Objective
Contingency management (CM) interventions that reinforce attendance have rarely been evaluated in terms of reducing drug use. Using a sequential randomized design, this study examined the efficacy of three attendance CM conditions compared to usual care (UC) on drug use outcomes. It evaluated whether the duration (6 versus 12 weeks) and timing (early versus later treatment) of CM delivery impact treatment response. Method: Upon initiating outpatient treatment, patients with cocaine use disorders (N = 360) were randomized to UC or CM for attending treatment for 6 weeks. At week 6, patients (n = 308) were re-randomized to UC or CM for another 6 weeks, with assignment stratified on current functioning. Samples were screened for illicit drugs twice weekly for 12 weeks. Results: Patients randomized to CM at both time- points attended more sessions and achieved more abstinence than those never randomized to CM. Relative to UC, receiving attendance CM in weeks 1–6 only was not efficacious, but those receiving attendance CM in weeks 7–12 only evidenced some benefits compared to those who never received CM. Twelve weeks of attendance CM was more efficacious than six weeks. No between-group differences in drug use were noted at follow-ups, but days attended treatment and proportion negative samples during treatment were associated with long-term cocaine abstinence. Conclusions: Attendance-based CM increases treatment participation and reduces drug use, with beneficial effects noted when CM is delivered over longer durations and during later phases of outpatient care.
Keywords: contingency management, cocaine, outpatient substance abuse treatment
Public health significance: This study suggests that reinforcing patients for attending substance abuse treatment is effective in increasing retention in care and reducing drug use. However, benefits are most likely when reinforcers are provided for longer periods of time and during the later phases of outpatient treatment.
Contingency management (CM) is the psychosocial treatment for substance use disorders with the largest effect size (Dutra et al., 2008). In controlled trials, CM interventions typically reinforce submission of negative toxicology samples (Higgins, Wong, Badger, Ogden, & Dantona, 2000; Petry et al., 2004; Petry, Alessi, et al., 2006; Petry, Alessi, Barry, & Carroll, 2015; Petry, Alessi, Hanson, & Sierra, 2007; Petry, Alessi, Marx, Austin, & Tardif, 2005; Petry, Martin, & Simcic, 2005; Petry, Pierce, et al., 2005). Reinforcing drug-negative samples is highly efficacious (Lussier, Heil, Mongeon, Badger, & Higgins, 2006; Prendergast, Podus, Finney, Greenwell, & Roll, 2006), but the substance use field lags in implementation of evidence-based practices (Reickmann, Abraham, Zwick, Rasplica, & McCarty, 2015).
Although CM is increasingly applied in practice settings (Henggeler, Sheidow, Cunningham, Donohue, & Ford, 2008; Kellogg et al., 2005; Petry, DePhilippis, Rash, Drapkin, & McKay, 2014; Ruan, Bullock, & Reger, 2017), clinicians often deviate from methods applied in research studies (Rash et al., 2012; Walker et al., 2010). In non-research settings, collecting and reinforcing urine samples multiple times weekly is often an implementation barrier (Petry & Simcic, 2002; Sinclair, Burton, Ashcroft, & Priebe, 2011). Reinforcing attendance is logistically simpler and better aligned with usual care practices. Reinforcing attendance is also less costly as it does not require funds, or clinician time, for toxicology testing. Reinforcing attendance also may be clinically significant because some patients do not submit positive samples while engaged in treatment (Petry et al., 2004; Petry, Weinstock, & Alessi, 2011; Petry, Alessi, & Ledgerwood, 2012; Petry, Barry, Alessi, Rounsaville, & Carroll, 2012). In a multicenter study of CM at psychosocial clinics (i.e., non-methadone) throughout the country, for example, over 88% of samples tested negative (Petry, Peirce, et al., 2005).
Attendance CM clearly enhances retention (Alessi, Hanson, Wieners, & Petry, 2007; Branson, Barbuti, Clemmey, Herman, & Bhutia, 2012; Ledgerwood, Alessi, Hanson, Godley, & Petry, 2008; Petry, Martin, & Finocche, 2001; Sigmon & Stitzer, 2005), but only a few studies of attendance CM have assessed substance use outcomes directly. Petry, Barry, et al. (2012) found that attendance CM resulted in longer durations of abstinence than usual care, but it also led to lower proportions of drug-negative samples submitted relative to abstinence-based CM, with patients in the latter condition submitting 90.2% + 20.3% negative samples versus 84.1% + 24.3% for those in the CM attendance condition, p < .05. One purpose of this study was to ascertain whether this effect replicated in another sample, because if attendance CM is harmful in any way, it clearly should not be implemented. On the other hand, if an attendance CM approach improves outcomes then efforts should be expended to implement it more widely. Thus, this study addresses the important and clinically relevant question as to whether reinforcing attendance alone is efficacious in improving drug use outcomes.
There is also a question as to the optimal timing and duration of CM, but few studies have examined these issues empirically. Long-term administration of abstinence-based CM extends its benefits to up to one year (Higgins et al., 2003, 2007; Silverman, Robles, Mudric, Bigelow, & Stitzer, 2004), but longer durations of CM are also more costly. Furthermore, in most research studies patients begin receiving CM when they initiate outpatient care, but the benefits appear in the later phases. For example, retention curves in reinforced and non- reinforced patients are similar during early weeks, and not until the latter stages of treatment do the groups diverge (Petry, Alessi, et al., 2012; Petry, Martin, Cooney, & Kranzler, 2000). This study also evaluated the efficacy of short-term CM, delivered during the initial stages of care compared to delayed delivery after patients had been engaged in treatment for six weeks.
This study used an adaptive research design (Collins, Murphy, & Strecher, 2007; Murphy, Collins, & Rush, 2007; TenHave, Coyne, Salzer, & Katz, 2003), which more closely mimics how care is provided in clinical settings than single randomization designs. In clinical settings, clinicians adjust treatments based on patients’ symptoms and functioning, whereas in traditional randomized trials, patients are assigned to one intervention and it is delivered throughout the study period regardless of whether or not the patient responds favorably. In this study, patients were randomized at two points: treatment initiation and 6 weeks later, and treatment assignment at each point was balanced on key indicators of patient functioning. In this manner, the study addressed the main questions of interest. The primary hypothesis was that 12 weeks of attendance CM would increase attendance at treatment and lead to improved substance use outcomes compared to no CM. The study also was designed to evaluate whether 6 weeks of attendance CM, during the earlier (weeks 1–6) or later (weeks 7–12) phases of care, would improve outcomes relative to usual care. In addition, the study examined if longer-term attendance CM (12 weeks) was more efficacious than short-term attendance CM (6 weeks).
Methods
Participants
This study recruited 360 patients beginning substance abuse treatment at four clinics spanning two states in the Hartford and Bridgeport, CT, and Springfield, MA, regions between 2009 and 2015. The clinics provided similar outpatients services and treated similar populations. Inclusion criteria were initiating outpatient care (without being in a controlled environment), age 18 or older, English speaking, and met Diagnostic and Statistical Manual of Mental Disorders-IV (American Psychiatric Association, 2000) criteria for cocaine use disorder. Most patients had other substance use diagnoses as well, which did not exclude participation to enhance generalization of findings. Exclusion criteria were inability to understand the study, uncontrolled psychotic or suicidal symptoms, and in recovery for gambling disorder. This latter criterion was applied because prize CM has an element of chance, although no increases in gambling are reported (Petry & Alessi, 2010; Petry, Kolodner, et al., 2006). The University’s Institutional Review Board approved the study, and all participants signed written informed consent.
Sample size was based on a power analysis. A meta-analysis of prize CM (Benishek et al., 2014) found an overall effect size of d = 0.46. Using a Type I error rate of alpha =.05 and a Type II error rate of Beta =.20, about 78 patients per group can detect this effect size between any two conditions. Because about 20% of patients were expected to not undergo the second randomization, we recruited an additional 48 patients, enrolling a total of 360 patients.
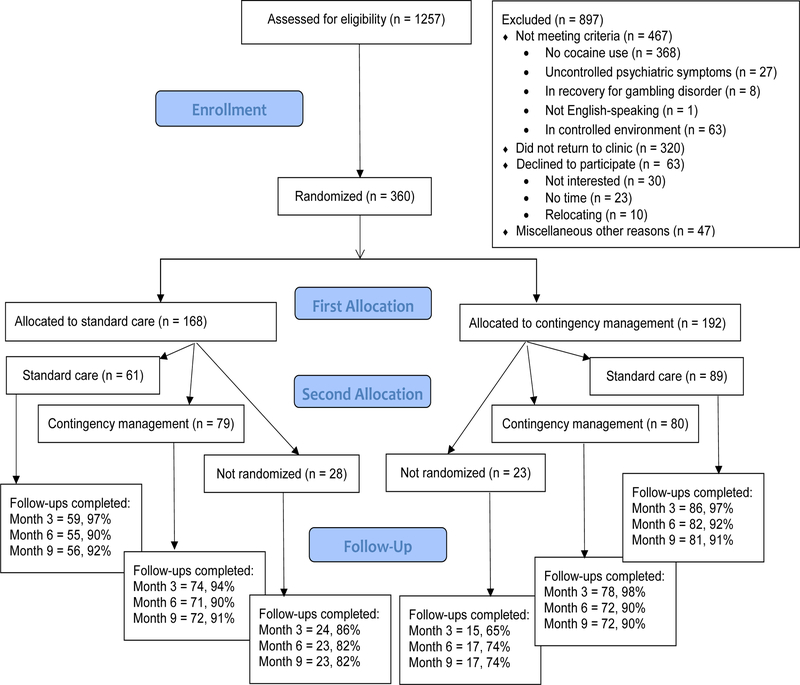
Research assistants (RAs) evaluated new admissions for eligibility criteria (Figure 1) and obtained consent and randomized 360 patients. The remainder were ineligible, primarily related to not having a cocaine use disorder or failing to return to the clinic after initial screening. All eligible consented patients (N = 360) were randomized initially, and 308 were randomized at week 6, when they were scheduled for their first post-baseline evaluation. Additional evaluations were scheduled at months 3, 6, and 9; patients received $20 for completing the baseline and $35 for each subsequent evaluation. Follow-up completion rates were lower overall in patients who did not undergo the second randomization versus those who did (ps < .001), but there were no differences between groups randomized at both time points in follow-up completion (ps > .54). The demographic and substance use characteristics of the sample is similar to that reported in other substance abuse treatment clinics in the region (Evaluation, Quality Management and Improvement Division, 2017).
Figure 1. CONSORT Flow Diagram.
Procedures
After obtaining informed consent, RAs interviewed patients using the Addiction Severity Index (ASI; Bovasso, Alterman, Cacciola, & Cook, 2001; Kosten, Rounsaville, & Kleber, 1983). The ASI provides severity ratings of 0 to 1 in seven domains: employment, drug, alcohol, medical, family/social, legal, and psychiatric. Patients also provided urine and breath samples which were tested for cocaine, methamphetamine, and amphetamine, opioids, and marijuana using OnTrak TesTstiks Varian, Inc., Walnut Creek, CA) and for alcohol using an Intoximeter Breathalyzer (Intoximeters, St. Louis, Mo).
Randomizations
A computerized program randomized patients to usual care or usual care with attendance CM after the baseline evaluation. The program (Stout, Wirtz, Carbonari, & Del Boca, 1994) balanced assignment on baseline urine and breath sample results (positive for alcohol or any drug tested vs. negative for all) and whether the patient had been in a controlled environment (e.g., jail, detoxification unit) in the prior month (yes/no). These variables were employed because baseline toxicology results are a strong predictor of outcomes (Petry et al., 2004; Preston, et al., 1998) and controlled environments impact recent drug use. Each clinic had its own program, thereby stratifying on clinic as well. Due to the nature of the intervention, it was not possible to blind patients or providers to treatment conditions; however, primary outcomes were based on objective indices (i.e., urine sample results).
During week 6, patients were scheduled for a mid-treatment evaluation, at which they were re-randomized to one of the same two conditions outlined below. Again, a computerized urn randomization procedure at each clinic conducted randomizations (Stout et al., 1994), stratifying patients based upon mid-treatment sample results (positive for any substance vs. negative for all) and whether or not they attended any groups at the clinic in the past 7 days.
Treatment conditions
Usual care (UC) included group therapy sessions focusing on time management, life skills, motivational enhancement, relapse prevention, AIDS education, and 12-step. Intensive care (up to 5 hours/day, 3–5 days/week) was provided up to six weeks, and then frequency of care decreased. Aftercare, available for 12 months, consisted of 1–2 groups per week.
Study patients submitted up to 24 urine and breath samples: 2 per week for 12 weeks. Sample collection occurred on days that patients were scheduled to attend the clinic for groups at the beginning and end of each week (e.g., M-Th, T-F). Patients received $2 for submitting each sample, and RAs congratulated them for each substance for which they tested negative and encouraged them to discuss any use in group. To keep testing procedures as consistent with usual care as possible, RAs did not disclose study sample results to clinicians. These clinics rarely tested urine samples and usually only upon suspicions of use; any clinic requested sample testing was independent of study testing.
Attendance CM
These patients received usual care and sample monitoring above, along with the chance to draw from a bowl, and possibly win a prize, for attending groups at the clinic. Draw days were twice weekly coinciding with days patients were scheduled to attend groups at the beginning and end of the week (e.g., M-Th, T-F); this schedule kept reinforcement frequency consistent regardless of the number of scheduled days of treatment (e.g., some patients came M,T,W,Th,F, while others only M,F). Patients earned at least one draw if they attended at least one group that day, and they earned bonus draws if they attended all scheduled group sessions since their previous draw day. Bonus draws started at one and increased by one for each successive period of perfect attendance, up to a maximum of 10 draws per day after 5 full weeks of attendance. The bonus reset if patients had an unexcused absence from group (excused absences included court appearances or other commitments cleared 24 hours in advance by primary therapist according to clinic procedures). Research assistants checked clinic attendance records to ensure the appropriate number of draws and informed patients verbally and in writing at each draw session of how many draws they earned that day and why, and how many were possible at the next draw day if they attended all scheduled sessions. To ensure exposure to large prizes during the initial phase when number of draws is relatively low, patients received a priming reinforcer in the form of a guaranteed large prize (see below) the first time they attended 2 consecutive weeks of groups.
The bowl from which patients drew contained 500 cards, and 50% of them were associated with a prize. Of these, 204 were small prizes (choice of fast food coupons, toiletries, food items, or bus tokens, etc.). Forty-five were large prizes worth up to $20 in value (choice of movie theater tickets, CDs, phone cards, gift cards, watches, etc.), and one was a jumbo prize worth up to $100 (choice of small stereo, television, or five large prizes). Cards were replaced after draw sessions, so that chances of winning remained constant. Patients who attended all sessions over 6 weeks could earn a total of 75 draws, resulting in an average expected maximum earning of $190 in prizes (including the guaranteed large prize). This magnitude is consistent with that applied over 6 weeks in other prize CM studies reinforcing abstinence (Petry, Peirce, et al., 2005; Petry, Alessi, et al., 2006, 2012).
For patients assigned to the CM condition both times, draws reset to one at the time of the second randomization and then escalated as before, along with the guaranteed large prize after the first two full weeks of attendance in weeks 7–12. Thus, CM was identical whether one received it initially, later, or twice, with the exception that patients with only one scheduled group session per week in later weeks had only weekly (instead of twice weekly) draw sessions.
Adherence to intended treatments
Four bachelors- to masters-level research staff provided all study interventions over the course of the study. They received didactic training and completed written tests and role plays of CM administration, as well as usual care monitoring, prior to study initiation. Ongoing supervision consisted of regular review of treatment notes and audiotapes (over 95% of patients consented to audiotaping). Using the Contingency Management Competence Scale for Attendance (Petry, Alessi, Ledgerwood, & Sierra, 2010), independent raters coded about 10% of randomly selected audiotapes across conditions, implementers and patients on the 12 items that comprise this scale, rated on a Likert scale (e.g., 1 = not at all/poor, 3 = somewhat/ adequate, 5 = very good, 7 = extensive/excellent). The CMCS includes items such as, “To what extent did the therapist inform the patient of the reinforcement possible at the next session?” and “To what extent did the therapist tie attendance and the CM program to abstinence and other treatment goals?” Overall inter-rater reliability, assessed by the intraclass correlation coefficient, was 0.98. In CM sessions conducted with participants during weeks 1–6 versus those conducted during weeks 7–12, overall means were 5.3 (0.5) and 5.2 (0.5), respectively, p = .94, reflecting similar “good” adherence regardless of the timing of CM delivery. Reviews of usual care tapes found no content related to reinforcement.
Data analyses
Analysis of variance and chi-square tests examined differences in baseline characteristics in patients assigned to the treatment conditions. Some data were log or square root transformed as needed to normalize distributions, but raw values are presented for ease of interpretation.
Primary outcome data, defined a priori, included: days attended groups, proportion of scheduled days attended groups, longest duration of time attended all scheduled groups, longest consecutive period of objectively determined abstinence (LDA), and proportions of negative samples using number of samples submitted in the denominator (making no assumptions about missing samples) as well as with 24 samples in the denominator (assuming missing samples were positive). For longest duration outcomes, unexcused missed groups or samples reset values, while excused absences did not, consistent with the reinforcement schedule.
For substance use outcomes, samples were considered negative if urine samples tested negative for stimulants (cocaine, methamphetamine and amphetamine), opioids, and marijuana concurrently, and the breath sample was negative for alcohol as well. The rationale for focusing on total abstinence is that it is the most conservative index and most clinicians are interested in it. Additionally, this index is less impacted by ceiling effects than substance use outcomes focusing on any single substance. The majority of positive samples were for cocaine, with 17.5% of samples cocaine positive. Marijuana was the next most commonly used substance, with 12.0% of samples positive. Overall, 7.2% of samples were opioid positive. Less than 1% of samples collected tested positive for alcohol, methamphetamine or amphetamine.
The primary outcomes were available for all randomized patients, allowing for an intent- to-treat approach. Analyses included patients who did not undergo the second randomization when appropriate (e.g., examination of efficacy of short-term early exposure to CM included patients assigned to CM in weeks 1–6 even if they were not re-randomized in weeks 7–12).
Analysis of variance evaluated differences between groups to address primary aims. First, we compared those who received CM for 12 weeks (randomized to CM at baseline and week 6) to those who never received CM to ascertain whether 12 weeks of attendance CM improved retention and drug use outcomes compared to usual care. Second, we examined whether short- term CM in weeks 1–6 only improved outcomes relative to no exposure to CM; persons not randomized at week 6 were included in these analyses. Third, we evaluated whether short-term exposure to CM in the later stages of care only (weeks 7–12) improved outcomes. Across these comparisons, patients who never received CM were the comparator group. We also analyzed whether longer-term CM improved outcomes relative to shorter-term CM by comparing those randomized to CM twice to those randomized to CM once (at either baseline or week 6).
Logistic regression identified predictors of abstinence from all tested substances at Months 6 and 9. In step 1, independent variables were clinic, age, income, gender and baseline toxicology results for cocaine, marijuana, and opioids. Toxicology results for other substances were not entered into the model because some cell sizes were less than 5 for use of other substances. Other than age and income (continuous variables), all other variables were included as categorical. In step 2, the treatment condition contrast addressing the primary aims, along with days attended treatment and proportion of submitted samples testing negative, were entered. These latter two variables were selected for inclusion in the model as they were the least correlated outcome measures. Because not all patients completed follow-ups, analyses were conducted twice—both excluding non-completers, and including them as testing positive for substances. SPSS (Version 15) performed analyses, with two-tailed alpha < 0.05 significant.
Results
Demographic and baseline characteristics
Table 1 shows baseline characteristics for patients randomized to the UC and CM conditions at the two time points, as well as for those not randomized at week 6. Groups were generally similar at baseline. The two exceptions were that ASI-legal scores and DSM-IV marijuana dependence status differed overall across groups in the omnibus tests. However, no two groups differed significantly from one another in post-hoc analyses for ASI-legal scores, and the group differences in marijuana dependence status related to those who only underwent the initial randomization; the other four groups were similar, χ2 (3) = 3.00, p = .39. No other baseline variables differed by treatment assignment.
Table 1. Demographic and baseline characteristics.
| Variable | CMNR | UCNR | UCUC | CMCM | UCCM | CMUC | Statistical test, p |
|---|---|---|---|---|---|---|---|
| N | 23 | 28 | 61 | 80 | 79 | 89 | |
| Clinic, % (n) | χ2 (15) = 20.92, .14 | ||||||
| A | 21.7 (5) | 10.7 (3) | 37.7 (23) | 28.8 (23) | 30.4 (24) | 29.2 (26) | |
| B | 17.4 (4) | 10.7 (3) | 9.8 (6) | 8.8 (7) | 7.6 (6) | 7.9 (7) | |
| C | 47.8 (11) | 60.7 (17) | 23.0 (14) | 35.0 (28) | 36.7 (29) | 44. 9 (40) | |
| D | 13.0 (3) | 17.9 (5) | 29.5 (18) | 27.5 (22) | 25.3 (20) | 18.0 (16) | |
| Age | 40.1 (2.1) | 34.9 (9.0) | 40.6 (9.8) | 37.4 (10.0) | 37.3 (9.4) | 38.3 (10.3) | F(5,354)=1.53, .18 |
| Male, % (n) | 43.5 (10) | 60.7 (17) | 45.9 (28) | 45.0 (36) | 48.1 (38) | 48.3 (43) | χ2 (5) = 2.40, .79 |
| Years of education | 12.0 (1.9) | 12.3 (1.6) | 11.5 (1.7) | 11.8 (1.8) | 11.7 (1.9) | 11.9 (2.0) | F(5,352)=0.80, .55 |
| Never married, % (n) | 60.9 (14) | 64.3 (18) | 50.8 (31) | 58.8 (47) | 64.6 (51) | 62.9 (56) | χ2 (5) = 3.41, .64 |
| Income | $9,009 (17,826) |
$13,768 (20,723) |
$4,391 (9,230) |
$5,841 (10,470) |
$6,220 (16,339) |
$6,504 (14,604) |
F(5,354) =1.91, .09 |
| Race, % (n) | χ2 (10) = 12.81, .24 | ||||||
| African American | 34.8 (8) | 28.6 (8) | 41.0 (25) | 23.8 (19) | 25.3 (20) | 37.1 (33) | |
| European American | 65.2 (15) | 64.3 (18) | 47.5 (29) | 62.5 (50) | 67.1 (53) | 53.9 (48) | |
| Other | 0 (0) | 7.1 (2) | 11.5 (7) | 13.8 (11) | 7.6 (6) | 9.0 (8) | |
| Hispanic ethnicity, % (n) | 17.4 (4) | 14.3 (4) | 14.8 (9) | 26.3 (21) | 20.3 (16) | 19.1 (17) | χ2 (10) = 7.42, .69 |
| Past year DSM-IV dependence, % (n) | |||||||
| Cocaine | 95.7 (22) | 96.4 (27) | 96.7 (59) | 97.5 (78) | 96.2 (76) | 98.9 (88) | χ2 (5) = 1.56, .91 |
| Opioids | 56.5 (13) | 60.7 (17) | 44.3 (27) | 63.8 (51) | 51.9 (41) | 51.7 (46) | χ2 (5) = 6.29, .28 |
| Marijuana | 8.7 (2) | 46.4 (13) | 31.1 (19) | 20.0 (16) | 21.5 (17) | 27.0 (24) | χ2 (5) = 13.00, .02 |
| Alcohol | 43.5 (10) | 53.6 (15) | 45.9 (28) | 36.3 (29) | 50.6 (40) | 51.7 (46) | χ2 (5) = 5.53, .35 |
| Sample positive, % (n) | |||||||
| Cocaine | 30.4 (7) | 21.4 (6) | 14.8 (9) | 20.0 (16) | 16.5 (13) | 18.0 (16) | χ2 (5) = 3.24, .66 |
| Opioids | 8.7 (2) | 14.3 (4) | 4.9 (3) | 8.8 (7) | 8.9 (7) | 9.0 (8) | χ2 (5) = 2.23, .82 |
| Marijuana | 8.7 (2) | 28.6 (8) | 11.5 (7) | 8.8 (7) | 10.1 (8) | 15.7 (14) | χ2 (5) = 9.06, .11 |
| Alcohol (breath sample) | 0 | 0 | 0 | 0 | 0 | 0 | -- |
| Days of use in past 30 | |||||||
| Cocaine | 4.5 (6.1) | 4.4 (6.7) | 5.0 (8.5) | 2.8 (5.5) | 4.6 (6.6) | 4.5 (7.0) | F(5,354)=0.93, .46 |
| Opioids | 2.4 (4.3) | 3.1 (6.9) | 0.5 (1.7) | 2.0 (5.2) | 1.8 (4.6) | 2.3 (6.0) | F(5,354)=1.49, .19 |
| Marijuana | 0.7 (2.3) | 4.9 (9.6) | 2.1 (5.6) | 1.5 (4.5) | 2.1 (6.1) | 3.4 (8.1) | F(5,354)=1.90, .09 |
| Alcohol | 1.6 (2.7) | 3.4 (6.2) | 2.6 (5.8) | 1.5 (3.7) | 2.5 (4.5) | 2.6 (5.1) | F(5,354)=0.99, .43 |
| Addiction Severity Index scores | |||||||
| Medical | 0.20 (0.34) | 0.31 (0.41) | 0.27 (0.38) | 0.30 (0.38) | 0.34 (0.39) | 0.33 (0.39) | F(5,354)=0.70, .62 |
| Employment | 0.83 (0.25) | 0.72 (0.28) | 0.76 (0.25) | 0.74 (0.28) | 0.82 (0.25) | 0.76 (0.28) | F(5,354)=1.20, .31 |
| Alcohol | 0.12 (0.20) | 0.15 (0.23) | 0.11 (0.21) | 0.09 (0.17) | 0.14 (0.22) | 0.18 (0.25) | F(5,354)=1.62, .16 |
| Drug | 0.15 (0.11) | 0.22 (0.12) | 0.15 (0.13) | 0.18 (0.11) | 0.17 (0.11) | 0.18 (0.12) | F(5,354)=1.65, .15 |
| Legal | 0.16 (0.22) | 0.08 (0.16) | 0.10 (0.18) | 0.06 (0.14) | 0.14 (0.22) | 0.18 (0.25) | F(5,354)=3.67, .003 |
| Family/social | 0.19 (0.23) | 0.27 (0.24) | 0.17 (0.22) | 0.20 (0.23) | 0.16 (0.21) | 0.21 (0.24) | F(5,352)=1.00, .42 |
| Psychiatric | 0.27 (0.24) | 0.34 (0.23) | 0.31 (0.24) | 0.33 (0.21) | 0.30 (0.22) | 0.28 (0.24) | F(5,354)=0.61, .69 |
Notes. Values are means (with standard deviations in parentheses) unless noted. CM = contingency management. NR = not randomized at week 6. UC = usual care.
During treatment attendance and substance use outcomes
Table 2 shows treatment outcomes and results of statistical tests comparing groups to address the four study aims. Patients who received 12 weeks of attendance CM came to treatment more days, attended a higher proportion of scheduled groups, and remained in treatment for a longer consecutive period of time than patients who were never randomized to CM, with effect sizes ranging from d = 0.54 to 0.60. The longest duration of time in which patients submitted all negative samples and proportion of samples testing negative when the 24 expected samples were included as the denominator also differed significantly, with attendance CM patients demonstrating improvements on these indices (d = 0.37 – 0.51). Using submitted samples in the denominator, however, no differences in proportions of negative samples were noted between those receiving 12 weeks of attendance CM and those who never received CM.
Table 2. Primary attendance and substance use outcomes.
| Means (standard deviations) | Statistical tests | |||||||
|---|---|---|---|---|---|---|---|---|
|
Outcome variables |
No CM | CM weeks 1-12 |
CM weeks 1-6 only |
CM weeks 7-12 only |
CM weeks 1-12 v no CM, t, p, d |
CM weeks 1-6 only v no CM |
CM weeks 7-12 only v no CM |
12 weeks CM v 6 weeks CM |
| Days attended | 12.2 (8.8) | 17.2 (7.7) | 14.2 (8.9) | 15.0 (7.8) |
t = 3.92, p < .001 d = 0.60 |
t = 1.60, p = .11 d = 0.23 |
t = 2.09, p < .05 d = 0.34 |
t = 2.47, p < .02 d = 0.32 |
| % Days attended | 68.4 (26.7) | 81.7 (22.2) | 70.4 (24.0) | 75.6 (22.3) |
t = 3.50, p < .001 d = 0.54 |
t = 0.56, p = .58 d = 0.08 |
t = 1.69, p = .09 d = 0.29 |
t = 3.07, p < .01 d = 0.38 |
| Longest attendance (days) | 25.3 (25.2) | 40.6 (26.7) | 29.0 (23.6) | 29.7 (24.9) |
t = 3.83, p < .001 d = 0.59 |
t = 1.07, p = .28 d = 0.15 |
t = 1.06, p = .29 d = 0.18 |
t = 3.46, p < .001 d = 0.44 |
| Longest total abstinence (days) |
23.2 (27.8) | 33.6 (28.9) | 24.5 (23.9) | 29.6 (26.7) |
t = 2.38, p < .02 d = 0.37 |
t = 0.37, p = .71 d = 0.05 |
t = 1.36, p = .17 d = 0.23 |
t = 2.07, p < .05 d = 0.22 |
| % Negative for all substances (of 24 samples) |
31.1 (30.1) | 46.6 (30.9) | 35.4 (29.1) | 41.7 (29.6) |
t = 3.09, p < .01 d = 0.51 |
t = 0.79, p = .43 d = 0.15 |
t = 1.98, p < .05 d = 0.36 |
t = 2.22, p < .03 d = 0.25 |
| % Negative for all substances (of submitted) |
66.4 (40.8) | 66.3 (38.3) | 65.3 (41.6) | 69.1 (37.1) |
t = 0.02, p = .94 d = 0.00 |
t = 0.19, p = .85 d = −0.03 |
t = 0.43, p = .67 d = 0.07 |
t = 0.13, p = .91 d = 0.02 |
Notes: Samples were tested for stimulants, opioids and marijuana via urine tests along with breath tests for the presence of alcohol. Results were similar when controlling for baseline differences between groups, clinic, and baseline urine sample results.
Early and short term exposure to attendance CM in weeks 1–6 only did not result in any differences relative to UC (Table 2). However, patients who were randomized to attendance CM in the latter stages of care only (weeks 7–12) had improved outcomes relative to patients never receiving CM on days attended treatment and proportion of negative samples when all expected samples were included in the denominator, with effect sizes of 0.34 and 0.36, respectively.
Receiving attendance CM for 12 weeks also yielded some improvements relative to receiving it for 6 weeks (Table 2; far right). Patients randomized to CM twice attended treatment for more days, a higher proportion of days expected at treatment, and longer durations than those randomized to CM only once. Patients randomized to CM twice also achieved longer durations of abstinence and had higher proportions of negative samples of the 24 expected samples. Compared to 6 weeks of CM, 12 weeks of CM yielded effect sizes ranging from 0.22 to 0.44 on these indices.
The results described above remained similar when controlling for baseline differences between groups, as well as clinic and baseline urinalysis results (data not shown; available from authors upon request).
Post-treatment abstinence
At the Month 6 follow-up, raw proportions of patients (N = 296) who tested negative for alcohol, stimulants, opioids, and marijuana concurrently were 52.8% (28 of 53), 49.2% (32 of 65), 53.3% (40 of 75), and 53.6% (37 of 69) for those randomized to UCUC, UCCM, CMUC, and CMCM, respectively. For those who were not randomized at week 6, 57.1% (8 of 14) of those randomized to CM initially and 40.0% (8 of 20) of those assigned to UC initially had a negative sample at Month 6. Logistic regressions evaluated predictors of abstinence. In examining contrasts addressing the four study aims, none were associated with submitting a negative sample at the Month 6 follow-up (data not shown). Because treatment assignment was not significant and to include the greatest number of patients in the analyses (as each between- group contrast excluded some randomized patients due to the nature of the contrast), treatment assignment contrasts were removed from the model. Step 1 with clinic and baseline characteristics was significant, χ2 (9) = 33.93, p < 0.001, and Step 2, adding days attended treatment and proportion of negative samples submitted, improved the model, χ2 (2) = 17.72, p < 0.001. The overall model was significant, χ2 (11) = 51.65, p < 0.001. Table 3 shows results from the final model (top). Days attended groups and percent negative samples submitted during treatment were significantly associated with abstinence at the 6 Month follow-up, with odds ratios of 1.04 and 1.02, respectively. Thus, each additional day attended treatment related to a 4% greater likelihood of abstinence at follow-up, and each increased percent negative samples during treatment was associated with a 2% increased likelihood of abstinence at follow-up.
Table 3. Results from logistic regression predicting substance free samples at follow-up evaluations.
| Follow-up completers only | Follow-up non-completers as relapsed | |||||
|---|---|---|---|---|---|---|
| Variable | Beta (SE) | Wald (df) | p value | Beta (SE) | Wald (df) | p value |
| Baseline variables | N = 296 | Month 6 | N = 360 | |||
| Site | 4.74 (3) | .19 | 4.07 (3) | .25 | ||
| Male gender | −0.31 (0.27) | 1.34 (1) | .25 | −0.46 (0.24) | 3.62 (1) | .06 |
| Age | −0.02 (0.01) | 2.76 (1) | .10 | −0.01 (0.01) | 1.07 (1) | .30 |
| Income | 0.00 (0.00) | 3.52 (1) | .06 | 0.00 (0.00) | 3.40 (1) | .07 |
| Cocaine negative | 0.30 (0.39) | 0.63 (1) | .43 | 0.09 (0.37) | 0.05 (1) | .82 |
| Opioid negative | 0.03 (0.54) | 0.00 (1) | .96 | 0.06 (0.51) | 0.01 (1) | .91 |
| THC negative | 0.78 (0.46) | 2.87 (1) | .09 | 0.49 (0.45) | 1.19 (1) | .27 |
| During treatment | variables | |||||
| Days attended | 0.04 (0.02) | 4.01 (1) | < .05 | 0.05 (0.01) | 8.92 (1) | < .01 |
| % Negative | 0.02 (0.01) | 8.64 (1) | < .01 | 0.01 (0.00) | 7.98 (1) | < .01 |
| Baseline variables | N = 292 | Month 9 | N = 360 | |||
| Site | 7.06 (3) | .07 | 3.64 (3) | .45 | ||
| Male gender | 0.15 (0.26) | 0.00 (1) | .95 | −0.10 (0.24) | 0.17 (1) | .68 |
| Age | −0.01 (0.01) | 0.75 (1) | .39 | −0.00 (0.01) | 0.06 (1) | .81 |
| Income | 0.00 (0.00) | 3.57 (1) | .06 | 0.00 (0.00) | 2.73 (1) | .10 |
| Cocaine negative | 0.38 (0.39) | 0.97 (1) | .33 | 0.21 (0.37) | 0.32 (1) | .56 |
| Opioid negative | −0.06 (0.56) | 0.01 (1) | .92 | 0.14 (0.51) | 0.08 (1) | .78 |
| THC negative | 0.32 (0.45) | 0.50 (1) | .48 | 0.16 (0.43) | 0.13 (1) | .74 |
| During treatment | ||||||
| Days attended | 0.03 (0.02) | 3.80 (1) | < .05 | 0.04 (0.02) | 8.03 (1) | < .01 |
| % Negative | 0.01 (0.01) | 4.95 (1) | < .05 | 0.01 (0.01) | 4.70 (1) | < .03 |
Results were similar when patients who failed to attend the follow-up evaluation were included in the model. Again, no treatment condition contrasts were significant, but the overall model including Step 1, χ2 (9) = 28.26, p < 0.001, and Step 2, χ2 (2) = 22.77, p < 0.001, significantly predicted substance abstinence at the follow-up, with the overall model χ2 (11) = 50.92, p < 0.001. The odds ratio (95% CI) for days attended treatment and percent negative samples during treatment were 1.05 and 1.01, respectively. Table 3 (middle) shows results of analyses including participants who missed the follow-up as relapsed.
At the final Month 9 follow-up, proportions of follow-up completers who provided entirely negative samples were 57.1% (8 of 14), 30.0% (6 of 20), 50.9% (27 of 53), 48.5% (32 of 66), 52.1% (37 of 71), and 52.9% (36 of 68) for those randomized to CM and not re-randomized, UC and not re-randomized, UCUC, UCCM, CMUC, and CMCM, respectively. As with the earlier follow-up, no primary aim contrasts were significant, so group assignment was removed from subsequent analyses. In these models, Step 1 was significant, χ2 (9) = 23.26, p < 0.01, as was Step 2, χ2 (2) = 11.66, p < 0.01, and the overall model, χ2 (11) = 34.93, p < 0.001. Again, days attended treatment and proportion of negative samples submitted during treatment were significantly associated with abstinence 9 months later, with odds ratios (95% CI) of 1.03 and 1.01, respectively. Table 3 (mid-bottom) shows results from the final model.
Results were very similar (Table 3, bottom) when patients who failed to attend the last follow-up evaluation were included as relapsed. Again, no treatment contrasts predicted abstinence, but Step 1, χ2 (9) = 17.34, p < 0.05, and Step 2, χ2 (2) = 16.95, p < 0.001, were significant, as was the full model, χ2 (10) = 34.29, p < 0.001. Again, days attended treatment and proportion of negative samples submitted during treatment were associated with abstinence at Month 9, with odds ratios of 1.04 and 1.01, respectively.
Reinforcement earned and adverse effects
No study-related adverse effects occurred, and patients randomized to CM in weeks 1–6 earned a mean ± standard deviation of 43.1 ± 28.1 draws resulting in $110 ± $83 in prizes. Those randomized to CM in weeks 7–12 earned 23.7 ± 26.9 draws and $65 ± $81 in prizes.
Discussion
Results from this study indicate that reinforcing attendance increases retention in treatment and also improves some, although not all, drug use outcomes. Access to attendance CM for only the first 6 weeks of outpatient psychosocial treatment did not lead to significant improvements in any domain, but providing it in the later stages of care did, and receiving attendance CM for 12 weeks resulted in better outcomes than receiving it for only 6 weeks. These data are important because they demonstrate that clinicians who wish to implement CM can reinforce attendance on its own to improve patient outcomes, including drug use outcomes. Nevertheless, we caution strongly that abstinence-based CM yields consistent and even larger effect sizes on substance use outcomes than attendance CM, both in this study and a prior one (Petry, Barry, et al., 2012) as well as in meta-analyses (Lussier et al., 2006).
Primary substance use outcomes were based on abstinence from all tested substances concurrently. This is the most conservative approach, and it is a high bar to achieve for polysubstance users, who comprise the bulk of patients initiating care at psychosocial outpatient substance abuse treatment settings. It may be particularly difficult for chronic marijuana users to test negative for all substances, as samples can continue testing positive for THC for up to 30 days or more. Further, marijuana use is becoming increasingly legalized, although at the time of this study it was not legalized in the two states from which patients were recruited. Overall results were similar across the different substances whether or not THC was included in considering outcomes. Regardless of the specific substances that patients use, this study indicates that reinforcing attendance alone is safe and does not result in patients using drugs while coming to groups to obtain reinforcement.
However, reinforcing attendance at treatment did not yield benefits with respect to long- term substance use outcomes. Thus, clinicians should not expect that reinforcing attendance will extend to long-term reductions in drug use. As with most any psychotherapy or pharmacotherapy for substance use disorders, effects of CM are strongest while it is in effect, and effect sizes diminish as time elapses (Benishek et al., 2014). Nevertheless, about one third of CM studies do find that the effects of abstinence CM persist after reinforcement is removed (Davis et al., 2016), and there are no data indicating that patients who earlier received CM have poorer long term outcomes than patients who never received reinforcement. Furthermore, abstinence during treatment is strongly and consistently associated with long-term benefits (Higgins, Badger, & Budney, 2000; Higgins et al., 2007; Petry et al., 2007, 2011; Petry, Alessi, et al., 2006; Petry, Alessi, Ledgerwood, et al., 2010; Petry, Barry, et al., 2012; Petry, Martin, et al., 2005), and this study likewise found that days attended treatment and proportion of negative samples during the 12-week treatment period were significantly associated with abstinence at both long-term follow- ups. Thus, providing attendance CM may not help long-term abstinence, but it also will not hinder it. To the extent that attendance CM increases retention in treatment and reduces during treatment drug use, it is a reasonable intervention to adopt.
Implementation of CM has been impeded by logistical issues and costs (Petry, Alessi, Olmstead, Rash, & Zajac, 2017). Applying reinforcement for attending treatment is easier and less costly than reinforcing abstinence, which requires staff time and supplies for urine testing. Many psychosocial clinics, especially those without nursing and medical staff, do not regularly collect and screen urine samples, and frequent testing may not be reimbursed by some insurers or payers. These data suggest an entirely attendance CM can be considered in these cases.
Costs of the reinforcers themselves are another implementation barrier. This study used magnitudes of expected reinforcement similar to other abstinence-based CM interventions to ensure that if any differences relative to prior studies occurred they could not be attributed to differential magnitudes of reinforcement, which are known to impact efficacy (Prendergast et al., 2006; Lussier et al., 2006). This study arranged for average maximal reinforcement of about $380 over 12 weeks of treatment, a magnitude efficacious in improving outcomes (Petry, Alessi, et al., 2005; Petry, Martin, et al., 2005; Petry, Peirce, et al., 2005; Petry, Alessi, et al., 2006; Petry, Barry, et al., 2012). As CM is disseminated, clinician training and supervision remains paramount regardless of the target behavior being reinforced (DePhilippis et al., in press) and arranging lower magnitude reinforcers, even for attendance, may be insufficient. However, efforts are ongoing to reduce costs of attendance-based CM by bringing prize reinforcement into group settings (Alessi et al., 2007; Ledgerwood et al., 2008; Petry et al., 2001, 2011) or arranging interdependent group contingencies (Kirby, Kerwin, Carpenedo, & Rosenwasser, 2008; Meredith & Dallery, 2013; Meredith, Grabinski & Dallery, 2011), which ultimately may allow for reductions in reinforcement costs without impacting efficacy. Such group-based attendance reinforcement systems permit all patients to participate (i.e., not just those with cocaine use disorders). However, the substantially lower cost group-based attendance CM approach (Petry et al., 2001; Ledgerwood et al., 2008) has never been compared directly to individually applied attendance CM, the approach used in the present study.
Some limitations should be considered in interpreting these results. This study enrolled patients with a common diagnosis of a cocaine use disorder. Although it did not exclude patients for polydrug use diagnoses, which were common, it is possible that results may differ in patients with other primary substance use diagnoses. The study was conducted at four outpatient substance abuse treatment programs that spanned two states, but all these clinics served primarily uninsured and underinsured patients and relied heavily on state supported funding. Results may differ in other areas of the country and health care systems or with different populations.
This study regularly screened for substance use, and although study results were not shared with the clinical team, it is possible that patients may be more likely to use substances while attending treatment if samples are never or rarely collected and tested. If attendance CM is applied clinically, usual clinic rules should apply to patients who use, or who are suspected of using, during treatment. These may include occasional urine testing and referrals to higher levels of care and possibly even cessation of attendance-based CM treatment upon submission of repeated positive samples. Clinicians may consider switching the reinforcement target to abstinence in these cases. A potential concern is that about half the patients relapsed to drug use after the treatment period ended. Development and evaluation of interventions that prolong long- term abstinence are necessary to extend CM’s benefits.
The present study was conducted in community clinics, but well trained and supervised research staff provided the CM intervention. This arrangement was deliberate to ensure intended treatments were provided, but we (Petry et al., 2012) and others (e.g., Hartzler, Beadnell, & Donovan, 2017) have found that clinicians can implement CM with fidelity when appropriately trained and supervised. Consistent with typical reductions in care over time, participants in this study randomized to CM in weeks 7–12 received less care, and fewer reinforcers, than those receiving CM in the initial weeks, in which twice weekly attendance was more common. Still, CM provided in later phase of care engendered some significant improvements in outcomes.
Strengths of this study include the adaptive design to address whether timing of CM administration impacts patient outcomes, as well as the large sample size, high rates of follow-up participation, limited use of patient exclusion criteria, inclusion of objective indices of substance use, and implementation in multiple community substance abuse treatment clinics. All these features increase generalization of the findings. Consistent with national trends (Treatment Episode Data Set, 2017), over half the sample had an opioid along with a cocaine use diagnosis. Inclusion of these patients renders results generalize to polysubstance using patients. In this sample, rates of positive samples at baseline were low, likely because only a proportion of the full sample had these other substance use problems, some patients were recently released from controlled environments, and patients with more severe drug use problems were referred to higher levels of care and not enrolled in the outpatient programs. Assignment to conditions was stratified based on some of these variables, and they did not impact overall outcomes.
This study is important and provides unique data because it demonstrates the efficacy of an entirely attendance-based CM. CM interventions that reinforce attendance have advantages in terms of adoption, and community settings are already implementing such interventions clinically (Kellogg et al., 2005; Ledgerwood et al., 2008). Development and assessment of an even lower-cost prize procedure for reinforcing attendance in groups is underway (Alessi et al., 2007; Ledgerwood et al., 2008; Petry et al., 2001). This system may produce similar benefits at lower costs (e.g., $30/week for a 12-person group) because not all patients win prizes at every group, yet they directly observe others winning prizes thereby highlighting the odds of winning.
Cost-effectiveness analyses (Olmstead, Sindelar, & Petry, 2007a; Olmstead, Sindelar, & Petry, 2007b; Sindelar, Elbel, & Petry, 2007; Sindelar, Olmstead, & Peirce, 2007) indicate that collecting and screening urine samples add substantially to implementation costs of CM. Costs can be lowered by reinforcing attendance. These data indicate that reinforcing attendance, especially when applied over 12 week and during later stages of outpatient care, is an efficacious approach in clinics and settings in which retention is low. Although reinforcing drug negative urine samples is clearly efficacious (Lussier et al., 2006; Prendergast et al., 2006) and policy makers should urge for its coverage (Petry et al., 2017), reinforcing attendance can also lead to greater treatment participation and lower drug use during periods it is in effect.
Acknowledgments
Funding for this study and preparation of this report was provided by the NIH, awarded to Nancy Petry, P50-DA09241, P60-AA03510, R01-AA021446. We thank the patients and clinicians for participating in this study.
References
- Alessi SM, Hanson T, Wieners M, & Petry NM. (2007). Low-cost contingency management in community clinics: Delivering incentives partially in group therapy. Experimental and Clinical Psychopharmacology, 15(3), 293-300. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (2000). Diagnostic and Statistical Manual of Mental Disorders (4th ed., text rev.). Washington, DC: Author. [Google Scholar]
- Benishek LA, Dugosh KL, Kirby KC, Matejkowski J, Clements NT, Seymour BL, & Festinger DS. (2014). Prize‐based contingency management for the treatment of substance abusers: A meta‐analysis. Addiction, 109(9), 1426-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovasso GB, Alterman AI, Cacciola JS, & Cook TG. (2001). Predictive validity of the Addiction Severity Index's composite scores in the assessment of 2-year outcomes in a methadone maintenance population. Psychology of Addictive Behaviors, 15(3), 171-176. [PubMed] [Google Scholar]
- Branson CE, Barbuti AM, Clemmey P, Herman L, & Bhutia P. (2012). A pilot study of low‐cost contingency management to increase attendance in an adolescent substance abuse program. The American Journal on Addictions, 21(2), 126-129. [DOI] [PubMed] [Google Scholar]
- Collins LM, Murphy SA, & Strecher V. (2007). The multiphase optimization strategy (MOST) and the sequential multiple assignment randomized trial (SMART): New methods for more potent eHealth interventions. American Journal of Preventive Medicine, 32, S112-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis DR, Kurti AN, Skelly JM, Redner R, White TJ, & Higgins ST. (2016). A review of the literature on contingency management in the treatment of substance use disorders, 2009–2014. Preventive Medicine, 92, 36-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePhilippis D, Petry NM, Bonn-Miller MO, Rosenbach SB, McKay JR. (2018). The national implementation of Contingency Management (CM) in the Department of Veterans Affairs: Attendance at CM sessions and substance use outcomes. Drug and Alcohol Dependence, 185, 367-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, & Otto MW. (2008). A meta-analytic review of psychosocial interventions for substance use disorders. American Journal of Psychiatry, 165(2), 179-187. [DOI] [PubMed] [Google Scholar]
- Evaluation, Quality Management and Improvement Division (2017). Connecticut Department of Mental Health and Addiction Services Annual Statistical Report. Available at: http://www.ct.gov/dmhas/lib/dmhas/eqmi/annualreport2017.pdf
- Hartzler B, Beadnell B, & Donovan D. (2017). Predictive validity of addiction treatment clinicians' post-training contingency management skills for subsequent clinical outcomes. Journal of Substance Abuse Treatment, 72, 126-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henggeler SW, Sheidow AJ, Cunningham PB, Donohue BC, & Ford JD. (2008). Promoting the implementation of an evidence-based intervention for adolescent marijuana abuse in community settings: Testing the use of intensive quality assurance. Journal of Clinical Child & Adolescent Psychology, 37(3), 682-689. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Badger GJ, & Budney AJ. (2000). Initial abstinence and success in achieving longer term cocaine abstinence. Experimental and Clinical Psychopharmacology, 8(3), 377-386. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Heil SH, Dantona R, Donham R, Matthews M, & Badger GJ. (2007). Effects of varying the monetary value of voucher‐based incentives on abstinence achieved during and following treatment among cocaine‐dependent outpatients. Addiction, 102(2), 271-281. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Sigmon SC, Wong CJ, Heil SH, Badger GJ, Donham R, … & Anthony S. (2003). Community reinforcement therapy for cocaine-dependent outpatients. Archives of General Psychiatry, 60(10), 1043-1052. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Wong CJ, Badger GJ, Ogden DE, & Dantona RL. (2000). Contingent reinforcement increases cocaine abstinence during outpatient treatment and 1 year of follow-up. Journal of Consulting and Clinical Psychology, 68(1), 64-72. [DOI] [PubMed] [Google Scholar]
- Kellogg SH, Burns M, Coleman P, Stitzer M, Wale JB, & Kreek MJ. (2005). Something of value: The introduction of contingency management interventions into the New York City Health and Hospital Addiction Treatment Service. Journal of Substance Abuse Treatment, 28(1), 57-65. [DOI] [PubMed] [Google Scholar]
- Kirby KC, Kerwin ME, Carpenedo CM, Rosenwasser BJ, & Gardner RS. (2008). Contingency management for attendance to group substance abuse treatment administered by clinicians in community clinics. Journal of Applied Behavior Analysis, 41(4), 579-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TR, Rounsaville BJ, & Kleber HD. (1983). Concurrent validity of the Addiction Severity Index. The Journal of Nervous and Mental Disease, 171(10), 606-610. [DOI] [PubMed] [Google Scholar]
- Ledgerwood DM, Alessi SM, Hanson T, Godley MD, & Petry NM. (2008). Contingency management for attendance to group substance abuse treatment administered by clinicians in community clinics. Journal of Applied Behavior Analysis, 41(4), 517-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lussier JP, Heil SH, Mongeon JA, Badger GJ, & Higgins ST. (2006). A meta‐analysis of voucher‐based reinforcement therapy for substance use disorders. Addiction, 101(2), 192-203. [DOI] [PubMed] [Google Scholar]
- Meredith SE, & Dallery J. (2013). Investigating group contingencies to promote brief abstinence from cigarette smoking. Experimental and Clinical Psychopharmacology, 21(2), 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith SE, Grabinski MJ, & Dallery J. (2011). Internet-based group contingency management to promote abstinence from cigarette smoking: A feasibility study. Drug and Alcohol Dependence, 118(1), 23-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SA, Collins LM. & Rush AJ. (2007). Customizing treatment to the patient: Adaptive treatment strategies. Drug and Alcohol Dependence, 88, S1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmstead TA, Sindelar JL, & Petry NM. (2007. a). Clinic variation in the cost effectiveness of contingency management. The American Journal on Addictions, 16(6), 457-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmstead TA, Sindelar JL, & Petry NM. (2007. b). Cost-effectiveness of prize-based incentives for stimulant abusers in outpatient psychosocial treatment programs. Drug and Alcohol Dependence, 87(2), 175-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, & Alessi SM. (2010). Prize-based contingency management is efficacious in cocaine-abusing patients with and without recent gambling participation. Journal of Substance Abuse Treatment, 39(3), 282-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Alessi SM, Barry D, & Carroll KM. (2015). Standard magnitude prize reinforcers can be as efficacious as larger magnitude reinforcers in cocaine-dependent methadone patients. Journal of Consulting and Clinical Psychology, 83(3), 464-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Alessi SM, Carroll KM, Hanson T, MacKinnon S, Rounsaville B, & Sierra S. (2006). Contingency management treatments: Reinforcing abstinence versus adherence with goal-related activities. Journal of Consulting and Clinical Psychology, 74(3), 592-601. [DOI] [PubMed] [Google Scholar]
- Petry NM, Alessi SM, Hanson T, & Sierra S. (2007). Randomized trial of contingent prizes versus vouchers in cocaine-using methadone patients. Journal of Consulting and Clinical Psychology, 75(6), 983-991. [DOI] [PubMed] [Google Scholar]
- Petry NM, Alessi SM, & Ledgerwood DM. (2012). Contingency management delivered by community therapists in outpatient settings. Drug and Alcohol Dependence, 122(1), 86-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Alessi SM, Ledgerwood DM, & Sierra S. (2010). Psychometric properties of the contingency management competence scale. Drug and Alcohol Dependence, 109(1), 167-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Alessi SM, Marx J, Austin M, & Tardif M. (2005). Vouchers versus prizes: Contingency management treatment of substance abusers in community settings. Journal of Consulting and Clinical Psychology, 73(6), 1005-1014. [DOI] [PubMed] [Google Scholar]
- Petry NM, Alessi SM, Olmstead TA, Rash CJ, Zajac K. (2017). Contingency management treatment for substance use disorders: How far has it come, and where does it need to go? Psychology of Addictive Behaviors, 31(8), 897-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Barry D, Alessi SM, Rounsaville BJ, & Carroll KM. (2012). A randomized trial adapting contingency management targets based on initial abstinence status of cocaine-dependent patients. Journal of Consulting and Clinical Psychology, 80(2), 276-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, DePhilippis D, Rash CJ, Drapkin M, & McKay JR. (2014). Nationwide dissemination of contingency management: The Veterans Administration initiative. The American Journal on Addictions, 23(3), 205-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Kolodner KB, Li R, Peirce JM, Roll JM, Stitzer ML, & Hamilton JA. (2006). Prize-based contingency management does not increase gambling. Drug and Alcohol Dependence, 83(3), 269-273. [DOI] [PubMed] [Google Scholar]
- Petry NM, Martin B, Cooney JL, & Kranzler HR. (2000). Give them prizes and they will come: Contingency management for treatment of alcohol dependence. Journal of Consulting and Clinical Psychology, 68(2), 250-257. [DOI] [PubMed] [Google Scholar]
- Petry NM, Martin B, & Finocche C. (2001). Contingency management in group treatment: A demonstration project in an HIV drop-in center. Journal of Substance Abuse Treatment, 21(2), 89-96. [DOI] [PubMed] [Google Scholar]
- Petry NM, Martin B, & Simcic F. (2005). Prize reinforcement contingency management for cocaine dependence: Integration with group therapy in a methadone clinic. Journal of Consulting and Clinical Psychology, 73(2), 354-359. [DOI] [PubMed] [Google Scholar]
- Petry NM, Peirce JM, Stitzer ML, Blaine J, Roll JM, Cohen A, … & Kirby, K. C. (2005). Effect of prize-based incentives on outcomes in stimulant abusers in outpatient psychosocial treatment programs: A national drug abuse treatment clinical trials network study. Archives of General Psychiatry, 62(10), 1148-1156. [DOI] [PubMed] [Google Scholar]
- Petry NM, & Simcic F. (2002). Recent advances in the dissemination of contingency management techniques: Clinical and research perspectives. Journal of Substance Abuse Treatment, 23(2), 81-86. [DOI] [PubMed] [Google Scholar]
- Petry NM, Tedford J, Austin M, Nich C, Carroll KM, & Rounsaville BJ. (2004). Prize reinforcement contingency management for treating cocaine users: How low can we go, and with whom? Addiction, 99(3), 349-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Weinstock J, & Alessi SM. (2011). A randomized trial of contingency management delivered in the context of group counseling. Journal of Consulting and Clinical Psychology, 79(5), 686-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast M, Podus D, Finney J, Greenwell L, & Roll J. (2006). Contingency management for treatment of substance use disorders: A meta‐ analysis. Addiction, 101(11), 1546-1560. [DOI] [PubMed] [Google Scholar]
- Preston KL, Silverman K, Higgins ST, Brooner RK, Montoya I, Schuster CR, Cone EJ. (1998). Cocaine use early in treatment predicts outcome in a behavioral treatment program. Journal of Consulting and Clinical Psychology, 66, 691-696. [DOI] [PubMed] [Google Scholar]
- Rash CJ, Petry NM, Kirby KC, Martino S, Roll J, & Stitzer ML. (2012). Identifying provider beliefs related to contingency management adoption using the contingency management beliefs questionnaire. Drug and Alcohol Dependence, 121(3), 205-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieckmann T, Abraham A, Zwick J, Rasplica C, McCarty D. (2015). A longitudinal study of state strategies and policies to accelerate evidence-based practices in the context of systems transformation. Health Services Research, 50(4), 1125-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan H, Bullock CL, & Reger GM. (2017). Implementation of contingency management at a large VA addiction treatment center. Psychiatric Services, in press. [DOI] [PubMed] [Google Scholar]
- Sigmon SC, & Stitzer ML. (2005). Use of a low-cost incentive intervention to improve counseling attendance among methadone-maintained patients. Journal of Substance Abuse Treatment, 29(4), 253-258. [DOI] [PubMed] [Google Scholar]
- Silverman K, Robles E, Mudric T, Bigelow GE, & Stitzer ML. (2004). A randomized trial of long-term reinforcement of cocaine abstinence in methadone-maintained patients who inject drugs. Journal of Consulting and Clinical Psychology, 72(5), 839-854. [DOI] [PubMed] [Google Scholar]
- Sinclair JMA, Burton A, Ashcroft R, & Priebe S. (2011). Clinician and service user perceptions of implementing contingency management: A focus group study. Drug and Alcohol Dependence, 119(1), 56-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindelar J, Elbel B, & Petry NM. (2007). What do we get for our money? Cost‐effectiveness of adding contingency management. Addiction, 102(2), 309-316. [DOI] [PubMed] [Google Scholar]
- Sindelar JL, Olmstead TA, & Peirce JM. (2007). Cost‐effectiveness of prize‐based contingency management in methadone maintenance treatment programs. Addiction, 102(9), 1463-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout RL, Wirtz PW, Carbonari JP, & Del Boca FK. (1994). Ensuring balanced distribution of prognostic factors in treatment outcome research. Journal of Studies on Alcohol, Supplement, (12), 70-75. [DOI] [PubMed] [Google Scholar]
- TenHave TR, Coyne J, Salzer M, & Katz I. (2003). Research to improve the quality of care for depression: Alternatives to the simple randomized clinical trial. General Hospital Psychiatry, 25(2), 115-123. [DOI] [PubMed] [Google Scholar]
- Treatment Episode Data Set (2017). Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. Treatment Episode Data Set (TEDS): 2005-2015. [Google Scholar]
- Walker R, Rosvall T, Field CA, Allen S, McDonald D, Salim Z, … & Adinoff B. (2010). Disseminating contingency management to increase attendance in two community substance abuse treatment centers: Lessons learned. Journal of Substance Abuse Treatment, 39(3), 202-209. [DOI] [PMC free article] [PubMed] [Google Scholar]