Short abstract
Objective
Intraductal papillary neoplasm of the bile duct (IPNB) has been increasingly recognized and reported. However, its clinical features are still controversial because of its low incidence. In the present study, we investigated the characteristics of IPNB.
Methods
In total, 28 patients with IPNB were treated at our institution from January 2000 to December 2016. Clinical data were collected and a retrospective accurate database was constructed. Demographic characteristics, perioperative management, and prognosis were retrospectively analyzed.
Results
Abdominal discomfort was the most common symptom. Preoperative imaging revealed biliary tract dilatation in 23 patients. Left lateral or left hepatic lobectomy was the most frequently performed surgical procedure. Histological analysis revealed malignancy in 17 patients. Eighty-eight lymph nodes were swept from the patients with malignant disease, but only three were metastatic. Twenty-one patients were followed up for 3 to 60 months (mean, 29.4 ± 18.2 months). Seven patients died during the follow-up period. Patients with benign tumors had significantly greater disease-free survival.
Conclusions
IPNB is a rare biliary disease that occurs mainly in patients of advanced age. The most common symptom is abdominal discomfort. Lymphatic metastasis is uncommon. Patients with benign tumors may have a better prognosis than those with malignant tumors.
Keywords: Intraductal papillary neoplasm of the bile duct, biliary tract, lymph node, treatment, survival, rare
Introduction
Intraductal papillary neoplasm of the bile duct (IPNB) has been increasingly recognized as a unique type of biliary neoplasm.1,2 It is characterized by dilated bile ducts that are filled with noninvasive papillary or villous biliary neoplasms covering delicate fibrovascular stalks.3 Lesions can involve both intrahepatic and extrahepatic bile ducts. Although approximately 40% to 80% of resectable IPNBs contain invasive components, the prognosis is more satisfactory than that of other cholangiocarcinomas.4–6 Because of the low incidence and incomplete understanding of IPNB, most studies of IPNB are designed as case reports and retrospective reviews involving small samples. Compared with intraductal papillary mucinous neoplasms of the pancreas (P-IPMN), the clinical features, surgical patterns, and prognosis of IPNB remain controversial and imprecise.7
In the present study, we retrospectively assessed 28 patients with IPNB treated at our hospital. We analyzed the clinical features of IPNB and explored the diagnostic and surgical patterns. Differences between patients with malignant and benign tumors were compared and analyzed. This study was performed to increase the understanding of the clinicopathologic characteristics of IPNB among the medical community.
Materials and methods
Patients
The data of patients with biliary tract tumors treated at our single institution from January 2000 to December 2016 were evaluated for inclusion in the present study. All pathological specimens were studied by two independent pathologists and classified in accordance with the guidelines of the World Health Organization.8,9 The cancer staging manual of the American Joint Committee on Cancer (AJCC) (7th edition) was used for tumor staging. IPNB was classified into the following four stages according to the depth of invasion and degree of dysplasia: I, low- to intermediate-grade dysplasia; II, high-grade dysplasia; III, intraductal growth type cholangiocarcinoma, AJCC T1; and IV, intraductal growth type cholangiocarcinoma, AJCC ≥T2.10 Stages III and IV were defined as malignant. Patients with mucinous cystic neoplasms of the liver, periampullary carcinoma, and P-IPMN were excluded. Discordant pathological reviews were resolved through discussion. Finally, patients with IPNB were included and divided into two groups: those with malignant tumors and those with benign tumors. All patients or their legal guardian provided written informed consent. This study was approved by the Peking Union Medical College Hospital Institutional Review Board for studies of humans.
Clinical materials were collected from both inpatient and outpatient medical records. The patients’ demographic characteristics, clinical symptoms, perioperative management, pathology, and prognosis were retrospectively analyzed.
Treatment and follow-up
Patients who agreed to undergo surgery underwent operations under general anesthesia in the supine position. Prophylactic antibiotics were administered via a peripheral vein before anesthetic induction. Frozen section was used to ensure negative margins. Hepatic function parameters and the serum bilirubin level were measured every 3 days for the first 2 weeks and weekly thereafter until discharge. One patient who did not agree to undergo surgery was diagnosed via endoscopic retrograde cholangiography with biopsy. Postoperative complications were defined as abnormal events recorded within 30 days postoperatively. Fever was defined as a body temperature of >38.5ºC. Biliary tract infection was diagnosed based on the presence of chills, fever, and an abnormally increased bilirubin level and leukocyte count with or without the presence of abdominal pain. Telephone calls, letters, and outpatient interviews were used for follow-up. Blood tests were performed every 3 months after surgery. Computed tomography (CT) was performed every 6 months during the first 2 years and once a year thereafter. The study endpoint was defined as death or the last follow-up time (December 2017).
Statistical analysis
The Statistical Package for the Social Sciences, version 19.0 (IBM Corp., Armonk, NY, USA) was used for the statistical analysis. All continuous data are presented as mean ± standard deviation. Differences between the study groups were analyzed using Fisher’s exact test and Student’s t test as appropriate. Survival probability was estimated using the Kaplan–Meier method with the log-rank test. A p value of <0.05 was considered statistically significant.
Results
Twenty-eight of 2243 patients (1.2%) admitted from January 2000 to December 2016 were diagnosed with IPNB. Eleven (39.3%) were men. Their mean age was 60.1 ± 14.1 years (range, 19–78 years). Abdominal discomfort was the most common symptom, occurring in 13 patients (46.4%); this was followed closely by jaundice, which occurred in 12 patients. All 28 patients were further divided into 2 groups according to the pathological results: those with malignant tumors (17 patients) and those with benign tumors (11 patients). There were no significant differences in the sex distribution, mean age, or clinical presenting symptoms between the two groups (Table 1).
Table 1.
Demographic data and clinical presenting symptoms of the two groups.
| Total(n = 28) | Malignant(n = 17) | Benign(n = 11) | p value | |
|---|---|---|---|---|
| Male/female | 11/17 | 6/11 | 5/6 | 0.70 |
| Age (years) | 60.1 ± 14.1 | 63.6 ± 7.4 | 54.8 ± 20.0 | 0.19 |
| Symptoms | ||||
| Abdominal discomfort | 13 | 9 | 4 | 0.46 |
| Jaundice | 12 | 6 | 6 | 0.44 |
| Weight loss | 7 | 5 | 2 | 0.67 |
| Fever | 7 | 5 | 2 | 0.67 |
| None | 5 | 2 | 3 | 0.35 |
Data are presented as number of patients or mean ± standard deviation
A median of 35 days (range, 10 days to 1 year) was required to diagnose IPNB. Laboratory tests performed in all 28 patients included measurement of hepatic function parameters, the serum bilirubin concentration, and tumor markers. Hepatic function parameters were abnormally increased in 20 patients (71.4%), and the serum bilirubin level was abnormally increased in 12 (42.9%). Ultrasonography was performed in all 28 patients, CT was performed in 22, magnetic resonance imaging was performed in 11, endoscopic retrograde cholangiography was performed in 10, and endoscopic ultrasonography was performed in 3. Characteristic findings of the imaging examinations are shown in Figure 1. Bile duct dilatation was observed in 23 patients (82.1%), an intraluminal mass in 18 (64.3%), and biliary stones in 5 (17.9%). Detailed results of the blood and imaging tests are shown in Table 2. The distributions in both groups were similar.
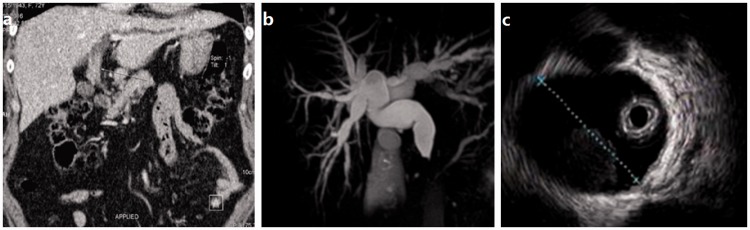
Figure 1.
Characteristic findings of imaging examinations. (a) Computed tomography showed an intraluminal lesion that originated from the extrahepatic bile duct (arrow). (b) Magnetic resonance imaging revealed extensively dilated bile ducts. (c) Endoscopic ultrasonography showed a hypoechoic occupancy lesion around the bile duct.
Table 2.
Laboratory and imaging test results of the two groups.
| Total (n = 28) | Malignant (n = 17) | Benign (n = 11) | p value | |
|---|---|---|---|---|
| Laboratory tests | ||||
| ALT (>50 U/L) | 17 | 10 | 7 | >0.99 |
| ALP (>125 U/L) | 8 | 6 | 2 | 0.42 |
| GGT (>60 U/L) | 13 | 8 | 5 | >0.99 |
| High bilirubin | 12 | 6 | 6 | 0.44 |
| CEA (>5 ng/mL) | 9 | 6 | 3 | >0.99 |
| Imaging features | ||||
| Bile duct dilatation | 23 | 15 | 8 | 0.35 |
| Intraluminal mass | 18 | 13 | 5 | 0.13 |
| Biliary stones | 5 | 4 | 1 | 0.62 |
| Bile duct diameter (cm) | 1.6 ± 0.9 | 1.5 ± 0.6 | 1.8 ± 1.2 | 0.36 |
Data are presented as number of patients or mean ± standard deviation.
High bilirubin: total bilirubin level of >44.4 µmol/L or direct bilirubin level of >13.6 µmol/L.
ALT, alanine aminotransferase; ALP, alkaline phosphatase; GGT, gamma-glutamyltransferase; CEA, carcinoembryonic antigen.
Left or left lateral hepatic lobectomy was performed in 15 patients, pancreaticoduodenectomy in 6, local excision of the biliary tract in 5, and combined lobectomy of the liver in 1. Partial resection and reconstruction of the portal vein was performed in one patient to achieve R0 resection. However, one patient refused to undergo surgery because she was not ready to accept the diagnosis and treatment plan. Among the patients who underwent surgery, 16 had lesions in the intrahepatic bile ducts and 11 had lesions in the extrahepatic bile ducts. In the patient who did not undergo surgery, endoscopic retrograde cholangiography with biopsy was performed to establish a diagnosis and estimate the tumor extent. This patient had lesions in both the intrahepatic and extrahepatic bile ducts. All patients were pathologically diagnosed with IPNB (Figure 2). Eighty-eight lymph nodes were swept from the patients with malignant disease. However, only three metastatic lymph nodes were found. The surgical details were compared between the two groups (Table 3). Among the 16 patients with malignant disease who underwent surgery, 7 had stage T1N0M0 tumors, 4 had stage T2N0M0, 1 had stage T2N1M0, 3 had stage T3N0M0, and 1 had stage T4N0M0. R0 resection was achieved in 15 patients with malignant disease and 10 with benign disease.
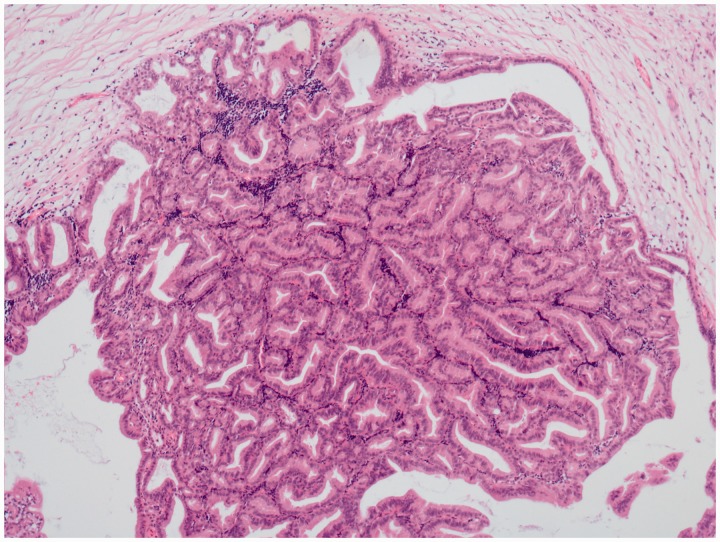
Figure 2.
Microscopic histopathology findings. Microscopic histopathology showed the papillary mucinous gland and mild to moderate heteromorphism.
Table 3.
Distribution of surgery details in the two groups.
| Total (n = 27)* | Malignant (n = 16) | Benign (n = 11) | p value | |
|---|---|---|---|---|
| Surgical procedures | ||||
| Left or left lateral hepatic lobectomy | 15 | 10 | 5 | |
| Pancreaticoduodenectomy | 6 | 4 | 2 | |
| Local excision of biliary tract | 5 | 1 | 4 | |
| Combined lobectomy of liver | 1 | 1 | 0 | |
| Lesion location | ||||
| Intrahepatic/extrahepatic bile ducts | 16/11 | 11/5 | 5/6 | 0.26 |
| Operative time (min) | 275.7 ± 89.0 | 287.8 ± 101.6 | 257.5 ± 70.7 | 0.54 |
| Bleeding amount (mL) | 556.8 ± 480.4 | 750.0 ± 543.6 | 325.0 ± 260.6 | 0.04 |
| Hospital stay (d) | 20.4 ± 8.7 | 22.1 ± 10.6 | 17.8 ± 4.4 | 0.22 |
| Postoperative hospital stay (d) | 13.6 ± 6.0 | 15.0 ± 7.3 | 11.5 ± 2.5 | 0.09 |
Data are presented as number of patients or mean ± standard deviation.
Because 1 patient refused surgery, the total number was 27.
Hepatic function and the serum bilirubin level returned to normal 2 weeks after surgery. Postoperative complications were acceptable. Within 1 week after surgery, three patients had developed a fever and two patients had developed vomiting. Within 1 month after surgery, two patients developed biliary tract infections that were cured with antibiotics. One patient developed seroperitoneum that was treated with interventional CT-guided percutaneous catheter drainage. According to the Clavien–Dindo classification of surgical complications,11 five patients had grade I complications, two had grade II, and one had grade IIIa.
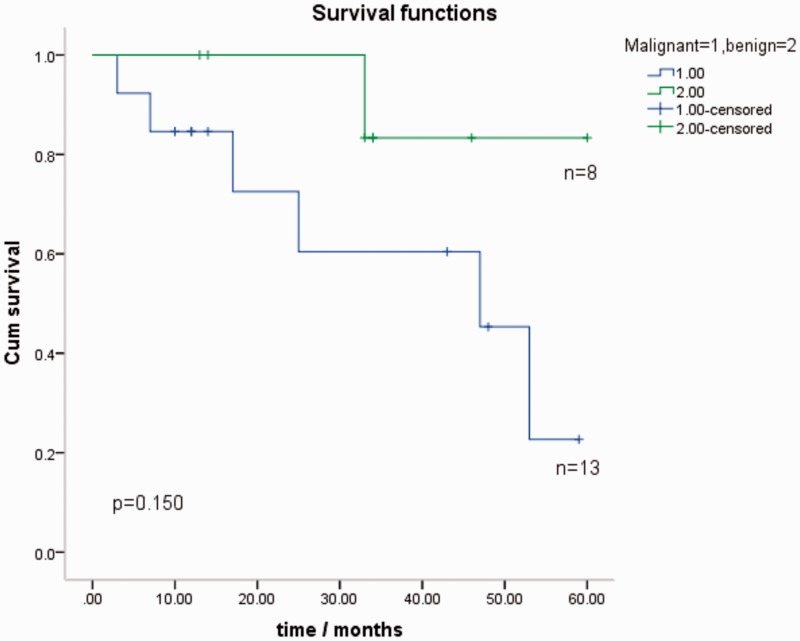
Twenty-one patients (75.0%) were followed up for 3 to 60 months (mean, 29.4 ± 18.2 months). Only two patients with malignant disease agreed to undergo chemotherapy. The others did not need chemotherapy or rejected it because of the adverse effects. Seven patients died during the follow-up period. One patient with benign disease died of biliary obstruction and infection 33 months after surgery. Six patients with malignant disease died of their tumor or a tumor-related disease. These six patients developed intrahepatic or hepatic portal metastasis 3, 6, 12, 18, 42, and 48 months after surgery, and they died 3, 7, 17, 25, 47, and 53 months after surgery. The Kaplan–Meier curve shows the survival for both patients with benign and malignant IPNB (Figure 3). The mean survival period was 44.3 months for the entire cohort. Patients with benign tumors had slightly longer survival than those with malignant tumors (55.5 vs. 38.3 months, respectively); however, the difference was not statistically significant. The detailed follow-up information is shown in Table 4. The benign group had significantly greater disease-free survival (p = 0.03). Among the patients with malignant disease, only those with stage T1 (5/7) and T2 (2/5) tumors survived. The two patients who did not achieve R0 resection died (malignant) or survived with the tumor (benign).
Figure 3.
Kaplan–Meier survival curve for patients with malignant (n = 13) and benign (n = 8) intraductal papillary neoplasm of the bile duct. The test showed a p value of 0.15, which was not statistically significant, but patients with benign tumors had somewhat longer survival. More samples were needed for a more meaningful result.
Table 4.
Follow-up status of the two groups.
| Total (n = 21) | Malignant (n = 13) | Benign (n = 8) | p value | |
|---|---|---|---|---|
| Death toll | 7 | 6 | 1 | 0.17 |
| Disease-free survival | 9 | 3 | 6 | 0.03 |
| Survival with tumor | 5 | 4 | 1* | – |
Data are presented as number of patients
The positive margin was confirmed by postoperative pathology in one patient with benign disease. This patient chose observation instead of reoperation because of severe underlying disease
Patients with lesions in the intrahepatic bile ducts were compared with patients with lesions in the extrahepatic bile ducts. One patient with lesions in both the intrahepatic and extrahepatic bile ducts was excluded. The detailed results are shown in Table 5. Patients with lesions in the extrahepatic bile ducts showed significantly more jaundice. However, a larger sample size is required to increase the statistical power of our findings.
Table 5.
Clinical data of patients with intraductal papillary neoplasm of the bile duct with different lesion locations.
| Total (n = 27)* | Lesion location |
p value | ||
|---|---|---|---|---|
| Intrahepatic (n = 16) | Extrahepatic (n = 11) | |||
| Male/female | 11/16 | 7/9 | 4/7 | >0.99 |
| Age (years) | 60.4 ± 14.3 | 62.4 ± 13.0 | 57.4 ± 16.3 | 0.38 |
| Abdominal discomfort | 12 | 9 | 3 | 0.24 |
| Jaundice | 12 | 4 | 8 | 0.02 |
| Imaging features | ||||
| Bile duct dilatation | 22 | 12 | 10 | 0.62 |
| Intraluminal mass | 17 | 11 | 6 | 0.69 |
| Biliary stones | 5 | 4 | 1 | 0.62 |
| Bile duct diameter (cm) | 1.6 ± 0.9 | 1.5 ± 0.7 | 1.9 ± 1.2 | 0.27 |
| Benign/malignant | 11/16 | 5/11 | 6/5 | 0.26 |
| Follow-up/survival | 21/14 | 13/7 | 8/7 | 0.17 |
Data are presented as number of patients or mean ± standard deviation.*Because 1 patient who had lesions in both intrahepatic and extrahepatic bile ducts was excluded, the total number was 27
Discussion
IPNB was recognized as a distinct biliary tract disease by the World Health Organization in 2010.4 Depending on whether macroscopically visible mucin secretion exists, IPNB can be divided into two different pathological types.12 IPNB and P-IPMN are sometimes regarded as two different patterns of one disease.13 However, some studies have indicated that significant differences in genetic backgrounds and biological pathways exist between IPNB and P-IPMN.7,14–16 Therefore, no widespread consensus regarding the pathogenesis of IPNB has been reached. Clonorchiasis and hepatolithiasis are possible causes in Far Eastern areas, where IPNB has mainly been found.17 Five (17.9%) cases of hepatolithiasis were found in the present study. However, the reported percentage approaches 63%.18
IPNB is commonly reported in patients of advanced age.1,4,19,20 In the present study, the mean age of patients with IPNB was 60.1 ± 14.1 years. Most of these patients (23/28; 82.1%) were 50 to 75 years old. No significant difference in the sex distribution was found in either previous reports or in the present study.4,7 The most common symptom in our study was abdominal discomfort. However, jaundice, fever, and weight loss were not rare. All of these symptoms are nonspecific; hence, early diagnosis was quite difficult. Interestingly, patients with malignant and benign tumors showed no significant differences in sex, age, or symptom distribution.
Lesions located in the intrahepatic bile duct and porta hepatis were more common than those located in the extrahepatic bile duct.4,17 The preoperative diagnosis was mostly dependent upon imaging examinations such as ultrasonography, CT, and magnetic resonance imaging.21 Dilated bile ducts, intraluminal mucin, and lesions were the most common abnormal findings.22 Similar to diffuse pancreatic duct dilation in patients with P-IPMN, the extensively dilated bile ducts had diagnostic significance.10,18 This abnormality was observed in 23 patients (82.1%) in our study.
The major differential diagnoses included hepatocellular carcinoma with a bile duct embolus, hepatoliths with cholangitis, and a biliary intraductal tubulopapillary neoplasm. Preoperative imaging was helpful in some cases. Biliary intraductal tubulopapillary neoplasm was radiologically differentiated from IPNB by a high proportion of intraductal soft tissue and a lack of intraluminal mucin.23 However, the precise differentiation was based on the postoperative histological examination.
Surgery is considered the most suitable treatment modality for patients with IPNB without distant metastasis.24,25 Complete excision is very important, and R0 resection might lead to better survival.4 Interestingly, one patient in the present study with a benign lesion and without R0 resection had survived for 14 months as of December 2017. Some other variables, such as the presence of a benign lesion, might play a role in the prognosis. Different operative approaches should be chosen for different tumor sites and extents. Hepatectomy is suitable for lesions of the intrahepatic bile duct and porta hepatis. Local excision of the biliary tract is suitable for lesions located at the middle part of the extrahepatic bile duct. Pancreaticoduodenectomy is the most suitable treatment modality for tumors at the distal extrahepatic bile duct. If the portal vein is involved, then vascular resection and reconstruction can be performed to achieve R0 resection.13 Frozen pathology of the surgical margin should be routinely performed. Intraoperative choledochoscopy is sometimes quite useful for estimating the surgical extent.26 In the present study, patients with malignant and benign tumors had no significant differences in the tumor location, operating time, or hospital stay. However, the amount of bleeding was significantly higher in the malignant group. These results remind surgeons that although the differential diagnoses of malignant and benign lesions do not affect the choice of surgical procedure, great attention should be focused on avoiding vascular damage when managing patients with possibly malignant tumors.
Much less lymph node metastasis is present in patients with IPNB than in those with conventional cholangiocarcinoma.13 In the present study, 88 lymph nodes were swept from the patients with malignant disease. However, only three metastatic lymph nodes were confirmed in 1 patient. Nevertheless, regional lymphadenectomy should be performed, especially for lesions located at the hilum and distal extrahepatic bile duct.27
IPNB has a better prognosis than conventional bile duct cholangiocarcinoma.27–29 Furthermore, patients with invasive carcinoma can live longer after suitable treatment. Compared with patients with malignant tumors, those with benign tumors had somewhat longer survival and significantly greater disease-free survival (p = 0.03), implying that patients with benign tumors might have a better prognosis than those with malignant tumors. More samples are needed to make strong conclusions.
In summary, IPNB is a rare biliary disease that occurs mainly in patients of advanced age. The most common symptom is abdominal discomfort. Surgery is the first-choice treatment. Patients with benign tumors may have a better prognosis than those with malignant tumors. This study had some limitations, including its retrospective design and small sample size. Because of the increasing understanding of IPNB, more reports involving larger series are expected in the next few years. These studies will be useful for a deeper understanding of IPNB.
Authors’ contributions
Xin Wu and Binglu Li designed the study; Xin Wu, Chaoji Zheng, and Xiaoyan Chang analyzed the data and drafted the manuscript; Binglu Li revised the manuscript for important intellectual content; and Taiping Zhang, Xiaodong He, and Yupei Zhao collected and analyzed important data. All authors approved the final manuscript.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Barton JG, Barrett DA, Maricevich MA, et al. Intraductal papillary mucinous neoplasm of the biliary tract: a real disease? HPB (Oxford) 2009; 11: 684–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sclabas GM, Barton JG, Smyrk TC, et al. Frequency of subtypes of biliary intraductal papillary mucinous neoplasm and their MUC1, MUC2, and DPC4 expression patterns differ from pancreatic intraductal papillary mucinous neoplasm. J Am Coll Surg 2012; 214: 27–32. [DOI] [PubMed] [Google Scholar]
- 3.Nakanuma Y, Jang KT, Fukushima N, et al. A statement by the Japan-Korea expert pathologists for future clinicopathological and molecular analyses toward consensus building of intraductal papillary neoplasm of the bile duct through several opinions at the present stage. J Hepatobiliary Pancreat Sci 2018; 25: 181–187. [DOI] [PubMed] [Google Scholar]
- 4.Rocha FG, Lee H, Katabi N, et al. Intraductal papillary neoplasm of the bile duct: a biliary equivalent to intraductal papillary mucinous neoplasm of the pancreas? Hepatology 2012; 56: 1352–1360. [DOI] [PubMed] [Google Scholar]
- 5.Jung G, Park KM, Lee SS, et al. Long-term clinical outcome of the surgically resected intraductal papillary neoplasm of the bile duct. J Hepatol 2012; 57: 787–793. [DOI] [PubMed] [Google Scholar]
- 6.Jonas S, Thelen A, Benckert C, et al. Extended liver resection for intrahepatic cholangiocarcinoma: A comparison of the prognostic accuracy of the fifth and sixth editions of the TNM classification. Ann Surg 2009; 249: 303–309. [DOI] [PubMed] [Google Scholar]
- 7.Minagawa N, Sato N, Mori Y, et al. A comparison between intraductal papillary neoplasms of the biliary tract (BT-IPMNs) and intraductal papillary mucinous neoplasms of the pancreas (P-IPMNs) reveals distinct clinical manifestations and outcomes. Eur J Surg Oncol 2013; 39: 554–558. [DOI] [PubMed] [Google Scholar]
- 8.Nakanuma Y, Curado M-P, Franceschi S, et al. Intrahepatic cholangiocarcinoma In: Bosman FT, Carneiro F, Hruban RH, et al. (eds) World Health Organization classification of tumours of the digestive system. Lyon: International Agency for Research on Cancer, 2010, pp.217–224. [Google Scholar]
- 9.Albores-Saavedra J, Adsay NV, Crawford JM, et al. Carcinoma of the gallbladder and extrahepatic bile ducts In: Bosman FT, Carneiro F, Hruban RH, et al. (eds) World Health Organization classification of tumours of the digestive system. Lyon: International Agency for Research on Cancer, 2010, pp.266–273. [Google Scholar]
- 10.Wan XS, Xu YY, Qian JY, et al. Intraductal papillary neoplasm of the bile duct. World J Gastroenterol 2013; 19: 8595–8604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004; 240: 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohtsuka M, Kimura F, Shimizu H, et al. Similarities and differences between intraductal papillary tumors of the bile duct with and without macroscopically visible mucin secretion. Am J Surg Pathol 2011; 35: 512–521. [DOI] [PubMed] [Google Scholar]
- 13.Yeh TS, Tseng JH, Chiu CT, et al. Cholangiographic spectrum of intraductal papillary mucinous neoplasm of the bile ducts. Ann Surg 2006; 244: 248–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furukawa T, Kuboki Y, Tanji E, et al. Whole-exome sequencing uncovers frequent GNAS mutations in intraductal papillary mucinous neoplasms of the pancreas. Sci Rep 2011; 1: 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu J, Matthaei H, Maitra A, et al. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci Transl Med 2011; 3: 92ra66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matthaei H, Wu J, Dal Molin M, et al. GNAS codon 201 mutations are uncommon in intraductal papillary neoplasms of the bile duct. HPB (Oxford) 2012; 14: 677–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim KM, Lee JK, Shin JU, et al. Clinicopathologic features of intraductal papillary neoplasm of the bile duct according to histologic subtype. Am J Gastroenterol 2012; 107: 118–125. [DOI] [PubMed] [Google Scholar]
- 18.Wang X, Cai YQ, Chen YH, et al. Biliary tract intraductal papillary mucinous neoplasm: report of 19 cases. World J Gastroenterol 2015; 21: 4261–4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zen Y, Fujii T, Itatsu K, et al. Biliary papillary tumors share pathological features with intraductal papillary mucinous neoplasm of the pancreas. Hepatology 2006; 44: 1333–1343. [DOI] [PubMed] [Google Scholar]
- 20.Kloek JJ, van der Gaag NA, Erdogan D, et al. A comparative study of intraductal papillary neoplasia of the biliary tract and pancreas. Hum Pathol 2011; 42: 824–832. [DOI] [PubMed] [Google Scholar]
- 21.Takanami K, Yamada T, Tsuda M, et al. Intraductal papillary mucinous neoplasm of the bile ducts: multimodality assessment with pathologic correlation. Abdom Imaging 2011; 36: 447–456. [DOI] [PubMed] [Google Scholar]
- 22.Lim JH, Yoon KH, Kim SH, et al. Intraductal papillary mucinous tumor of the bile ducts. Radiographics 2004; 24: 53–66. [DOI] [PubMed] [Google Scholar]
- 23.Wu CH, Yeh YC, Tsuei YC, et al. Comparative radiological pathological study of biliary intraductal tubulopapillary neoplasm and biliary intraductal papillary mucinous neoplasm. Abdom Radiol (NY) 2017; 42: 2460–2469. [DOI] [PubMed] [Google Scholar]
- 24.Kim JK, Hwang HK, Park JS, et al. Left hemihepatectomy and caudate lobectomy and complete extrahepatic bile duct resection using transduodenal approach for hilar cholangiocarcinoma arising from biliary papillomatosis. J Surg Oncol 2008; 98: 139–142. [DOI] [PubMed] [Google Scholar]
- 25.Wang M, Deng BY, Wen TF, et al. An observational and comparative study on intraductal papillary mucinous neoplasm of the biliary tract and the pancreas from a Chinese cohort. Clin Res Hepatol Gastroenterol 2016; 40: 161–168. [DOI] [PubMed] [Google Scholar]
- 26.Ohtsuka M, Shimizu H, Kato A, et al. Intraductal papillary neoplasms of the bile duct. Int J Hepatol 2014; 2014: 459091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jarnagin WR, Bowne W, Klimstra DS, et al. Papillary phenotype confers improved survival after resection of hilar cholangiocarcinoma. Ann Surg 2005; 241: 703–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tajima Y, Kuroki T, Fukuda K, et al. An intraductal papillary component is associated with prolonged survival after hepatic resection for intrahepatic cholangiocarcinoma. Br J Surg 2004; 91: 99–104. [DOI] [PubMed] [Google Scholar]
- 29.Fu Y, Yang W, Wu W, et al. Radiofrequency ablation for postoperative recurrences of intrahepatic cholangiocarcinoma. Chin J Cancer Res 2011; 23: 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]