Abstract
BACKGROUND:
Carcinogenesis, a multistep process involves sequential changes during neoplastic transformation. The various hallmarks of cancer aid in cell survival, proliferation, and dissemination. Aberrant glycosylation, a recently defined hallmark of cancer, is influenced by glycosylation enzymes during carcinogenesis. Therefore, the present study measured α-2,3 and α-2,6 sialyltransferase (ST), sialidase, and α-L-fucosidase activity in patients with oral precancerous conditions (OPC) and oral cancer patients.
SUBJECTS:
The study enrolled 100 oral cancer patients, 50 patients with OPC, 100 healthy controls, and 46 posttreatment follow-ups of oral cancer patients. Blood and saliva were collected from all the participants.
MATERIALS AND METHODS:
Sialidase activity was measured by spectrofluorimetric method, α-2,3 and α-2,6 ST by ELISA using biotinylated lectins, and α-L-fucosidase by spectrophotometric method.
RESULTS:
The results depicted increased levels of sialidase, α-2,3 and α-2,6 ST, α-L-fucosidase in patients with OPC and oral cancer patients. Receiver operating characteristic curve indicated significant discriminatory efficacy in distinguishing controls and oral cancer patients for serum and salivary sialidase and α-L-fucosidase activity, and serum α-2,6 ST. Furthermore, serum and salivary α-L-fucosidase activity and serum sialidase activity significantly distinguished controls and patients with OPC. Serum and salivary sialidase, α-L-fucosidase, and serum α-2,3 ST activity were higher in patients with metastasis as compared to nonmetastatic patients. Higher values of serum α-L-fucosidase activity were significantly associated with low-overall survival.
CONCLUSION:
The increased levels of enzymes correlated with tumor initiation, progression, and metastasis in oral cancer patients. The alterations in glycosyltransferases/glycosidases thus support the view of glycosylation as a hallmark of cancer.
Keywords: Fucosidase, fucosyltransferase, glycosylation, glycosyltransferase, hallmark, oral cancer, saliva, sialidase, sialyltransferase
Introduction
The development of a cancer phenotype involves tumorigenesis and ultimately metastasis. Initially, Hanahan and Weinberg proposed six hallmark capabilities for the understanding of neoplastic process.[1] Gradually, there were subsequent additions of newly identified hallmarks.[2] Glycosylation, the most abundant posttranslational modification of proteins, is also considered as a new hallmark of cancer.[2,3]
Sialylation and fucosylation are the most abundant forms of glycosylation known to play a significant role in tumor formation and metastasis.[4,5,6,7] Sialylation is regulated mainly by sialyltransferase (ST) and sialidase enzymes, which regulates the formation of sialoglycoconjugates.[5] Similarly, fucosylation is regulated by fucosyltransferase (FUT) and α-L-fucosidase enzymes, which regulate the formation of fucoproteins. One of the mechanisms associated with altered glycosylation in cancer includes altered glycosidase and glycosyltransferase enzymes, which supports the view of Dall’Olio et al.[8] It has been suggested that the relationship between altered glycosylation and altered signal transduction is bidirectional. The cancer-associated alterations in signal transduction pathways lead to increased expression of specific glycosyltransferase which is responsible for altered glycosylation pattern.[9] The alterations of these glycosyltransferase enzymes might be by dysregulation at the transcriptional level, dysregulation of chaperone function as well as altered glycosidase activity.[10] Our recent review considered various aspects in view of glycosylation as a new hallmark of cancer and effectively summarized various studies on aberrant glycosylation including alterations in glycosyltransferases and glycosidases in various cancers. It also highlighted the correlation of glycosylation with other various hallmark capabilities involved in tumor progression and malignant transformation.[3] Earlier studies have reported the altered expression of sialylation and fucosylation enzymes in various cancers.[3,5,6] However, most of the studies have used tissues or serum sample source. Moreover, all sialylation and fucosylation enzyme alterations have not been evaluated together in patients with oral precancerous conditions (OPC), oral cancer patients, and posttreatment follow-ups. According to GLOBOCAN 2012, the estimated incidence of oral cancer is 300, 373 (2.1%), and mortality is 145,353 (1.8%).[11] As per recent reports, there is a changing trend in incidence and prevalence of oral cancer with more youngsters and women being affected by oral cancer.[12] The major problem is metastasis which occurs even at the diagnosis of the disease; however, it is left undetected due to the lack of biomarkers early disease. There is also a need for a noninvasive tool for early detection and easy monitoring of oral cancer. Salivary diagnostics is getting increasing attention due to its noninvasiveness, easy availability, and possibility of repeated sampling during follow-ups for clinically useful biomarker detention.[13] The present study was therefore focused on enzymes involved in glycosylation and evaluated serum and salivary sialidase, α-2,3 and α-2,6 ST, and α-L-fucosidase to understand its utility in early detection and monitoring of OPC and oral cancer.
Materials and Methods
Subjects
The study was approved by Institutional Review Board of the Institute. Due consent was obtained from all the participants to participate in the study. The participants consisted of 100 healthy individuals (controls) who had no major illness in the past, 50 patients with OPC and 100 histopathologically proven untreated oral cancer patients. The patients with OPC and oral cancer patients were enrolled from the outpatients' department of the Institute. Out of 50 patients with OPC, 39 patients were with oral submucous fibrosis and 11 patients were with leukoplakia. Pathological tumor, node, and metastasis staging of malignant diseases was performed as per the American Joint Committee on Cancer norms.[14] The clinicopathological details of oral cancer patients are as depicted in Supplementary Table 1 (361.5KB, tif) . The patients were followed after anticancer treatment. A total of 46 posttreatment follow-up samples were collected, and they were divided into complete responders (CR) and nonresponders (NR) based on the clinical and radiological findings as described by Therasse et al.[15] CR (n = 38) were those who showed good response to anticancer treatment and nonresponders NR (n = 8), the patients with the stable progressive disease or with no response to anticancer treatment.
Clinicopathological details of oral cancer patients
Fasting blood samples were collected by venipuncture from all participants and serum was separated and stored at −80°C until analyzed. For collection of saliva spit, the participants were asked to rinse their mouth with water and expel out. The patients were then asked to spit unstimulated whole saliva into a 50 mL falcon tube and the tube was put on ice, while more saliva in a final volume of 5 mL was collected. Saliva was processed immediately after sample collection. Saliva samples were centrifuged at 2600 g for 15 min at 4°C, supernatant was collected and after protease inhibitors addition,[10] the samples were stored at −80°C until analyzed.
Methodology
Estimation of total proteins
Total protein levels from saliva supernatant were determined using the Lowry's method.[14] Total protein estimation from serum was performed by Biuret method.[15]
Sialidase assay
Sialidase activity was performed by spectrofluorometric method as described by Vajaria et al.[14]
10 μl enzyme source (serum/saliva supernatant) was taken for the assay. Finally, the released fluorescent substrate 4-methylumbelliferone (MU) was recorded spectrofluorometrically using excitation at 365 nm and fluorescence emission at 450 nm. The results were expressed as mU/mg protein.
Biotinylation of lectins
Biotinylation of lectins Sambucus nigra agglutinin and Maackia amurensis agglutinin (Sigma, USA) was performed according to the procedure of sulfo-NHS Biotinylation kit (Pierce, IL, USA). Biotin conjugated lectins were used for detection of α-2,6 and α-2,3 linkage specific STs.
α-2,6 and α-2,3 sialyltransferase activity
Serum and salivary α-2,6 and α-2,3 ST activities were assessed by ELISA method as described by Hakomori et al. and Yeh and Cummingsm, respectively.[14] The absorption of microplate was read at 405 nm. The results were depicted as mU/mg protein.
α-L-fucosidase activity
α-L-fucosidase activity was measured using spectrophotometric method as described by Vajaria et al.[16]20 μl of enzyme source (serum/saliva supernatant) was used in the assay. The enzyme activity was expressed as a specific activity: MU/mg protein. The unit was defined as micromoles of product formed per minute per mg of protein.
Statistical analysis
Statistical analysis was performed using SPSS statistical software version 15.0. Student's independent ‘t’-test was performed to compare the levels between different groups. Student's Paired ‘t’-test was performed to compare levels in pretreatment (PT) and CR, and PT and NR groups. Receiver operating characteristic (ROC) curves were constructed to analyze the distinguishing capability of the markers and also to obtain an optimal cutoff point for survival analysis. Kaplan–Meier's survival analysis was used to analyze the correlation of the markers with overall survival and significance of differences in survival rates was analyzed by Log-rank test. The values were expressed as the mean ± standard error of mean and “P” ≤ 0.05 was considered as statistically significant.
Results
Serum and salivary enzymes are progressively increased from controls, patients with oral precancerous conditions and oral cancer patients
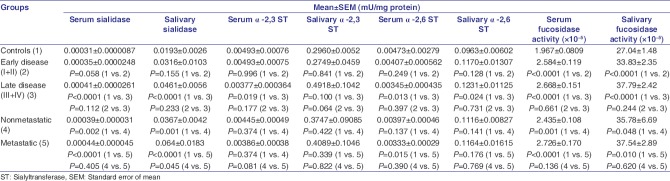
Figure 1a, b and Table 1 depict levels of serum and salivary sialidase, α-2,3 ST, α-2,6 ST, and α-L-fucosidase enzymes in controls, patients with OPC and oral cancer patients with a statistical significance of the alterations. It was observed that the levels of serum and salivary sialidase activity depicted an increasing trend from controls to patients with OPC to oral cancer patients. In addition, serum and salivary levels could significantly (P < 0.0001) discriminate controls from oral cancer patients. Moreover, salivary sialidase activity significantly (P = 0.002) distinguished patients with OPC and oral cancer patients. Our broadened view was to study glycosylation enzymes from serum, saliva, and tissues. In connection with our present results, we have earlier studied glycosylation enzymes from tissues.[5,17] Our earlier data have indicated significant (P = 0.05) increased levels of sialidase activity in malignant tissues as compared to adjacent normal tissues. In the present study, the results depicted an increasing trend of serum and salivary α-2,3 and α-2,6 ST from controls to patients with OPC to oral cancer patients. Furthermore, the levels of α-2,3 and α-2,6 ST were found to be significantly elevated (P < 0.0001) in saliva as compared to serum. Salivary α-2,6 ST significantly (P = 0.045) distinguished controls and oral cancer patients, and patients with OPC and oral cancer patients (P = 0.045). Our earlier study in a different group of participants has shown that the levels of tissue α-2,6 and α-2,3 ST were significantly elevated in malignant tissues as compared to adjacent normal tissues.[5] In the present study, serum and salivary α-L-fucosidase activity was significantly (P < 0.0001 and P = 0.001, respectively) higher in oral cancer patients as compared to controls. Serum α-L-fucosidase also showed significantly higher levels (P < 0.0001) in patients with OPC as compared to controls. Earlier results from our laboratory have also indicated that tissue α-L-fucosidase activity was significantly (P < 0.001) higher in malignant tissue as compared to adjacent normal tissues.[6] The results from our laboratory have also indicated significantly (P < 0.05) elevated serum FUT (P < 0.05) in patients with OPC and oral cancer patients in comparison with controls.[5]
Figure 1.
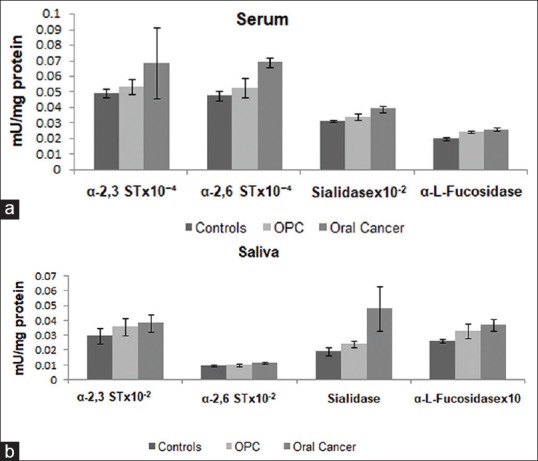
(a) Levels of serum sialidase, α-2,3 and α-2,6 sialyltransferase and α-L-fucosidase enzymes in controls, patients with oral precancerous conditions and oral cancer patients. The values are expressed as mean ± standard error of mean in mU/mg protein. (b) Levels of salivary sialidase, α-2,3 sialyltransferase, α-2,6 sialyltransferase, and α-L-fucosidase enzymes in controls, patients with oral precancerous conditions and oral cancer patients. The values are expressed as Mean ± standard error of mean in mU/mg protein
Table 1.
Statistical significance of the enzymes levels for discriminating controls, patients with oral precancerous conditions and oral cancer patients
Receiver operating characteristic curve analysis of the enzymes exhibited significant discriminatory efficacy between controls, patients with oral precancerous conditions and oral cancer patients
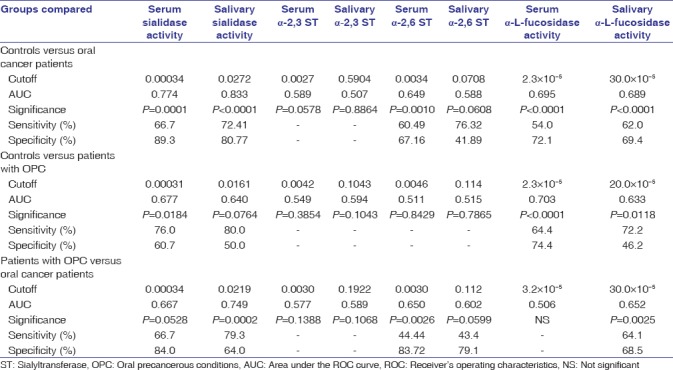
ROC curve analysis of the enzymes is shown in Table 2. The data analysis depicted significant discriminatory efficacy of serum sialidase activity (P = 0.0001), salivary sialidase activity (P < 0.0001), serum α-2,6 ST (P = 0.0010), serum α-L-fucosidase activity (P < 0.0001), and salivary α-L-fucosidase activity (P < 0.0001) in distinguishing between controls and oral cancer patients. ROC curve analysis also indicated significant discriminatory efficacy of serum sialidase activity (P = 0.0184), serum α-L-fucosidase activity (P < 0.0001), and salivary α-L-fucosidase activity (P = 0.0118) in distinguishing controls and patients with OPC. Moreover, ROC curve analysis could significantly distinguish patients with OPC and oral cancer patients by means of the alterations in levels of serum sialidase activity (P = 0.0528), salivary sialidase activity (P = 0.0002), serum α-2,6 ST (P = 0.0026), and salivary α-L-fucosidase (P = 0.0025).
Table 2.
Receiver's operating characteristics curve analysis of enzymes to discriminate between controls, patients with oral precancerous conditions and oral cancer patients
The glycosylation enzymes levels were increased with the disease advancement
As shown in Table 3, it was observed that the levels of serum and salivary fucosidase activity, serum and salivary sialidase activity, and salivary α-2,3 ST and α-2,6 ST were higher in advanced stage as compared to early stage disease. It was observed that the levels of salivary sialidase activity were significantly (P = 0.045) higher in metastatic disease as compared to nonmetastatic disease. Earlier studies from our laboratory have also indicated that tissue α-2,6 and α-2,3 ST enzyme activities were increased from Stage I to Stage IV of disease.[5]
Table 3.
Levels of enzymes in early and advanced disease as well as patients with and without lymph node metastasis
The alterations in enzymes levels showed significant correlation with treatment outcome
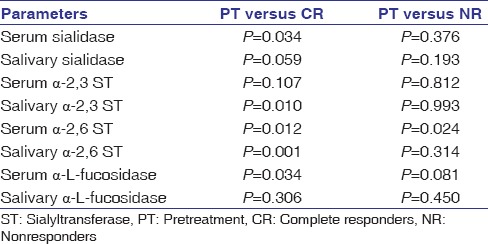
The enzymes levels were compared between PT, CR, and NR patients and results are in bar charts [Figure 2a and b]. The statistical significance of the data is documented in Table 4. It was observed that levels of serum sialidase, salivary α-2,3 ST, salivary α-2,6 ST, and serum α-L-fucosidase activity were significantly (P = 0.034, P = 0.010, P = 0.001, and P = 0.034, respectively) decreased in CR as compared to PT levels. Serum α-2,6 ST activity was observed to be significantly (P = 0.012) decreased in CR while levels were significantly (P = 0.024) elevated in NR as compared to PT levels.
Figure 2.
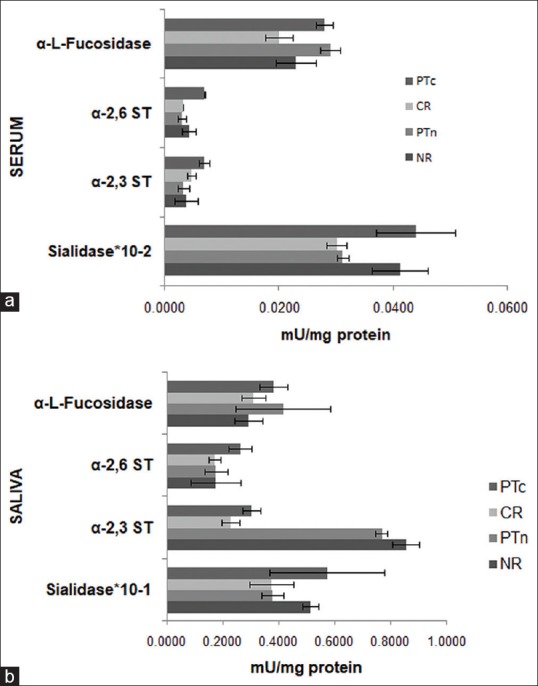
(a) Serum α-L-fucosidase, α-2,3 and α-2,6 sialyltransferase and sialidase in PT, complete responder and nonresponders patients. The values are expressed as mean ± standard error of mean in mU/mg protein PTc: Pretreatment levels of CR, PTn: Pretreatment levels of nonresponders. (b) Salivary α-L-fucosidase, α-2,3 and α-2,6 sialyltransferase and sialidase in pretreatment, complete responder and nonresponders patients. The values are expressed as mean ± standard error of mean in mu/mg protein PTc: Pretreatment levels of CR, PTn: Pretreatment levels of nonresponders
Table 4.
Statistical significance of the enzyme levels for discriminating pretreatment, complete responders and nonresponders follow-up patients
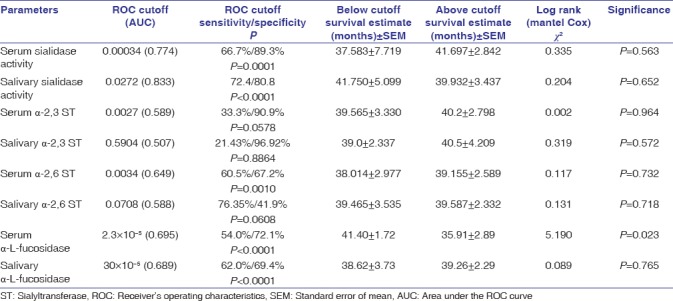
Survival analysis was also performed for all the enzymes, and ROC cutoff was used as an optimal cutoff point for calculating overall survival. Our study revealed that higher values (above ROC cutoff) of serum α-L-fucosidase activity were significantly (P = 0.023) associated with lower overall survival as depicted in Table 5.
Table 5.
Kaplan–Meier's Survival analysis of enzymes involved in glycosylation
Discussion
Sialylation and fucosylation enzymes being the key players in regulating glycosylation machinery of the cell the study was focused on enzymes involved sialylation and fucosylation in patients with OPC and oral cancer patients. The results depicted an increasing trend of serum and salivary sialidase activity from controls to patients with OPC to oral cancer patients and significantly discriminated controls and oral cancer patients. Furthermore, salivary sialidase activity significantly distinguished patients with OPC and oral cancer patients, which was also supported by ROC curve analysis. In concordance with our results, Reuter et al. have reported elevated levels of sialidase activity in oral secretions of patients with upper aerodigestive tract tumors.[18] Our results also depicted significantly increased levels of sialidase activity in malignant oral cancer tissues as compared to adjacent normal tissues. Previous studies have observed higher sialidase activity or different sialidases such as Neuraminidase (Neu) 1, Neu2, Neu3, and Neu4 in various cancers.[19,20,21] The elevated levels of sialic acid in cancer patients might be due to increased sialidase activity. In a different study, we have also reported increased salivary total sialic acid in patients with OPC and oral cancer patients.[14] Increased sialic acid also contributes to the process of metastasis. We observed higher levels of serum and salivary sialidase activities in patients with LN-metastasis and advanced disease as compared to patients without LN-metastasis and early-stage disease, respectively. Earlier studies have documented the significant difference in serum and tissue sialidase activity between different tumor grades of tumor in breast cancer.[19,20] It was interesting to note that serum and salivary sialidase activity were significantly decreased in CR as compared to PT levels and the salivary sialidase activity was elevated in NR as compared to their corresponding PT levels. It was remarkable that levels increased before clinical detection of recurrence and decreased in patients with remission. To the best of our knowledge, there are no studies on serum and salivary sialidase activity in posttreatment follow-ups of oral cancer patients.
We observed an increasing trend of serum and salivary α-2,3 and α-2,6 ST from controls to patients with OPC to oral cancer patients. The α-2,3 and α-2,6 ST levels were significantly elevated in saliva as compared to serum. Salivary α-2,6 ST significantly distinguished patients with OPC and oral cancer patients. ROC curve analysis indicated that serum α-2,6 ST significantly distinguished patients with OPC and oral cancer patients. Our study on a different group of patients has also indicated significantly elevated levels of α-2,6 and α-2,3 ST in malignant tissues as compared to adjacent normal tissues. Several studies have documented de-regulated expression of serum/tissue ST enzyme in various cancers such as colorectal, liver, gliomas, and oral cancer.[5,22,23,24] Our earlier reports have reported increased ST3GAL1 mRNA expression in malignant oral cancer tissues as compared to adjacent normal tissues, which depicted it is important role in oral cancer pathogenesis.[18] The increased mRNA expression of ST3GAL1 indicated the elevation of ST activity in oral cancer patients. We observed higher levels of salivary α-2,3 ST in patients with LN-metastasis/advanced disease as compared to the patients without LN-metastasis/early disease. Earlier studies have found higher serum/tissue ST activity in metastatic disease in various cancers.[5,25]
The progress made in the development of drugs inhibiting glycosylation may also prove wonders in the inhibition of cancer metastasis and invasion. There is much advancement in inhibitors of ST and FUT, which plays an important role in inhibiting overall sialylation process.[26] Our recent review also summarized the details on inhibitors of pathways of sialylation.[16] The inhibitors included various lithocholic derivatives, fluorinated analogs, and other glycomimetics and inhibition using antisense or small hairpin RNA. Glycosyltransferases have been reported as potent drug targets for inhibiting glycosylation.[27] Various STs and FUTs subtypes are involved in the formation of SLex antigens. Studies have shown that alterations in tumor antigens play an important role in tumorigenesis.[3,28]
It was observed that the levels of serum and salivary α-2,6 ST along with salivary α-2,3 ST were found to be significantly decreased in CR as compared to PT levels. The results strengthened our earlier studies in a different group of participants that depicted significantly decreased levels of serum α-2,6 ST and α-2,3 ST in CR as compared to PT levels.[5] The result highlights the importance of STs in treatment monitoring of oral cancer patients during follow-ups.
Our study revealed a significant increase in serum and salivary α-L-fucosidase activity in patients with OPC and oral cancer patients. The results also highlighted its usefulness in treatment monitoring of oral cancer patients. The ROC curve analysis revealed that serum and salivary α-L-fucosidase activity could significantly discriminate controls and oral cancer patients as well as controls and patients with OPC. Salivary α-L-fucosidase activity also significantly distinguished patients with OPC and oral cancer patients. The alterations in patients with OPC signify its usefulness in screening for early detection of disease. The increased activity of α-L-fucosidase in serum/tissues is reported in various malignancies.[5,16,29] Our earlier study has correlated serum and salivary α-L-fucosidase activity with total sialic acid.[16] The present study also observed the higher activity of serum and salivary α-L-fucosidase activities in advanced and metastatic disease as compared to early and nonmetastatic disease, respectively. Increased serum α-L-fucosidase activity has been earlier observed to be correlated with stage of disease.[5,29] A significant decrease in serum α-L-fucosidase activity was noticed in CR as compared to the corresponding PT value. In concordance with our data, previous investigations have also reported decreased serum α-L-fucosidase activity after treatment in cancer.[5,29] Ayude et al. have observed significant clinical utility of serum α-L-fucosidase activity in diagnosis, prognosis, early detection of recurrence and overall management of colorectal cancer.[30] Our study revealed that higher values of serum α-L-fucosidase activity were significantly associated with lower overall survival. However, there is a dearth of reports on correlation with overall survival in oral cancer patients. Our earlier study has documented alterations in serum and tissue FUT in oral cancer patients. Similar alteration was also observed in other cancers.[4,6] In the present study, we observed alterations of sialylation and fucosylation enzymes in serum and saliva, which was an extension of our earlier work which reported increased levels in tissues. The levels were observed to be increased in saliva as compared to serum; this increase might be due to direct contact of saliva with oral cancer lesions.
Conclusion
Our study revealed alterations in glycosylation enzymes in serum, saliva, and tissues of patients with OPC and oral cancer patients, which highlights that the changes in serum/tissues are also reflected in saliva. Thus, it signifies the importance of altered glycosylation enzymes in diagnosis, prognostication, and treatment monitoring and serves as clinically relevant biomarker in oral cancer. It may hold up the conception that glycosylation is a new hallmark of cancer. Further, the mechanism of the altered enzymes leading to altered glycan structures responsible for controlling cellular cancer phenotype can be evaluated.
Financial support and sponsorship
This study was supported by The Gujarat Cancer and Research Institute, Ahmedabad, Gujarat, India.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Vajaria BN, Patel PS. Glycosylation: A hallmark of cancer? Glycoconj J. 2017;34:147–56. doi: 10.1007/s10719-016-9755-2. [DOI] [PubMed] [Google Scholar]
- 4.Miyoshi E, Uozumi N, Sobajima T, Takamatsu S, Kamada Y. In: Mechanisms of Malignant Phenotypes. Springer: Japan; 2016. Roles of fucosyltransferases in cancer phenotypes. Glycosignals in cancer; pp. 3–16. [Google Scholar]
- 5.Shah MH, Telang SD, Shah PM, Patel PS. Tissue and serum α2-3-and α2-6-linkage specific sialylation changes in oral carcinogenesis. Glycoconj J. 2008;25:279–90. doi: 10.1007/s10719-007-9086-4. [DOI] [PubMed] [Google Scholar]
- 6.Shah M, Telang S, Raval G, Shah P, Patel PS. Serum fucosylation changes in oral cancer and oral precancerous conditions: Alpha-L-fucosidase as a marker. Cancer. 2008;113:336–46. doi: 10.1002/cncr.23556. [DOI] [PubMed] [Google Scholar]
- 7.Rodrigues JG, Balmaña M, Macedo JA, Poças J, Fernandes Â, de-Freitas-Junior JC, et al. Glycosylation in cancer: Selected roles in tumour progression, immune modulation and metastasis. Cell Immunol. 2018 doi: 10.1016/j.cellimm.2018.03.007. pii: S0008-8749(18)30121-7. [DOI] [PubMed] [Google Scholar]
- 8.Dall’Olio F, Malagolini N, Trinchera M, Chiricolo M. Mechanisms of cancer-associated glycosylation changes. Front Biosci (Landmark Ed) 2012;17:670–99. doi: 10.2741/3951. [DOI] [PubMed] [Google Scholar]
- 9.Ohtsubo K, Marth JD. Glycosylation in cellular mechanisms of health and disease. Cell. 2006;126:855–67. doi: 10.1016/j.cell.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 10.Pinho SS, Reis CA. Glycosylation in cancer: Mechanisms and clinical implications. Nat Rev Cancer. 2015;15:540–55. doi: 10.1038/nrc3982. [DOI] [PubMed] [Google Scholar]
- 11.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. Lyon, France: International Agency for Research on Cancer; 2013. Cancer Incidence and Mortality Worldwide: IARC Cancer Base No.11. GLOBOCAN 2012 v1.0. [Google Scholar]
- 12.Gupta N, Gupta R, Acharya AK, Patthi B, Goud V, Reddy S, et al. Changing trends in oral cancer – A global scenario. Nepal J Epidemiol. 2016;6:613–9. doi: 10.3126/nje.v6i4.17255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nayyar AS, Khan M, Deosarkar B, Deosarkar SB, Chalapathi KV, Kartheek G, et al. Saliva: Newer avenues in the era of molecular biology, diagnostic and prognostic application. J Med Sci. 2018;38:7. [Google Scholar]
- 14.Vajaria BN, Patel KR, Begum R, Patel JB, Shah FD, Joshi GM, et al. Salivary glyco-sialylation changes monitors oral carcinogenesis. Glycoconj J. 2014;31:649–59. doi: 10.1007/s10719-014-9561-7. [DOI] [PubMed] [Google Scholar]
- 15.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 16.Vajaria BN, Patel KR, Begum R, Shah FD, Patel JB, Shukla SN, et al. Evaluation of serum and salivary total sialic acid and α-l-fucosidase in patients with oral precancerous conditions and oral cancer. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115:764–71. doi: 10.1016/j.oooo.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Munkley J, Elliott DJ. Hallmarks of glycosylation in cancer. Oncotarget. 2016;7:35478–89. doi: 10.18632/oncotarget.8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reuter G, Struwe R, Feige J, Brede R, Bumm P, Schauer R, et al. Analysis of carbohydrate composition and sialidase activity in oral secretions of patients with tumors in the upper aerodigestive tract. Eur Arch Otorhinolaryngol. 1992;249:5–11. doi: 10.1007/BF00175662. [DOI] [PubMed] [Google Scholar]
- 19.Sönmez H, Süer S, Güngör Z, Baloglu H, Kökoglu E. Tissue and serum sialidase levels in breast cancer. Cancer Lett. 1999;136:75–8. doi: 10.1016/s0304-3835(98)00295-x. [DOI] [PubMed] [Google Scholar]
- 20.Miyagi T, Yamaguchi K. Mammalian sialidases: Physiological and pathological roles in cellular functions. Glycobiology. 2012;22:880–96. doi: 10.1093/glycob/cws057. [DOI] [PubMed] [Google Scholar]
- 21.Forcella M, Mozzi A, Stefanini FM, Riva A, Epistolio S, Molinari F, et al. Deregulation of sialidases in human normal and tumor tissues. Cancer Biomark. 2018;21:591–601. doi: 10.3233/CBM-170548. [DOI] [PubMed] [Google Scholar]
- 22.Venkitachalam S, Guda K. Altered glycosyltransferases in colorectal cancer. Expert Rev Gastroenterol Hepatol. 2017;11:5–7. doi: 10.1080/17474124.2017.1253474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vajaria BN, Patel KR, Begum R, Patel PS. Sialylation: An avenue to target cancer cells. Pathol Oncol Res. 2016;22:443–7. doi: 10.1007/s12253-015-0033-6. [DOI] [PubMed] [Google Scholar]
- 24.Vajaria BN, Patel KR, Begum R, Shah FD, Patel JB, Joshi GM, et al. Expression of glycosyltransferases; ST3GAL1, FUT3, FUT5, and FUT6 transcripts in oral cancer. Glycobiol Insights. 2014;2014:7–14. [Google Scholar]
- 25.Wang PH, Li YF, Juang CM, Lee YR, Chao HT, Ng HT, et al. Expression of sialyltransferase family members in cervix squamous cell carcinoma correlates with lymph node metastasis. Gynecol Oncol. 2002;86:45–52. doi: 10.1006/gyno.2002.6714. [DOI] [PubMed] [Google Scholar]
- 26.Wang L, Liu Y, Wu L, Sun XL. Sialyltransferase inhibition and recent advances. Biochim Biophys Acta. 2016;1864:143–53. doi: 10.1016/j.bbapap.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 27.Sun XL. Glycosyltransferases as potential drug targets. Med Chem. 2013;3:e106. [Google Scholar]
- 28.Carvalho AS, Harduin-Lepers A, Magalhães A, Machado E, Mendes N, Costa LT, et al. Differential expression of alpha-2,3-sialyltransferases and alpha-1,3/4-fucosyltransferases regulates the levels of sialyl Lewis a and sialyl Lewis x in gastrointestinal carcinoma cells. Int J Biochem Cell Biol. 2010;42:80–9. doi: 10.1016/j.biocel.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 29.Wei X, Wang S, Rui J. The value of serum alpha-L-fucosidase activity in the diagnosis of primary liver cancer. Zhonghua Zhong Liu Za Zhi. 2000;22:148–50. [PubMed] [Google Scholar]
- 30.Ayude D, Páez De La Cadena M, Martínez-Zorzano VS, Fernández-Briera A, Rodríguez-Berrocal FJ. Preoperative serum alpha-L-fucosidase activity as a prognostic marker in colorectal cancer. Oncology. 2003;64:36–45. doi: 10.1159/000066521. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clinicopathological details of oral cancer patients