Short abstract
Retroperitoneal malignant schwannomas are extremely rare. Only a few cases have been reported, only one of which occurred in a child. We herein report a case of retroperitoneal malignant schwannoma in a 2-year-old boy who presented with a painless mass in the right lumbar region. The mass had gradually enlarged during a 1-year period, and it was about the size of the patient’s fist at the time of consultation. Whole-abdomen computed tomography revealed a space-occupying lesion in the retroperitoneum infiltrating from the L1 to L4 spinal canal. A preoperative diagnosis of a retroperitoneal tumor was made, and complete tumorectomy was performed. Postoperative pathological examination showed a malignant schwannoma. The tumor recurred 1 month after the first operation, and a second complete excision was carried out; the postoperative pathologic examination findings were similar to the previous findings. The patient recovered well and continued to undergo close follow-up.
Keywords: Retroperitoneal schwannoma, malignant, childhood, computed tomography, tumorectomy, recurrence
Introduction
Schwannomas are usually benign, and most of them are located in the head and neck region.1 Only 0.5% to 5.0% of all schwannomas occur in the retroperitoneum.2 Retroperitoneal malignant schwannomas are extremely rare, and only a few cases have been reported. Moreover, only one such case has involved a child;3 the present report describes the second case of retroperitoneal malignant schwannoma in a child. We also present a review of the published literature on the epidemiology, pathogenesis, diagnosis, treatment, and prognosis of this rare condition to enhance the awareness and cognition of this disease among physicians in clinical practice.
Case report
This article was a case report and literature review; it involved no ethical issues and did not require ethics approval. We obtained written informed consent from the patient’s parents. This consent statement is provided in the supplementary material.
A 2-year-old boy presented to our hospital in August 2017 for examination of a mass located in the right lumbar region. It had been discovered 1 year previously and had gradually enlarged without apparent pain. The mass was about the size of the patient’s fist at the time of consultation. Physical examination showed no obvious abnormalities of the skin, and the boy was otherwise well. A routine examination showed that the mass had a soft, smooth surface with a fixed base. Whole-abdomen computed tomography (CT) revealed a space-occupying lesion in the retroperitoneum infiltrating from the L1 to L4 spinal canal (Figure 1). Examination of tumor markers showed a neuron-specific enolase level of 29.150 ng/mL, which was slightly high; all other markers were normal. A retroperitoneal tumor was diagnosed, and surgical tumorectomy was carried out. During the operation, we found that the parenchyma of the tumor had an integrated capsule with its roots entering the spinal canal of the right L2 and L3 intervertebral foramens. The whole tumor was macroscopically resected during the operation. Postoperative pathologic examination showed a malignant tumor composed of spindle cells with large areas of necrosis. Immunohistochemical examination revealed that vimentin, SOX10, and S100 were strongly positive (Figure 2) while Olig-2, P53, EMA, Neu-N, and Syn were negative. Finally, the patient was diagnosed with a malignant schwannoma and recovered well. He was discharged from the hospital on postoperative day 8. One month later, however, the tumor recurred and presented as a lumbar mass. He presented to our hospital again, and abdominal CT showed both an intraspinal and paraspinal space-occupying lesion with bone destruction of the vertebral body and its appendage (Figure 3). Consequently, a second complete retroperitoneal macroscopic tumorectomy was performed. During the operation, we found that the mass was hard and rugged, measured about 7 × 6 × 3 cm, and was located behind the psoas muscle, clinging to the spine without apparent infiltration to the abdominal cavity or kidney. The tumor was completely resected again, and the pathological examination results were very similar to the previous findings, indicating a retroperitoneal malignant schwannoma (Figure 4). The postoperative period was uneventful. Unfortunately, the tumor recurred for the second time about 5 weeks after the second operation. At this time, the patient visited a high-class hospital in Shanghai and underwent a third complete resection followed by histological confirmation. Although we were unable to obtain the specific examination results and postoperative pathology findings of the third hospitalization, the patient recovered well and had been followed up for 5 months at the time of this writing.
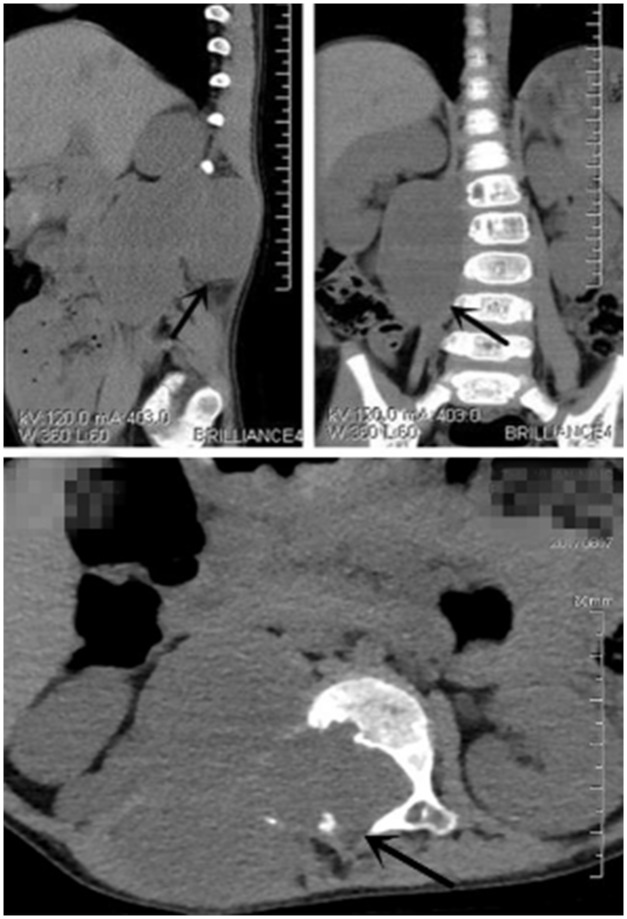
Figure 1.
Whole-abdomen computed tomography before the first operation revealed a space-occupying lesion in the retroperitoneum infiltrating from the L1 to L4 spinal canal.
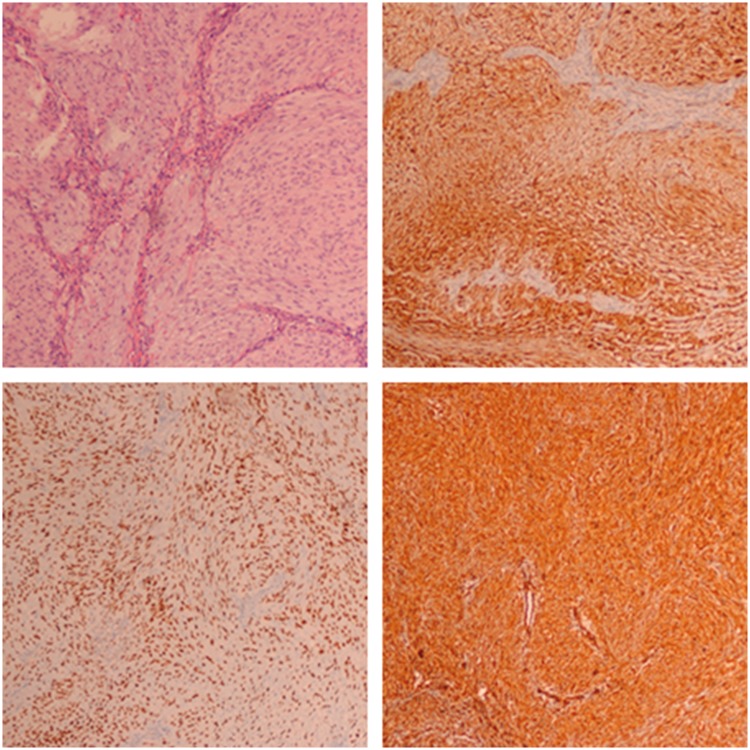
Figure 2.
Postoperative pathologic examination after the first operation showed a malignant tumor composed of spindle cells with large areas of necrosis, and immunohistochemical examination revealed that vimentin, SOX10, and S100 were strongly positive.
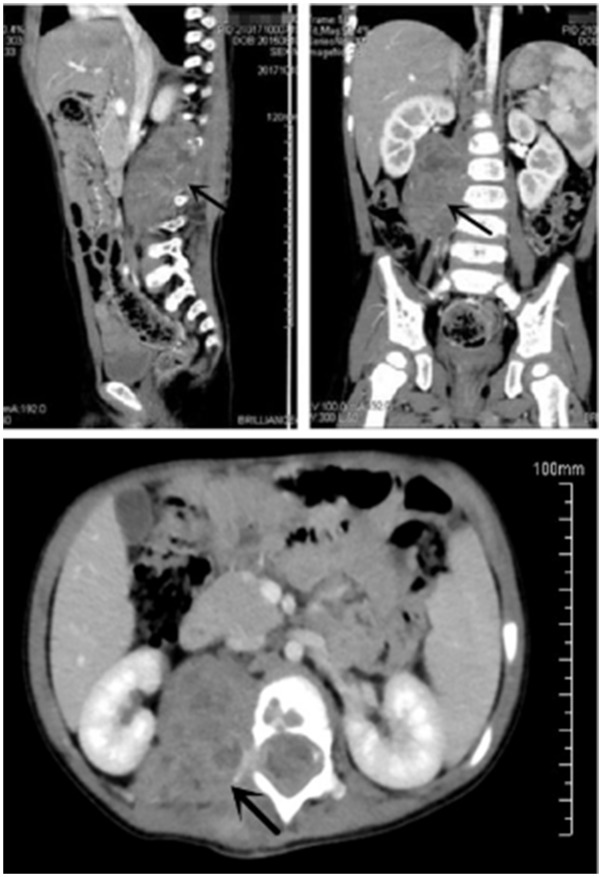
Figure 3.
Abdominal computed tomography before the second operation showed both an intraspinal and paraspinal space-occupying lesion with bone destruction of the vertebral body and its appendage.
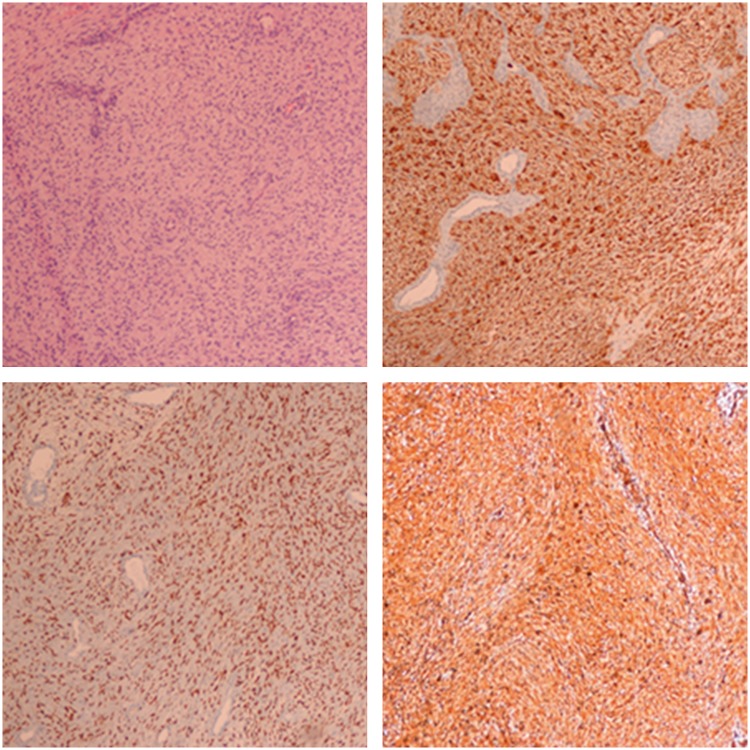
Figure 4.
Postoperative pathologic examination after the second operation showed a low-degree malignant tumor composed of spindle cells, and immunohistochemical examination revealed that vimentin, SOX10, and S100 were strongly positive.
Discussion
Schwannomas arise from Schwann cells of the peripheral nerve sheath.4 These tumors are usually benign and grow slowly.5 Most of them are located in the peripheral, motor, sympathetic, or cranial nerves of the head and neck region.1 Schwannomas have been found at almost every location of the body, including the lower extremities, retroperitoneum, spinal column, mediastinum, abdominal cavity, and pelvis. However, these tumors rarely occur in the retroperitoneum; this location accounts for about 0.5% to 5.0% of all schwannomas.2 Schwannomas in the retroperitoneum occur most commonly from 40 to 60 years of age with a male:female ratio of 2:3.6 Malignant retroperitoneal schwannomas, especially in children, are extremely rare; only one such tumor has previously been reported in a child.3
The molecular pathogenesis of schwannomatosis may be attributed to co-involvement of multiple tumor suppressor genes such as SMARCB1, LZTR1, and others.7 Malignant schwannomas are frequently associated with neurofibromatosis type 1 (NF-1), also called von Recklinghausen’s disease, while NF-1–associated malignant schwannomas have clinical features that differ from those of sporadic schwannomas and require more careful management.8 In addition, because of the larger size of extracranial schwannomas, Cury et al.5 considered that these tumors were likely to manifest secondary to degenerative changes such as cysts and calcification.
Preoperative diagnosis of retroperitoneal schwannomas is challenging because these tumors can mimic many common primary lesions.1 Numerous studies involving large-sample analysis have been performed.9,10 The symptoms of retroperitoneal schwannomas are variable due to the flexibility of the retroperitoneal cavity, and most affected patients are asymptomatic; this often results in the discovery of a substantially large tumor at the time of consultation.11 Preoperative diagnosis mainly depends on radiological examinations, such as CT and magnetic resonance imaging (MRI).1,12,13 CT scans often show cystic changes, which are more common in retroperitoneal schwannomas (about 66%) and rare in other retroperitoneal tumors.12 MRI can show low signal intensity on T1-weighted images and heterogeneous high signal intensity on T2-weighted images.1,13 Although both CT and MRI are nonspecific in the diagnosis of retroperitoneal schwannomas, they are of a certain clinical value in the establishment of differential diagnoses as well as distinguishing malignant from benign lesions.1,10 Fine needle aspiration and CT- or ultrasound-guided biopsy have been proven invalid for this tumor because of the high cellular pleomorphism and increased risk of hemorrhage, infection, and tumor seeding.5,14,15
The definitive diagnosis is dependent upon pathological examination.1 The diagnosis of retroperitoneal malignant schwannomas lacks standardized criteria, but it is characterized by features such as dense fascicules in a “marble-like” pattern consisting of asymmetrically tapered spindle cells and histological mitosis, pleomorphism, and blood vessel infiltration.5,16 Immunohistochemistry is of significant importance to the final diagnosis, and the most accurate positive markers mainly consist of S100, vimentin, GFAP, SOX10, and a few others.11,17,18
The treatment of retroperitoneal malignant schwannomas is challenging.19 Surgery is the primary treatment method because schwannomas are insensitive to radiation and chemotherapy.20 Although whether it is necessary to resect negative soft tissue margins of retroperitoneal schwannomas is controversial, complete excision remains the optimal method5,21 for tumors suspected to be malignant; these can be generally diagnosed by preoperative examination and intraoperative rapid pathology. Additionally, laparoscopic resection22 and robotic laparoscopic resection23 are promising operative excision techniques.
The prognosis of retroperitoneal malignant schwannomas is not optimistic. Factors influencing the prognosis may include incomplete excision,11 association with NF-1,8 and hypoxia-inducible factor 1 alpha.24 The most common problem is recurrence, which is much higher for malignant than benign tumors.17 Moreover, a relatively high proportion of patients require several operations, and fewer than half of these patients survive for more than a few years.25 The prognosis in children appears to be overwhelmingly negative. Both our patient and the one reported previously developed recurrence within 1 month of the first operation. Our patient underwent a second operation 6 weeks after the first operation, and the other child died 13 weeks after the first operation.
Conclusion
Retroperitoneal malignant schwannomas are extremely rare; only a few cases have been reported. The present report describes the second known retroperitoneal malignant schwannoma occurring in a child. The preoperative diagnosis of retroperitoneal malignant schwannomas mainly depends on radiological examinations and can only be definitively confirmed by pathology. Complete excision is currently the only potentially curative treatment method. However, the prognosis is not optimistic and may be particularly poor in children. Recurrence and death are the most serious problems after surgery, and close follow-up is absolutely essential.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research was supported by Wuxi Young Medical Talents (Grant No. QNRC023) and the Medical Innovation Team of Jiangsu Province (Grant No. CXTDB2017016).
References
- 1.Lee NJ, Hruban RH, Fishman EK. Abdominal schwannomas: review of imaging findings and pathology. Abdom Radiol (NY) 2017; 42: 1864–1870. [DOI] [PubMed] [Google Scholar]
- 2.Kasperlik-Zaluska AA, Roslonowska E, Slowinska-Srzednicka J, et al. 1,111 patients with adrenal incidentalomas observed at a single endocrinological center: incidence of chromaffin tumors. Ann N Y Acad Sci 2006; 1073: 38–46. [DOI] [PubMed] [Google Scholar]
- 3.Moazam F, Rogers BM, Talbert JL. Retroperitoneal malignant schwannoma: a case report. J Pediatr Surg 1983; 18: 189–192. [DOI] [PubMed] [Google Scholar]
- 4.Singh V, Kapoor R. Atypical presentation of benign retroperitoneal schwannoma: report of three cases with review of literature. Int Urol and Nephrol 2005; 37: 547–549. [DOI] [PubMed] [Google Scholar]
- 5.Cury J, Coelho RF, Srougi M. Retroperitoneal schwannoma: case series and literature review. Clinics (Sao Paulo) 2007; 62: 359–362. [DOI] [PubMed] [Google Scholar]
- 6.Ohigashi T, Nonaka S, Nakanoma T, et al. Laparoscopic treatment of retroperitoneal benign schwannoma. Int J Urol 1999; 6: 100–103. [DOI] [PubMed] [Google Scholar]
- 7.Kehrer-Sawatzki H, Farschtschi S, Mautner VF, et al. The molecular pathogenesis of schwannomatosis, a paradigm for the co-involvement of multiple tumour suppressor genes in tumorigenesis. Hum Genet 2017; 136: 129–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hwang IK, Hahn SM, Kim HS, et al. Outcomes of treatment for malignant peripheral nerve sheath tumors: different clinical features associated with neurofibromatosis type 1. Cancer Res Treat 2017; 49: 717–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Q, Gao C, Juzi JT, et al. Analysis of 82 cases of retroperitoneal schwannoma. ANZ J Surg 2007; 77: 237–240. [DOI] [PubMed] [Google Scholar]
- 10.Ghosh BC, Luna G, Huvos AG, et al. Malignant schwannoma. A clinicopathologic study. Cancer 1973; 31: 184–190. [DOI] [PubMed] [Google Scholar]
- 11.Chen W, Dang C, Zhu K, et al. Preoperative management of giant retroperitoneal schwannoma: a case report and review of the literature. Oncol Lett 2016; 11: 4030–4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang YM, Lei PF, Chen MN, et al. CT findings of adrenal schwannoma. Clin Radiol 2006; 71: 464–470. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki K, Nakanishi A, Kurosaki Y, et al. Adrenal schwannoma: CT and MRI findings. Radiat Med 2007; 25: 299–302. [DOI] [PubMed] [Google Scholar]
- 14.Neifer R, Nguyen GK. Aspiration cytology of solitary schwannoma. Acta Cytol 1985; 29: 12–14. [PubMed] [Google Scholar]
- 15.Kudo T, Kawakami H, Kuwatani M, et al. Three cases of retroperitoneal schwannoma diagnosed by EUS-FNA. World J Gastroenterol 2011; 17: 3459–3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen KT, Latorrace R, Fubich D, et al. Malignant schwannoma: a light microscopy and ultrastructural study. Cancer 1980; 45: 1583–1593. [DOI] [PubMed] [Google Scholar]
- 17.Li ZQ, Wang HY, Li J, et al. Recurrent retroperitoneal Schwannomas displaying different differentiation from primary tumor: case report and literature review. World J Surg Oncol 2010; 8: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu H, Sha N, Li HW, et al. A giant pelvic malignant schwannoma: a case report and literature review. Int J Clin Exp Pathol 2015; 8: 15363–15368. [PMC free article] [PubMed] [Google Scholar]
- 19.Kerezoudis P, Bydon M, Spinner RJ. Peripheral nerve sheath tumors: the “orphan disease” of national databases. World Neurosurg 2017; 103: 948–949. [DOI] [PubMed] [Google Scholar]
- 20.Kapan M, Onder A, Gümüş M, et al. Retroperitoneal schwannoma. J Surg Case Rep 2011; 2011: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daneshmand S, Youssefzadeh D, Chamie K, et al. Benign retroperitoneal schwannoma: a case series and review of literature. Urology 2003; 62: 993–997. [DOI] [PubMed] [Google Scholar]
- 22.Ji JH, Park JS, Kang CM, et al. Laparoscopic resection of retroperitoneal benign neurilemmoma. Ann Surg Treat Res 2017; 92: 149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deboudt C, Labat JJ, Riant T, et al. Pelvic schwannoma: robotic laparoscopic resection. Neurosurgery 2013; 72(1 Suppl Operative): 2–5; discussion 5. [DOI] [PubMed] [Google Scholar]
- 24.Fukushima S, Endo M, Matsumoto Y, et al. Hypoxia-inducible factor 1 alpha is a poor prognostic factor and potential therapeutic target in malignant peripheral nerve sheath tumor. PLoS One 2017; 12: e0178064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.White HR., Jr. Survival in malignant schwannoma. An 18-year study. Cancer 1971; 27: 720–729. [DOI] [PubMed] [Google Scholar]