Abstract
Purpose
This retrospective analysis evaluated treatment with trabectedin plus pegylated liposomal doxorubicin (PLD) in 34 heavily pretreated patients (median number of previous lines, 3; range, 2-10) with platinum-sensitive relapsed ovarian cancer (ROC) at a single center in Italy.
Methods
Trabectedin/PLD treatment consisted of trabectedin administered every 3 weeks as a 3-hour intravenous (i.v.) infusion at a dose of 1.1 mg/m2, immediately after PLD 30 mg/m2 i.v. infusion. Study objectives were the evaluation of the objective response rate (ORR), progression-free survival (PFS) and overall survival (OS).
Results
Three complete responses and 8 partial responses were observed, with an ORR of 32.4% (95% CI, 17.450.5%). Median PFS was 6.1 months (95% CI, 4.4-8.9 months). Median OS was 16.3 months (95% CI, 6.8-23.5). Most responses (9 of 11) were found in patients with partially platinum-sensitive disease (ORR 40.9% in this subset; median PFS 6.8 months and median OS 20.8 months). Grade 3 treatment-related adverse events consisted of nausea/vomiting (n = 5; 14.7%), mucositis (n = 2; 5.9%), alanine aminotransferase increase, anemia and neutropenia (n = 1 each; 2.9%).
Conclusions
The overall findings appear consistent with those previously observed in a randomized controlled clinical trial, and support the use of trabectedin/PLD in heavily pretreated patients with platinum-sensitive ROC, especially those with partially platinum-sensitive disease.
Keywords: Ovarian cancer, Trabectedin, PLD, Clinical practice
Introduction
Trabectedin (Yondelis®) is a marine-derived antineoplastic agent that was initially isolated from the Caribbean tunicate Ecteinascidia turbinata and is currently produced by chemical synthesis. In early phase II trials, trabectedin showed encouraging antitumor activity as a single-agent therapy in relapsed ovarian cancer (ROC) (1-4). Trabectedin plus doxorubicin showed synergy in vitro (5-7) and, in a phase I trial, trabectedin plus pegylated liposomal doxorubicin (PLD) was well tolerated and provided clinical benefit in pretreated patients with diverse solid tumor types including epithelial ovarian cancer (8). A randomized multicenter phase III trial (OVA-301) evaluated trabectedin/PLD versus PLD alone in ROC (9). Trabectedin/PLD improved progression-free survival (PFS) over PLD alone, with a 21% risk reduction of disease progression or death (hazard ratio [HR] 0.79; 95% confidence interval [CI] 0.65-0.96; p = 0.0190; median 7.3 vs. 5.8 months) and adequate tolerability. In the platinum-sensitive stratum (platinum-free interval [PFI] ≥6 months), the risk reduction of PD or death was 27% (HR 0.73; 95% CI 0.56-0.95; p = 0.0170; median PFS 9.2 vs. 7.5 months). Based on these results, trabectedin/PLD received approval for the treatment of patients with relapsed, platinum-sensitive ovarian cancer in the EU in 2009 and in around 70 countries. Further data obtained from the OVA-301 study after a median follow-up of 47.4 months showed a median overall survival (OS) for the trabectedin/PLD and PLD arms of 22.2 and 18.9 months, respectively (HR 0.86; 95% CI 0.72-1.02; p = 0.0835) (10).
Apart from information obtained in clinical trials, few data are available about the use of trabectedin for ROC in clinical practice. Two retrospective analyses evaluated trabectedin as a single agent in patients with ROC (11, 12), but to date little information is available on the use of trabectedin in combination with PLD. This retrospective analysis was conducted on heavily pretreated patients with platinum-sensitive ROC receiving trabectedin/PLD treatment at a single center in Italy consecutively from May 2012 to January 2015.
Methods
Written informed consent to treatment and use of clinical data for scientific purposes was obtained from all patients at the time of chemotherapy administration. Given the retrospective design of this analysis, approval of local ethics committees to retrieve data from clinical charts was not required, but the principles outlined in the Declaration of Helsinki were followed.
Patients were adult women with histologically confirmed epithelial ovarian cancer, previously treated with at least 1 platinum-based regimen, and with radiological evidence of disease progression, Eastern Cooperative Oncology Group (ECOG) performance status (PS) ≤2 and adequate hematological (hemoglobin ≥9 g/dL; absolute neutrophil count ≥1.5 × 109/L; platelets ≥100 × 109/L), renal (serum creatinine ≤1.5 mg/dL) and hepatic function (bilirubin ≤ upper limit of normal [ULN]; aspartate aminotransferase [AST]/ alanine aminotransferase [ALT] ≤2.5 × ULN; alkaline phosphatase [AP] ≤2.5 × ULN [if total AP >2.5 × ULN, AP liver fraction and/or gamma glutamyltransferase and/or 5′-nu-cleotidase had to be ≤ULN], and albumin >25 g/L).
Trabectedin/PLD treatment consisted of trabectedin administered every 3 weeks as a 3-hour intravenous (i.v.) infusion at a dose of 1.1 mg/m2, immediately after i.v. infusion of PLD at a dose of 30 mg/m2. To minimize the risk of PLD infusion reactions, the initial dose was administered at a rate no greater than 1 mg/minute and, if no infusion reaction was observed, subsequent PLD infusions were administered over a 1-hour period. All patients received i.v. prophylactic medication with corticosteroids (dexamethasone 20 mg or equivalent) 30 minutes prior to PLD infusion.
Tumor response and PFS were assessed according to the Response Evaluation Criteria in Solid Tumors, RECIST v. 1.1 (13). Patients were also followed up for OS analysis. PFS and OS were calculated from the first day of trabectedin/PLD treatment to the date of disease progression or death.
The safety of trabectedin/PLD treatment was evaluated by recording of adverse events (AEs), laboratory test results, physical examinations and vital signs. AEs and laboratory abnormalities were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE), v.3.0.
Descriptive statistics were used for this retrospective analysis. Noncontiguous variables are described in frequency tables using counts and percentages. Continuous variables are described by median, minimum and maximum. The binomial exact estimator and its 95% CI was calculated for the evaluation of categorical efficacy variables (e.g., RECIST response). The Kaplan-Meier method was used for time-to-event variables (PFS and OS). Exploratory log-rank tests were utilized to estimate the relationship of time-to-event variables (PFS and OS) with tumor grade, histology, BRCA mutation status, number of prior surgical procedures, number of prior chemotherapy lines or PFI.
Results
Patient characteristics
Thirty-four patients with ROC were treated with trabect-edin/PLD in the evaluated period. Their main demographic and baseline characteristics are shown in Table I. Most of them (67.6%) had serous adenocarcinoma and differentiation grade 3 tumors (76.5%) at time of diagnosis. All patients had undergone surgery; the initial tumor was optimally debulked in 73.5% of cases. BRCA mutation was found in 3 of 13 patients with available assessment: 1 in the BRCA1 gene and 2 in BRCA2.
Table I.
Demographic and baseline characteristics (n = 34)
| No. | % | ||
|---|---|---|---|
| Age (years) | Median (range) | 60 (26-79) | |
| Time from diagnosis (months) | Median (range) | 30.0 (11.0-124.9) | |
| Histological type | Serous | 23 | 67.6 |
| adenocarcinoma | |||
| Othera | 9 | 26.5 | |
| Undetermined | 2 | 5.9 | |
| Tumor grade at diagnosis | 2 | 5 | 14.7 |
| 3 | 26 | 76.5 | |
| 4 | 1 | 2.9 | |
| UK | 2 | 5.9 | |
| Residual tumor | Optimally debulked <1b | 25 | 73.5 |
| Non-optimally debulkedc | 9 | 26.5 | |
| BRCA | Wild type | 10 | 29.4 |
| Mutated | 3d | 8.8 | |
| Unknown | 21 | 61.8 | |
| Number of previous lines of chemotherapy | Median (range) | 3 (2-10) | |
| 2 | 10 | 29.4 | |
| 3 | 10 | 29.4 | |
| ≥4 | 14 | 41.2 | |
| Platinum-free interval (months) | 6-12 | 22 | 64.7 |
| >12 | 12 | 35.3 | |
UK = unknown.
Adenocarcinoma not otherwise specified (NOS), clear cell, endometrioid (n = 4), mucinous, primitive peritoneal and yolk sac tumor.
Residual tumor <1 cm.
Residual tumor ≥1 cm (n = 7) or localized peritoneal seeding (n = 2).
BRCA1 (n = 1) and BRCA2 (n = 2).
The evaluated population had been heavily pretreated; the median number of previous chemotherapy lines was 3 (range, 2-10 lines), and 41.2% of patients had received 4 or more lines. Most patients (64.7%) had partially platinum-sensitive disease (i.e., PFI was 6-12 months) (Tab. I).
Trabectedin/PLD treatment
The median number of trabectedin/PLD cycles received per patient was 5 (range, 1-16), although those patients less heavily pretreated were able to receive trabectedin/PLD for longer periods: patients with 2 prior chemotherapy lines had a median of 9 cycles administered.
Response to treatment
Thirty-one patients were evaluable for response (Tab. II). Three complete responses (CRs) and 8 partial responses (PRs) were observed, with an objective response rate (ORR) of 32.4% (95% CI 17.4-50.5). All but 2 responses were in patients with partially platinum-sensitive disease (ORR was 40.9% in this subset of patients). One CR was found in a patient with partially platinum-sensitive disease and BRCA2 mutation. Ten of these 11 tumor responses were observed in patients with serous histology, 9 in patients with grade 3 tumors, 8 in patients with optimally debulked residual tumor, and 7 in patients with only 1 previous surgical procedure.
Table II.
Response rate according to RECIST (n = 34)
| No. | % | |
|---|---|---|
| Complete response | 3 | 8.8 |
| Partial response | 8 | 23.5 |
| Stable disease | 16 | 47.1 |
| Progressive disease | 4 | 11.8 |
| Not evaluable | 3 | 8.8 |
| Objective response rate, % (95% CI) | 32.4 (17.4-50.5) | |
RECIST = Response Evaluation Criteria in Solid Tumors; CI = confidence interval.
Progression-free survival
Median PFS was 6.1 months (95% CI 4.4-8.9). The PFS rates at 6 and 12 months were 52.5% and 12.4%, respectively (Tab. III). There was no statistically significant difference according to the number of previous chemotherapy lines, although a trend to a better outcome was observed in those patients less pretreated (Fig. 1). Median PFS was 6.8 months in patients with partially platinum-sensitive disease. PFS was significantly longer in patients with serous histology (8.9 vs. 5.2 months in those with other histologies; p = 0.0433).
Table III.
Progression-free survival (n = 30)
| Progression-free survival | |
|---|---|
| Censored, n (%) | 8 (26.7%) |
| Median (95% CI) (months) | 6.1 (4.4-8.9) |
| PFS at 6 months, % (95% CI) | 52.5% (33.5-71.5) |
| PFS at 12 months, % (95% CI) | 12.4% (0-27.5) |
CI = confidence interval; PFS = progression-free survival.
Fig. 1.
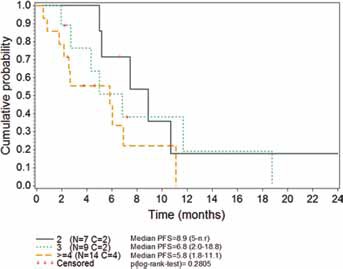
Kaplan-Meier plot of progression-free survival (PFS) according to the number of previous chemotherapy lines received. Data shown are medians (95% CI). C = censored; CI = confidence interval; N = number of patients; n.r. = not reached.
Overall survival
The majority of patients were alive at the time of this retrospective analysis (58% of patients censored) and therefore OS data were very immature. Median OS was 16.3 months (95% CI 6.8-23.5). The OS rates at 6 and 12 months were 76.3% and 68.9%, respectively. A statistically significant difference (p = 0.0318) was found according to the number of previous chemotherapy lines (Fig. 2).
Fig. 2.
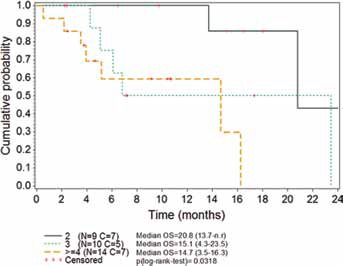
Kaplan-Meier plot of overall survival (OS) according to the number of previous chemotherapy lines received. Data shown are medians (95% CI). C = censored; CI = confidence interval; N = number of patients; n.r. = not reached.
Safety
No grade 4 treatment-related AEs were reported. Grade 3 treatment-related AEs included nausea/vomiting (n = 5; 14.7%), mucositis (n = 2; 5.9%), ALT increase, anemia and neutropenia (n = 1 each; 2.9%) (Tab. IV).
Table IV.
Treatment-related adverse events (worst grade per patient; n = 34)
| NCI-CTCAE grade |
||||
|---|---|---|---|---|
| 1/2 |
3 |
|||
| n | % | n | % | |
| Alanine aminotransferase increase | - | - | 1 | 2.9 |
| Anemia | 1 | 2.9 | 1 | 2.9 |
| Asthenia | 6 | 17.6 | - | - |
| Fever | 1 | 2.9 | - | - |
| Intolerance to antiemetics | 1 | 2.9 | - | - |
| Mucositis | 3 | 8.8 | 2 | 5.9 |
| Myalgia | 1 | 2.9 | - | - |
| Nausea/vomiting | 12 | 35.3 | 5 | 14.7 |
| Neutropenia | 5 | 14.7 | 1 | 2.9 |
| Pancreatitis | 1 | 2.9 | - | - |
| Phlebitis | 1 | 2.9 | - | - |
| Sensorial peripheral neuropathy | 2 | 5.9 | - | - |
| Thrombocytopenia | 1 | 2.9 | - | - |
NCI-CTCAE = National Cancer Institute Common Terminology Criteria for Adverse Events.
Discussion
This retrospective analysis evaluated the efficacy and safety outcomes of the trabectedin/PLD combination when used in patients with ROC in daily clinical practice. This analysis was conducted in patients with platinum-sensitive disease (64.7% had partially platinum-sensitive disease) and showed ORR = 32.4%, median PFS = 6.1 months and median OS = 16.3 months. In the pivotal OVA-301 trial, data obtained from the platinum-sensitive subset of patients showed ORR = 35.3%, median PFS = 9.2 months and median OS = 27.0 months (Tab. V). The ORR obtained in the present study is similar to that of OVA-301, although in that trial an independent review was implemented. In addition to the difficulties of retrospective comparisons, the differences in PFS and OS with the pivotal trial may be partially explained by the immaturity of the data in the present study (27% and 58% of patients were censored for PFS and OS, respectively). Nevertheless, the overall data obtained seem to be consistent with those observed in a controlled, randomized clinical trial, and support that trabectedin/PLD maintains antitumor activity when administered as a third or further chemotherapy line. Trabectedin/PLD activity appears to be unrelated to the number of previous chemotherapy lines. A retrospective study on 98 heavily treated ROC patients (median number of previous lines, 4; range, 1-6 lines) who received single-agent trabectedin as salvage treatment showed an ORR of 27.5%. This rate did not vary with the number of previously administered chemotherapy lines (14). The present results obtained in heavily pretreated patients (median of 3 previous lines, with some patients having received up to 10 lines) are similar to those obtained in OVA-301, where the inclusion criteria allowed only 1 previous line of treatment.
Table V.
Efficacy outcomes in patients with platinum-sensitive relapsed ovarian cancer treated with trabectedin 1.1 mg/m2 plus PLD 30 mg/m2 3-hour q3wk infusion.
| First author (year) | Type of study | No. | Number of previous lines (median, range) | Platinum-sensitive, n (%) | cycles, median (range) | ORR (%) | Median PFS (months) | Median OS (months) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PFla |
|||||||||||
| >6 | 6-12 | >6 | 6-12 | >6 | 6-12 | ||||||
| Monk (2010, 2012) (9, 10) | Phase III clinical trial, randomized Independent review | 337 | 1b | 278 (64.7%) | 6 (1-21) | 35.3% | NA | 9.2 | 7.4 | 27.0 | NA |
| Current study | Single-center retrospective analysis | 34 | 3 (2-10) | 34 (100.0%) | 5 (1-16) | 32.4% | 40.9% | 6.1 | 6.8 | 16.3c | 20.8c |
ORR = objective response rate; OS = overall survival; PFI = platinum-free interval; PFS = progression-free survival; PLD = pegylated liposomal doxorubicin; q3wk = every 3 weeks.
Platinum-sensitive disease (PFI >6 months) and partially platinum-sensitive disease (PFI 6-12 months).
No data on pretreatment were available, but the inclusion criteria of this study allowed only 1 previous line of treatment.
The majority of patients were alive at the time of this retrospective analysis (58% of patients censored) and therefore OS data were very immature.
In the present single-center study, patients with partially platinum-sensitive disease obtained more clinical benefit in terms of ORR, PFS and OS. This is an expected finding as previous studies showed that the efficacy of the trabectedin/PLD combination appears particularly optimized in the partially platinum-sensitive ROC population (7, 15, 16).
The safety profile was as expected for trabectedin/PLD treatment; no new safety signals were reported. The median number of cycles received per patient was similar to that reported in the pivotal OVA-301 trial in a less heavily pretreated population (Tab. V).
In conclusion, this retrospective analysis shows that trabectedin/PLD is an effective treatment for ROC patients in daily clinical practice, especially patients with partially platinum-sensitive disease. Our findings were consistent with those of a previous randomized trial and further support that trabectedin/PLD maintains antitumor activity when administered as a third or further chemotherapy line.
Disclosures
Financial support: PharmaMar provided financial support for statistical analyses and manuscript writing.
Conflict of interest: Pilar Lardelli, Antonio Nieto, Vicente Alfaro and Claudia Rigamonti are employees at PharmaMar. Other authors did not declare any conflict of interest.
References
- 1.Sessa C., De Braud F., Perotti A. et al. Trabectedin for women with ovarian carcinoma after treatment with platinum and taxanes fails. J Clin Oncol. 2005; 23(9): 1867–1874. [DOI] [PubMed] [Google Scholar]
- 2.Krasner C.N., McMeekin D.S., Chan S. et al. A Phase II study of trabectedin single agent in patients with recurrent ovarian cancer previously treated with platinum-based regimens. Br J Cancer. 2007; 97(12): 1618–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Del Campo J.M., Roszak A., Bidzinski M. et al. ; Yondelis Ovarian Cancer Group. Phase II randomized study of trabectedin given as two different every 3 weeks dose schedules (1.5 mg/m2 24 h or 1.3 mg/m2 3 h) to patients with relapsed, platinum-sensitive, advanced ovarian cancer. Ann Oncol. 2009; 20(11): 1794–1802. [DOI] [PubMed] [Google Scholar]
- 4.del Campo J.M., Sessa C., Krasner C.N. et al. Trabectedin as single agent in relapsed advanced ovarian cancer: results from a retrospective pooled analysis of three phase II trials. Med Oncol. 2013; 30(1): 435. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi N., Li W.W., Banerjee D., Scotto K.W., Bertino J.R. Sequence-dependent enhancement of cytotoxicity produced by ecteinascidin 743 (ET-743) with doxorubicin or paclitaxel in soft tissue sarcoma cells. Clin Cancer Res. 2001; 7(10): 3251–3257. [PubMed] [Google Scholar]
- 6.Meco D., Colombo T., Ubezio P. et al. Effective combination of ET-743 and doxorubicin in sarcoma: preclinical studies. Cancer Chemother Pharmacol. 2003; 52(2): 131–138. [DOI] [PubMed] [Google Scholar]
- 7.Poveda A., Vergote I., Tjulandin S. et al. Trabectedin plus pe-gylated liposomal doxorubicin in relapsed ovarian cancer: outcomes in the partially platinum-sensitive (platinum-free interval 6-12 months) subpopulation of OVA-301 phase III randomized trial. Ann Oncol. 2011; 22(1): 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.von Mehren M., Schilder R.J., Cheng J.D. et al. A phase I study of the safety and pharmacokinetics of trabectedin in combination with pegylated liposomal doxorubicin in patients with advanced malignancies. Ann Oncol. 2008; 19(10): 1802–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monk B.J., Herzog T.J., Kaye S.B. et al. Trabectedin plus pegylated liposomal doxorubicin in recurrent ovarian cancer. J Clin Oncol. 2010; 28(19): 3107–3114. [DOI] [PubMed] [Google Scholar]
- 10.Monk B.J., Herzog T.J., Kaye S.B. et al. Trabectedin plus pegylated liposomal doxorubicin (PLD) versus PLD in recurrent ovarian cancer: overall survival analysis. Eur J Cancer. 2012; 48(15): 2361–2368. [DOI] [PubMed] [Google Scholar]
- 11.Ferrandina G., Salutari V., Vincenzi B. et al. Trabectedin as single agent in the salvage treatment of heavily treated ovarian cancer patients: a retrospective, multicenter study. Gynecol Oncol. 2013; 130(3): 505–510. [DOI] [PubMed] [Google Scholar]
- 12.Martella F., Marchetti C., Pisano C. et al. A retrospective analysis of trabectedin (T) use in ovarian cancer patients: a multicentric Italian experience. Int J Gynecol Cancer. October 2013; 23(8 Suppl): 494. [Google Scholar]
- 13.Eisenhauer E.A., Therasse P., Bogaerts J. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45(2): 228–247. [DOI] [PubMed] [Google Scholar]
- 14.Mascilini F., Amadio G., Di Stefano M.G. et al. Clinical utility of trabectedin for the treatment of ovarian cancer: current evidence. Onco Targets Ther. 2014; 7: 1273–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sehouli J., Alfaro V., González-Martín A. Trabectedin plus pegylated liposomal doxorubicin in the treatment of patients with partially platinum-sensitive ovarian cancer: current evidence and future perspectives. Ann Oncol. 2012; 23(3): 556–562. [DOI] [PubMed] [Google Scholar]
- 16.Colombo N. Optimizing treatment of the partially platinum-sensitive ovarian cancer patient. Future Oncol. 2013; 9(12 Suppl): 19–23. [DOI] [PubMed] [Google Scholar]


