Short abstract
Objective
To investigate the prognostic effect of pre-diagnosis preserved vegetable consumption on oesophageal squamous cell carcinoma (ESCC) in Yanting County, China.
Methods
This prospective cohort study enrolled consecutive patients with ESCC. The pre-diagnosis diet consumption data were collected using a food frequency questionnaire at baseline. Preserved vegetable consumption was categorized into two groups: < 1/week and ≥1/week. Kaplan–Meier survival curve analysis with a log-rank test and a Cox proportional hazard regression model analysis were undertaken to compare the two consumption groups.
Results
The study enrolled 185 patients (121 males and 64 females) with ESCC. Patients consuming preserved vegetables ≥1/week had a median survival time of 41 months, but patients consuming preserved vegetables <1/week did not achieve a median survival time. The adjusted hazard ratio (HR) for an intake of ≥1/week was 1.58 (95% confidence interval [CI] 1.01, 2.47). Among ‘ever smokers’, the HR increased to 2.04 (95% CI 1.10, 3.77) and among ‘ever alcohol drinkers’, the HR increased to 2.50 (95% CI 1.33, 4.73). Among ‘never smokers’ or ‘never alcohol drinkers’, no significant association was observed.
Conclusion
A high consumption of preserved vegetables was associated with a poorer prognosis among patients with ESCC.
Keywords: Oesophageal squamous cell carcinoma, preserved vegetables, prospective cohort study, prognosis, high-risk area in China
Introduction
China has one of the highest burdens of oesophageal cancer (EC), accounting for nearly 50% of new cases and specific deaths worldwide in 2012.1 From 2003, EC incidence and mortality have been declining in China.2 In cancer registration areas, the age-standardized incidence rate decreased from 39.5/100 000 to 23.0/100 000 from 1989 to 2008, with an mean annual percentage change of –3.3% (95% confidence interval [CI] –2.8%, –3.7%).2 Several factors are thought to have contributed to the reduction of EC occurrence, such as construction of a cancer registration system, improved economic status, screening, and dietary interventions. However, EC incidence shows a geographic variation in China; populations from high-risk areas are still suffering from oesophageal squamous cell carcinoma (ESCC), the main pathological type of EC in China.3 Exploring factors that are significantly associated with the development of ESCC is meaningful for vulnerable people in these high-risk areas.
Preserved vegetables are a traditional food in the Yanting area of China and local residents consume them during several months each year. Consumption of preserved vegetables was reported to be associated with a high risk of ESCC and pre-cancerous lesions from case–control studies.4,5 In addition, a diet that contained preserved vegetables, pickled vegetables and salted meat, was associated with an odds ratio of 2.84 for ESCC.6 Preserved vegetable consumption might be one of the aetiological factors for ESCC, however, its contribution to the prognosis of patients remains unclear. This study aimed to investigate the prognostic effect of preserved vegetable consumption among patients with ESCC.
Patients and methods
Study population and design
This prospective cohort study recruited consecutive patients with ESCC between October 2009 and March 2010 at the Department of Cancer Early Detection and Early Treatment, Yanting Cancer Hospital, Mianyang, Sichuan Province, China. All participants lived in Yanting County and were diagnosed with a resectable tumour. All patients had a pathological diagnosis of ESCC and were incident cases with the interval between diagnosis and interview < 3 months. The exclusion criteria were as follows: (i) survival length < 3 months; (ii) > 70 years old; (iii) migrants to Yanting County; (iv) dementia; (v) memory dysfunction; (vi) American Joint Committee on Cancer stage > II.
Two interviewers (F.S. and L.Z.) collected demographic and other related factors, and baseline dietary data from 1 year before ESCC occurrence. Interviewers used a modified food frequency questionnaire to collect dietary information1 year prior to disease diagnosis.7 The all cause death registry centre in Yanting was responsible for undertaking follow-up of all patients to 31 December 2016. The overall survival was the primary outcome and was calculated from the date of ESCC diagnosis.
This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human patients were approved by the Ethical Committee of Beijing Shijitan Hospital, Capital Medical University, Beijing, China (no. 2016061). Written informed consent was obtained from all study participants.
Study variables
Preserved vegetables are a particular type of processed food used in Yanting County and the production process involves boiling and keeping fresh vegetables in a sealed container for fermentation for about 2 weeks. Body mass index (BMI) was estimated by dividing weight in kilograms by current height in squared meters. ‘Ever smoker’ was defined as smoking more than 100 cigarettes or equivalent use of pipes in their lifetime.8 ‘Ever alcohol drinkers’ were individuals that drank alcohol at least once per month.9 Family cancer history was cancer occurrence among their first-degree genetic relatives (parents, brothers, sisters and offspring).
Data collection
Two health workers from Yanting Cancer Hospital attended a training workshop on data collection using a questionnaire. The questionnaire recorded the preserved vegetable, vegetable and fruit consumption frequency as less than once per month, once to three times per month, once to three times per week, four to six times per week, once per day and twice or more per day.7 Intake frequency of preserved vegetables was categorized into two groups: <1/week and ≥1/week. The repeatability and validity of the modified questionnaire had been previously tested among the rural population.7 The study hypothesis was blinded to health workers and participants during the data collection process.
Statistical analyses
All statistical analyses were performed using the SPSS® statistical package, version 17.0 (SPSS Inc., Chicago, IL, USA) for Windows®. The age and BMI of the two preserved vegetable intake groups were analysed using Student’s t-test. Smoking, alcohol drinking, total vegetable consumption and family cancer history in the two preserved vegetable intake groups were compared using χ2-test. Total fruit consumption and mean annual family income in the two preserved vegetable intake groups were compared using Wilcoxon rank-sum test. Median overall survival was estimated using the Kaplan–Meier curve method with log-rank test between the two preserved vegetable intake groups. Hazard ratio (HR) and 95% CI were estimated using a Cox proportional hazard regression model, with further adjustments for age, sex, smoking status and alcohol drinking status. Stratified analysis was conducted by smoking and alcohol drinking stratification. A P-value < 0.05 was considered statistically significant.
Results
This prospective cohort study enrolled 185 patients (121 males and 64 females) with ESCC. A total of 72 patients consumed preserved vegetables <1/week and 113 consumed preserved vegetables ≥1/week (Table 1). The age of patients with a preserved vegetable consumption ≥1/week was significantly higher than patients with an intake <1/week (P = 0.015). Females accounted for 42.5% of patients (48/113) that consumed preserved vegetables ≥1/week compared with 22.2% female patients (16/72) that consumed preserved vegetables <1/week (P < 0.05). Forty-nine of 113 patients (43.4%) with a preserved vegetable intake ≥1/week were smokers compared with 48 of 72 patients (66.7%) who consumed preserved vegetables <1/week being smokers (P < 0.05). The proportion of patients who consumed preserved vegetables ≥1/week that also had an alcohol drinking history was significantly lower than patients who consumed preserved vegetables <1/week (45 of 113 [39.8%] versus 44 of 72 [61.1%], respectively; P < 0.05). There were no significant differences between the two preserved vegetable consumption groups in terms of BMI, total vegetable consumption, total fruit consumption, family cancer history and mean annual family income.
Table 1.
Demographic and clinical characteristics of patients (n = 185) with oesophageal squamous cell carcinoma categorized according to their frequency of consumption of preserved vegetables in the year prior to diagnosis.
| Characteristic |
Preserved vegetable consumption rate |
Statistical significancea | |
|---|---|---|---|
| <1/weekn = 72 | ≥1/weekn = 113 | ||
| Age, years | 60.1 ± 8.07 | 62.8 ± 7.05 | P = 0.015 |
| Sex | P = 0.005 | ||
| Male | 56 (77.8) | 65 (57.5) | |
| Female | 16 (22.2) | 48 (42.5) | |
| Body mass index, kg/m2 | 22.5 ± 2.62 | 23.0 ± 3.35 | NS |
| Ever smoker | P = 0.002 | ||
| No | 24 (33.3) | 64 (56.6) | |
| Yes | 48 (66.7) | 49 (43.4) | |
| Ever alcohol drinker | P = 0.005 | ||
| No | 28 (38.9) | 68 (60.2) | |
| Yes | 44 (61.1) | 45 (39.8) | |
| Total vegetable consumption | NS | ||
| ≤1/day | 38 (52.8) | 68 (60.2) | |
| >1/day | 34 (47.2) | 45 (39.8) | |
| Total fruit consumption | NS | ||
| <1/month | 40 (55.6) | 60 (53.1) | |
| <1/week | 21 (29.2) | 32 (28.3) | |
| ≥1/week | 11 (15.3) | 21 (18.6) | |
| Family cancer historyb,c | NS | ||
| No | 44 (62.0) | 61 (54.5) | |
| Yes | 27 (38.0) | 51 (45.5) | |
| Mean annual family income, RMBc | NS | ||
| <600 | 20 (30.3) | 46 (41.8) | |
| ≥600 to <1200 | 13 (19.7) | 27 (24.5) | |
| ≥1200 to <3000 | 25 (37.9) | 21 (19.1) | |
| ≥3000 | 8 (12.1) | 16 (14.5) | |
Data presented as mean ± SD or n of patients (%).
aContinuous variables were compared using Student’s t-test and categorical variables were compared using χ2-test, except total fruit consumption and mean annual family income, which were compared using Wilcoxon rank-sum test; NS, no significant between-group difference (P ≥ 0.05).
bAmong first-degree genetic relatives.
cNot all study participants were able to recall their family cancer history or were prepared to disclose their mean annual family income.
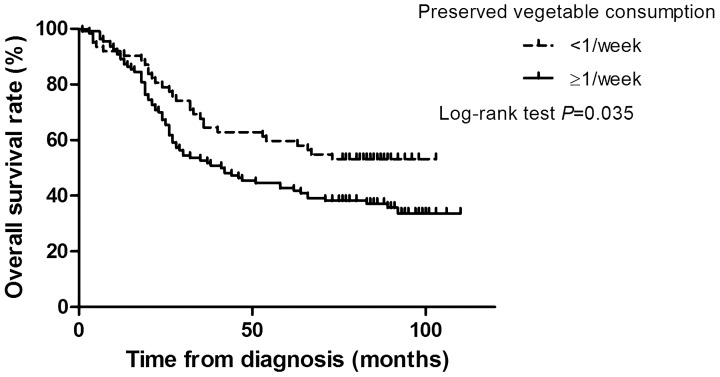
The median follow-up time was 49 months. Median overall survival time of patients consuming preserved vegetables ≥1/week was 41 months. Patients who consumed preserved vegetables <1/week did not achieve a median overall survival time (P = 0.035; Figure 1). The association between survival and preserved vegetable intake was reanalysed by reclassifying the intake frequency into <1/week, <1/day and ≥1/day; and the median overall survival times were not achieved (<1/week), 47 months (<1/day) and 26 months (≥1/day), respectively (P = 0.032).
Figure 1.
Kaplan–Meier survival curve analysis of patients (n = 185) with oesophageal squamous cell carcinoma categorized according to their frequency of consumption of preserved vegetables in the year prior to diagnosis (<1/week versus ≥1/week).
More frequent consumption of preserved vegetables was associated with overall survival; with the adjusted HR for the intake frequency ≥1/week being 1.58 (95% CI 1.01, 2.47) (Table 2). Among patients without a smoking history, preserved vegetable consumption was not associated with overall survival. Among patients with a smoking history, consuming preserved vegetables ≥1/week increased their risk of death by 104% (adjusted HR 2.04; 95% CI 1.10, 3.77). The adjusted HR for consuming preserved vegetables ≥1/week was 0.88 and 2.50 among patients without alcohol drinking and with alcohol drinking (P < 0.05 for with alcohol drinking), respectively.
Table 2.
Results of Cox proportional hazard regression model analysis of the effect of the rate of preserved vegetable consumption on overall survival.
| Subgroup | Preserved vegetable consumption rate | HR crude | 95% CI | HR adjusteda | 95% CI |
|---|---|---|---|---|---|
| <1/week | 1 | — | 1 | — | |
| ≥1/week | 1.56 | 1.02, 2.39 | 1.58 | 1.01, 2.47 | |
| Never smoker | <1/week | 1 | — | 1 | — |
| ≥1/week | 0.96 | 0.52, 1.78 | 0.98 | 0.53, 1.81 | |
| Ever smoker | <1/week | 1 | — | 1 | — |
| ≥1/week | 2.31 | 1.29, 4.14 | 2.04 | 1.10, 3.77 | |
| Never drinker | <1/week | 1 | — | 1 | — |
| ≥1/week | 0.85 | 0.48, 1.52 | 0.88 | 0.49, 1.59 | |
| Ever drinker | <1/week | 1 | — | 1 | — |
| ≥1/week | 2.72 | 1.47, 5.05 | 2.50 | 1.33, 4.73 |
aAdjusted for age and sex.
HR, hazard ratio; CI, confidence interval.
Discussion
This current prospective cohort study demonstrated that more frequent consumption of preserved vegetables was associated with a poorer survival among patients with ESCC who also had a history of smoking or drinking alcohol. The higher rate of preserved vegetable consumption (i.e. ≥1/week) resulted in a 58% increased risk of death among patients with ESCC in Yanting County, China.
Dietary consumption has previously been associated with the prognosis of patients with various malignancies. For example, similar results were observed in non-Hodgkin lymphoma patients: among ‘ever smokers’, pre-diagnosis exposure to α-carotene foods improved their overall survival (HR 0.4, 95% CI 0.2, 0.9), but among ‘never smokers’, no beneficial effect was observed (HR 1.5; 95% CI 0.6, 3.6).10 In this present study, frequent intake of preserved vegetables had a significant relationship with overall survival among ‘ever smokers’. In ‘ever alcohol drinkers’, the association remained significant. Non-Hodgkin lymphoma patients consuming high amounts of vegetables and fruits pre-diagnosis experienced a 42% (HR 0.58; 95% CI 0.38, 0.99) and 27% (HR 0.73; 95% CI 0.54, 0.99) reduction in the risk of death, respectively.11 High quartile consumption of processed meat was associated with a poorer overall survival for patients with colon cancer (HR 2.13; 95% CI 1.03, 4.43) compared with the lowest quartile processed meat consumption, and it resulted in a 2.29-fold higher recurrence risk for the patients (95% CI 1.19, 4.40).12 Another cohort study with 2522 postmenopausal breast cancer patients showed that an unhealthy pre-diagnosis diet (high intake of red meat, processed meat and deep-fried fat) produced a 269% higher non-breast cancer death risk (HR 3.69; 95% CI 1.66, 8.17) among the patients.13 A systematic review pooled the risk ratios of cohort studies to summarize the prognostic effects of diets on cancers and the pooled analysis showed dietary intake significantly affected patients' survival and disease progression.14 Fish consumption was associated with a 15% reduction in death risk.14 Alcohol drinking was associated with a poorer survival for hepatocellular carcinoma (relative risk [RR] 1.21), non-Hodgkin lymphoma (RR 1.33), laryngeal and pharyngeal cancer (RR 1.48) and head and neck cancer (RR 1.39).14 In addition, alcohol consumption increased the breast cancer recurrence risk to 1.21-fold high (95% CI 1.06, 1.39).14 Pre-diagnosis food intake also affected the prognosis of invasive ovarian cancer patients: high consumption of green leafy vegetables was correlated with a 30% reduced risk of death, high consumption of fish was associated with a 26% reduced risk of death, high intake of fibre was associated with a 31% reduced risk of death, but a high glycaemic index was associated with an increased risk of death (HR 1.28: 95% CI 1.01, 1.65).15 However, the dietary effects on ESCC survival has been less well reported. ESCC is the main pathological type of EC in China and the composition of the Chinese diet is different compared with Western diets.16 Exploring the prognostic effects of local foods on ESCC is important in order to be able to improve patient survival and quality of life. The poorer survival among patients with ESCC who had consumed preserved vegetables in a high-risk area was first reported by this study. This current study reanalysed the association between survival and preserved vegetable intake by reclassifying the intake frequency into < 1/week, < 1/day and ≥1/day; and the correlation was still significant.
Preserved vegetable consumption has been shown to be associated with ESCC risk and precancerous lesions.4–6 These processed foods are the source of carcinogenic and mutagenic agents, such as N-nitroso compounds.17 These chemicals possess carcinogenetic effects in animals and promote the development of cancer cells.18 Even among epidemiological studies, high exposure to N-nitroso compounds resulted in increased mortality in cancer patients.19,20 People from high-risk areas for ESCC are exposed to high levels of N-nitroso compounds from water and foods.21 N-nitroso compounds can promote the expression of inducible nitric oxide synthase and cause DNA damage.22 In rat oesophagus, N-nitroso compounds induced a transitional mutation on the Ha-ras codon 12 G–A; and the activation played a functional role in tumour progression.23 N-nitroso compounds were capable of inducing over-expression of cyclin E1, cyclin D1, transforming growth factor-alpha and epidermal growth factor receptor in rat oesophagus tissues.24,25 The consumption of preserved vegetables provides the possibility of being exposed to carcinogenic/mutagenic chemicals, which might result in tumour progression.
The current study had several limitations. First, there were insufficient adjustments made for confounding factors, such as clinical characteristics and related treatments. Secondly, data about tumour recurrence were not available among these patients with resectable tumours.
In conclusion, this current prospective cohort study demonstrated that more frequent consumption of preserved vegetables was associated with a poorer survival among patients with ESCC. These current findings suggest that a public health strategy to reduce the consumption of preserved vegetables should be implemented in areas at high-risk of ESCC.
Declaration of conflicting interests
The authors declare that there are no conflicts of interest.
Funding
This study was supported by the organization department of Beijing Municipal Committee (no. 2014000021469G252) and Beijing Shijitan Hospital, Capital Medical University (no. 2014-C01). The organization department of Beijing Municipal Committee and Beijing Shijitan Hospital, Capital Medical University had no role in the design, data collection, analysis, interpretation of the data or writing of this manuscript.
References
- 1.Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. Available from: http://globocan.iarc.fr, accessed on 11/07/2015. [Google Scholar]
- 2.Zhao J, He YT, Zheng RS, et al. Analysis of esophageal cancer time trends in China, 1989–2008. Asian Pac J Cancer Prev 2012; 13: 4613–4617. [DOI] [PubMed] [Google Scholar]
- 3.He J, Zhao P, Chen W. Chinese Cancer Registry Annual Report 2011. Beijing: Military Medical Science Press, 2011. [Google Scholar]
- 4.Song QK, Zhao L, Li J, et al. Adverse effects of preserved vegetables on squamous cell carcinoma of esophagus and precancer lesions in a high risk area. Asian Pac J Cancer Prev 2013; 14: 659–663. [DOI] [PubMed] [Google Scholar]
- 5.Song Q, Wang X, Yu IT, et al. Processed food consumption and risk of esophageal squamous cell carcinoma: A case-control study in a high risk area. Cancer Sci 2012; 103: 2007–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu X, Wang X, Lin S, et al. Dietary patterns and the risk of esophageal squamous cell carcinoma: A population-based case-control study in a rural population. Clin Nutr 2017; 36: 260–266. [DOI] [PubMed] [Google Scholar]
- 7.Liu X, Wang X, Lin S, et al. Reproducibility and validity of a food frequency questionnaire for assessing dietary consumption via the dietary pattern method in a Chinese rural population. PloS One 2015; 10: e0134627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pandeya N, Williams GM, Sadhegi S, et al. Associations of duration, intensity, and quantity of smoking with adenocarcinoma and squamous cell carcinoma of the esophagus. Am J Epidemiol 2008; 168: 105–114. [DOI] [PubMed] [Google Scholar]
- 9.Pandeya N, Williams G, Green AC, et al. Alcohol consumption and the risks of adenocarcinoma and squamous cell carcinoma of the esophagus. Gastroenterology 2009; 136: 1215–1224. [DOI] [PubMed] [Google Scholar]
- 10.Ollberding NJ, Aschebrook-Kilfoy B, Caces DB, et al. Dietary intake of fruits and vegetables and overall survival in non-Hodgkin lymphoma. Leuk Lymphoma 2013; 54: 2613–2619. [DOI] [PubMed] [Google Scholar]
- 11.Han X, Zheng T, Foss F, et al. Vegetable and fruit intake and non-Hodgkin lymphoma survival in Connecticut women. Leuk Lymphoma 2010; 51: 1047–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu Y, Wu H, Wang PP, et al. Dietary patterns and colorectal cancer recurrence and survival: a cohort study. BMJ Open 2013; 3: pii: e002270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vrieling A, Buck K, Seibold P, et al. Dietary patterns and survival in German postmenopausal breast cancer survivors. Br J Cancer 2013; 108: 188–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwedhelm C, Boeing H, Hoffmann G, et al. Effect of diet on mortality and cancer recurrence among cancer survivors: a systematic review and meta-analysis of cohort studies. Nutr Rev 2016; 74: 737–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Playdon MC, Nagle CM, Ibiebele TI, et al. Pre-diagnosis diet and survival after a diagnosis of ovarian cancer. Br J Cancer 2017; 116: 1627–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lambert R and, Hainaut P. Esophageal cancer: cases and causes (part I). Endoscopy 2007; 39: 550–555. [DOI] [PubMed] [Google Scholar]
- 17.Siddiqi MA, Tricker AR, Kumar R, et al. Dietary sources of N-nitrosamines in a high-risk area for oesophageal cancer–Kashmir, India. IARC Sci Publ 1991; 105: 210–213. [PubMed] [Google Scholar]
- 18.Fong LY, Farber JL, Magee PN. Zinc replenishment reduces esophageal cell proliferation and N-nitrosomethylbenzylamine (NMBA)-induced esophageal tumor incidence in zinc-deficient rats. Carcinogenesis 1998; 19: 1591–1596. [DOI] [PubMed] [Google Scholar]
- 19.Sullivan PA, Eisen EA, Woskie SR, et al. Mortality studies of metalworking fluid exposure in the automobile industry: VI. A case-control study of esophageal cancer. Am J Ind Med 1998; 34: 36–48. [DOI] [PubMed] [Google Scholar]
- 20.Straif K, Weiland SK, Bungers M, et al. Exposure to high concentrations of nitrosamines and cancer mortality among a cohort of rubber workers. Occup Environ Med 2000; 57: 180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ribeiro U, Jr., Posner MC, Safatle-Ribeiro AV, et al. Risk factors for squamous cell carcinoma of the oesophagus. Br J Surg 1996; 83: 1174–1185. [PubMed] [Google Scholar]
- 22.Chen T, Stoner GD. Inducible nitric oxide synthase expression in N-nitrosomethylbenzylamine (NMBA)-induced rat esophageal tumorigenesis. Mol Carcinog 2004; 40: 232–240. [DOI] [PubMed] [Google Scholar]
- 23.Liston BW, Gupta A, Nines R, et al. Incidence and effects of Ha-ras codon 12 G–>A transition mutations in preneoplastic lesions induced by N-nitrosomethylbenzylamine in the rat esophagus. Mol Carcinog 2001; 32: 1–8. [DOI] [PubMed] [Google Scholar]
- 24.Wang QS, Sabourin CL, Wang H, et al. Overexpression of cyclin D1 and cyclin E in N-nitrosomethylbenzylamine-induced rat esophageal tumorigenesis. Carcinogenesis 1996; 17: 1583–1588. [DOI] [PubMed] [Google Scholar]
- 25.Wang QS, Sabourin CL, Bijur GN, et al. Alterations in transforming growth factor-alpha and epidermal growth factor receptor expression during rat esophageal tumorigenesis. Mol Carcinog 1996; 15: 144–153. [DOI] [PubMed] [Google Scholar]