SUMMARY
Setting and Objective:
Current anti-tuberculosis (TB) formulations necessitate uninterrupted treatment to cure TB, but are characterised by sub-optimal adherence, jeopardising therapeutic efficacy. Long-acting injectable (LAI) formulations or implants could address these associated issues. This study aimed to use physiologically-based pharmacokinetic (PBPK) modelling to simulate potential LAI administration strategies for four anti-TB agents - isoniazid, rifapentine, bedaquiline and delamanid in adults for latent TB (LTBI) treatment.
Design:
PBPK models were developed and qualified against available clinical data by integrating drug physicochemical properties, in vitro and population pharmacokinetic data into a mechanistic description of drug distribution. Combinations of optimal dose and release rates were simulated such that the plasma concentrations were maintained over the epidemiological cut-off or minimum inhibitory concentration for the dosing interval.
Results:
The PBPK model identified 1500 mg of delamanid and 250 mg of rifapentine as sufficient doses for monthly intramuscular (IM) administration, if a formulation or device can deliver the required release kinetics ranging from 0.001 – 0.0025 h−1 and 0.0015 – 0.0025 h−1 respectively. Bedaquiline and isoniazid would require weekly to biweekly IM dosing.
Conclusion:
These findings identify the theoretical doses and release rates of LAI anti-TB formulations. Such a strategy could ease the problem of sub-optimal adherence provided the associated technological complexities are addressed for LTBI treatment.
Keywords: PBPK, pharmacokinetics, anti-TB, long-acting, intramuscular
1. INTRODUCTION
Tuberculosis (TB) remains a global pandemic and is one of the top ten leading causes of death worldwide, however early diagnosis and anti-TB therapy have saved over 49 million lives between 2000 and 2015.1 Individuals with latent TB infection (LTBI) along with immunosuppression, including neonates, infants, elderly and patients co-infected with HIV are at higher risk of developing active TB infection.2 Progression of LTBI to active TB is 12 to 20 times higher in HIV-infected persons than in those who are HIV-uninfected.3
Treatment for LTBI is with isoniazid alone, rifampin alone or isoniazid in combination with rifampin or rifapentine.4 Regimens vary in duration from 3 to 9 months, while dosing frequency varies from daily to weekly administration. Patients co-infected with HIV may experience higher rates of adverse effects, and co-treatment is complicated by drug-drug interactions with antiretrovirals.5 Suboptimal adherence and low completion rates have been reported in patients with LTBI due to numerous factors.6 Although directly observed therapy has success rates > 80%7, DOTS (Directly Observed Therapy, Short-course) requires patients to be physically present during therapy which is resource intensive and an additional burden to patients. By the year 2050, the target of one TB case per million individuals (currently 1280 per million, a reduction by a factor of thousand) can be achieved only if higher numbers of patients infected with both latent and active TB could be succesfully treated.8
In low- and middle-income countries, studies show that poor adherence is a major obstacle to TB treatment success and simplification of the administration of anti-TB drugs is essential to address these problems.9 Existing drugs could be reformulated or loaded into drug-delivery implants to achieve long-acting (LA) exposure, thus simplifying therapy.10 Reducing the frequency of administration decreases pill fatigue, thus improving patient adherence and achieving higher treatment completion rates. Long-acting injectable (LAI) nanoformulations represent a valuable technological platform to address the problem of sub-optimal adherence, reducing the overall amount of drug used and simplifying DOTS. In several therapeutic areas – antipsychotics, contraceptives, antiretrovirals -- characterised by the need for chronic dosing, LAI formulations have been successfully introduced, motivated by their multiple advantages over conventional oral administration strategies.11
Physiologically-based pharmacokinetic (PBPK) modelling is a mathematical representation of drug pharmacokinetics (PK) in humans with potential application in multiple drug/formulation development and clinical scenarios. PBPK modelling is a bottom-up approach that collates various allometric and anthropometric equations to describe concurrent physiological processes affecting drug disposition, in addition to key PK processes – absorption, distribution, metabolism and elimination (ADME). PBPK modelling incorporates the anatomy and physiology of humans, drug physiochemical parameters, in vitro data such as apparent gastrointestinal and cellular permeability, intrinsic clearance, and population PK data such as apparent clearance to predict drug concentrations over time through the simulation of ADME processes.12
In this present study, the LA potential of isoniazid and rifapentine - drugs used in LTBI treatment and novel agents active against MDR-TB - bedaquiline and delamanid were chosen due to their favourable characteristics for LA formulations. The aim of this study was to simulate the LA administration of select anti-TB drugs – bedaquiline, delamanid, isoniazid and rifapentine to assess their potential use in a modified 4-weekly intramuscular (IM) regimen for treatment of LTBI.
2. METHODS
A previously published compartmental whole-body PBPK model was used to evaluate the LA potential of anti-TB drugs.13 Simbiology® v.4.3.1., a product of MATLAB® v.8.2 (MathWorks, Natick, MA, USA 2013) was used as a platform for the design of the PBPK models. Movement of drug from the blood circulation into tissues was blood-flow limited, with instant drug distribution in tissues and organs. For orally-administered drug simulations, no drug reabsorption from the large intestine was assumed. One hundred simulated healthy male adults (n=3) aged 18–60 years were included in each test group. Ethical approval was not required for this study since the data is computer generated.
2.1. PBPK Model
Age (18–60 years) and weight (40–100 kg) of the virtual individuals were defined initially and other key characteristics such as body mass index (BMI), body surface area (BSA), height (1.5–2.0 m), organ and tissue weights, and volumes and blood flow rates were computed using appropriate anthropometric equations available from the literature.13 Absorption rate, log P, pKa, protein binding, blood-to-plasma ratio and plasma clearance were obtained from various literature sources (as shown in Table 1). For the current simulations, healthy volunteers were selected as the population of interest as they are more likely to represent patients with LTBI, and also considering that potential future dose finding studies for LAI formulations will be conducted in this population. An additional compartment representing the IM depot with first-order release kinetics was introduced to describe the drug release from the IM depot to the surrounding blood capillaries, as described in our previous publication.13
Table 1.
Physicochemical properties, in vitro and population pharmacokinetic data of anti-TB drugs
| Bedaquiline | Delamanid | Isoniazid | Rifapentine | |
|---|---|---|---|---|
| log Po:w | 6.37 28 | 5.53 29 | −0.7 30 | 4.0 31 |
| pKa | 13.61, 8.91 28 | 3.99 29 | 1.82 30 | 7.17, 7.01 31 |
| Blood-to-plasma ratio | *4.04 | *3.92 | *0.80 | 0.56 32 |
| Protein binding (%) | 99.9 28 | 99.55 33 | 10 30 | 97.7 31 |
| Plasma clearance | 0.04 ± 0.002 34 | 0.729 ± 0.121 15 |
‡0.142 ± 0.042 35 |
0.028 ± 0.009 17 |
| Absorption rate (h−1) | 1.03 ± 0.19 36 | §0.96 37 | 2.28 35 | #0.42 37 |
| Vd (L/kg) | 2.18 34 | 7.32 15 | 0.21 38 | 0.69 17 |
| Bioavailability (%) | ∆10034 | 36 (25-47) 15 | ∆10035 | φ45 (40-50)39 |
log Po:w – Partition coefficient between octanol and water; pKa – logarithmic value of the dissociation constant; Vd – volume of distribution;
blood-to-plasma ratios were computed using equations from Paixão et al.40;
Computed from the PBPK model; Plasma clearance values are given for adults and are represented as L/h/kg,
Value provided is apparent clearance of slow acetylators,
Computed from polar surface area and hydrogen bond donor values using the equation from Gertz et al.41,
Computed from Caco-2 permeability using the equation from Gertz et al.41,
Bioavailability has been fixed to 100 in the population pharmacokinetic studies.
A bioavailabity between 40-50% was assumed based on the relative bioavailility of 70% for the oral formulation compared to oral formulation
2.2. Model qualification
PBPK models for all the four drugs were constructed using the parameters mentioned in Table 1. The models were assumed to be qualified if the mean simulated PK parameters Cmax (maximum plasma concentration), Ctrough (trough plasma concentration) and AUC (area under the curve) were within 50% of the mean observed clinical value.
Steady-state PK values were collected from the literature where available and, if not available, single-dose PK data was used for PBPK model qualification. Model validation was performed against 10, 30, 100, 300, 450 and 700 mg single dose oral clinical formulation of bedaquiline.14 The PBPK qualification of delamanid was performed at steady-state with 100 mg thrice daily, 150 mg twice daily and 300 mg once daily oral formulations.15 Clearance of isoniazid varies with acetylator status, determined by NAT2 genotype. Hence pharmacokinetics of 300 mg single dose in intermediate acetylators, 300, 600 and 900 mg single dose in rapid acetylators were used for the validation of the PBPK model.16 The rifapentine PBPK model was qualified by comparing predictions with oral doses of 5, 10, 15 and 20 mg/kg at steady-state.17 The assumptions used and visual predictive checks for model qualification are described in the appendix.
2.3. Dose and release rate optimisation
Various combinations of dose and release rates were evaluated and the trough concentrations were recorded on the 28th day from the start of the IM administration. For all the simulations, six different release rates – 0.0005, 0.001, 0.0015, 0.002, 0.0025 and 0.003 h−1 were used. The variability in release rate was assumed to be ± 10% from the mean reported value. A maximum dose of 2000 mg was considered for a single IM injection. Epidemiological cut-off (ECOFF) value is the minimum inhibitory concentration (MIC) value identifying the upper limit for the wild-type population.18 The ECOFF value for each drug, if available, was used as the target trough concentrations in the simulations. If ECOFF values were not available, MIC or critical concentration (CC) values were used. The reported target trough concentrations are adjusted for protein binding. The reported ECOFF value for bedaquiline and delamanid were 1.6 mg/L and 0.04 mg/L respectively.18 The CC of high-level isoniazid resistance (0.125 mg/L) was chosen as the target concentration.19 Thrice the MIC value of rifapentine was chosen as the target concentration to include wild-type TB.20 For a dosing interval, the minimum dose at a particular release rate maintaining plasma concentrations over the target concentrations was considered as the optimal release rate. A combination of different doses and release rates were simulated to support sustained exposure such that the plasma concentrations in at least 95 out of 100 virtual individuals (n=3) exceeded the target concentration for the entire dose interval (4 weeks).
3. RESULTS
The validation of the four anti-TB drugs against clinical formulations is shown in Table 2.
Table 2.
Validation of the PBPK model for various anti-TB drugs against oral clinical formulations in adults
| Clinical | Simulated | ||||||
|---|---|---|---|---|---|---|---|
| Drug | Dose (mg) | Cmax | Ctrough | AUC | Cmax | Ctrough | AUC |
| Bedaquiline (single dose)14 |
10 mg | 0.069 ± 0.015 | - | *1.248 ± 0.233 | 0.072 ± 0.011 | - | *1.421 ± 0.337 |
| 30 mg | 0.276 ± 0.064 | - | *4.418 ± 1.424 | 0.213 ± 0.038 | - | *4.226 ± 0.956 | |
| 100 mg | 0.854 ± 0.283 | - | *13.604 ± 5.115 | 0.718 ± 0.126 | - | *14.117 ± 3.218 | |
| 300 mg | 2.547 ± 1.305 | - | *38.737 ± 15.584 | 2.142 ± 0.359 | - | *42.063 ± 9.163 | |
| 450 mg | 3.755 ± 1.165 | - | *64.530 ± 26.927 | 3.220 ± 0.545 | - | *62.687 ± 13.772 | |
| 700 mg | 6.747 ± 2.210 | - | *97.816 ± 38.074 | 5.001 ± 0.784 | - | *98.131 ± 20.868 | |
| Delamanid (day 10)15 |
100 mg TID | 0.606 ± 0.168 | 0.349 ± 0.049 | †11.8 ± 2.08 | 0.425 ± 0.119 | 0.276 ± 0.099 | †8.654 ± 2.638 |
| 300 mg BD | 0.512 ± 0.081 | 0.333 ± 0.068 | †10.2 ± 1.57 | 0.434 ± 0.121 | 0.222 ± 0.088 | †7.994 ± 2.467 | |
| 300 mg QD | 0.412 ± 0.051 | 0.146 ± 0.041 | †5.84 ± 0.99 | 0.330 ± 0.085 | 0.093 ± 0.045 | †4.739 ± 1.421 | |
| Isoniazid (single dose)16 |
300 mg IA | 6.77 ± 2.121 | - | ‡14.24 ± 1.753 | 6.23 ± 1.31 | - | ‡17.1 ± 6.59 |
| 300 mg RA | 5.72 ± 2.064 | - | ‡9.54 ± 2.404 | 5.35 ± 1.16 | - | ‡12.41 ± 4.53 | |
| 600 mg RA | 13.92 ± 3.422 | - | ‡24.99 ± 4.299 | 11.99 ± 2.53 | - | ‡30.27 ± 9.89 | |
| 900 mg RA | 21.49 ± 5.402 | - | ‡48.19 ± 5.204 | 19.15 ± 4.17 | - | ‡54.88 ± 18.17 | |
| Rifapentine (OD, day 14)17 |
5 mg/kg | 15.7 (13.0–17.7) | - | †218 (142-251) | 13.2 (12.0-14.5) | 6.7 (5.7-7.5) | †237 (215-262) |
| 10 mg/kg | 21.7 (21.3-22.2) | - | †330 (284-340) | 21.8 (19.9-23.8) | 8.8 (7.4-10.0) | †367 (326-397) | |
| 15 mg/kg | 35.9 (25.1-39.4) | - | †560 (401-735) | 37.8 (34.8-41.3) | 22.9 (19.8- 25.3) |
†732 (664-803) | |
| 20 mg/kg | 34.1 (29.7-42.9) | - | †483 (414-546) | 35.6 (32.6-38.5) | 14.9 (12.4- 16.8) |
†594 (540-644) | |
Values are represented as arithmetic mean ± standard deviation, Rifapentine clinical and simulated values are represented as median (IQR), AUC – area under the concentration-time curve, Cmax – maximum plasma concentration, Ctrough – trough plasma concentration at 24 h after administration; Cmax and Ctrough are expressed as mg/L and AUC is expressed as mg × h/L;
AUC0-144,
AUC0-24,
AUC0-12.
OD – once daily, BD – twice daily, TID – thrice daily.
3.1. Bedaquiline
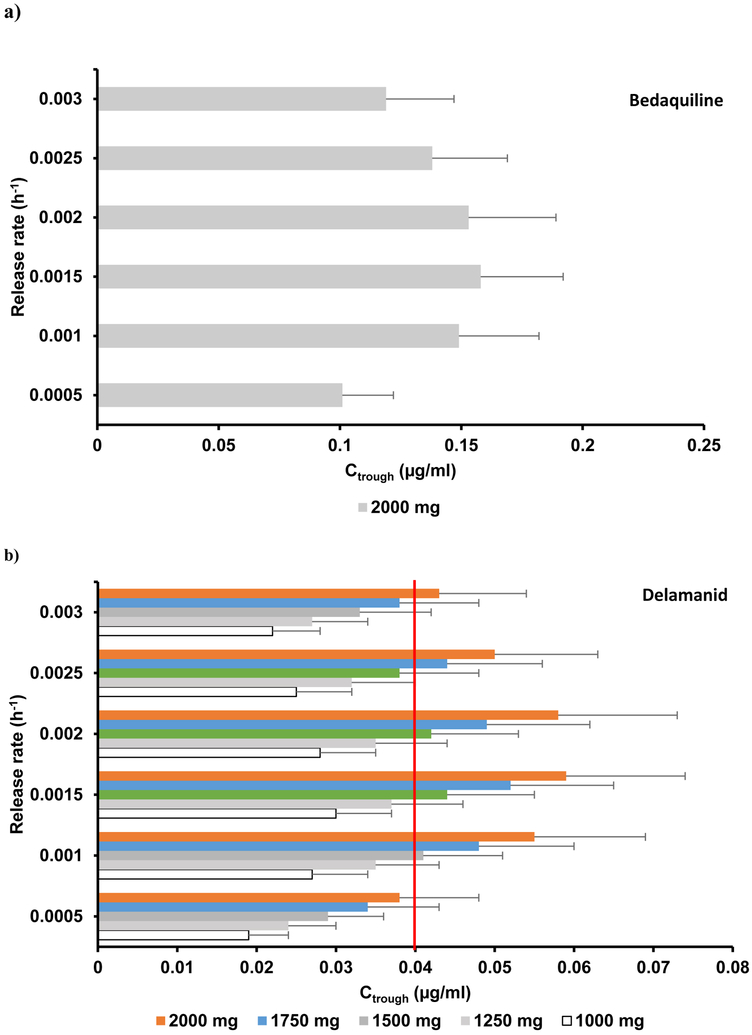
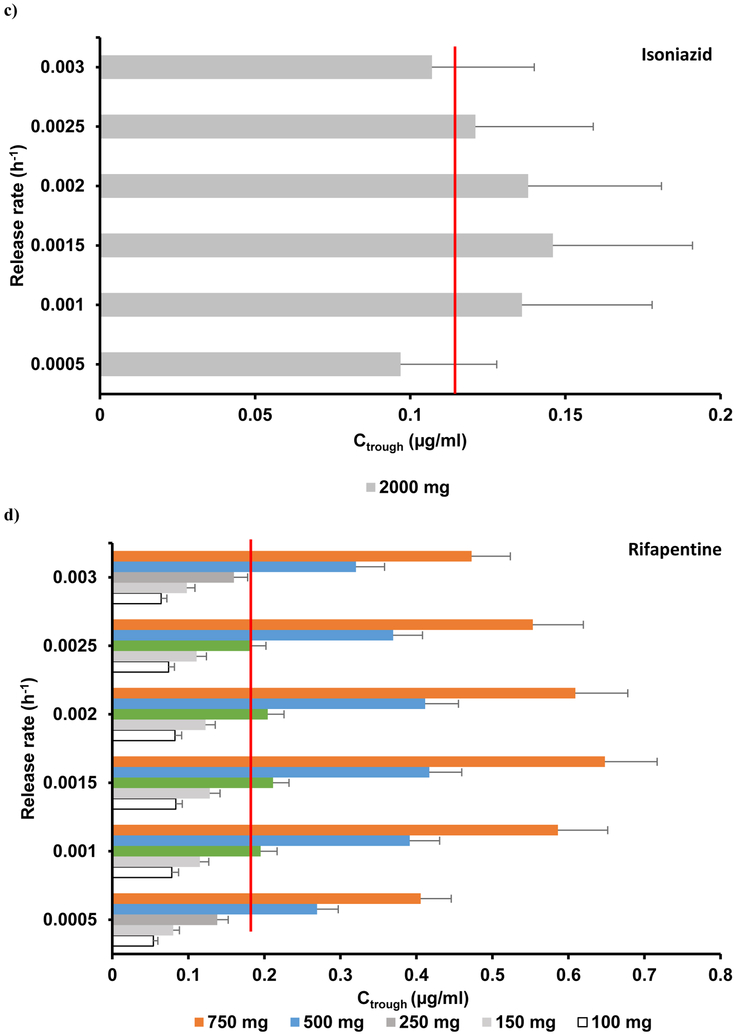
The simulated PK parameters AUC and Cmax deviated less than 18% from the mean reported values.14 A range of release rates were simulated for a single 2000 mg IM dose and the optimal release rate was identified as 0.0015 h−1 (Figure 1a). However, the maximum Ctrough obtained was 0.17 mg/L – approximately ten times below the ECOFF value (Figure 2a, Table 3).
Figure 1.
Plasma trough concentrations of a) bedaquiline, b) delamanid, c) isoniazid and d) rifapentine after the administration of a single intramuscular injection on day 28 for various doses and release rates in adults. The red line represents the target trough concentration. The target concentration for bedaquiline is beyond the indicated scale, therefore not visible on the chart.
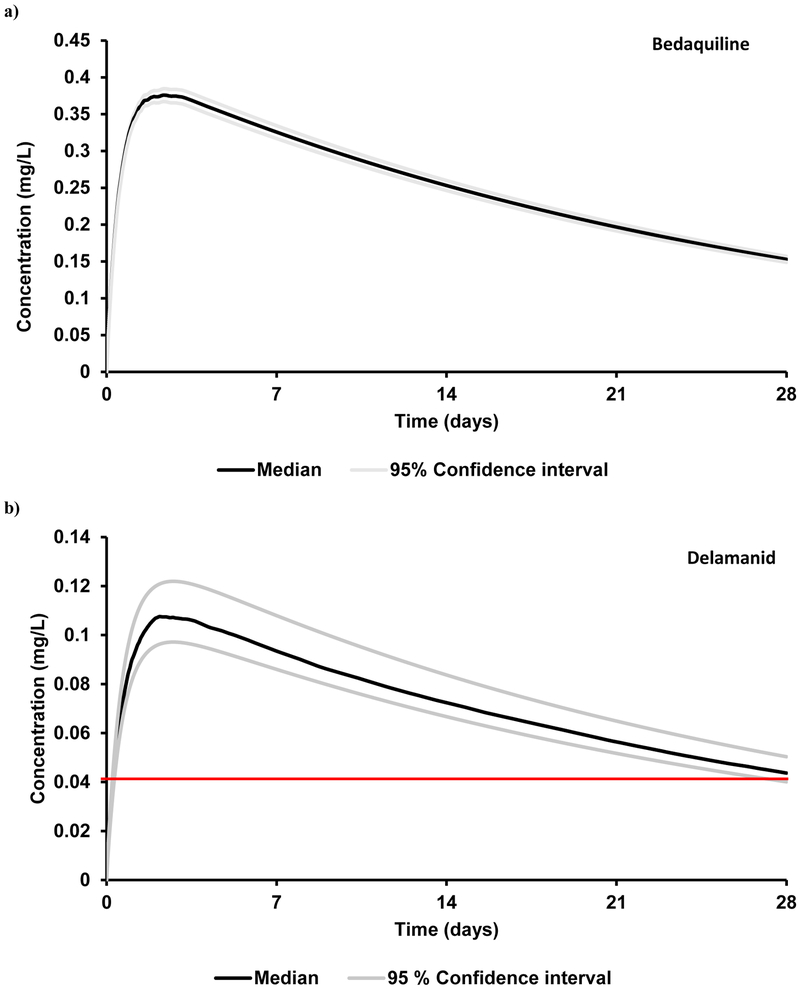
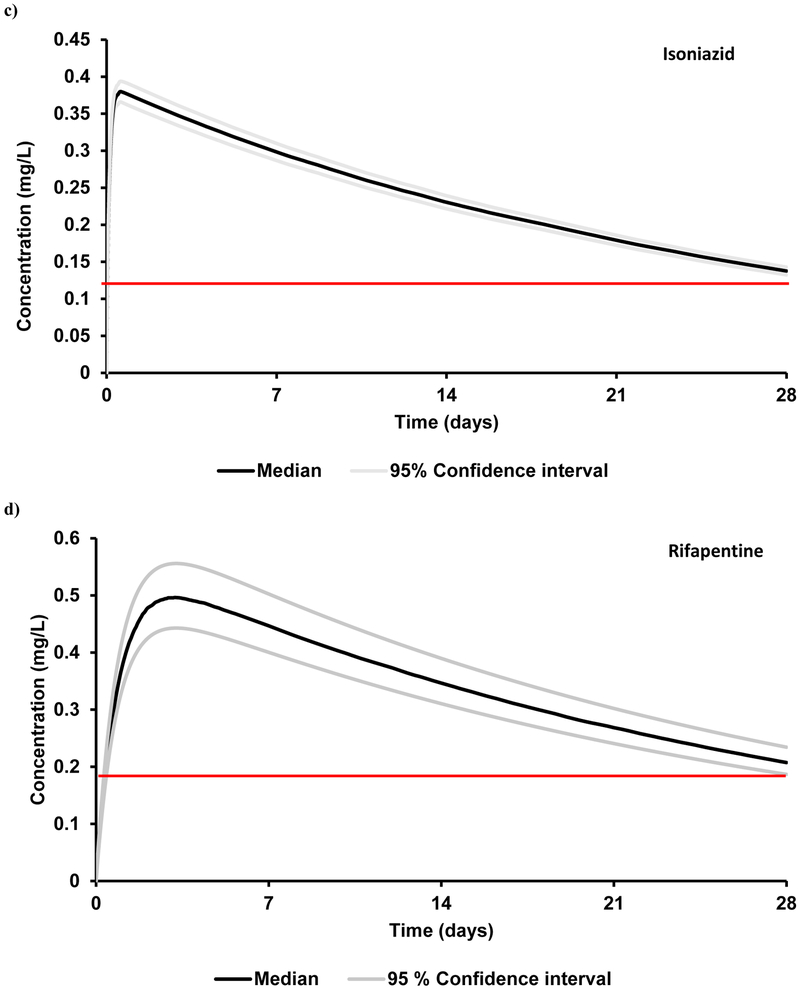
Figure 2.
Plasma concentrations of a single dose intramuscular long-acting anti-TB drugs with an optimal release rate of 0.0015 h−1 in healthy adults. a) bedaquiline (2000 mg), b) delamanid (1500 mg), c) isoniazid (2000 mg) and d) rifapentine (250 mg). The red line represents the target trough concentration. The target concentrations for bedaquiline is beyond the indicated scale, therefore not visible in the graph.
Table 3.
Pharmacokinetic prediction of a single intramuscular injection of anti-TB agents at an optimal release rate of 0.0015 h−1 using a qualified PBPK model.
| Drug | Dose | Cmax | Ctrough | AUC | Target concentration |
|---|---|---|---|---|---|
| Bedaquiline | 2000 | 0.39 ± 0.08 | 0.16 ± 0.03 | 177 ± 36 | 1.6 (ECOFF) 18 |
| Delamanid | 1500 | 0.11 ± 0.03 | 0.044 ± 0.011 | 50 ± 12 | 0.04 (ECOFF) 18 |
| Isoniazid | 2000 | 0.39 ± 0.13 | 0.15 ± 0.05 | 170 ± 56 | 0.125 (CC) 19 |
| Rifapentine | 250 | 0.50 ± 0.06 | 0.21 ± 0.02 | 232 ± 21 | 0.18 (MIC*3) 20 |
PBPK – physiologically-based pharmacokinetic, dose is represented in mg, Cmax – maximum plasma concentration, Ctrough – trough plasma concentration 4 weeks after administration, AUC – area under the plasma concentration curve. Cmax, Ctrough and target concentration are expressed as mg/L, AUC is expressed as mg.h/L; ECOFF – epidemiological cut-off, CC – critical concentration, MIC – minimum inhibitory concentration
3.2. Delamanid
The mean simulated AUC, Cmax and Ctrough values deviated from clinical data by less than 18%.15 Five different doses (1000 mg, 1250 mg, 1500 mg, 1750 mg and 2000 mg) were simulated, each with six different release rates ranging from 0.0005 – 0.003 h-1. Ctrough values obtained for various dose and release rate combinations are shown in Figure 1b. A Ctrough of 0.044 mg/L at the end of a 4-weekly single IM dose at a dose of 1500 mg with a release rate between 0.001 – 0.0025 h−1 was determined as the optimal condition (Figure 1b, Table 3). Therapeutic concentrations were obtained within 8 h after IM administration.
3.3. Isoniazid
The mean simulated AUC and Cmax deviated from clinical values by a maximum of 30%.16 Clearance of slow acetylators was used initially to evaluate LAI pharmacokinetics. An IM dose of 2000 mg was simulated at various release rates. The maximum target concentration at the end of 4 weeks was achieved with a release rate of 0.0015 h−1 and gave a Ctrough of 0.141 mg/L, with only 60 % of the simulated population over the CC (Figure 1c, 2c, Table 3) indicating that the monthly formulation of isoniazid is not feasible, although biweekly administration might be.
3.4. Rifapentine
The mean simulated AUC and Cmax deviated from clinical values by less than 31%.17 IM doses of 100 mg, 150 mg, 250 mg, 500 mg and 750 mg were simulated at varying release rates (Figure 1d). An optimal dose of 250 mg with a release rate between 0.0015 – 0.0025 h−1 resulted in a simulated Ctrough over the target trough concentration (Figure 2d, Table 3) with rifapentine plasma concentrations reaching targets within 10 h after IM administration.
4. DISCUSSION
The current study has evaluated the potential reformulation of existing oral anti-TB drugs – bedaquiline, delamanid, isoniazid and rifapentine – into 4-weekly IM LAI formulations using PBPK models. Bedaquiline and delamanid have potential utility for TB preventive therapy and isoniazid and rifapentine are currently approved for this indication.21 Potential use of these anti-TB agents as IM LAI formulations would be an attractive option for LTBI treatment.
For the 4-weekly IM LAI predictions, it was observed that the optimal release rate was 0.0015 h−1 for all the four investigated drugs. Delamanid and rifapentine were identified as potential LAI candidates with an optimal dose less than 2000 mg. For rifapentine, an arbitrary Ctrough, about three times higher than the MIC value, was chosen to include the inhibition of wild-type TB strains. Rifapentine is characterised by a combination of favourable ADME characteristics with low blood-to-plasma ratio (< 1), low plasma clearance and high potency (0.06 mg/L). The predictions report that only 250 mg is needed for a monthly LAI thus having the potential for a quarterly administration with less than 1000 mg. Delamanid is characterised by a lower ECOFF value (0.04 mg/L, 40 times lower than bedaquiline), high log P value and blood-to-plasma ratio and relatively low clearance, therefore having a more favourable profile for a monthly IM injection.
Bedaquiline is characterised by high octanol-water partition-coefficient (log P >5), high blood-to-plasma ratio (>1), high protein binding (>99%) and relatively low clearance, resulting in a long half-life that sustains higher plasma concentrations. However, the predicted Ctrough could not achieve the high ECOFF value, even when a 2000 mg IM dose was simulated. Loading dose prior to IM administration would nevertheless drop the plasma concentrations significantly and would require at least a weekly IM dose. Isoniazid plasma concentrations were below the high-level isoniazid resistance critical concentration in less than 15 days after an IM dose of 2000 mg (Figure 2). Nevertheless, the predicted Ctrough satisfied the defined CC of the low-level isoniazid resistance value of 0.0312 mg/L.19 Isoniazid is a water soluble hydrophilic compound with low log P (<0), low protein binding value (<50%) and a lower volume of distribution, resulting in a sharper PK profile following IM administration. Due to its high renal clearance, the resulting plasma concentrations are lower than the CC, suggesting that isoniazid is not a suitable candidate for LA strategies.
These simulated dose and release rates could represent a theoretical guideline in the design of IM LAI formulations. However the PBPK model is characterised by several limitations that could affect the overall drug PK profile. Action of drug transporters at the IM depot could not be captured due to the absence of relevant data. Active transport could have an effect on drug release from the IM depot, and subsequent diffusion into the systemic distribution could also affect the release profile. Evidence of granuloma formation at the injection site as observed for paliperidone LAI could alter drug PK.22 Highly lipophilic drugs concentrate in the lymphatic circulation preferentially to blood/plasma circulation, resulting delay attaining maximum plasma concentrations and potentially altering Ctrough.23 Also, MIC of anti-TB drugs ranges between the MIC of the lowest known resistant strains and the highest MIC of wild-type strains. This is the major limitation to identify appropriate target concentrations as it complicates the pharmacokinetic –pharmacodynamic relationship (i.e. AUC/MIC, Cmax /MIC or T>MIC).24 Standardised target concentrations are not currently available for the treatment of LTBI and are likely to be very different from active/MDR or XDR-TB. Consequently the identification of a suitable benchmark for the simulated pharmacokinetics for LA administration represent a substantial challenge. The selected approach aimed at achieving plasma concentrations > ECOFF (or 3 times the MIC) for the entire dosing interval. Higher exposure limits could be taken into consideration further limiting the applicability of LAI formulations for active TB treatment, however this approach is more likely to be of value in LTBI treatment where shorter and simplified therapies are needed for successful therapy.25 Additional investigation of the pharmacodynamics of anti-TB agents represents an essential element to support a better understanding of predictive parameters that correlates best with efficacy for LTBI treatment and the target exposures necessary for optimal response. An additional barrier for potential application of these regimens would be the administration of a combination of two anti-TB agents defining challenges in terms of co-formulation, volume of injection and patient acceptability.
The presented data represent a theoretical approach and the concept of LAI formulations would be plausible provided formulation and technological complexities are addressed. Providing self-administered IM injectables to infected individuals could help in TB treatment in resource poor settings. Initially, stability assessment of anti-TB drug and associated excipients in the interstitial fluid at body temperature for prolonged time period is essential. Isoniazid is reported to be highly unstable in plasma which further marks this drug unsuitable as an LAI formulation. The development of formulations with desirable characteristics could be a challenge with existing technology. Anti-TB drugs are associated with a number of side effects including nausea, vomiting, rash, peripheral neuropathy and hepatotoxicity that could be less serious during oral than with parenteral administration.26 High dose and prolonged contact of drug formulation at the site of injection could trigger unwanted adverse reactions and discontinuation of an IM injection during therapy could complicate administration. Drug-drug interactions are another important consideration, especially in patients co-infected with HIV and receiving antiretroviral therapy.27 IM LAIs could effectively address problems of suboptimal adherence and low completion rates and pave the way for innovative administration strategies in the treatment of LTBI.
5. CONCLUSION
PBPK models were successfully qualified against selected oral clinical data for four anti-TB drugs in adult population. These models were used for optimal dose and release rate prediction to inform suitable monthly IM doses for the chosen anti-TB drugs. Delamanid and rifapentine were suitable for a monthly administration however more frequent dosing would be necessary for bedaquiline and isoniazid to have plasma concentrations over the indicated target trough concentrations. Various drug specific characteristics influence pharmacokinetic profiles and in silico modelling approach could effectively inform drug formulation design for various routes of administration and also identify novel drugs for anti-TB therapy.
Supplementary Material
Acknowledgements:
Source of Funding:
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number R24 AI 118397.
Conflicts of Interest:
Rajith KR Rajoli, Anthony T. Podany and Darren M Moss have no conflicts of interest to declare. Susan Swindells reports research grants to her institution from ViiV and Merck. Charles Flexner reports serving as a consultant for GlaxoSmithKline, Merck Laboratories, Mylan Pharmaceuticals, and ViiV Healthcare. Andrew Owen has received research funding from ViiV Healthcare, Merck, and Janssen, has conducted consultancy for Merck, is a co-inventor of patents relating to drug delivery, and is a founder of Tandem Nano Ltd. Marco Siccardi has received research funding from ViiV and Janssen.
REFERENCES
- 1.World Health Organisation. Tuberculosis: Fact sheet 2016. Available from: http://www.who.int/mediacentre/factsheets/fs104/en/.
- 2.CDC. Tuberculosis prevention 2017. [25/04/2017]. Available from: https://www.cdc.gov/tb/topic/basics/tbprevention.htm.
- 3.Luetkemeyer AF. Current Issues in the Diagnosis and Management of Tuberculosis and HIV Coinfection in the United States. Topics in Antiviral Medicine. 2010;18(4):143–8. [PubMed] [Google Scholar]
- 4.CDC. Treatment Regimens for Latent TB Infection (LTBI) 2017. [25/04/2017]. Available from: https://www.cdc.gov/tb/topic/treatment/ltbi.htm.
- 5.TBFACTS.ORG. TB & HIV – Co-infection, statistics, diagnosis & treatment 2017. [24/04/2017)]. Available from: http://www.tbfacts.org/tb-hiv/.
- 6.Hirsch-Moverman Y, Daftary A, Franks J, Colson PW. Adherence to treatment for latent tuberculosis infection: systematic review of studies in the US and Canada [Review article]. The International Journal of Tuberculosis and Lung Disease. 2008;12(11):1235–54. [PubMed] [Google Scholar]
- 7.World Health Organisation. Tuberculosis: DOTS treatment success. 2006. Available from: http://www.who.int/whosis/whostat2006TuberculosisDOTSTreatmentSuccess.pdf.
- 8.Dye C, Glaziou P, Floyd K, Raviglione M. Prospects for Tuberculosis Elimination In: Fielding JE, editor. Annual Review of Public Health, Vol 34 Annual Review of Public Health. 342013. p. 271–86. [DOI] [PubMed] [Google Scholar]
- 9.Thiam S, LeFevre AM, Hane F, et al. Effectiveness of a strategy to improve adherence to tuberculosis treatment in a resource-poor setting: A cluster randomized controlled trial. JAMA. 2007;297(4):380–6. [DOI] [PubMed] [Google Scholar]
- 10.Gordin FM. Rifapentine for the treatment of tuberculosis - Is it all it can be? American Journal of Respiratory and Critical Care Medicine. 2004;169(11):1176–7. [DOI] [PubMed] [Google Scholar]
- 11.Owen A, Rannard S. Strengths, weaknesses, opportunities and challenges for long acting injectable therapies: Insights for applications in HIV therapy. Advanced Drug Delivery Reviews. 2016;103:144–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siccardi M, Rajoli RKR, Curley P, Olagunju A, Moss D, Owen A. Physiologically based pharmacokinetic models for the optimization of antiretroviral therapy: recent progress and future perspective. Future Virol 2013;8(9):871–90. [Google Scholar]
- 13.Rajoli RKR, Back DJ, Rannard S, Freel Meyers CL, Flexner C, Owen A, et al. Physiologically Based Pharmacokinetic Modelling to Inform Development of Intramuscular Long-Acting Nanoformulations for HIV. Clinical Pharmacokinetics. 2015;54(6):639–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Heeswijk RPG, Dannemann B, Hoetelmans RMW. Bedaquiline: a review of human pharmacokinetics and drug–drug interactions. Journal of Antimicrobial Chemotherapy. 2014;69(9):2310–8. [DOI] [PubMed] [Google Scholar]
- 15.EMA. Assessment report : Deltyba 2014. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002552/WC500166234.pdf.
- 16.Kubota R, Ohno M, Hasunuma T, Iijima H, Azuma J. Dose-escalation study of isoniazid in healthy volunteers with the rapid acetylator genotype of arylamine N-acetyltransferase 2. European Journal of Clinical Pharmacology. 2007;63(10):927–33. [DOI] [PubMed] [Google Scholar]
- 17.Dooley KE, Bliven-Sizemore EE, Weiner M, Lu Y, Nuermberger EL, Hubbard WC, et al. Safety and Pharmacokinetics of Escalating Daily Doses of the Antituberculosis Drug Rifapentine in Healthy Volunteers. Clinical Pharmacology & Therapeutics. 2012;91(5):881–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keller PM, Hömke R, Ritter C, Valsesia G, Bloemberg GV, Böttger EC. Determination of MIC Distribution and Epidemiological Cutoff Values for Bedaquiline and Delamanid in Mycobacterium tuberculosis Using the MGIT 960 System Equipped with TB eXiST. Antimicrobial Agents and Chemotherapy. 2015;59(7):4352–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gumbo T New Susceptibility Breakpoints for First-Line Antituberculosis Drugs Based on Antimicrobial Pharmacokinetic/Pharmacodynamic Science and Population Pharmacokinetic Variability. Antimicrobial Agents and Chemotherapy. 2010;54(4):1484–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dickinson JM, Mitchison DA. In vitro properties of rifapentine (MDL473) relevant to its use in intermittent chemotherapy of tuberculosis. Tubercle. 1987;68(2):113–8. [DOI] [PubMed] [Google Scholar]
- 21.CDC. Latent Tuberculosis Infection: A Guide for Primary Health Care Providers 2017. [23/03/2017)]. Available from: https://www.cdc.gov/tb/publications/ltbi/treatment.htm.
- 22.Darville N, van Heerden M, Vynckier A, De Meulder M, Sterkens P, Annaert P, et al. Intramuscular Administration of Paliperidone Palmitate Extended-Release Injectable Microsuspension Induces a Subclinical Inflammatory Reaction Modulating the Pharmacokinetics in Rats. Journal of Pharmaceutical Sciences. 2014;103(7):2072–87. [DOI] [PubMed] [Google Scholar]
- 23.Tegenge M, Mitkus R. A physiologically-based pharmacokinetic (PBPK) model of squalene-containing adjuvant in human vaccines. J Pharmacokinet Pharmacodyn. 2013;40(5):545–56. [DOI] [PubMed] [Google Scholar]
- 24.Kim SJ. Drug-susceptibility testing in tuberculosis: methods and reliability of results. European Respiratory Journal. 2005;25(3):564–9. [DOI] [PubMed] [Google Scholar]
- 25.Susan Swindells RR, Gupta Amita, Benson Constance A., Leon-Cruz Jorge T., Omoz-Oarhe Ayotunde, Marc Antoine Jean Juste, Lama Javier R., Valencia Javier A., Badal-Faesen Sharlaa, Moran Laura E., Fletcher Courtney V., Nuermberger Eric, Chaisson Richard E., editor One Month of Rifapentine/Isoniazid to Prevent TB in People With HIV: BRIEF-TB/A5279 Conference of retroviruses and opportunistic infections; 2018 March 4–7 2018; Boston. [Google Scholar]
- 26.Nahid P, Dorman SE, Alipanah N, Barry PM, Brozek JL, Cattamanchi A, et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice Guidelines: Treatment of Drug-Susceptible Tuberculosis. Clinical Infectious Diseases. 2016;63(7):e147–e95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.U.S. Department of Health and Human Services. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents 2016. [12/06/2017)]. Available from: https://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-treatment-guidelines/0/.
- 28.DrugBank. Bedaquiline 2017. [31/01/2017)]. Available from: https://www.drugbank.ca/drugs/DB08903.
- 29.ChEMBL. Compound report card - CHEMBL218650 2017. [02/02/2017)]. Available from: https://www.ebi.ac.uk/chembl/compound/inspect/CHEMBL218650.
- 30.DrugBank. Isoniazid 2017. [31/01/2017)]. Available from: https://www.drugbank.ca/drugs/DB00951.
- 31.DrugBank. Rifapentine 2017. [31/01/2017)]. Available from: https://www.drugbank.ca/drugs/DB01201.
- 32.Reith K, Keung A, Toren PC, Cheng L, Eller MG, Weir SJ. Disposition and Metabolism of 14C-Rifapentine in Healthy Volunteers. Drug Metabolism and Disposition. 1998;26(8):732–8. [PubMed] [Google Scholar]
- 33.Patterson S, Wyllie S, Norval S, Stojanovski L, Simeons FRC, Auer JL, et al. The anti-tubercular drug delamanid as a potential oral treatment for visceral leishmaniasis. eLife. 2016;5:e09744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McLeay SC, Vis P, van Heeswijk RPG, Green B. Population Pharmacokinetics of Bedaquiline (TMC207), a Novel Antituberculosis Drug. Antimicrobial Agents and Chemotherapy. 2014;58(9):5315–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kinzig-Schippers M, Tomalik-Scharte D, Jetter A, Scheidel B, Jakob V, Rodamer M, et al. Should We Use N-Acetyltransferase Type 2 Genotyping To Personalize Isoniazid Doses? Antimicrobial Agents and Chemotherapy. 2005;49(5):1733–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Svensson EM, Dosne AG, Karlsson MO. Population Pharmacokinetics of Bedaquiline and Metabolite M2 in Patients With Drug-Resistant Tuberculosis: The Effect of Time-Varying Weight and Albumin. CPT: Pharmacometrics & Systems Pharmacology. 2016;5(12):682–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lakshminarayana SB, Huat TB, Ho PC, Manjunatha UH, Dartois V, Dick T, et al. Comprehensive physicochemical, pharmacokinetic and activity profiling of anti-TB agents. Journal of Antimicrobial Chemotherapy. 2014;70(3):857–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seng K-Y, Hee K-H, Soon G-H, Chew N, Khoo SH, Lee LS-U. Population Pharmacokinetic Analysis of Isoniazid, Acetylisoniazid, and Isonicotinic Acid in Healthy Volunteers. Antimicrobial Agents and Chemotherapy. 2015;59(11):6791–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanofi-Aventis U.S. LLC. Priftin® (rifapentine) tablets [prescribing information]. Bridgewater, NJ: 1998. Available from: http://products.sanofi.us/priftin/priftin.pdf. [Google Scholar]
- 40.Paixão P, Gouveia LF, Morais JAG. Prediction of drug distribution within blood. European Journal of Pharmaceutical Sciences. 2009;36(4–5):544–54. [DOI] [PubMed] [Google Scholar]
- 41.Gertz M, Harrison A, Houston JB, Galetin A. Prediction of Human Intestinal First-Pass Metabolism of 25 CYP3A Substrates from In Vitro Clearance and Permeability Data. Drug metabolism and disposition: the biological fate of chemicals. 2010;38(7):1147–58. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.