Abstract
Glycoprotein non-metastatic melanoma protein B (GPNMB) is a transmembrane protein enriched on the cell surface of cancer cells, including melanoma, glioblastoma, and triple-negative breast cancer. There is growing evidence identifying GPNMB as a tumor-promoter; however, despite its biological and clinical significance, the molecular mechanisms engaged by GPNMB to promote tumorigenesis are not well understood. GPNMB promotes aggressive behaviors such as tumor cell proliferation, migration, and invasion. The extracellular domain of GPNMB shed from the cell surface interacts with integrins to facilitate in the recruitment of immune-suppressive and pro-angiogenic cells to the tumor microenvironment, thereby enhancing tumor migration and invasion. GPNMB also modulates receptor tyrosine kinases and integrin signaling in a cell autonomous fashion, leading to downstream kinase signaling that in turn triggers the expression and secretion of tumorigenic factors such as matrix metalloproteinases (MMPs) and cytokines. Therefore, GPNMB exerts its pro-tumorigenic role both intracellularly and in a paracrine fashion through shedding its extracellular domain. This review highlights the importance of GPNMB in cancer progression and discusses molecular mediators of GPNMB-induced tumor growth and invasion.
Keywords: GPNMB, Melanoma, Breast cancer, Invasion, Matrix metalloproteinase, Antibody-drug conjugate
1. Introduction
Glycoprotein non-metastatic melanoma protein B (GPNMB), also known as osteoactivin (OA; rat ortholog), dendritic cell-heparin integrin ligand (DC-HIL; mouse ortholog), or hematopoietic growth factor inducible neurokinin-1 type (HGFIN), is a type 1 transmembrane protein [1]. Although GPNMB is a relatively recently described protein with therefore limited information about its function, recent studies reveal an interesting biological role for GPNMB in various cancers. Intriguingly, GPNMB appears to have an inhibitory role in some cancers whereas it may promote metastasis in others. In fact, GPNMB was first characterized as a gene expressed in normal mammary epithelial cells with tumor suppressor properties in breast cancer [2]. Similarly, it was originally described in melanoma cell lines in a study that compared phenotypes of low and high metastatic cell lines and found GPNMB to be preferentially expressed in the low-metastatic or “non-metastatic” cell lines [1,3]. In this early study, transfection of GPNMB cDNA into BLM cells, a highly metastatic melanoma cell line, resulted in slowed subcutaneous tumor growth and reduced metastasis in nude mice [3]. However, successive transcriptional profiling and tumor microarray studies in primary melanoma and breast cancers established GPNMB as an aggressive pro-metastatic protein [4–6]. GPNMB is now known to be highly expressed in multiple tumor types, including triple negative breast cancers (TNBC), uveal and cutaneous melanoma, glioblastomas, hepatocellular carcinoma, prostate cancer, osteosarcoma, lung cancer, bladder cancer, and lymphangioleiomyomatosis (LAM) [7–11], making it a critical protein in tumor biology and an attractive target for therapeutic interventions.
Research is currently being performed to understand the molecular complexities of GPNMB and the heterogeneity in its expression. The goals of this review are to shed light on current GPNMB discoveries, including its structure and function as it relates to both autocrine and paracrine actions, its role in both normal physiological processes as well as in cancer progression, and to review the current clinical studies targeting GPNMB-expressing cancers.
2. GPNMB structure and function
The GPNMB gene encodes a type 1a transmembrane protein that has close sequence homology to a common melanocytic protein called premelanosome protein (PMEL17 or gp100) [3]. It is a heavily glycosylated protein with sites for both N- and O- glycosylation [12]. GPNMB is localized on the plasma membrane as well as in subcellular locations in various cell types. In normal cells, it is preferentially localized intracellularly, such as in melanosomes and endosomal/lysosomal compartments [13–16]. However, in cancer cells, including melanoma,TNBC, and glioblastomas, overall GPNMB expression increases and a greater proportion becomes plasma membrane-localized [5,8,17]. There are two GPNMB mRNA isoforms encoding 560 and 572 amino acid proteins. Both isoforms consist of a larger extracellular domain (ECD; also called the ectodomain), a single pass transmembrane domain, and a shorter cytoplasmic tail [18].
GPNMB has been reported to have multifaceted roles, including facilitating cell-cell adhesion and migration, promoting tissue-repair, stimulating kinase signaling, and regulating cell growth and differentiation [17–21]. It has certain vital domains that regulate these processes. GPNMB harbors an RGD (integrin-binding) motif and a polycystic kidney disease (PKD) domain within the ECD, as well as a half immunoreceptor tyrosine-based activation (hemITAM) motif in the cytoplasmic tail [12]. The RGD motif, composed of three amino acids, R-arginine, G-glycine, and D-aspartate, mediates GPNMB-driven cell-cell adhesion in several cellular contexts [15,21,22]. In contrast, the function of GPNMB’s PKD domain is still unclear. However based on its structure, it may facilitate protein-protein and protein-carbohydrate interactions [23]. Additionally, in melanocytes and melanoma cells, glycosylation of the PKD domain influences differential sorting and localization patterns between GPNMB and its closest homolog PMEL17 [12], and therefore may influence expression on the plasma membrane. With regard to other domains, the hemITAM motif of GPNMB is a highly conserved single YxxI sequence in the cytoplasmic tail suggested to play a role in cell-intrinsic Src-mediated signaling [24]. Lastly, GPNMB consists of a dileucine motif in the cytoplasmic tail with a D/ExxxLL sequence, which is commonly associated with functions such as rapid receptor internalization and subsequent lysosomal/endosomal targeting [25]. If either of the leucine residues is mutated, GPNMB is retained at the plasma membrane, as seen with quail GPNMB [12]. However, the significance of these domains in cancer is still unknown.
3. GPNMB is involved in physiological processes in normal tissues
GPNMB is expressed in a broad range of normal tissue types including bone, skin, immune cells, and the hematopoietic system, suggesting that GPNMB regulates a variety of physiologic processes. For example, within bone, it has been shown to promote the differentiation of both osteoclasts and osteoblasts [16,22,26,27]. Specifically, GPNMB associates with integrin complexes in osteoclasts and mediates osteoclast differentiation and fusion to generate multi-nucleated osteoclasts [22]. Importantly, inhibiting GPNMB in developing osteoblasts hinders their differentiation process as well as weakens their ability to form bone matrix [26,28]. Therefore, GPNMB appears to be involved in bone development.
In addition to bone, GPNMB is expressed in immune cells, such as macrophages and dendritic cells [14,29], and may impair T-cell activation to down-modulate anti-tumor immune responses [13,30,31]. Expression of GPNMB in antigen presenting cells such as macrophages and dendritic cells is well documented, which explains its designation as ‘dendritic cell-heparin integrin ligand’ (DC-HIL) [21]. Interestingly, the PKD domain of GPNMB suppresses T-cell activation [32,33]; however, in contrast to this immunosuppressive role, phosphorylation of GPNMB’s hemITAM tyrosine residue in dendritic cells induces increased cytokine secretion, particularly tumor necrosis factor alpha (TNFα) and interleukin-1β (IL-1β), augmenting the activation of nearby naïve T-cells [34].
Within the skin, GPNMB is predominantly expressed in melanocytes, which are found in the skin’s basal layer [12,35]. GPNMB localizes to stage III and IV melanosomes and plays a putative role in melanosome maturation. Furthermore, GPNMB is expressed in other cell types within the skin, including keratinocytes and Langerhans cells [35], with data suggesting that GPNMB may regulate cell-cell adhesion between melanocytes and keratinocytes [15,35].
4. GPNMB in cancer
GPNMB is expressed in a wide array of tumors and is thus emerging as a therapeutic target. However, the role of GPNMB in cancers is complex, since it has both anti- and pro-tumorigenic properties. GPNMB is expressed at a higher level in numerous malignant cancers relative to normal tissues [5,9,17,36,37], and is associated with reduced disease-free and overall survival [9,17]. GPNMB expression often correlates with increased proliferation, migration, invasion, and decreased tumor cell apoptosis [4,10,17,18,37–39] in cancers, including melanoma, triple-negative breast cancer, gliomas, prostate cancer, lung cancer and bladder cancer. In parallel to this observation, as described above, GPNMB’s wide range of physiological roles center around its ability to promote a variety of processes, including cell adhesion and differentiation, both of which are important phenotypes observed in cancer metastasis. GPNMB has diverse subcellular localizations depending on cell type, including the plasma membrane of cancer cells, lysosomal vesicles in osteoclasts, and melanosomes in melanocytes. Importantly, as mentioned, plasma membrane localized GPNMB markedly increases in cancer cells compared to benign cells. Notably, this membrane-bound GPNMB can be proteolytically cleaved from the cell surface, primarily in a matrix metalloproteinase (MMP)-dependent fashion, yielding a soluble extracellular domain of GPNMB containing the RDG motif. Some studies suggest that this shed extracellular domain may account for many of GPNMBs pro-tumorigenic roles (see below).
4.1. GPNMB promotes tumor growth and cancer cell invasion
The functional role of GPNMB in the tumor microenvironment has been extensively studied in culture and in various mouse models, especially with regard to breast cancer and melanoma. In contradiction to previous reports on breast cancer characterizing GPNMB as a tumor suppressor expressed in normal epithelial cells, recent reports have identified it as a driver of primary tumor growth and invasion, promoting endothelial cell recruitment and potentiating lung and bone metastasis [17,18,20]. GPNMB was first implicated as a promoter of breast cancer metastasis when it was detected in elevated levels in select subpopulations of 4 T1 mouse mammary carcinoma cells that aggressively spread to the bone, lungs, and liver [4,18]. Moreover, GPNMB overexpression in poorly metastatic 66cl4 mouse mammary carcinoma cells sufficiently increased their invasion in culture and stimulated bone metastasis in vivo [4]. Furthermore, GPNMB expression enhanced tumor growth in vivo in GPNMB-expressing 66cl4-derived mammary tumors compared to control mammary tumors, although this effect was attributed to decreased apoptosis rather than a difference in proliferation. Additionally, vascular density of these tumors, as quantified by positive CD31 staining, was significantly higher in GPNMB-expressing mammary tumors, indicating that angiogenesis may increase in the presence of GPNMB [18]. Notably, tissue microarray data show that GPNMB is found in the tumor-epithelium of approximately 10% of human breast cancers and in the stroma of nearly 70% of breast cancers [17]. It is primarily overexpressed on the cell surface in basal type breast cancers, particularly triple negative breast cancer (TNBC) [17,36], as well as in cells of the tumor stroma [12].
Since GPNMB is a melanocytic marker predominantly expressed in melanocytes, investigating its role in melanoma is of considerable interest. Malignant melanoma is an extremely aggressive cancer and the most life-threatening skin cancer [1]. Despite widespread efforts to discover treatment options, it is unresponsive to conventional agents, though immunotherapy is becoming the primary approach toward treatment of melanoma [5]. GPNMB was found to be highly expressed in human melanoma cell lines as well as melanoma patient samples [5]. Transcript expression data showed that GPNMB mRNA co-expressed with other melanocytic markers such as PMEL-17, MART-1, and tyrosinase. In addition, flow cytometry confirmed that GPNMB is expressed on the cell surface of most melanoma cell lines [5].
In a recent study, short hairpin (sh) RNA-mediated suppression of GPNMB in B16 melanoma cells reduced subcutaneous tumor growth when injected into syngeneic mice. However, this result was not obtained when these cells were injected into immunodeficient mice. This observation led to the hypothesis that GPNMB may exert an inhibitory function on T-cell activation to promote tumor growth. Hence, GPNMB may impair melanoma-reactive T-cell activation, thereby allowing cancer cells to evade immunologic recognition and destruction [40]. This is, however, only one of the many mechanisms through which GPNMB exerts its pro-tumorigenic properties.
Fiorentini et al. demonstrated that silencing GPNMB reduced the proliferation rate by 32.5% and migratory potential by 63% in DU145 human metastatic prostate cancer cells [41]. Moreover, GPNMB dramatically increased the number of apoptotic cells, similar to mammary tumor models, suggesting that GPNMB-induced impairment of apoptosis may be a key process involved in prostate cancer cell growth regulation [41]. Similar migration results were obtained in PC3 prostate cancer cells after transiently knocking down GPNMB; however, the reduction in proliferation rate was not dramatic. Likewise, Zhang et al. evaluated the functional role of GPNMB in 5637 and BIU87 bladder cancer cells. Their results demonstrated that downregulating GPNMB significantly suppressed bladder cancer cell proliferation, migration, and invasion. Strikingly, cox multivariate analysis indicates that high GPNMB expression is a risk factor of poor prognosis for bladder cancer patients [42].
In a recently published study, Prizant et al. introduced GPNMB as a novel biomarker of lymphangioleiomyomatosis (LAM), a rare and devastating progressive cystic lung cancer. This study demonstrated positive GPNMB staining in LAM-like tumor cells in the lymph nodes and uterus, as well as uniform staining within LAM nodules and the walls of lung cysts in lung LAM from five different patient samples [7].
Taken together, these studies present evidence that GPNMB has a multifaceted role in various types of cancers, and modulates the tumor microenvironment such that it facilitates tumor growth and metastasis via enhanced cancer cell invasion.
4.2. Mechanisms of GPNMB actions in cancer
There are primarily two proposed mechanisms through which GPNMB may promote metastatic processes and modulate the tumor microenvironment (Fig. 1):
Fig. 1.
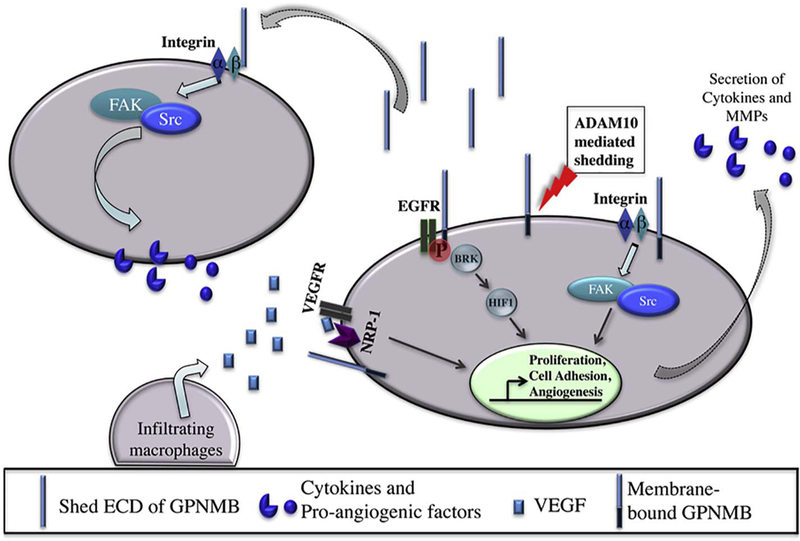
Potential pro-tumorigenic functions of GPNMB. GPNMB can exert both autocrine and paracrine effects to enhance tumor cell growth and cancer cell metastasis. GPNMB extracellular domain (ECD) is shed most likely in an ADAM10-mediated fashion. Tumor-specific integrin α5β1 interacts with the extracellular RGD domain of GPNMB to drive cancer cell metastasis by triggering the expression and secretion of tumorigenic factors such as MMPs and cytokines, most likely via the FAK/Src pathway, as well as by recruiting immune-suppressive cells (such as macrophages) to the tumor microenvironment. Crosstalk between GPNMB and Receptor Tyrosine Kinase signaling includes (1) GPNMB/NRP-1/VEGF receptor signaling that leads to increased tumor cell proliferation and invasion, (2) EGFR:GPNMB heterodimer complex formation enables interaction with LINK-A (long intergenic non-coding RNA for kinase activation, not shown) activating BRK, leading to HIF1α phosphorylation and stabilization, thereby, upregulation of its tumorigenic target genes. NRP-1, Neuropilin-1; ECD, extracellular domain/ectodomain of GPNMB; ADAM10, A Disintegrin and metalloproteinase protein 10; VEGF, Vascular endothelial growth factor; VEGFR, Vascular endothelial growth factor receptor; BRK, Breast tumor kinase or Protein-tyrosine kinase-6; HIF1α, Hypoxia-inducible factor 1-alpha.
-
(1)
Paracrine signaling: recent mechanistic studies focus on membrane-bound GPNMB shedding its RGD- containing extracellular domain, which may act in a paracrine fashion to interact with integrins to directly promote growth, invasion, and metastasis, as well as to facilitate in the recruitment of immune-suppressive and pro-angiogenic cells to the tumor microenvironment.
-
(2)
Autocrine signaling: another possible mechanism is that GPNMB modulates receptor tyrosine kinases (e.g., Epidermal Growth Factor Receptor (EGFR) or Vascular Endothelial Growth Factor Receptor (VEGFR)) and integrin signaling in a cell autonomous fashion, leading to downstream kinase signaling triggering the expression and secretion of tumorigenic factors such as MMPs and cytokines. The following sections elaborate on these potential mechanisms in detail.
GPNMB ectodomain shedding:
A vital process underlying GPNMB’s pro-tumorigenic effects is its ability to be shed from the plasma membrane. While the exact mechanisms governing this process are still not known, evidence suggests that ADAM10 (A Disintegrin and Metalloproteinase), belonging to the MMP family of proteolytic enzymes, is responsible for GPNMB cleavage. Once cleaved by ADAM10, the shed ectodomain (which contains the RGD domain) is a biologically active and soluble molecule that appears to at least in part mediate most pro-tumorigenic phenotypes, including cell-cell adhesion, migration and invasion [11,13,18,30,31]. The level of GPNMB ECD released into the conditioned media of GPNMB-expressing BT549 and basal-like MDA-MB-468 breast cancer cells was prominently diminished after transient siRNA mediated knockdown of ADAM10 [18].
Regulation of MMPs:
In addition to being a target of MMPs, GPNMB (potentially both the intact protein and ectodomain) is also known to activate MMPs, a process that promotes cancer cell migration and invasion. A recent study showed that GPNMB increased invasiveness of DU145 and PC3 through increased MMP2/9 activity. Notably, ERK phosphorylation was not modified by GPNMB silencing in both DU145 and PC3 prostate cancer cell lines [41], suggesting that the regulation of MMP2/9 by GPNMB was independent of ERK. In contrast, LPS treatment upregulated GPNMB expression in activated microglia, inducing a GPNMB-dependent increase in MMP3 expression through the ERK pathway [43]. Moreover, in this model, knocking down GPNMB via siRNA or treatment with an MMP3 inhibitor in microglia BV2 cells suppressed expression of pro-inflammatory factors, including TNFα, IL-1β, NO (nitric oxide), and iNOS (inducible nitric oxide synthase) [43], consistent with previous reports suggesting that GPNMB is pro-in-flammatory through regulation of T-cell activation.
Interaction with integrins and other cell surface receptors:
A study on breast cancer metastasis shed light on a novel interaction between the tumor-specific integrin α5β1 and the extracellular RGD domain of GPNMB to drive breast cancer cell metastasis, most likely via the FAK/Src pathway [20]. Plating BT549 breast cancer cells on fibronectin enhanced GPNMB/α5β1 interactions and FAK/Src signaling, as the integrin α5β1 serves as a receptor for fibronectin [20]. Of note, mutations in both the cytoplasmic tail (cytoplasmic tail truncation) and the extracellular RGD domain (RGD to RAA substitution) eliminated this process, suggesting that full length, membrane-bound GPNMB may be needed to mediate breast cancer cell invasion and growth in vitro. In contrast, only the intact RGD domain was required to facilitate lung metastasis in vivo [20], suggesting that the RGD-containing extra-cellular domain may be the integrin-interacting part of GPNMB, and that the shed ectodomain may be most important during metastasis.
In fact, another study showed that the GPNMB ectodomain engaged with RGD-containing β1 integrins, leading to formation of focal adhesions, followed by increased FAK and ERK activity [44]. In parallel with these observations, a GPNMB ECD-coated plate behaved similarly to a fibronectin plate in terms of promoting lung cancer cell adhesion, confirming that GPNMB binding to integrin in any context (direct cell-cell contact or via the shed ectodomain) is key for cell growth and invasion [8,11].
Therefore, the GPNMB extracellular domain exerts both paracrine and autocrine effects by interacting with integrins and stimulating downstream signaling in neighboring tumor cells and stromal cells to enhance tumor metastasis. One possible interpretation of these data is that the tumor intrinsic functions of GPNMB are important primarily for tumor growth, and require both intracellular and extracellular domains. In contrast, the shed GPNMB ECD and its extracellular domain consisting of the RGD motif may be driving tumor microenvironment modulation and metastasis [8].
Crosstalk between GPNMB and Receptor Tyrosine Kinase Signaling:
Apart from GPNMB’s interactions with integrins, which may occur via both membrane-bound GPNMB and its shed ectodomain, recent studies reveal interactions between membrane-bound GPNMB and receptor tyrosine kinases. Neuropilin-1 (NRP-1; a co-receptor for VEGF) co-expresses with GPNMB in breast cancer cells [20]. GPNMB increases neuropilin-1 expression in multiple human and mouse breast cancer cell lines, which induces VEGF receptor signaling and primary mammary tumor growth, possibly through ERK or AKT signaling pathways. In this model, upon GPNMB mediated upregulation of NRP-1 expression, NRP-1/VEGF receptor signaling leads to recruitment of macrophages and myeloid cells into the microenvironment. In a positive feedback loop, VEGF produced by the infiltrating macrophages and surrounding endothelial cells in the tumor stroma further activates the NRP1/VEGFR2 signaling axis, promoting increased downstream signals that amplify tumor cell growth.
As another example of GPNMB-receptor tyrosine kinase interactions, a recent mechanistic study demonstrated an important role for intact membrane-bound GPNMB engaging with cell surface EGFR receptors in triple-negative breast cancer. In this study, GPNMB was associated with an HB-EGF-stimulated EGFR:GPNMB heterodimer complex [45]. Upon HB-EGF stimulation, EGFR phosphorylated the tyrosine residue in GPNMB’s hemITAM motif, leading to heterodimer formation and interaction with LINK-A (long intergenic non-coding RNA for kinase activation), a cytoplasmic long non-coding RNA (lncRNA). This complex formation then activated BRK (Breast tumor kinase), which mediated phosphorylation of hypoxia-inducible factor 1-alpha (HIF1α), allowing for its stabilization and upregulation of target genes, thereby promoting glycolysis reprogramming and tumorigenesis [45].
GPNMB promotes epithelial-mesenchymal transition (EMT):
Finally, GPNMB may promote metastasis by augmenting EMT of breast cancer cells. Using a microarray analysis, GPNMB was identified as a MAFK (musculoaponeurotic fibrosarcoma oncogene family of small transcription factors) regulated gene in NMuMG cells [46]. Intriguingly, GPNMB or MAFK over-expression stimulated EMT, migration and invasion in vitro, as well as tumor growth in vivo [46]. This response was markedly abrogated by creating a GPNMB mutant whereby the Tyr529 residue within the hemITAM motif was replaced with a phenylalanine, with almost complete failure to induce GPNMB-driven tumor sphere-formation, tumor growth, and EMT [46]. Treatment with the Src inhibitor dasatinib plus the EGFR inhibitor AG1478 had a similar effect, suggesting that Src and the EGFR may be mediating phosphorylation of Tyr529 to promote GPNMB-mediated tumor progression [46].
5. GPNMB-targeted therapies
Selectively targeting tumor cells is a novel and valuable emerging therapeutic approach. One developing method is to optimize antibody-drug conjugates (ADCs). As the name suggests, ADCs consist of target-specific antibodies bound to cytotoxins. ADCs act as vehicles to deliver cytotoxins specifically into the tumor cells. There are currently more than 60 ADCs in clinical settings and each uses different approaches, primarily based on the biology of their target [36]. However, the efficacy of ADCs is limited by a number of factors, including intra/inter-tumoral heterogeneity and the level of the target protein expression in tumor tissue. Some other challenges may include selectivity of the ADC and its internalization rate [36].
GPNMB is an attractive therapeutic target not only due to its important role in driving cancer progression, but also its enhanced cell surface localization on cancer cells compared to its mostly intracellular localization in normal cells. In fact, an ADC targeting GPNMB called glembatumumab vedotin (GV, also called CDX-011 or CR011-vcMMAE) [8] is currently in clinical studies for various cancers, predominantly melanoma and advanced breast cancer.
The history of GV began with the development of a fully human monoclonal antibody named CR011 that was generated against the extracellular domain of GPNMB. However, CR011 did not inhibit the melanoma growth, possibly due to its rapid internalization. Interestingly, CR011 did effectively inhibit cell growth when it was combined with a toxin (saporin) -conjugated secondary antibody [5]. Consequently, this led to the development of CDX-011, which consists of the human GPNMB-specific monoclonal antibody CR011 conjugated to a highly potent anti-mitotic agent called monomethyl auristatin E (MMAE) [36]. This conjugation occurs via a valine-citrulline linker (vc; a protease-specific peptide linker). The mechanism of CDX-011 actions is a multi-step process that involves CR011 binding to cell surface GPNMB, internalization that is facilitated in part by the vc linker, and then cleavage within cellular endosomes, yielding the MMAE toxin that then binds to β -tubulin, resulting in the inhibition of mitosis followed by cell death due to impaired microtubule structure [5,47,48].
5.1. Preclinical studies
Notably, CDX-011 treatment produces anti-tumor effects on human melanoma both in vitro and in vivo [5]. In studies by Tse et al., CDX-011 potently and specifically inhibited growth of GPNMB-positive SK-MEL-2 and SK-MEL-5 melanoma cells in vitro, with IC50 values of 216 and 300 ng/mL, respectively; however, they observed no effects of CDX-011 on GPNMB-negative cells or on tumor cells in which GPNMB levels had been knocked down (IC50 > 1000 ng/mL) [5]. Furthermore, over-expression of full-length GPNMB in HEK293 cells that do not endogenously express GPNMB [5] rendered transfected, but not parent, cells susceptible to CDX-011. Thus, GPNMB expression appears to be both necessary and sufficient to sensitize cells to CDX-011 [5]. Additionally, CDX-011 produced dose-dependent inhibition of melanoma tumor growth in a human melanoma SK-MEL-2 xenograft model at a very low dose (1.25 mg/kg) [5], and regressed tumors did not regrow through a post-treatment duration of 200 days. Importantly, CDX-011-associated deaths, toxicities, abnormal behavior, or weight loss were not observed [5].
These studies were confirmed by Pollack et al., who also observed that CDX-011 produced potent cytotoxic effects in human SK-MEL-2 cells in vitro [1]. They further showed that CDX-011 induced regression of established SK-MEL-2 and SK-MEL-5 xenografts in athymic mice in a dose-dependent manner, with a half-life of approximately 10 days [1]. Both short-term (inhibition of tumor growth) and long-term (complete regression) effects were observed. Furthermore, Kobb et al. tested the activity of CDX-011 in GPNMB-positive osteosarcoma xenograft models [49], finding that CDX-011 induced tumor growth inhibition in these xenografts, whereas GPNMB-negative rhabdomyosarcoma xenograft models showed limited response to CDX-011.
Although data presented in these preclinical models is promising, it is important to note that the CR011 antibody does not recognize and bind to mouse GPNMB (expressed by normal tissues). Thus, in vivo studies performed in mice may underestimate the toxicities induced by CDX-011. Moreover, most of these studies were conducted in immunocompromised mice, which negates the possibility of potential immunoregulatory responses against the ADC, which could impact the pharmacokinetics of the drug [1]. Nonetheless, based on the outcomes of these different studies, examining the relevance of CDX-011 in a clinical setting and continued evaluation of CDX-011 for the treatment of GPNMB-positive cancers is merited.
5.2. Clinical studies
Based on the aforementioned preclinical data, CDX-011 has been evaluated in phase I/II clinical trials for patients with melanoma and advanced breast cancer [50,51].
The first clinical study was performed in melanoma by Ott et al., who investigated various doses of CDX-011 treatment in advanced melanoma patients. They reported 1.88 mg/kg once every 3 weeks as the recommended phase II dose. Based on the success of this clinical trial, which resulted in a promising objective response rate (ORR) of 15%, subsequent clinical trials with CDX-011 have been conducted [50].
Accordingly, 42 patients with metastatic breast cancer were recruited for a Phase I/II study, in which the same dose of CDX-011 resulted in an ORR of 12%. In this study, confirmed expression of GPNMB was not required [36]. Promisingly, a small subset of TNBC patients with GPNMB-expressing tumors had an ORR of 25% [37]. Interestingly, cell death in response to CDX-011 treatment was observed regardless of whether GPNMB was expressed on the tumor cell or detected only in the tumor stroma, suggesting a “bystander tumor cell killing” effect of CDX-011 [36]. It is possible that cells undergoing apoptosis release toxins causing neighboring cancer cells to undergo apoptosis.
Due to the encouraging results from this phase I/II study, the EMERGE study was initiated. This phase II randomized study was primarily designed to evaluate CDX-011 activity specifically in GPNMB-expressing breast cancer patients (patients whose breast cancer expressed GPNMB in at least 5% of tumor epithelium or stromal cells), and secondly to identify any correlation between CDX-011 activity and GPNMB expression pattern [37]. Unfortunately, there was no significant advantage of CDX-011 treatment. However, there was significant benefit of CDX-011 treatment in patients with TNBC (ORR of 40%), since more than 25% of these tumors expressed GPNMB. A shortcoming of this study was the small sample size, making it difficult to generalize the conclusions. To address this limitation, a pivotal ongoing phase II clinical trial called METRIC (NCT01997333) focusing on CDX-011 treatment specifically in patients with TNBC with tumors overexpressing GPNMB [37]. Although these clinical trials established significant antitumor activity by CDX-011, there were frequent side effects observed in patients due to CDX-011 associated toxicity, including rash, fatigue, diarrhea, neuropathy, and myalgia [50,51].
5.3. New directions for drug targeting
Confounding factors impacting the activity of ADC’s include inter/intra tumoral heterogeneity in the target protein expression, and shedding of plasma membrane proteins generating a soluble form of the target protein that may compete with it for ADC binding [6]. Therefore, studies were undergone to determine how to increase cell surface GPNMB expression in melanoma and glioblastoma cell lines.
Melanoma cell lines harboring either NRAS or BRAF mutations have constitutive ERK signaling that maintains cells in a dedifferentiated state by suppressing the expression of differentiation-associated genes such as GPNMB [6]. Hence, in cancer cells with constitutively active ERK signaling, GPNMB might be subjected to partial repression. In vitro studies demonstrate that blocking the MAPK pathway using MEK inhibitors leads to increased GPNMB expression in BRAF or NRAS mutated melanoma cells, but not in melanoma cells with wild-type NRAS/BRAF [6]. Thus, pretreatment with a MEK inhibitor may sensitize melanoma cells to the growth-inhibitory activity of CDX-011 by enhancing cell surface GPNMB expression [6].
In addition to MEK inhibition, other compounds have been shown to increased GPNMB expression, including imatinib (an tyrosine kinase inhibitor specific to Abl), ammonium chloride (which inhibits protein degradation in lysosomes/endosomes), chloroquine (may also affect protein degradation), and GM6001 (a general metalloproteinase inhibitor that my eliminate shedding of the ectodomain) [6].
6. Conclusions
Current understanding about GPNMB has grown tremendously in the past several years, and collectively recent literature emphasizes GPNMB’s critical role in promoting cancer progression. However, there is no true agreement on GPNMB’s underlying mechanism(s) with regard to promoting tumor progression. For example, the reason why GPNMB localization at the membrane is enhanced in cancer cells is not fully elucidated. Furthermore, the contributions of the membrane-bound full length GPNMB, intracellular GPNMB, versus shed GPNMB ectodomain with regard to tumor growth, invasion, and metastasis, have still not been delineated. Hence, more work is required to fully understand GPNMB’s paracrine and autocrine actions. As our understanding of these processes improves, our ability to design better GPNMB-targeted therapeutic approaches will expand, which may lead to further improvements in the treatment of GPNMB-expressing cancers.
Acknowledgements
This work was supported by the National Institutes of Health (R01CA193583).
References
- [1].Pollack VA, et al. , Treatment parameters modulating regression of human melanoma xenografts by an antibody-drug conjugate (CR011-vcMMAE) targeting GPNMB, Cancer Chemother. Pharmacol 60 (3) (2007) 423–435. [DOI] [PubMed] [Google Scholar]
- [2].Rose AA, Siegel PM, Osteoactivin/HGFIN: is it a tumor suppressor or mediator of metastasis in breast cancer? Breast Cancer Res 9 (6) (2007) 403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Weterman MA, et al. , nmb, a novel gene, is expressed in low-metastatic human melanoma cell lines and xenografts, Int. J. Cancer 60 (1) (1995) 73–81. [DOI] [PubMed] [Google Scholar]
- [4].Rose AA, et al. , Osteoactivin promotes breast cancer metastasis to bone, Mol. Cancer Res 5 (10) (2007) 1001–1014. [DOI] [PubMed] [Google Scholar]
- [5].Tse KF, et al. , CR011, a fully human monoclonal antibody-auristatin E conjugate, for the treatment of melanoma, Clin. Cancer Res 12 (4) (2006) 1373–1382. [DOI] [PubMed] [Google Scholar]
- [6].Qian X, et al. , Pharmacologically enhanced expression of GPNMB increases the sensitivity of melanoma cells to the CR011-vcMMAE antibody-drug conjugate, Mol Oncol 2 (1) (2008) 81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Prizant H, et al. , Estrogen maintains myometrial tumors in a lymphangioleiomyomatosis model, Endocr. Relat. Cancer 23 (4) (2016) 265–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Rose AAN, et al. , Targeting GPNMB with glembatumumab vedotin: current developments and future opportunities for the treatment of cancer, Pharmacol. Ther (2017). [DOI] [PubMed] [Google Scholar]
- [9].Kuan CT, et al. , Glycoprotein nonmetastatic melanoma protein B, a potential molecular therapeutic target in patients with glioblastoma multiforme, Clin. Cancer Res 12 (7 Pt 1) (2006) 1970–1982. [DOI] [PubMed] [Google Scholar]
- [10].Li YN, et al. , Glycoprotein nonmetastatic B as a prognostic indicator in small cell lung cancer, APMIS 122 (2) (2014) 140–146. [DOI] [PubMed] [Google Scholar]
- [11].Oyewumi MO, et al. , Osteoactivin (GPNMB) ectodomain protein promotes growth and invasive behavior of human lung cancer cells, Oncotarget 7 (12) (2016) 13932–13944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Maric G, et al. , Glycoprotein non-metastatic b (GPNMB): a metastatic mediator and emerging therapeutic target in cancer, Onco Targets Ther 6 (2013) 839–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zhou LT, et al. , Gpnmb/osteoactivin, an attractive target in cancer immunotherapy, Neoplasma 59 (1) (2012) 1–5. [DOI] [PubMed] [Google Scholar]
- [14].Ripoll VM, et al. , Gpnmb is induced in macrophages by IFN-gamma and lipopolysaccharide and acts as a feedback regulator of proinflammatory responses, J. Immunol 178 (10) (2007) 6557–6566. [DOI] [PubMed] [Google Scholar]
- [15].Tomihari M, et al. , Gpnmb is a melanosome-associated glycoprotein that contributes to melanocyte/keratinocyte adhesion in a RGD-dependent fashion, Exp. Dermatol 18 (7) (2009) 586–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ripoll VM, et al. , Microphthalmia transcription factor regulates the expression of the novel osteoclast factor GPNMB, Gene 413 (1–2) (2008) 32–41. [DOI] [PubMed] [Google Scholar]
- [17].Rose AA, et al. , Glycoprotein nonmetastatic B is an independent prognostic indicator of recurrence and a novel therapeutic target in breast cancer, Clin. Cancer Res 16 (7) (2010) 2147–2156. [DOI] [PubMed] [Google Scholar]
- [18].Rose AA, et al. , ADAM10 releases a soluble form of the GPNMB/Osteoactivin extracellular domain with angiogenic properties, PLoS One 5 (8) (2010) e12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Li B, et al. , The melanoma-associated transmembrane glycoprotein Gpnmb controls trafficking of cellular debris for degradation and is essential for tissue repair, FASEB J 24 (12) (2010) 4767–4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Maric G, et al. , GPNMB cooperates with neuropilin-1 to promote mammary tumor growth and engages integrin alpha5beta1 for efficient breast cancer metastasis, Oncogene 34 (43) (2015) 5494–5504. [DOI] [PubMed] [Google Scholar]
- [21].Shikano S, et al. , Molecular cloning of a dendritic cell-associated transmembrane protein, DC-HIL, that promotes RGD-dependent adhesion of endothelial cells through recognition of heparan sulfate proteoglycans, J. Biol. Chem 276 (11) (2001) 8125–8134. [DOI] [PubMed] [Google Scholar]
- [22].Sheng MH, et al. , Osteoactivin is a novel osteoclastic protein and plays a key role in osteoclast differentiation and activity, FEBS Lett 582 (10) (2008) 1451–1458. [DOI] [PubMed] [Google Scholar]
- [23].Ibraghimov-Beskrovnaya O, et al. , Strong homophilic interactions of the Ig-like domains of polycystin-1, the protein product of an autosomal dominant polycystic kidney disease gene, PKD1, Hum. Mol. Genet 9 (11) (2000) 1641–1649. [DOI] [PubMed] [Google Scholar]
- [24].Bradshaw JM, The Src, Syk, and Tec family kinases: distinct types of molecular switches, Cell. Signal 22 (8) (2010) 1175–1184. [DOI] [PubMed] [Google Scholar]
- [25].Bonifacino JS, Traub LM, Signals for sorting of transmembrane proteins to endosomes and lysosomes, Annu. Rev. Biochem 72 (2003) 395–447. [DOI] [PubMed] [Google Scholar]
- [26].Selim AA, et al. , Anti-osteoactivin antibody inhibits osteoblast differentiation and function in vitro, Crit. Rev. Eukaryot. Gene Expr 13 (2–4) (2003) 265–275. [DOI] [PubMed] [Google Scholar]
- [27].Abdelmagid SM, et al. , Osteoactivin, an anabolic factor that regulates osteoblast differentiation and function, Exp. Cell Res 314 (13) (2008) 2334–2351. [DOI] [PubMed] [Google Scholar]
- [28].Abdelmagid SM, et al. , Osteoactivin acts as downstream mediator of BMP-2 effects on osteoblast function, J. Cell Physiol 210 (1) (2007) 26–37. [DOI] [PubMed] [Google Scholar]
- [29].Ahn JH, et al. , Identification of the genes differentially expressed in human dendritic cell subsets by cDNA subtraction and microarray analysis, Blood 100 (5) (2002) 1742–1754. [PubMed] [Google Scholar]
- [30].Selim AA, Osteoactivin bioinformatic analysis: prediction of novel functions, structural features, and modes of action, Med. Sci. Monit 15 (2) (2009) MT19–33. [PubMed] [Google Scholar]
- [31].Singh M, et al. , Functional roles of osteoactivin in normal and disease processes, Crit. Rev. Eukaryot. Gene Expr 20 (4) (2010) 341–357. [DOI] [PubMed] [Google Scholar]
- [32].Chung JS, et al. , Syndecan-4 mediates the coinhibitory function of DC-HIL on T cell activation, J. Immunol 179 (9) (2007) 5778–5784. [DOI] [PubMed] [Google Scholar]
- [33].Chung JS, et al. , DC-HIL is a negative regulator of T lymphocyte activation, Blood 109 (10) (2007) 4320–4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Chung JS, et al. , Binding of DC-HIL to dermatophytic fungi induces tyrosine phosphorylation and potentiates antigen presenting cell function, J. Immunol 183 (8) (2009) 5190–5198. [DOI] [PubMed] [Google Scholar]
- [35].Hoashi T, et al. , Glycoprotein nonmetastatic melanoma protein b, a melanocytic cell marker, is a melanosome-specific and proteolytically released protein, FASEB J 24 (5) (2010) 1616–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Trail PA, Dubowchik GM, Lowinger TB, Antibody drug conjugates for treatment of breast cancer: novel targets and diverse approaches in ADC design, Pharmacol. Ther (2017). [DOI] [PubMed] [Google Scholar]
- [37].Yardley DA, et al. , EMERGE: a randomized phase II study of the antibody-drug conjugate glembatumumab vedotin in advanced glycoprotein NMB-expressing breast cancer, J. Clin. Oncol 33 (14) (2015) 1609–1619. [DOI] [PubMed] [Google Scholar]
- [38].Rich JN, et al. , Bone-related genes expressed in advanced malignancies induce invasion and metastasis in a genetically defined human cancer model, J. Biol. Chem 278 (18) (2003) 15951–15957. [DOI] [PubMed] [Google Scholar]
- [39].Onaga M, et al. , Osteoactivin expressed during cirrhosis development in rats fed a choline-deficient, L-amino acid-defined diet, accelerates motility of hepatoma cells, J. Hepatol 39 (5) (2003) 779–785. [DOI] [PubMed] [Google Scholar]
- [40].Tomihari M, et al. , DC-HIL/glycoprotein Nmb promotes growth of melanoma in mice by inhibiting the activation of tumor-reactive T cells, Can. Res 70 (14) (2010) 5778–5787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Fiorentini C, et al. , GPNMB/OA protein increases the invasiveness of human metastatic prostate cancer cell lines DU145 and PC3 through MMP-2 and MMP-9 activity, Exp. Cell Res 323 (1) (2014) 100–111. [DOI] [PubMed] [Google Scholar]
- [42].Zhang YX, et al. , Knocking down glycoprotein nonmetastatic melanoma protein B suppresses the proliferation, migration, and invasion in bladder cancer cells, Tumour Biol 39 (4) (2017) 1010428317699119. [DOI] [PubMed] [Google Scholar]
- [43].Shi F, et al. , Induction of matrix metalloproteinase-3 (MMP-3) expression in the microglia by lipopolysaccharide (LPS) via upregulation of glycoprotein nonmeta-static melanoma B (GPNMB) expression, J. Mol. Neurosci 54 (2) (2014) 234–242. [DOI] [PubMed] [Google Scholar]
- [44].Moussa FM, et al. , Osteoactivin promotes osteoblast adhesion through HSPG and alphavbeta1 integrin, J. Cell. Biochem 115 (7) (2014) 1243–1253. [DOI] [PubMed] [Google Scholar]
- [45].Lin A, et al. , The LINK-A lncRNA activates normoxic HIF1alpha signalling in triple-negative breast cancer, Nat. Cell Biol 18 (2) (2016) 213–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Okita Y, et al. , The transcription factor MAFK induces EMT and malignant progression of triple-negative breast cancer cells through its target GPNMB, Sci. Signal 10 (474) (2017). [DOI] [PubMed] [Google Scholar]
- [47].Wu AM, Senter PD, Arming antibodies: prospects and challenges for immunoconjugates, Nat. Biotechnol 23 (9) (2005) 1137–1146. [DOI] [PubMed] [Google Scholar]
- [48].Carter PJ, Senter PD, Antibody-drug conjugates for cancer therapy, Cancer J 14 (3) (2008) 154–169. [DOI] [PubMed] [Google Scholar]
- [49].Kolb EA, et al. , Initial testing (stage 1) of glembatumumab vedotin (CDX-011) by the pediatric preclinical testing program, Pediatr. Blood Cancer 61 (10) (2014) 1816–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ott PA, et al. , Phase I/II study of the antibody-drug conjugate glembatumumab vedotin in patients with advanced melanoma, J. Clin. Oncol 32 (32) (2014) 3659–3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Bendell J, et al. , Phase I/II study of the antibody-drug conjugate glembatumumab vedotin in patients with locally advanced or metastatic breast cancer, J. Clin. Oncol 32 (32) (2014) 3619–3625. [DOI] [PubMed] [Google Scholar]


