Short abstract
Objectives
This study aimed to examine a simple, effective, time-saving, and low-cost protein microarray method for detecting serum alpha-fetoprotein (AFP) and AFP-L3 levels.
Methods
Serum samples from patients with hepatocellular carcinoma (HCC) (n = 33) and control subjects (n = 39) were collected and evaluated for the presence of AFP using a novel protein microarray. Glycoprotein (including AFP-L3) was enriched from crude samples by a Hotgen Biotech glycosyl capture spin column and then detected by protein microarray. An electrochemiluminescence immunoassay (ECLIA) was used to validate the measured values.
Results
Neither AFP levels lower than 20 ng/mL in the HCC group nor AFP levels higher than 20 ng/mL in the control group were found when tested by the ECLIA and protein microarray. The kappa test showed good consistency in the diagnostic performance of measuring serum AFP levels and the percentage of AFP-L3 in total AFP by the ECLIA and protein microarray. Protein microarray had advantages of smaller sample size required, low cost, and convenience compared with the ECLIA.
Conclusion
The protein microarray assay that was developed in the present study shows potential as an economic and convenient technique for detecting AFP and AFP-L3 levels in serum samples from patients with HCC.
Keywords: Protein microarray, alpha-fetoprotein (AFP), AFP-L3, hepatocellular carcinoma, electrochemiluminescence immunoassay, glycoprotein, Lens culinaris agglutinin
Introduction
The World Health Organization has estimated that the third leading cause of cancer-related global death and the fifth most common cancer is hepatocellular carcinoma (HCC). Most HCCs originate from hepatitis B and C virus infections.1–3 The incidence of HCC is especially high in eastern and south-eastern Asia. In these areas, there has been a large increase in the prevalence of chronic hepatitis B and C virus infections, which has resulted in a large group of patients with liver cirrhosis.
For the diagnosis of HCC, many types of technology, including imaging tools, are available for doctors to use. These technologies include resonance imaging and computed tomography, and application of tumor biomarkers, such as alpha-fetoprotein (AFP), Golgi protein 73,4,5 and vitamin K absence or antagonist-II.6 Currently, serum AFP levels are widely used by many physicians for diagnosing HCC in clinical practice. However, serum AFP levels can also be increased in benign liver diseases, such as liver cirrhosis and hepatitis, and AFP levels in up to 40% of patients with HCC can be normal. Therefore, the poor specificity of AFP is an inherent concern for its application in diagnosis of cancer.
One approach to greatly improve the selectivity of AFP is to clarify and identify its subtypes. According to the percentage of Lens culinaris agglutinin (LCA)-reactive AFP, AFP can be classified into three categories, including AFP-L1 (LCA nonreactive), AFP-L2 (LCA low-reactive), and AFP-L3 (LCA reactive).7,8 Fucosylation of biantennary sugar chains that are associated with various biological events can be observed in AFP-L3. AFP-L3 is widely modified at posttranslational levels and its changes are closely related to specific for diagnosing HCC.9,10 The percentage of AFP-L3 in total AFP is called the fucosylation index of AFP or AFP-L3%. Therefore, serum AFP-L3 levels and AFP-L3% can be effectively applied to discriminate HCC from benign liver diseases and then for diagnosing HCC early in the clinic.
For detecting AFP-L3, several methods are available, such as the affinity adsorption assay,11 immune electrophoresis,12,13 enzyme-linked immunoassay, the electrochemical technique,14 affinity chromatography, and affinity imprinting. However, all of these methods have some disadvantages, including being time-consuming, complex to perform, having low sensitivity, requiring a large sample size, and requiring sophisticated instruments. Therefore, a method that is highly sensitive, low cost, only needs a small sample size, and is time-effective is required. Protein microarrays or protein chips, based on a protein–protein interaction, are a robust and versatile method in cancer proteomics research. This method has advantages, including high sensitivity, low sample consumption, high throughput, and multiple proteins are able to be measured.15–18 Therefore, protein microarrays have served as a useful tool for screening of tumor biomarkers.
In this study, we developed a protein microarray method for detecting AFP and AFP-L3 levels. The Hotgen Biotech glycosyl capture spin column was used to enrich glycoprotein (including AFP-L3) from crude samples.
Materials and methods
Serum samples
The clinical specimens were collected from patients at Beijing YouAn Hospital. Patients were divided into the HCC (Cancer Classification of Malignant Tumors [TNM] stages I–IV) group or the control group. All of the patients with HCC were diagnosed by histological findings or imaging technological characteristics and classified using the American Joint Committee on TNM. Moreover, control subjects were screened by resonance imaging to exclude potential HCC. Each individual provided 5 mL of blood or this study. Sample collection was approved by the institutional ethics committee of Beijing YouAn Hospital and informed consent was obtained from each patient before collection. All blood specimens were centrifuged at 3500 rpm/minute for 30 minutes after clotting. Serum samples were then coded, sub-packed, and stored at −80°C. Each aliquot was not thawed infrequently before use.
Antibodies and reagents
Mouse monoclonal AFP antibody, rabbit horseradish peroxidase (HRP)-labeled polyclonal AFP antibody, and recombinant AFP standard solution were obtained from Fapon Biotech Inc. (Shenzhen, China). Bovine serum albumin (BSA) and normal goat serum were purchased from Abcam Company Ltd. (Cambridge, MA, USA). The Hotgen Biotech glycosyl capture spin column was supplied by Beijing Hotgen Biological Technology Co., Ltd. (Beijing, China). Chemiluminescent HRP substrate RapidStep™ ECL was purchased from Merck KgaA Ltd. (Darmstadt, Germany).
Protein microarray fabrication
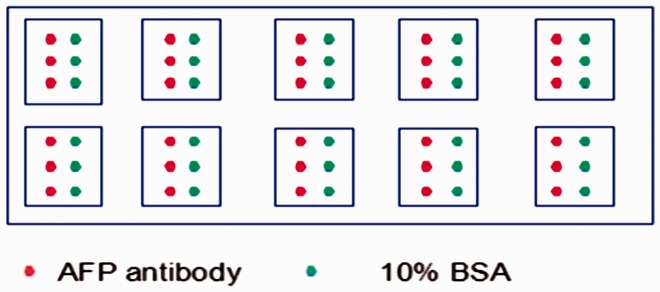
The GeSiM Nano-Plotter™ Micropipetting System (Radeberg, Germany) was used to spot approximately 100 pL of mouse monoclonal AFP antibody solution (1.25 mg/mL) and 10% BSA on the surface of aldehyde-coated microscope slides (Shanghai Baiao Co., Shanghai, China) in triplicate (Figure 1). Spotted BSA was applied as control protein. The detailed spotting process was performed according to the manufacturer’s instructions and settings. The slides were incubated at 4°C for at least 24 hours after being imprinted, and then they were blocked in a coupling buffer (10% normal goat serum with 0.1% NaN3) at 37°C for 2 hours. The slides were then rinsed briefly with 1 × phosphate-buffered saline with 0.1% Tween-20 three times for 3 minutes each. After the slides were rinsed, they were dried by centrifugation in plastic and then were ready to be used at 4°C.
Figure 1.
Diagram of AFP antibody and BAS solution spotted on a protein microarray.
AFP: alpha-fetoprotein; BSA: bovine serum albumin.
Separation of serum AFP-L3
Each AFP-L3 fraction solution was separated from all serum samples by using the Hotgen Biotech glycosyl capture spin column, which is widely used for glycosyl detection in Chinese hospitals. The basic principle of this spin column is that LCA is preloaded in the column to capture fucosylated materials, and then a simple sugar is applied to compete for binding to LCA with the captured fucosylated materials. Therefore, serum fucosylated materials, including AFP-L3, are finally eluted out of the spin column.
Detection of AFP and AFP-L3
All serum samples and corresponding separated AFP-L3 solutions were measured by the prepared protein microarray and an electrochemical instrument (Hitachi-Technologies Corporation, Tokyo, Japan), respectively. For the protein microarray, all detected samples were diluted tenfold or to a suitable dilution. A total of 15 µL of diluted samples were added to each slide and incubated at 37°C for 30 minutes. After rinsing the slides with 1 × phosphate-buffered saline with 0.1% Tween-20 three times for 3 minutes each, 15 µL of 1 mg/mL HRP-labeled polyclonal AFP antibody was added to each slide and incubated at 37°C for 30 minutes again. After washing as mentioned above, the chemiluminescent HRP substrate was added to each slide and then scanned by a chemiluminescent scanner (Chemi Doc™ MP System; Bio-Rad, Hercules, CA, USA). Image Lab software Version 5 (Bio-Rad Laboratories, Inc., Hercules, CA, USA) was used to quantify the image data. Recombinant AFP protein was used as the positive control and to create the standard curve of AFP. The electrochemical instrument was used according to the manufacturer’s instructions.
Statistical analysis
IBM SPSS version 22.0 (IBM Corp., Armonk, NY, USA) and GraphPad Software version 7 (GraphPad Software, Inc., San Diego, CA, USA) were used to analyze the data. The Mann–Whitney test and Student’s t test were applied to compare differences between values. The weighted kappa test was used to measure agreement of the results tested by two different methods. P < 0.05 was considered as statistically significant.
Results
Table 1 shows the clinical features of all 72 patients who were enrolled, including serum levels of alanine aminotransferase, aspartate aminotransferase, and total bilirubin. There were 48 men and 24 women. There were 33 patients in the HCC group and 39 patients in the control group, including four healthy people, nine patients with hepatitis, and six patients with cirrhosis. There was no significant difference in age between the HCC and control groups. With regard to laboratory findings in this study, serum levels of aspartate aminotransferase (P = 0.04) and total bilirubin (P = 0.02) were significantly higher in the HCC group than in the control group.
Table 1.
Characteristics of the study population
| Clinical features | HCC group | Control group | P value |
|---|---|---|---|
| Men | 23 | 25 | |
| Women | 10 | 14 | |
| Age, years | 57 ± 9 | 51 ± 12 | 0.17 |
| Types of disease | |||
| Cancer | 33 | ||
| Cirrhosis | 6 | ||
| Hepatitis | 29 | ||
| Healthy | 4 | ||
| Stage of cancer | |||
| I | 6 | ||
| II | 8 | ||
| III | 12 | ||
| IV | 7 | ||
| ALT, U/L | 38.1 ± 21.1 | 31.8 ± 19.4 | 0.37 |
| AST, U/L | 81.1 ± 83.3 | 30.2 ± 14.3 | 0.04 |
| Total bilirubin, mg/dL | 34.1 ± 31.3 | 13.9 ± 5.7 | 0.02 |
HCC: hepatocellular carcinoma; ALT: alanine aminotransferase; AST: aspartate aminotransferase.
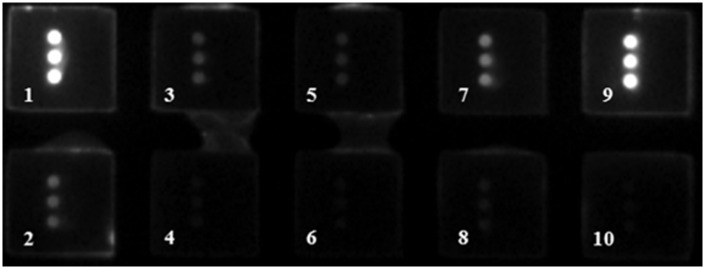
Among the 72 serum samples tested by ECLIA, AFP serum levels of the 33 patients in the HCC group were higher than 20 ng/mL and those of the 39 individuals in the control group were lower than 20 ng/mL (Table 2). The results detected by protein microarray were similar to those of ECLIA. The standard curve of AFP used in the protein microarray method is shown in Figure 2. When AFP-L3% was measured by ECLIA, AFP-L3% in one patient in the control group was higher than 10% and no patients in the HCC group showed AFP-L3% less than 10%. When AFP-L3% was measured by protein microarray, three patients in the control group showed AFP-L3% higher than 10% and one patient in the HCC group showed AFP-L3% less than 10%. Figure 3 shows that there was a considerable difference in serum AFP levels before and after pretreatment of the Hotgen Biotech glycosyl capture spin column. The kappa test showed good consistency in the diagnostic performance of measuring serum AFP levels and AFP-L3% by the ECLIA and protein microarray (Table 3).
Table 2.
Detection of serum AFP levels and AFP-L3% in the HCC and control groups
| Method | AFP levels ≥ 20 ng/mL | AFP levels > 0 and < 20 ng/mL | AFP-L3% ≥10% | AFP-L3% > 0% and < 10% | |
|---|---|---|---|---|---|
| ECLIA | HCC group | 33 | 0 | 33 | 0 |
| Control group | 0 | 39 | 1 | 38 | |
| Protein microarray | HCC group | 33 | 0 | 32 | 1 |
| Control group | 0 | 39 | 3 | 36 | |
Values represent the number of patients in each group. AFP: alpha-fetoprotein; HCC: hepatocellular carcinoma; AFP-L3%: percentage of AFP-L3 in total AFP; ECLIA: electrochemiluminescence immunoassay.
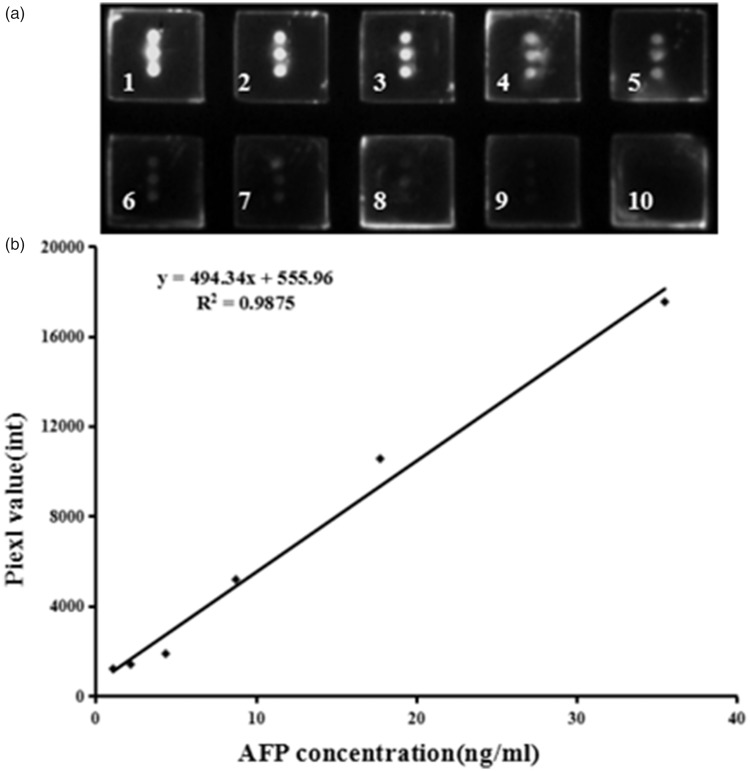
Figure 2.
(a) Image of a protein microarray for detecting AFP antigen standards. Different concentrations of AFP antigen standards were added to wells one to six (35.5, 17.8, 8.8, 4.4, 2.2, and 1.1 ng/mL). Serum samples from the control group were added to wells seven to nine and well 10 was used as a negative control. (b) Standard curve used in the protein microarray for determining AFP levels. AFP: alpha-fetoprotein.
Figure 3.
Image of a protein microarray for detecting serum samples before and after pretreatment of the Hotgen Biotech glycosyl capture spin column. Four pairs of serum samples were added to wells one and two, three and four, five and six, and seven and eight. Wells nine and 10 were used as a positive and negative control, respectively.
Table 3.
Detection performance of the ECLIA and protein microarray in measuring serum AFP levels and AFP-L3%
|
ECLIA |
||||||
|---|---|---|---|---|---|---|
| AFP levels ≥20 ng/mL | AFP levels > 0 and < 20 ng/mL | Total | Kappa value | P value | ||
| Protein microarray | AFP levels ≥20 ng/mL | 33 | 0 | 33 | 1 | <0.001 |
| AFP levels > 0 and< 20 ng/mL | 0 | 39 | 39 | |||
| Total | 33 | 39 | 72 | |||
|
ECLIA |
||||||
|
|
|
AFP-L3% ≥10% |
AFP-L3% > 0% and < 10% |
Total |
Kappa value |
P value |
| Protein microarray | AFP-L3% ≥10% | 33 | 2 | 35 | 0.97 | <0.001 |
| AFP-L3% > 0% and < 10% | 1 | 36 | 37 | |||
| Total | 34 | 38 | 72 | |||
The Kappa test showed that there was good consistency between the protein microarray and ECLIA in detecting AFP levels and AFP-L3%. ECLIA: electrochemiluminescence immunoassay; AFP: alpha-fetoprotein; AFP-L3%: percentage of AFP-L3 in total AFP.
Discussion
AFP, a 70-kD major fetal serum glycoprotein, is one of the most commonly tested tumor biomarkers in clinical practice. AFP is synthesized in the liver, yolk sac, or fetal gastrointestinal tract with an unknown function. A slight elevation in serum AFP levels can be seen in either patients with liver cirrhosis and hepatitis or pregnant women.19 In patients with HCC and non-seminomatous testicular cancer, serum AFP levels can be greatly increased and then aid in diagnosis, classification, staging, and monitoring of these diseases.20 Because of the low specificity of AFP, finding a new strategy for AFP that can screen HCC from other benign diseases is required. Previous studies have indicated that AFP can be fractionated into three glycoforms on the basis of reactivity with LCA.8,21 These glycoforms include the AFP-L3 isoform, which can strongly contact with LCA via an α1-6 fucose residue that is attached at the reducing terminus of N-acetylglucosamine.
AFP-L3 is produced by the enzyme of α1-6 fucosyltransferase with GDP-fucose. Previous studies showed that α1-6 fucosyltransferase levels were positively correlated with the degree of dedifferentiation in HCC tissues and then with a subsequent increment of AFP-L3.22 This finding indicates that AFP-L3 levels are closely related to behavior and prognosis of cancer. Therefore, AFP-L3 is more specific to malignant tumors rather than benign diseases, and can be useful for screening and monitoring patients at a high risk of progression to HCC.
The protein microarray is an excellent protein research method, and has been extensively applied to measure AFP levels, including AFP-L3.18 Serum AFP levels can be reliably detected by protein microarray with a sensitivity of 91.67% and a specificity of 93.24%.23 However, many issues remain in measuring AFP-L3% directly by protein microarray, including a low sensitivity, high background, and complex preparation. The main issue is that fabricating a unique AFP-L3 antibody with high specificity for protein microarray use is difficult because antigen activity of glycan on AFP-L3 protein is low. Several studies have reported that anti-AFP-L3 was used to measure AFP-L3 effectively with simultaneous AFP detection.24 However, the specificity of anti-AFP-L3 is still unclear and an antibody is not available.
One strategy to solve this issue is to apply lectins, which are the main method of investigating glycosylation with high specificity. We had previously proposed a chemiluminescent protein chip to detect serum AFP-L3 levels and AFP-L3% based on an LCA-AFP-L3-AFP antibody sandwich reaction.25,26 This method avoids the AFP-L3 antibody, but there are still some limitations, such as a low specificity of LCA, strict performance, and low sensitivity, which impede its wide used in the clinic.27
Another strategy is to apply the antibody array technology for detecting AFP-L3 on the basis of the anti-AFP-AFP-LCA principle. However, to avoid LCA lectin binding to glycans on antibodies, this method needs to chemically derivate glycans on the spotted antibodies. This may affect the affinity of the spotted antibody and greatly add to the preparation time.28,29 Moreover, when detecting serum AFP-L3 levels, non-AFP-L3 may compete against AFP-L3 to bind with the spotted AFP antibody and then effect the accuracy of detecting AFP-L3. This is an important issue that must be addressed if this method is used in the clinical setting. Considering these issues, AFP-L3 must be isolated from total serum AFP before detection by protein microarray to more accurately determine serum AFP-L3 levels.
Therefore, in the present study, serum AFP levels and AFP-L3% were detected by an antibody array combined with the Hotgen Biotech glycosyl capture spin column pretreatment technology. The most striking advantage of this method is that it can simultaneously quantify AFP-L3% and serum AFP levels. This method also has many merits, such as being efficient, and having a high-throughput analysis and low amount of clinical sample required compared with conventional methods.
The electrochemical method is the most commonly used technology for measuring AFP-L3% in clinical practice. We used the electrochemical method to validate the results of serum AFP values and AFP-L3% as tested by antibody array combined with the Hotgen Biotech glycosyl capture spin column. We found no significant difference in results between these two methods. However, an antibody array combined with the Hotgen Biotech glycosyl capture spin column system still has some limitations. These limitations include non-automation and this method is not simple. However, we believe that these problems will be solved in future investigations.
In summary, we developed a new strategy to simultaneously detect AFP serum levels and AFP-L3%. We believe that this unique strategy will potentially be widely used in future medicine.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This study was funded by the Beijing Municipal Science & Technology Commission (D171100003117004).
References
- 1.Giunchi F, Vasuri F, Fiorentino M. Epidemiology of hepatocellular carcinoma. In: Loda M, Mucci L, Mittelstadt M, Van Hemelrijck M and Cotter M. (eds) Pathology and Epidemiology of Cancer Springer, Cham, 2017, pp.447–454. DOI: 10.1007/978-3-319-35153-7_23
- 2.Levrero M, Zucman-Rossi J. Mechanisms of HBV-induced hepatocellular carcinoma. J Hepatol 2016; 64(1 Suppl): S84–S101. [DOI] [PubMed] [Google Scholar]
- 3.Nagaratnam N, Nagaratnam K, Cheuk G. Hepatocellular carcinoma. In: Geriatric Diseases Cham: Springer, 2017, pp.1–4.
- 4.Jiang K, Li W, Zhang Q, et al. GP73 N-glycosylation at Asn144 reduces hepatocellular carcinoma cell motility and invasiveness. Oncotarget 2016; 7: 23530–23541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao Y, Wang M, Cui C, et al. Significance of combined tests of serum golgi glycoprotein 73 and other biomarkers in diagnosis of small primary hepatocellular carcinoma. Cancer Biomarkers:Section A of Disease Markers 2015; 15: 677–683. [DOI] [PubMed] [Google Scholar]
- 6.Gao J, Song P. Combination of triple biomarkers AFP, AFP-L3, and PIVAKII for early detection of hepatocellular carcinoma in China: expectation. Drug Discov Ther 2017; 11: 168–169. [DOI] [PubMed] [Google Scholar]
- 7.Korekane H, Hasegawa T, Matsumoto A, et al. Development of an antibody-lectin enzyme immunoassay for fucosylated α-fetoprotein. Biochim Biophys Acta 2012; 1820: 1405–1411. [DOI] [PubMed] [Google Scholar]
- 8.Kumada T, Toyoda H, Tada T, et al. High-sensitivity Lens culinaris agglutinin-reactive alpha-fetoprotein assay predicts early detection of hepatocellular carcinoma. J Gastroenterol 2014; 49: 555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asazawa H, Kamada Y, Takeda Y, et al. Serum fucosylated haptoglobin in chronic liver diseases as a potential biomarker of hepatocellular carcinoma development. Clin Chem Lab Med 2015; 53: 95–102. [DOI] [PubMed] [Google Scholar]
- 10.Matsuda M, Asakawa M, Amemiya H, et al. Lens culinaris agglutinin-reactive fraction of AFP is a useful prognostic biomarker for survival after repeat hepatic resection for HCC. J Gastroenterol Hepatol 2011; 26: 731–738. [DOI] [PubMed] [Google Scholar]
- 11.Yamagata Y, Shimizu K, Nakamura K, et al. Simultaneous determination of percentage of Lens culinaris agglutinin-reactive alpha-fetoprotein and alpha-fetoprotein concentration using the LiBASys clinical auto-analyzer. Clinica Chimica Acta 2003; 327: 59–67. [DOI] [PubMed] [Google Scholar]
- 12.Ma H, Sun X, Chen L, et al. Multiplex immunochips for high-accuracy detection of AFP-L3% based on surface-enhanced Raman scattering: implications for early liver cancer diagnosis. Anal Chem 2017; 89: 8877–8883. [DOI] [PubMed] [Google Scholar]
- 13.Katoh H, Nakamura K, Tanaka T, et al. Automatic and simultaneous analysis of Lens culinaris agglutinin-reactive alpha-fetoprotein ratio and total alpha-fetoprotein concentration. Anal Chem 1998; 70: 2110–2114. [DOI] [PubMed] [Google Scholar]
- 14.Li J, Gao T, Gu S, et al. An electrochemical biosensor for the assay of alpha-fetoprotein-L3 with practical applications. Biosens Bioelectron 2017; 87: 352–357. [DOI] [PubMed] [Google Scholar]
- 15.Huang Y, Zhu H. Protein array-based approaches for biomarker discovery in cancer. Genomics Proteomics Bioinformatics 2017; 15: 73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haab BB, Partyka K, Cao Z. Using antibody arrays to measure protein abundance and glycosylation: considerations for optimal performance. Curr Protoc Protein Sci 2013; 73: Unit 27.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen S, Haab BB. Analysis of glycans on serum proteins using antibody microarrays. Methods Mol Biol 2009; 520: 39–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haab BB. Antibody-lectin sandwich arrays for biomarker and glycobiology studies. Expert Rev Proteomics 2010; 7: 9–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tomasi TB., Jr. Structure and function of alpha-fetoprotein. Annu Rev Med 1977; 28: 453–465. [DOI] [PubMed] [Google Scholar]
- 20.Ertle JM, Heider D, Wichert M, et al. A combination of α-fetoprotein and des-γ-carboxy prothrombin is superior in detection of hepatocellular carcinoma. Digestion 2013; 87: 121–131. [DOI] [PubMed] [Google Scholar]
- 21.Oka H, Saito A, Ito K, et al. Multicenter prospective analysis of newly diagnosed hepatocellular carcinoma with respect to the percentage of Lens culinaris agglutinin-reactive alpha-fetoprotein. J Gastroenterol Hepatol 2001; 16: 1378–1383. [DOI] [PubMed] [Google Scholar]
- 22.Aoyagi Y, Mita Y, Igarashi H, et al. The fucosylation index of serum alpha-fetoprotein as useful prognostic factor in patients with hepatocellular carcinoma in special reference to chronological changes. Tumor Biology 2002; 21: 38. [DOI] [PubMed] [Google Scholar]
- 23.Zhao Y, Zhang Y, Lin D, et al. Protein microarray with horseradish peroxidase chemiluminescence for quantification of serum α-fetoprotein. J Int Med Res 2015; 43: 639–647. [DOI] [PubMed] [Google Scholar]
- 24.Wu J, Zhu J, Yin H, et al. Analysis of glycan variation on glycoproteins from serum by the reverse lectin-based ELISA assay. J Proteome Res 2014; 13: 2197–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang A, Skog S, Wang S, et al. A chemiluminescent protein microarray method for determining the seroglycoid fucosylation index. Sci Rep 2016; 6: 31132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li D, Chiu H, Zhang H, et al. Analysis of serum protein glycosylation by a differential lectin immunosorbant assay (dLISA). Clin Proteomics 2013; 10: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haab BB. Using lectins in biomarker research: addressing the limitations of sensitivity and availability. Proteomics Clin Appl 2012; 6: 346–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu C, Wonsidler JL, Li J, et al. Chemically-blocked antibody microarray for multiplexed high-throughput profiling of specific protein glycosylation in complex samples. J Vis Exp 2012; (63): e3791. DOI:10.3791/3791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen S, LaRoche T, Hamelinck D, et al. Multiplexed analysis of glycan variation on native proteins captured by antibody microarrays. Nat Methods 2007; 4: 437–444. [DOI] [PubMed] [Google Scholar]