Short abstract
Background
To investigate the relationship between the levels of nuclear factor (NF)-κB p50 and NF-κB p65 and tumour characteristics in patients with thyroid carcinoma.
Methods
This prospective study enrolled consecutive patients with thyroid carcinoma. Tumour samples were collected and the levels of NF-κB p50 and NF-κB p65 protein and mRNA were measured using immunohistochemistry and quantitative real-time reverse transcription–polymerase chain reaction (qRT–PCR).
Results
A total of 73 patients with thyroid carcinoma were included in the study (20 males; 53 females; mean ± SD age, 44.8 ± 12.7 years, range, 18–76 years). There were no significant differences in sex, age and pathological type between the NF-κB p50 positive group and the NF-κB p50 negative group, but tumour diameter and lymph node metastasis were significantly higher in the NF-κB p50 positive group compared with the NF-κB p50 negative group. Similar findings were observed for NF-κB p65. The levels of NF-κB p50 were positively correlated with NF-κB p65 in samples of thyroid carcinoma (rs = 0.653).
Conclusion
The levels of NF-κB p50 and NF-κB p65 in samples of thyroid carcinoma were positively associated with tumour diameter and the presence of lymph node metastasis.
Keywords: Thyroid cancer, nuclear factor-κB p50, nuclear factor-κB p65, immunohistochemistry, quantitative real-time reverse transcription–polymerase chain reaction
Introduction
Thyroid carcinoma has become one of the most common malignant tumours in the head, neck and endocrine system worldwide.1 Thyroid carcinoma is mainly diagnosed by pathological examination. However, this method has certain difficulties when diagnosing some types of atypical thyroid cancers or small thyroid cancers.2 With the development of technology, molecular biology methods have been applied in clinical practice, which may play an important role in the molecular diagnosis and therapeutic evaluation of thyroid cancer.3 Nuclear factor (NF)-κB is an important nuclear transcription factor. For example, it has been demonstrated that the loss of NF-κB is associated with malignant tumours, inflammation, viral infections, septic shock and other types of diseases.4,5 NF-κB has five subtypes in the human body, of which p50 and p65 are the most important subunits.6,7 This study aimed to investigate the levels of NF-κB p50 and p65 in thyroid carcinoma using both immunohistochemistry and quantitative real-time reverse transcription–polymerase chain reaction (qRT–PCR) and to determine their relationship with clinical malignancy and prognosis.
Patients and methods
Patient population
This prospective study enrolled consecutive patients with thyroid carcinoma in the Department of Head and Neck Surgery, Jiangxi Cancer Hospital, Nanchang, Jiangxi Province, China between January 2016 and March 2017. The inclusion criteria were as follows: (i) patients diagnosed using postoperative pathological histology; (ii) patients with no prior history of thyroid surgery. Demographic and clinical characteristics were recorded for all patients. The study was approved by Ethics Department of Jiangxi Cancer Hospital (no. 2016-019). All patients participating in the study provided written informed consent.
Immunohistochemical detection of NF-κB p50 and NF-κB p65
Formalin-fixed, paraffin-embedded tumour samples were obtained from each patient in order to undertake immunohistochemical staining for NF-κB p50 and NF-κB p65 proteins. In brief, tumour tissue was fixed in 30% formalin, dehydrated in ethanol and embedded in paraffin wax. All specimens were sectioned (4-µm thick) and the sections deparaffinized. After epitope (antigen) retrieval by proteinase K digestion (Thermofisher Scientific, Rockford, IL, USA), nonspecific sites were blocked using a blocking kit (Thermofisher Scientific). The sections were incubated with either rabbit antihuman NF-κB p50 or rabbit antihuman NF-κB p65 primary antibody (1:100; Cell Signaling Technology®, Danvers, MA, USA) at 4°C overnight. The sections were then washed in 10 mM phosphate-buffered saline (PBS; pH 7.4) (Thermofisher Scientific) at room temperature three times and then incubated with goat antirabbit horseradish peroxidase (HRP)-conjugated IgG secondary antibody (1:10000; Cell Signaling Technology®) at room temperature for 30 min. Then the sections were washed in 10 mM Tris-buffered saline (pH 8.4) three times for 2 min each at room temperature and 10 mM PBS (pH 7.4) three times at room temperature. The sections were then incubated with 200 µl 3,3'-diaminobenzidine substrate (Thermofisher Scientific) to completely cover the sample for 5 min at room temperature, followed by washing with distilled water at room temperature for 5 min. Immunohistochemistry, counterstaining and scoring were performed by a pathologist (F.L.). The level of immunostaining in the tissue samples was evaluated using a standard scoring system based on the proportion of positively stained cells and the level of staining intensity. In brief, 10 different sections were chosen randomly for each tissue sample and 100 cells were examined in each section. The scores were based on the proportion of positively-stained cells as follows: < 5% was scored 0, 5–25% was scored 1, 26–50% was scored 2, > 50% was scored 3. For staining intensity, no staining of the cytoplasm was scored 0, light brown was scored 1, brown was scored 2, and dark brown scored 3. The staining intensity and positivity scores were multiplied to give an overall score: 0–1 was negative, 2–3 was weakly positive, 4–6 was positive, and > 6 was strongly positive.
Tissue RNA preparation
Formalin-fixed, paraffin-embedded tumour samples were obtained using similar methods described above. Total RNA from 1 mg of thyroid tumour was extracted using TRIzol® reagent (Invitrogen, Carlsbad, CA, USA) and chloroform based on an organic extraction protocol that had been optimized in-house. The RNA quality and quantity were verified using a Bioanalyzer 2100 system (Agilent Technologies, Santa Clara, CA, USA). The RNA was reverse transcribed to cDNA using superscript IV with poly-dT selection (Thermofisher Scientific).
Real-time qRT–PCR
TaqMan® primer sets and the probes for NF-κB p50, NF-κB p65 and β-actin (internal reference control) were designed using Primer3 software and synthesized by Integrated DNA Technologies (San Diego, CA, USA). Primer3 is a widely used program for designing PCR primers. The primers were as follows: β-actin forward 5ʹ-TGAGAGGGAAATCGTGCGTG-3ʹ, reverse 5ʹ-TGCTTGCTGATCCACATCTGC-3ʹ; NF-κB p50 forward 5ʹ-TGGACAGCAAATCCGCCCTG-3ʹ, reverse 5ʹ-TGTTGTAATGAGTCGTCATCCT-3ʹ; NF-κB p65 forward 5ʹ-AGGCAAGGAATAATGCTGTCCTG-3ʹ, reverse 5ʹ-ATCATTCTCTAGTGTCTGGTTGG-3ʹ. The cDNA was mixed with TaqMan® universal gene expression buffer and the qRT–PCR cycle was undertaken as recommended by the manufacturer using an Applied Biosystems™ ABI 7900HT real-time thermal cycler (Thermofisher Scientific). The cycling programme involved preliminary denaturation at 95°C for 10 min, followed by 36 cycles of denaturation at 98°C for 30 s, annealing at 60°C for 60 s, and elongation at 72°C for 15 s, followed by a final elongation step at 72°C for 5 min. After qRT–PCR, the cycle threshold (CT) was calculated and the relative fold change was calculated using the standard delta CT method.
Statistical analyses
All statistical analyses were performed using IBM SPSS Statistics for Windows®, version 19.0 (IBM Corp, Armonk, NY, USA) by a dedicated statistician (F.L.).8 Demographic and clinical data were compared between the groups using χ2-test or Student’s t-test as appropriate. A Pearson correlation coefficient analysis of qRT–PCR and immunohistochemical protein levels was undertaken as an internal cross validation between the two methods (PCC = 0.97, data not shown). Spearman's rank correlation coefficient analysis was undertaken to determine if there was a significant correlation between NF-κB p50 and NF-κB p65 levels in thyroid carcinoma samples. A P-value < 0.05 was considered statistically significant.
Results
This study included 73 patients with thyroid carcinoma, of which 20 patients were male and 53 were female. The mean ± SD age was 44.8 ± 12.7 years (range, 18–76 years). The pathological types were as follows: 42 papillary carcinomas, 15 follicular carcinomas and 16 medullary carcinomas. The mean ± SD tumour diameter was 1.7 ± 1.5 cm (range, 0.4–3.5 cm). Of the 73 patients, there were 37 with lymph node metastasis and 36 without lymph node metastasis.
Tumour samples from patients were immunohistochemically stained for NF-κB p50 and NF-κB p65 proteins (Figure 1). A total of 53 of 73 patients (72.6%) were NF-κB p50 positive and 20 of 73 patients (27.4%) showed no staining of NF-κB p50 protein. A total of 61 of 73 patients (83.6%) were NF-κB p65 positive and 12 patients (16.4%) showed no staining of NF-κB p65 protein.
Figure 1.
Representative photomicrographs showing positive immunohistochemical staining (arrows) for nuclear factor (NF)-κB p50 (a) and NF-κB p65 (b) proteins in paraffin wax-embedded sections of thyroid carcinoma. Sections were counter-stained with haematoxylin. The colour version of this figure is available at: http://imr.sagepub.com. Scale bar 25 µm.
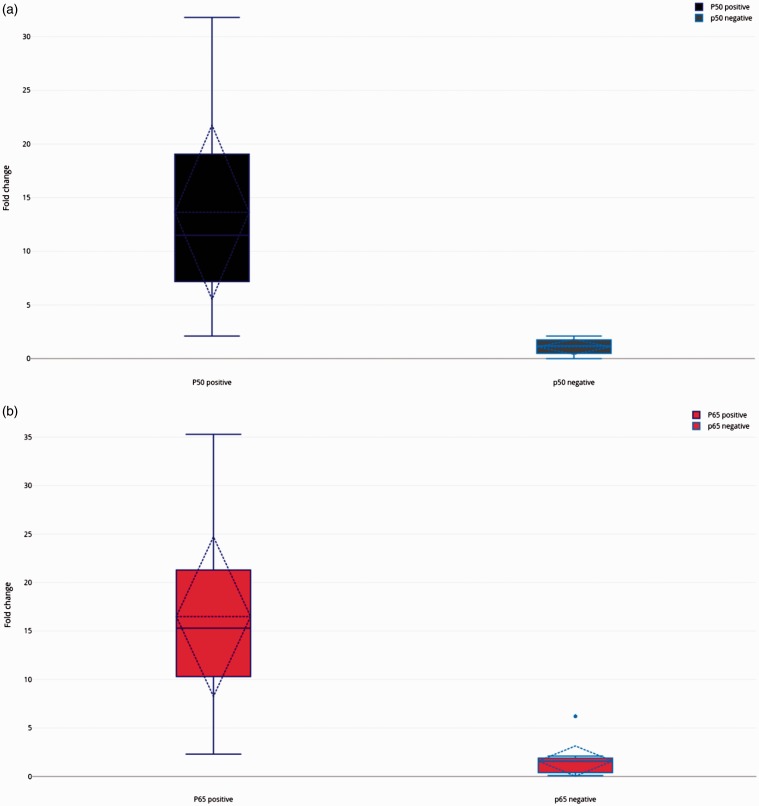
Using qRT–PCR, the levels of NF-κB p50 and NF-κB 65 relative to the β-actin reference control were determined (Figure 2). The NF-κB p50 or NF-κB p65 levels were considered positive when the relative fold change compared with β-actin was > 2. A total of 51 of 73 patients (70%) of patients showed positive levels of NF-κB p50 and 59 of 73 patients (81%) of the patients were NF-κB p65 positive.
Figure 2.
The levels of nuclear factor (NF)-κB p50 (a) and NF-κB p65 (b) mRNA as quantified by real-time reverse transcription–polymerase chain reaction relative to the β-actin reference control. The NF-κB p50 or NF-κB p65 levels were considered positive when the relative fold change compared with β-actin was > 2. The solid central horizontal line in each box is the median and the broken central horizontal line is the mean. The extremities of the boxes are the 25th and 75th percentiles. The diamond shape on each box represents the standard deviation. The circle above the p65 negative box in Figure B represents an extreme outlier.
There were no significant differences in sex, age and pathological type between the NF-κB p50 positive group and the NF-κB p50 negative group (Table 1). Tumour diameter and lymph node metastasis were significantly increased in the NF-κB p50 positive group compared with the NF-κB p50 negative group (P < 0.05 for both comparisons).
Table 1.
Demographic and clinical characteristics of patients with thyroid carcinomas (n = 73) stratified according to the immunohistochemical identification of positive staining of nuclear factor (NF)-kB p50 protein.
| Characteristic | NF-κB p50 positive group n = 53 | NF-κB p50 negative group n = 20 | Statistical significancea |
|---|---|---|---|
| Sex, male/female | 14/39 | 6/14 | NS |
| Age, years | 47.1 ± 13.4 | 46.3 ± 12.8 | NS |
| Pathological types, papillary/follicular/medullary carcinoma | 33/9/11 | 9/6/5 | NS |
| Tumour diameter, cm | 3.2 ± 1.7 | 2.0 ± 1.8 | P = 0.0100 |
| Lymph node metastasis, yes/no | 32/21 | 5/15 | P = 0.0074 |
Data presented as mean ± SD or n of patients.
aGroups were compared using χ2-test (pathological type and lymph node metastasis) or Student’s t-test (sex, age and tumour diameter) as appropriate.
NS, no statistically significant between-group difference (P ≥ 0.05).
There were no significant differences in sex, age and pathological type between the NF-κB p65 positive group and the NF-κB p65 negative group (Table 2). Tumour diameter and lymph node metastasis were significantly increased in the NF-κB p65 positive group compared with the NF-κB p65 negative group (P < 0.05 for both comparisons).
Table 2.
Demographic and clinical characteristics of patients with thyroid carcinomas (n = 73) stratified according to the immunohistochemical identification of positive staining of nuclear factor (NF)-kB p65 protein.
| Characteristic | NF-κB p65 positive group n = 61 | NF-κB p65 negative group n = 12 | Statistical significancea |
|---|---|---|---|
| Sex, male/female | 17/44 | 3/8 | NS |
| Age, years | 46.5 ± 14.7 | 48.8 ± 15.1 | NS |
| Pathological types, papillary/follicular/medullary carcinoma | 37/11/13 | 5/3/4 | NS |
| Tumour diameter, cm | 3.4 ± 1.6 | 1.8 ± 1.5 | P = 0.0021 |
| Lymph node metastasis, yes/no | 35/26 | 2/10 | P = 0.0099 |
Data presented as mean ± SD or n of patients.
aGroups were compared using χ2-test (pathological type and lymph node metastasis) or Student’s t-test (sex, age and tumour diameter) as appropriate.
NS, no statistically significant between-group difference (P ≥ 0.05).
There was a significant positive correlation between NF-κB p50 and NF-κB p65 levels in thyroid carcinoma samples using Spearman's rank correlation coefficient analysis (rs = 0.653, P < 0.05).
Discussion
In this present study, the levels NF-κB p50 and NF-κB p65 were measured in 73 patients with thyroid carcinoma using immunohistochemistry and qRT–PCR. The results showed that NF-κB p50 and NF-κB p65 levels in thyroid carcinoma samples were not related to sex, age or pathological type, but there were significant correlations with tumour diameter and the occurrence of lymph node metastasis. The reason for these findings might be that NF-κB p50 and NF-κB p65 play a synergistic role in the development of thyroid carcinoma and lymph node metastasis, but the specific mechanisms involved require further research.
Thyroid carcinoma is currently the most common thyroid cancer and thyroid cancers account for approximately 1% of all malignant tumours in the body.9 The incidence of thyroid cancer has increased rapidly in recent years, which has resulted in it becoming more common in clinical practice.10 Like other malignancies, pathological examination is the most important method for the diagnosis of thyroid cancer. However, this method is not applicable to some atypical thyroid cancers or small thyroid cancers. Molecular biology techniques may play an important role in thyroid cancer diagnosis.
Nuclear factor-κB is an important nuclear transcription factor in eukaryotic cells. For example, it plays a role in initiating gene transcription by binding to a fixed nucleotide sequence in the promoter region.11 NF-κB plays an important role in cell proliferation and differentiation, tumour formation, tumour invasion and tumour metastasis.12 In addition, NF-κB is also involved in the response of cells to stimuli such as free radicals, ultraviolet radiation, cytokines, bacterial or viral antigens; and it plays a key role in regulating the immune system's response to infection.13,14 At present, NF-κB is considered to be uncontrolled and associated with malignancy, inflammation, viral infections, septic shock and other types of diseases.4,5 NF-κB family members have five subunits in humans: Rel, RelB, p50, p52 and p65.15 Among the five subunits, p50 is the DNA binding site and p65 can regulate transcriptional activity and promote the binding of p50 and DNA, through the coordination of Rel homologous domains.6,7 Therefore NF-κB p50 and NF-κB p65 are the most important members of the NF-κB family.
The detection of NF-κB p50 and NF-κB p65 levels has been widely used in many malignant tumours such as breast cancer, liver cancer, oesophageal squamous cell carcinoma and gastric cancer, but there are few reports on its expression in thyroid cancer.16 A previous study reported that 91 of 122 patients (74.6%) with thyroid papillary carcinoma were positive for NF-κB p65.17 When the tumour diameter was > 1 cm, the rate of NF-κB p65 positivity was significantly higher.17 There were significant differences in thyroid extravasation, lymph node metastasis, and BRAF(V600E) mutation between the NF-κB p65 positive group and the NF-κB p65 negative group (P < 0.05).17 The positivity rates of NF-κB p50 and NF-κB p65 expression in thyroid carcinoma were reported to be 73.0% and 74.0%, respectively.18
This study had several limitations. First, the sample size was relatively small. Secondly, the immunohistochemistry might have been subject to false positive or false negative staining. Currently, patients are being enrolled into a larger cohort study to improve future analyses.
In conclusion, this current study demonstrated that there were significant associations between NF-κB p50 and NF-κB p65 levels and tumour diameter and the occurrence of lymph node metastasis in patients with thyroid carcinomas.
Declaration of conflicting interests
The authors declare that there are no conflicts of interest.
Funding
This work was supported by Health Planning Committee Support Project no. 20165396 from the Health Department of Jiangxi Province, China. The opinions, findings, conclusions or recommendations expressed in this publication are those of the authors and do not necessarily reflect those of the Jiangxi Provincial Education Department.
References
- 1.Pellegriti G, Frasca F, Regalbuto C, et al. Worldwide increasing incidence of thyroid cancer: update on epidemiology and risk factors. J Cancer Epidemiol 2013; 2013: 965212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klang K, Kamaya A, Tahvildari AM, et al. Atypical thyroid cancers on sonography. Ultrasound Q 2015; 31: 69–74. [DOI] [PubMed] [Google Scholar]
- 3.D'Cruz AK, Vaish R, Vaidya A, et al. Molecular markers in well-differentiated thyroid cancer. Eur Arch Otorhinolaryngol 2018; 275: 1375–1384. [DOI] [PubMed] [Google Scholar]
- 4.Karin M. NF-kappaB as a critical link between inflammation and cancer. Cold Spring Harb Perspect Biol 2009; 1: a000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amaya M, Keck F, Bailey C, et al. The role of the IKK complex in viral infections. Pathog Dis 2014; 72: 32–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Porta C, Ippolito A, Consonni FM, et al. Protumor Steering of Cancer Inflammation by p50 NF-κB Enhances Colorectal Cancer Progression. Cancer Immunol Res 2018; 6: 578–593. [DOI] [PubMed] [Google Scholar]
- 7.Li YZ, Zhao P. Expressions and clinicopathologic significance of Id2 and NF-κB/P65 in gastric cancer. Zhonghua Yi Xue Za Zhi 2018; 98: 846–850 [Article in Chinese; English abstract]. [DOI] [PubMed] [Google Scholar]
- 8.IBM Corp. Released. IBM SPSS Statistics for Windows, Version 19.0. Armonk, NY: IBM Corp, 2010. [Google Scholar]
- 9.Nguyen QT, Lee EJ, Huang MG, et al. Diagnosis and treatment of patients with thyroid cancer. Am Health Drug Benefits 2015; 8: 30–40. [PMC free article] [PubMed] [Google Scholar]
- 10.Morris LG, Sikora AG, Tosteson TD, et al. The increasing incidence of thyroid cancer: the influence of access to care. Thyroid 2013; 23: 885–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen F, Castranova V, and Shi X. New insights into the role of nuclear factor-kappaB in cell growth regulation. Am J Pathol 2001; 23: 387–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xia Y, Shen S, and Verma IM. NF-κB, an active player in human cancers. Cancer Immunol Res 2014; 2: 823–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Z, Gao M, Yang B, et al. Naringin attenuates MLC phosphorylation and NF-κB activation to protect sepsis-induced intestinal injury via RhoA/ROCK pathway. Biomed Pharmacother 2018; 103: 50–58. [DOI] [PubMed] [Google Scholar]
- 14.Riedlinger T, Haas J, Busch J, et al. The direct and indirect roles of NF-κB in cancer: lessons from oncogenic fusion proteins and Knock-in mice. Biomedicines 2018; 6: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schreiber J, Jenner RG, Murray HL, et al. Coordinated binding of NF-kappaB family members in the response of human cells to lipopolysaccharide. Proc Natl Acad Sci U S A 2006; 103: 5899–5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown M, Cohen J, Arun P, et al. NF-kappaB in carcinoma therapy and prevention. Expert Opin Ther Targets 2008; 12: 1109–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pyo JS, Kang G, Kim DH, et al. Activation of nuclear factor-κB contributes to growth and aggressiveness of papillary thyroid carcinoma. Pathol Res Pract 2013; 209: 228–232. [DOI] [PubMed] [Google Scholar]
- 18.Schwertheim S, Worm K, Schmid KW, et al. Valproic acid downregulates NF-κB p50 activity and IRAK-1 in a progressive thyroid carcinoma cell line. Horm Metab Res 2014; 46: 181–186. [DOI] [PubMed] [Google Scholar]