Abstract
BACKGROUND:
Obesity has frequently been associated with the dyslipidemic state and with the risk of various chronic diseases.
OBJECTIVE:
The objective of this study was to determine the relationship between obesity and blood lipids with a risk of colorectal cancer (CRC).
METHODOLOGY:
Histologically confirmed CRC patients from five local hospitals were matched with cancer-free controls for age, gender, and ethnicity (n = 140: 280). The study participants underwent physical assessment for the presence of obesity and 10 mL of fasting blood was drawn for blood lipid analysis.
RESULTS:
In this study, abdominal obesity significantly doubled the risk of CRC (adjusted odds ratio [AOR] =1.69, 95% confidence interval [CI] = 1–2.83). Hypercholesterolemia and low high-density lipoprotein cholesterol (HDL) increased the risk of CRC more than twofolds (AOR = 2.6, 95% CI = 1.7–3.9 and AOR = 3.8, 95% CI = 2.3–6.3, respectively). Abdominal obesity and hypercholesterolemia synergically doubled the risk of CRC (AOR = 2.0, 95% CI = 1–4). Low-HDL has shown no synergic association with other dyslipidemic states with an increased CRC risk.
CONCLUSION:
Improving abdominal obesity, hypercholesterolemia, and low HDL may be a clinically relevant strategy to reduce the risk of CRC among Malaysians.
Keywords: Abdominal obesity, colorectal cancer, hypercholesterolemia
Introduction
Obesity has been identified as a prime candidate for cardiovascular diseases, type II diabetes mellitus (T2DM), dyslipidemias, and a host of related complications.[1] According to the World Health Organization (WHO), more than 1.9 billion adults worldwide were overweight, with 650 million obese adults in 2016, and this number will increase if no remedial strategies are taken. The incidents are expected to escalate to 573 million by 2030. The WHO has estimated that 1.9 billion (39%) of adults aged 18 years and above were overweight. Of this total, approximately 650 million (13%) were diagnosed with obesity. The rate of mortality was higher among overweight and obese adults compared to those who were underweight.[2]
Few decades ago, excess body mass index (BMI) was identified as independent risk factor of cardiovascular disease (CVD) and T2DM. However, recent studies have hypothesized that increased body adipose tissue is a significant risk factor for various cancers, including colorectal cancer (CRC).[3] In obesity-pandemic regions, the medical consequences are already quantifiable, and obesity-related cancer risk is an important medical concern. In Malaysia, cancer is now the 4th leading cause of death, accounting for about 10.11% of all deaths. CRC is the first cause among males (56.5%) and third among females (43.5%).[4]
Obesity was hypothesized as being associated with tissue inflammation by regulating dyslipidemia, which is associated with the development of CRC.[5] Yet, its adverse effects on multiracial countries such as Malaysia are still unknown. It is important to measure the role of obesity on the risk of CRC among Malaysians because the lifestyle and dietary habits of the population are different, compared to other populations that have significant risks of CRC with the presence of obesity. Nevertheless, it is very important to identify a necessary, invasive screening tool to diagnose those at considerable risk of CRC. Therefore, this Malaysian multicentered case–control study aims to investigate the associations of obesity and blood lipids with a CRC risk.
Methodology
Study subjects
A total of 140 newly diagnosed CRC cases were recruited and two cancer-free controls were matched for age, sex, and ethnicity with each case (n = 280). The study was conducted from December 2009 to January 2012 in five selected public hospitals in Peninsular Malaysia. This study was approved by the Medical Research Ethics Committee of the Universiti Putra Malaysia and Ministry of Health (MREC; NMRR-09-505-3994). A written informed consent was taken from each individual that participated in the study.
Assessment of obesity
The body weight of each study participant was measured using TANITA HD-319 digital weight-scale (Taiwan Zhen-Xin medical equipment Co., Ltd, Taipei, ROC). Height was measured by a wall-mounted role-up measuring tape (Seca 206). BMI, which indicates overall obesity, was calculated and classified according to the WHO.[6] Those with BMI ≥30 k/g2 were categorized as obese. The waist circumference (WC), which indicates the abdominal obesity, was measured using a nonelastic measuring tape Seca 201 (Vogel and Halke GmbH and Co., Hamburg, Germany). Men with WC >102 cm and women with WC >88 cm were categorized as abdominally obese.[7] The waist-hip ratio (WHR), which indicates regional fat distribution, was calculated and categorized according to the WHO (2008),[7] where men with WHR ≥1.0 and women with WHR ≥0.85 were considered obese. Body fat percentage (BF%), which determines the percentage of BF, was assessed using the OMRON BF306 (HBF-306-E) electronic hand-held body fat monitor (Omron Healthcare Co., Ltd., Kyoto, 615-0085 Japan). Men with BF% >25% and women with BF% >35% were considered at risk of obesity.
Assessment of blood lipid
Ten milliliters of fasting blood sample of venous blood was collected by a trained and qualified paramedic from each hospital. The fasting blood sample was collected in the morning to control for circadian intra-individual variations. The collected blood samples were processed, separated, and stored at a temperature of −80°C on the same day. Plasma lipids (low-density lipoprotein (LDL)-cholesterol, high-density lipoprotein [HDL]-cholesterol, and triglyceride) and total cholesterol (TC) were determined using the Roche Hitachi 902, the automated clinical chemistry analyzer.[8]
Statistical analysis
Data were analyzed using Statistical Package for the Social Sciences (SPSS) software (Version 23.0. Armonk, NY: IBM Corp) and were described using frequencies (N), percentages (%), means, and standard deviations. Categorical variables were reported as absolute number and percentage. Chi-square distribution was used to determine the association between categorical variables. Odds ratio (OR) was determined by Cox regression analysis, and P < 0.05 was considered statistically significant.
Results
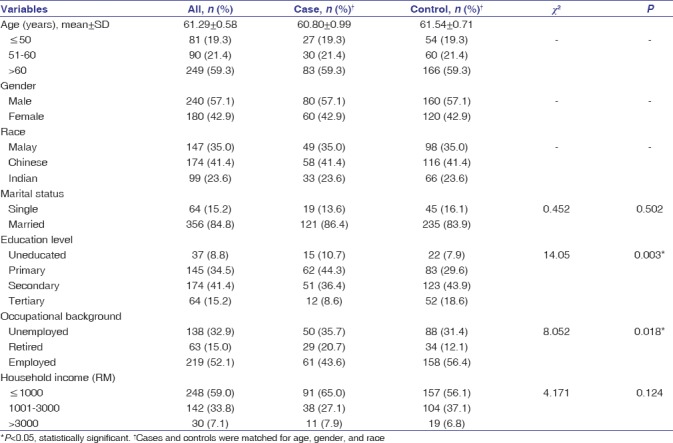
Majority of the study participants were above 60 years (59.3%). In this study, CRC was more prominent among males (57.1%) and among Chinese (41.4%). Most of the study participants were married (84.8%). A significantly higher proportion of cases were illiterate or educated up to primary only compared to controls (10.7% vs. 7.9% and 44.3% vs. 29.6%, respectively; χ2 = 14.05, P = 0.003). The proportion of cases who were unemployed or retired was significantly higher than controls (35.7% vs. 31.4% and 20.7% vs. 12.1%, respectively; χ2 = 8.05, P = 0.018). Majority of the study participants (59.0%) had low household income (≤RM 1000) [Table 1].
Table 1.
Distribution of sociodemographic background of the study participants
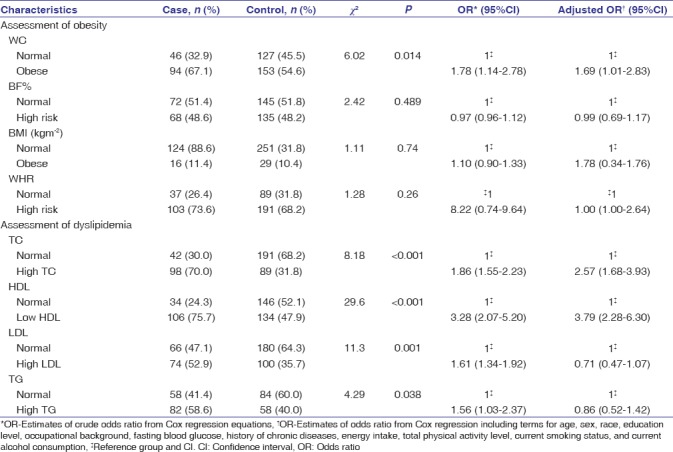
Using Cox regression models, CRC risk factors were examined in relation to four obesity and dyslipidemia variables [Table 2]. This study found that an independent presence of high WC (adjusted odds ratio [AOR] = 1.69, 95% confidence interval [CI] = 1.01–2.83) was significantly associated with an increased risk of CRC. Assessment of four variables of dyslipidemia showed that high TC and low HDL cholesterol independently and significantly increased the risk of CRC (AOR = 2.57, 95% CI = 1.68–3.93 and AOR = 3.79, 95% CI = 2.28–6.30, respectively).
Table 2.
Prevalence, odds ratio, and 95% confidence interval of obesity and dyslipidemia assessment for colorectal cancer
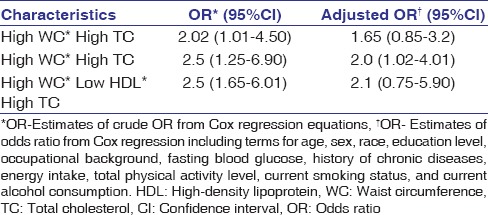
In addition, the assessment of synergic effects of WC, HDL cholesterol, and TC with the risk of CRC showed that high WC and high TC synergically doubled the risk of CRC (AOR = 2.0, 95% CI = 1.0–4.0). The presence of low HDL with other variables of dyslipidemia showed no synergic, independent, and significant association with an increased risk of CRC [Table 3].
Table 3.
Odds ratio and 95% confidence interval of synergic effects between total cholesterol, high-density lipoprotein cholesterol, and total cholesterol for colorectal cancer
Discussion
In this study, having abdominal obesity significantly doubled the risk of CRC. The current finding supports the fact that people who are abdominally obese are at a considerable risk of CRC, although they are diagnosed with high BMI.[9] The finding adds evidence that BMI measurements could not explain the overall obesity-related disease dimension.[10] This is because the difference between fat mass and muscle mass could not be assessed solely with BMI.[11] Studies have shown that abdominal obesity was highly correlated with metabolic and hormonal alterations.[10] This is based on the mounting evidence that abdominal obesity is significantly associated with insulin resistance, contributes to hypertension, increases TC concentration, and lowers HDL cholesterol concentration, hyperglycemia, and T2DM, which are also established risk factors of CRC.[12,13,14] Nevertheless, compelling evidence was found linking WC with cancer progression and the increased likelihood that central obesity is an early step in the pathophysiology of metabolic syndrome.[15]
Hypercholesterolemia and low HDL have significantly increased CRC risk independently, with ORs ranging from 2.5 to almost 4.0. Hypercholesterolemia acts as a marker of an altered metabolism, which results in excessive adiposity.[16] Besides that, adipose cells have been identified as the body's largest pool of free cholesterol.[17] Through various releases and absorptions of adipokines, like adiponectin and leptin, the adipocyte maintains the cellular cholesterol homeostasis by establishing intracell communication.[18] This fact supports the findings that CRC risk is more obvious with a synergic effect between abdominal obesity and hypercholesterolemia. A national study on obesity and overweightness reported that dyslipidemia was assessed among 14.5% of Malaysian adults. Low HDL cholesterol was significantly associated with the increased risk of overweightness and obesity, by 50% (OR = 1.47, 95% CI; 1.18–1.83).[19]
Several potential biologic mechanisms have been proposed to elucidate the underlying association between dyslipidemia and the risk of CRC. High serum cholesterol is involved in inflammation signaling and induces cellular proliferation as well as inhibits apoptosis, which potentially leads to CRC carcinogenesis and perhaps alters the gene expression.[20,21,22] On the other hand, compared to the normal cell, the plasma membrane of a cancer cell has a higher concentration of cholesterol-rich lipids that play a key role in the transduction of Akt signaling pathways, influencing the survival of cancer cells.[23] It is hypothesized that an increased HDL cholesterol concentration is associated with induction of interleukin 10 (IL-10), the anti-inflammatory cytokines that inhibit the production of pro-inflammatory cytokines, including IL-6, and tumor necrosis factors leading to the stimulation of cell growth and cellular proliferation as well as inhibiting apoptosis.[24] HDL cholesterol perhaps plays a role in antioxidative activities and modulates oxidative stress, as well as protects cells against the adverse effect of LDL cholesterol oxidation.[25] Dyslipidemia may occur in insulin resistance and metabolic syndrome, which have been significant risk factors for CRC.[26]
The findings of this study could not indicate the long-term variation of dyslipidemia before the diagnosis of the disease because the lipid concentration was assessed in a single plasma sample. However, being one of the largest hospital-based case–controlled retrospective studies in Malaysia dealing with the adjustment of detailed anthropometry, lifestyle, and dietary factors adds to the strength of the study.
Conclusion
This study suggested that a simple measurement of WC indicating abdominal obesity corresponded to the presence of a metabolic disorder and an increased risk of CRC. To address this association, Malaysia needs a set of obesity prevention actions at the individual and national levels, leading to meaningful changes at the societal level to reduce the risk of CRC and other chronic diseases. It is possible that obesity intervention could have beneficial effects on reducing hypercholesterolemia because it is strongly associated with an increased risk of CRC. This strategy can be conveyed easily to the public to reduce the prevalence of obesity and subsequently, the risk of CRC. Every health-care provider should assess WC as an additional anthropometry measurement because this can add value for the better prediction of CRC, other cancers, and other chronic diseases.
Financial support and sponsorship
This work was funded by Fundamental Research Grant Scheme, Universiti Putra Malaysia (04-11-08-625FR).
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors would like to acknowledge Fundamental Research Grant Scheme, Universiti Putra Malaysia (04-11-08-625FR), for funding.
References
- 1.Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: Risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53:1925–32. doi: 10.1016/j.jacc.2008.12.068. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Obesity and Overweight. 2018. [Last accessed on 2018 Feb 02]. Available from: http://www.who.int/news--room/fact--sheets/detail/obesity--and--overweight .
- 3.Roberts DL, Dive C, Renehan AG. Biological mechanisms linking obesity and cancer risk: New perspectives. Annu Rev Med. 2010;61:301–16. doi: 10.1146/annurev.med.080708.082713. [DOI] [PubMed] [Google Scholar]
- 4.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 5.Liu CS, Hsu HS, Li CI, Jan CI, Li TC, Lin WY, et al. Central obesity and atherogenic dyslipidemia in metabolic syndrome are associated with increased risk for colorectal adenoma in a Chinese population. BMC Gastroenterol. 2010;10:51. doi: 10.1186/1471-230X-10-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. WHO Technical Report Series 854. Geneva: World Health Organization; 1995. [Last accessed on 2012 Jun 25]. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. Available from: http://www.assessmentpsychology.com/icbmi.htm . [PubMed] [Google Scholar]
- 7.World Health Organization. Geneva: World Health Organization; 2008. Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation; pp. 8–11. [Google Scholar]
- 8.Nguyen QM, Srinivasan SR, Xu JH, Chen W, Hassig S, Rice J, et al. Elevated liver function enzymes are related to the development of prediabetes and type 2 diabetes in younger adults: The Bogalusa heart study. Diabetes Care. 2011;34:2603–7. doi: 10.2337/dc11-0919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rickles AS, Iannuzzi JC, Mironov O, Deeb AP, Sharma A, Fleming FJ, et al. Visceral obesity and colorectal cancer: Are we missing the boat with BMI? J Gastrointest Surg. 2013;17:133–43. doi: 10.1007/s11605-012-2045-9. [DOI] [PubMed] [Google Scholar]
- 10.Tchernof A, Després JP. Pathophysiology of human visceral obesity: An update. Physiol Rev. 2013;93:359–404. doi: 10.1152/physrev.00033.2011. [DOI] [PubMed] [Google Scholar]
- 11.Prabhat P, Tewari R, Natu SM, Dalela D, Goel A, Tandon P, et al. Is central obesity, hyperinsulinemia and dyslipidemia associated with high-grade prostate cancer? A descriptive cross-sectional study. Indian J Urol. 2010;26:502–6. doi: 10.4103/0970-1591.74440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen L, Xu WM, Zhang D. Association of abdominal obesity, insulin resistance, and oxidative stress in adipose tissue in women with polycystic ovary syndrome. Fertil Steril. 2014;102:1167–74. doi: 10.1016/j.fertnstert.2014.06.027. e4. [DOI] [PubMed] [Google Scholar]
- 13.Hall ME, do Carmo JM, da Silva AA, Juncos LA, Wang Z, Hall JE. Obesity, hypertension, and chronic kidney disease. Int J Nephrol Renovasc Dis. 2014;7:75–88. doi: 10.2147/IJNRD.S39739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsuda M, Shimomura I. Increased oxidative stress in obesity: Implications for metabolic syndrome, diabetes, hypertension, dyslipidemia, atherosclerosis, and cancer. Obes Res Clin Pract. 2013;7:e330–41. doi: 10.1016/j.orcp.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Gallagher EJ, LeRoith D. Epidemiology and molecular mechanisms tying obesity, diabetes, and the metabolic syndrome with cancer. Diabetes Care. 2013;36(Suppl 2):S233–9. doi: 10.2337/dcS13-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flock MR, Green MH, Kris-Etherton PM. Effects of adiposity on plasma lipid response to reductions in dietary saturated fatty acids and cholesterol. Adv Nutr. 2011;2:261–74. doi: 10.3945/an.111.000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu BL, Zhao SP, Hu JR. Cholesterol imbalance in adipocytes: A possible mechanism of adipocytes dysfunction in obesity. Obes Rev. 2010;11:560–7. doi: 10.1111/j.1467-789X.2009.00699.x. [DOI] [PubMed] [Google Scholar]
- 18.de Barros-Mazon S, Marin DM, Carvalho CP, Alegre SM. Anti-Obesity Drug Discovery and Development. Oak Park, IL: Bentham Science Publishers Ltd; 2011. Inflammatory and Metabolic Markers in Pre-and Post-Treatment of Obesity; pp. 49–66. [Google Scholar]
- 19.Mohamud WN, Musa KI, Khir AS, Ismail AA, Ismail IS, Kadir KA, et al. Prevalence of overweight and obesity among adult Malaysians: An update. Asia Pac J Clin Nutr. 2011;20:35–41. [PubMed] [Google Scholar]
- 20.Kitahara CM, Berrington de González A, Freedman ND, Huxley R, Mok Y, Jee SH, et al. Total cholesterol and cancer risk in a large prospective study in Korea. J Clin Oncol. 2011;29:1592–8. doi: 10.1200/JCO.2010.31.5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhuang L, Kim J, Adam RM, Solomon KR, Freeman MR. Cholesterol targeting alters lipid raft composition and cell survival in prostate cancer cells and xenografts. J Clin Invest. 2005;115:959–68. doi: 10.1172/JCI200519935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li YC, Park MJ, Ye SK, Kim CW, Kim YN. Elevated levels of cholesterol-rich lipid rafts in cancer cells are correlated with apoptosis sensitivity induced by cholesterol-depleting agents. Am J Pathol. 2006;168:1107–18. doi: 10.2353/ajpath.2006.050959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oh HY, Lee EJ, Yoon S, Chung BH, Cho KS, Hong SJ. Cholesterol level of lipid raft microdomains regulates apoptotic cell death in prostate cancer cells through EGFR-mediated Akt and ERK signal transduction. Prostate. 2007;67:1061–9. doi: 10.1002/pros.20593. [DOI] [PubMed] [Google Scholar]
- 24.Moraitis AG, Freeman LA, Shamburek RD, Wesley R, Wilson W, Grant CM, et al. Elevated interleukin-10: A new cause of dyslipidemia leading to severe HDL deficiency. J Clin Lipidol. 2015;9:81–90. doi: 10.1016/j.jacl.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruscica M, Botta M, Ferri N, Giorgio E, Macchi C, Franceschini G, et al. High density lipoproteins inhibit oxidative stress-induced prostate cancer cell proliferation. Sci Rep. 2018;8:2236. doi: 10.1038/s41598-018-19568-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giovannucci E. Metabolic syndrome, hyperinsulinemia, and colon cancer: A review. Am J Clin Nutr. 2007;86:s836–42. doi: 10.1093/ajcn/86.3.836S. [DOI] [PubMed] [Google Scholar]


