal of International Medical Research
Declaration of conflicting interests
The authors declare that there is no conflict of interest.
Funding
This research was funded by grants from the Scientific Development Program of Jilin Province (Nos., 3D5177743429, 3D516D733429 and 20170204004GX).
References
- 1.Kopterides P, Mayr FB, Yende S. Understanding the sepsis mortality belt: time to buckle down! Ann Transl Med 2016; 4: 319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.SepNet Critical Care Trials Group. Incidence of severe sepsis and septic shock in German intensive care units: the prospective, multicentre INSEP study. Intensive Care Med 2016; 42: 1980–1989. [DOI] [PubMed] [Google Scholar]
- 3.Pittard MG, Huang SJ, McLean AS, et al. Association of positive fluid balance and mortality in sepsis and septic shock in an Australian cohort. Anaesth Intensive Care 2017; 45: 737–743. [DOI] [PubMed] [Google Scholar]
- 4.Dellinger RP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med 2008; 36: 296–327. [DOI] [PubMed] [Google Scholar]
- 5.Kumar A, Ellis P, Arabi Y, et al. Initiation of inappropriate antimicrobial therapy results in a fivefold reduction of survival in human septic shock. Chest 2009; 136: 1237–1248. [DOI] [PubMed] [Google Scholar]
- 6.Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001; 345: 1368–1377. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Z, Hong Y, Smischney NJ, et al. Early management of sepsis with emphasis on early goal directed therapy: AME evidence series 002. J Thorac Dis 2017; 9: 392–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang TS, Huang SS, Shyu YC, et al. A procalcitonin-based algorithm to guide antibiotic therapy in secondary peritonitis following emergency surgery: a prospective study with propensity score matching analysis. PLoS One 2014; 9: e90539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phua J, Ngerng WJ, Lim TK. The impact of a delay in intensive care unit admission for community-acquired pneumonia. Eur Respir J 2010; 36: 826–833. [DOI] [PubMed] [Google Scholar]
- 10.Chalfin DB, Trzeciak S, Likourezos A, et al. Impact of delayed transfer of critically ill patients from the emergency department to the intensive care unit. Crit Care Med 2007; 35: 1477–1483. [DOI] [PubMed] [Google Scholar]
- 11.Rincon F, Mayer SA, Rivolta J, et al. Impact of delayed transfer of critically ill stroke patients from the Emergency Department to the Neuro-ICU. Neurocrit Care 2010; 13: 75–81. [DOI] [PubMed] [Google Scholar]
- 12.García-Gigorro R, de la Cruz Vigo F, Andrés-Esteban EM, et al. Impact on patient outcome of emergency department length of stay prior to ICU admission. Med Intensiva 2017; 41: 201–208. [DOI] [PubMed] [Google Scholar]
- 13.Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 2013; 39: 165–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.ARISE Investigators, ANZICS Clinical Trials Group, Peake SL, Delaney A, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med 2014; 371: 1496–1506. [DOI] [PubMed] [Google Scholar]
- 15.Angus DC, Barnato AE, Bell D, et al. A systematic review and meta-analysis of early goal-directed therapy for septic shock: the ARISE, ProCESS and ProMISe Investigators. Intensive Care Med 2015; 41: 1549–1560. [DOI] [PubMed] [Google Scholar]
- 16.Early Goal-Directed Therapy Collaborative Group of Zhejiang Province. The effect of early goal-directed therapy on treatment of critical patients with severe sepsis/septic shock: a multi-center, prospective, randomized, controlled study. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 2010; 22: 331–334 [in Chinese, English abstract]. [PubMed] [Google Scholar]
- 17.Chen ZQ, Jin YH, Chen H, et al. Early goal-directed therapy lowers the incidence, severity and mortality of multiple organ dysfunction syndrome. Nan Fang Yi Ke Da Xue Bao 2007; 27: 1892–1895 [in Chinese, English abstract]. [PubMed] [Google Scholar]
- 18.Johnson AE, Pollard TJ, Shen L, et al. MIMIC-III, a freely accessible critical care database. Sci Data 2016; 3: 160035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Z. Accessing critical care big data: a step by step approach. J Thorac Dis 2015; 7: 238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016; 315: 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Z, Hong Y. Development of a novel score for the prediction of hospital mortality in patients with severe sepsis: the use of electronic healthcare records with LASSO regression. Oncotarget 2017; 8: 49637–49645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Z, Hong Y, Liu N, et al. Association of do-not-resuscitate order and survival in patients with severe sepsis and/or septic shock. Intensive Care Med 2017; 43: 715–717. [DOI] [PubMed] [Google Scholar]
- 23.Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001; 29: 1303–1310. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Z. Missing data imputation: focusing on single imputation. Ann Transl Med 2016; 4: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Z. Univariate description and bivariate statistical inference: the first step delving into data. Ann Transl Med 2016; 4: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Z, Gayle AA, Wang J, et al. Comparing baseline characteristics between groups: an introduction to the CBCgrps package. Ann Transl Med 2017; 5: 484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burri E, Hochholzer K, Arenja N, et al. B-type natriuretic peptide in the evaluation and management of dyspnoea in primary care. J Intern Med 2012; 272: 504–513. [DOI] [PubMed] [Google Scholar]
- 28.Villar J, Ambrós A, Soler JA, et al. Age, PaO2/FIO2, and plateau pressure score: a proposal for a simple outcome score in patients with the acute respiratory distress syndrome. Crit Care Med 2016; 44: 1361–1369. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Z. Residuals and regression diagnostics: focusing on logistic regression. Ann Transl Med 2016; 4: 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harrell FE., Jr. rms v5.1-2: Regression Modeling Strategies. https://www.rdocumentation.org/packages/rms/versions/5.1-2 (2018).
- 31.Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med 2017; 43: 304–377. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Z, Xu X. Lactate clearance is a useful biomarker for the prediction of all-cause mortality in critically ill patients: a systematic review and meta-analysis*. Crit Care Med 2014; 42: 2118–2125. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Z. Big data and clinical research: perspective from a clinician. J Thorac Dis 2014; 6: 1659–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Z, Ni H, Xu X. Observational studies using propensity score analysis underestimated the effect sizes in critical care medicine. J Clin Epidemiol 2014; 67: 932–939. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Z, Ni H, Xu X. Do the observational studies using propensity score analysis agree with randomized controlled trials in the area of sepsis? J Crit Care 2014; 29: 886.e9–886.e15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material for Prompt admission to intensive care is associated with improved survival in patients with severe sepsis and/or septic shock by Qiang Li, Jiajiong Wang, Guomin Liu, Meng Xu, Yanguo Qin, Qin Han, He Liu, Xiaonan Wang, Zonghan Wang, Kerong Yang, Chaohua Gao, Jin-cheng Wang and Zhongheng Zhang in Journal of International Medical Research