Abstract
In light of the limited efficacy of current treatments for cardiac regeneration, tissue engineering approaches have been explored for their potential to provide mechanical support to injured cardiac tissues, deliver cardio-protective molecules, and improve cell-based therapeutic techniques. Injectable hydrogels are a particularly appealing system as they hold promise as a minimally invasive therapeutic approach. Moreover, injectable acellular alginate-based hydrogels have been tested clinically in patients with myocardial infarction (MI) and show preservation of the left ventricular (LV) indices and left ventricular ejection fraction (LVEF). Thus, avoiding adverse remodeling post MI. In this review, we provide an overview of recent developments that have occurred in the design and engineering of various injectable hydrogel systems for cardiac tissue engineering efforts, including a comparison of natural vs synthetic systems with emphasis on the ideal characteristics for biomimetic cardiac materials.
Keywords: injectable hydrogels, cardiac tissue engineering, hydrogels, cardiac regeneration
Graphical Abstract
Injectable hydrogels for cardiac tissue engineering can be used for in vitro models, in vivo preclinical purposes and for clinical trials.
1. Introduction
Heart failure (HF) affects around 6.5 million individuals over the age of 20 in the United States alone1, and a limited availability of donor hearts presents a significant hurdle for cardiac transplantation, which is currently the only definitive option for end-stage HF2–4. HF can be caused by myocardial infarction (MI)5,6, which occurs when a blocked coronary artery decreases or ceases blood flow to part of the heart, causing decreased oxygen supply to the heart muscle tissue and eventually necrosis7–9. The infarcted region initially becomes particularly damaged by the ischemic event, then post MI, the surrounding heart wall becomes thinner, leading to ventricular dilation and progression towards HF10–12. During HF, cardiomyocyte (CM) loss, cardiac matrix degradation, and fibrosis make the heart muscle unable to efficiently pump blood to the rest of the body13–16. HF is associated with poor quality of life, high healthcare costs, and a high mortality rate17–20. According to the American Heart Association, in recent years (2005–2013) there have been approximately 960,000 new cases annually in the United States, highlighting an urgent need for novel treatment approaches1.
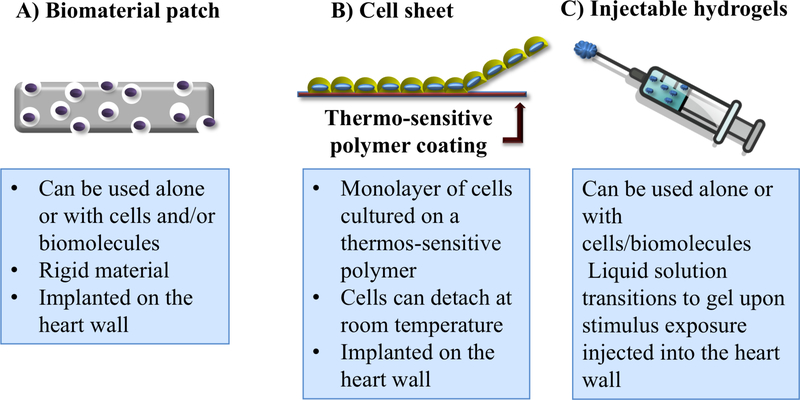
Due to the limited supply of donor hearts21–23 and the poor regenerative ability of the myocardium24–27, investigators have turned to therapeutic approaches aimed at improving myocardial function. Such approaches that have been explored include the direct injection of biomolecules28,29, chemokines30,31, and CMs32–34 for treating HF. However, each of these techniques face significant hurdles that have yet to be overcome, namely short biomolecule half-life in vivo, nonspecific cell/molecule delivery, low cell survival, and poor localization to the target area. Cardiac tissue engineering efforts have sought to overcome these limitations by utilizing biomaterials that can protect deliverables from degradation, improve targeted delivery, and increase cell viability35–37. These biomaterials may be composed of synthetic or natural materials, or may be a hybrid of the two. In addition, the biomaterials may be prepared and delivered in many formats, the primary ones most explored for cardiac tissue engineering efforts being injectable hydrogels38,39, patches40,41, and cell sheets42,43 (Figure 1). Injectable hydrogels utilize polymeric biomaterials that undergo a solution to gel phase transition and may incorporate embedded cells and/or active compounds. Patches are porous solid polymeric matrices which may contain cells and/or biomolecules attached to the biomaterial. Cell sheets are typically cell monolayers cultured on tissue culture plates coated with a temperaturesensitive material that enables cell detachment at room temperature. The various properties of each system should not only ideally improve the delivery and functionality of cells/molecules, but also provide mechanical support for damaged and weakened cardiac tissue44,45. In addition, biomaterials ideal for cardiac regeneration should be biomimetic, providing biological, mechanical, electrical, and chemical cues similar to those found in the native myocardium. Furthermore, the material should be biocompatible to help overcome immune rejection, potentially biodegradable, and able to be delivered through a minimally invasive procedure to reduce any additional damage incurred through surgical implantation. In some circumstances, it may be desirable to deliver material in a minimal invasive way to a specific localized area of the heart tissue, such as the infarcted region in individuals with cardiac infarcts46. Although patch-based and cell sheet systems have been widely studied and present promising results for cardiac tissue engineering, they require a more invasive surgery intervention. These procedures may be more complicated to translate into clinical applications in which minimally invasive procedures are more preferable47. Because injectable hydrogels can be deployed into the myocardium through minimal invasive approaches, such as catheter delivery, they are particularly appealing for cardiac regeneration. Moreover, they possess many of the criteria required for cardiac tissue engineering such as high biocompatibility, tunable physical and chemical properties and most important, they can be deployed in a minimally invasive manner 9, therefore they will be the focus of this review.
Figure 1.
Cardiac tissue engineering approaches. A) Biomaterial patches are scaffolds that can be implanted in the wall heart alone or in combination with cells and/or biomolecules. B) Cell sheets contain cell monolayers cultured on a thermos-sensitive hydrogel, poly(isopropylacrylamide) (PNIPAAm). C) Injectable hydrogels are polymeric liquid solutions that become gel after exposure to an external stimulus.
This review provides an overview of recent advances and hurdles that remain to be overcome in the biomaterial design and engineering of various injectable hydrogel systems, with emphasis placed on systems being used in cardiac tissue engineering efforts. The articles that comprise the focus of this review are shown in Table 148–75.
Table 1.
Natural and Synthetic materials used in injectable hydrogel systems for cardiac engineering.
| Material | Application | Ref. |
|---|---|---|
| Natural Materials | ||
| CNT dispersed in collagen type I hydrogel | Conductive hydrogel tested in vitro using (NRVM) | 48 |
| Porcine ECM cross-linked with genipin | Porcine ECM promoted endothelial differentiation of human MSCs. | 49 |
| Fullerenol/alginate | Antioxidant fullerenol was dispersed in alginate hydrogel and tested in vitro using BADSCs and in vivo in a MI rat model as BADSCs delivery. | 50 |
| Porcine ECM | Tested in vivo in a MI mini-pig model | 53 |
| Porcine ECM with mixed or conjugated doxycycline | Tested in vivo in a myocardium injection rat model. | 52 |
| Porcine ECM cross-linked with genipin or with chitosan | Tested in vitro using hMSCs and in vivo in a mouse subcutaneous injection model. | 53 |
| Type I collagen | Timing injection tested in vivo in a mouse MI model. | 54 |
| Decellularized cardiac and skeletal muscle ECM | Material characterization with potential to be used in MI | 55 |
| Chitosan chloride-RoY | Tested in vitro using HUVECs and in vivo in a rat MI model to promote angiogenesis | 56 |
| Fibrin gel with embedded GF and TIMP-3 | Tested in vivo in a Rat MI model as protein and cytokined delivery system | 57 |
| Chitosan gel with mixed gold nanoparticles | Tested in vitro using MSCs | 58 |
| pH sensitive chitosan hydrogel | Tested in vitro using hBMSCs and human hAMSCs | 59 |
| Synthetic Materials | ||
| CS-AT-di-benzaldehyde-terminatedPEG-DA | Conductive and antibacterial self-healing hydrogel tested in vitro and in vivo using murine myoblast cell lines. | 60 |
| PNIPAM-mPEGMA-MDO-MATA or PN-TA | Antioxidant and electrical hydrogel tested in vitro with myoblasts and subcutaneously in vivo. | 61 |
| PSHU-PNIPAAm-co-poly(L-lysine)CNT | Conductive hydrogel tested in vitro using NRVMs. | 62 |
| PEGylated silk fibroin functionalized with protein microspheres | Cross-linked hydrogel tested in vitro using human cardiac MSCs. | 63 |
| GelMA | Visible light cross-linkable hydrogel tested in vitro using primary rat CMs, ex vivo and in vivo using a MI rat model. | 64 |
| PEG hydrogel EPO | Tested in vivo in a rat MI model as iPSC-CM delivery system. | 65 |
| CTA-PLGA-PEG-PLGA-CTA | Conductive hydrogel tested in vitro using rat myoblast cell line and in vivo using rat subcutaneous injection. | 66 |
| PNIPAAm-co-HEMA-co-AOLA with MMP2 inhibitor | MMP2 inhibitor delivery system tested in vivo in MI rat model. | 67 |
| PNIPAAm-AA-HEMA-PCL functionalized with type I collagen | Thermosensitive hydrogel tested in vivo in a MI mouse model as a mouse MSCs delivery system. | 68 |
| PEGylated Fibrin | Tested in vitro using human CMs. | 69 |
| Single-wall CNT-PNIPAAm | Tested in vitro using ADSCs and in vivo using a MI rat model for delivery of ADSCs. | 70 |
| PNIPAAm-gelatin | Tested in vitro using NRVMs. | 71 |
| dex-PCL-HEMA-NIPAAm | Tested in vivo using MI rat model for delivery of ACE-shRNA. | 72 |
| PSHU-PNIPAAm-lysine | Tested in vitro using NRVMs. | 73 |
| Sulfonated PSHU-PNIPAAm | Tested in vitro for protein delivery. | 74 |
| PEGDMA-GelMA | Tested in vitro for muscle fiber formation | 75 |
Abbreviations: carbon nanotubes: CNT; neonatal rat ventricular cardiomyocytes: NRVMs; extracellular matrix: ECM; adipose-derived stem cells: ADSCs; mesenchymal stem cells: MSCs; human umbilical vein endothelial cells: HUVECs; growth factors: GFs; tissue inhibitor of metalloproteinases-3: TIMP-3; Growth factors: GF; human bone marrow MSC: hBMSCs; human adipose MSC (hAMSCs). chitosan-graft-aniline tetramer: CS-AT; polyethylene glycol: PEG; poly(N-isopropyl acrylamide-co-acrylamide): PNIPAM; methylene 1,3-dioxepane: MDO; N-isopropyl acrylamide: NIPAAm; methoxy PEG methacrylate: mPEGMA; methacrylic-tetraaniline: MATA; carbon nanotube: CNTs; poly(serinol hexamethylene urea)co-poly(N-isopropylacrylamide): PSHU-PNIPAAm; gelatin methacryloyl: GelMA; encapsulation erythropoietin: EPO; carboxyl tetra-aniline: CTA; poly(D,L-lactic acid-coglycolic acid): PLGA; hydroxyethyl methacrylate: HEMA; D,L-lactide oligolactide: AOLA; matrix metalloproteinase-2: MMP2; acrylic acid: AA; poly(ε-caprolactone): PCL; dextran: dex; neonatal rat ventricular cardiomyocytes: NRVMs; mesenchymal stem cells: MSCs; cardiomyocytes: CMs; induced pluripotent stem cell-derived CMs: iPSC-CMs; adipose-derived stem cells: ADSCs; short-hairpin RNA of angiotensin converting enzyme: ACE-shRNA; PEGDMA: PEG-dimethacrylate.
2. Overview of Injectable hydrogels for cardiac tissue engineering
Injectable hydrogels can assemble into a three-dimensional polymeric network with a highwater content. They have been used extensively as scaffolds for tissue engineering approaches or as delivery systems for therapeutic agents and cells. Injectable hydrogels function well in biological systems in general, owing to their high permeability, biodegradability, and biocompatibility, and have already been tested in two clinical trials. In one clinical pilot study, injectable acellular alginate-based hydrogels were tested in 27 patients with MI. All patients were treated with the hydrogel and all demonstrated tolerability of the procedure, with no adverse events. Moreover, echocardiographic results demonstrated that the left ventricular (LV) indices and the LVEF were preserved. There results make injectable hydrogels promising materials for such cardiac regeneration therapies76. However, a recent study by Anker et al. reported that in a large clinical trial using alginate hydrogels injected into the LV heart muscle of patients with advanced HF, while the treatment improved exercise capacity and mitigated HF symptoms, 8.6% of patients who received the hydrogel injection died within 30 days post injection. During this same time, no fatalities occurred within the control group (which received no surgery)77. These results suggest that further studies and additional efforts to improve biocompatible hydrogels and explore other possible issues to tolerance are still need.
In regards to compatibility specifically with the cardiac environment, injectable hydrogels are ideal as they can be altered to provide specific physical, chemical, and electrical properties, the latter of which may be important for supporting the conductive properties of the heart78–80. Their ability to provide structural support with varying stiffness may also enable compatibility with the contractions continually produced by the cardiac muscle38, an activity that makes it a particularly challenging tissue to physically repair. Hydrogels injected directly into damaged cardiac tissue can serve as a potential delivery vehicle for cells, growth factors, and therapeutic peptides or drugs, etc.55,56. Hydrogels may also be used to support various gene delivery systems, including viral and non-viral methods, enabling controlled delivery to the desired site and efficient localized therapy83,84. Such deliverables could be specifically sequestered to the target tissue, reducing nonspecific spreading to other nearby tissues.
While considerable efforts and accomplishments have been made recently by the scientific community in developing injectable hydrogels ideal for cardiac regeneration, progress remains to be made for these biomaterials to be fully optimized for regular clinical use. In particular, injectable hydrogels should ideally be designed to closely resemble cardiac muscle cues and possess properties that enable them to support cell viability and/or maintain biomolecule activity even in the harsh, damaged localized tissue environments that exist post MI85,86. Utilizing high degrees of biocompatibility, particularly including cues supportive of cell/biomolecule integration into the cardiac tissue87, and controlled degradation, so that the hydrogel may initially be supportive of new cell/biomolecule engraftment but may then later harmlessly degrade to leave behind no foreign materials within the body that could potentially trigger an autoimmune response88,89, may be essential for the optimization of such injectable hydrogels. In addition, tight control over the gelation properties and process is also important for these hydrogels to be successful90–92; it may be ideal for them to remain in a liquid state while inside a catheter to enable a smooth deployment, and then transition to a gel state only seconds after injection into the target area to support rapid integration of the hydrogel93 (with or without embedded cells/biomolecules) into the tissue. Being able to rapidly integrate into the tissue is especially important for cardiac muscle tissue as it is continually contracting and thus effectively in motion.
3. Natural Vs. Synthetic Material Considerations
Injectable scaffolds for cardiac engineering can be fashioned from natural materials, synthetic materials, or a hybrid of the two38. These different groups of materials offer their own unique set of advantages and disadvantages that will be discussed in this section, with emphasis on their relevance for designing and fabricating scaffold systems for cardiac tissue engineering.
3.1. Natural Polymers
Natural polymers have proven advantageous for tissue engineering applications as they preserve their biochemical and biological properties, increasing their biocompatibility with the host tissue94,95. Some common natural polymers used for cardiac tissue engineering include collagen, gelatin, laminin, Matrigel, hyaluronic acid (hyaluronan), alginate, and chitosan96. These natural materials are composed of proteins, and/or polysaccharides, which allow water absorption and swelling97. This absorptive property enables these materials to diffuse nutrients and waste easily through the scaffold, thus improving cell survival and cell motility into the surrounding tissue98. However, potential drawbacks for these materials include immune response complications, rapid degradation, long gelation times, poor mechanical properties, insufficient electrical conductivity, and lack of inherent antioxidant properties99,100. These disadvantages, and the aforementioned advantages, will be explored in the following subsections.
3.1.1. Collagen Polymers
Collagen IV forms a major protein component of the native cardiac EM, with other highly expressed ECM proteins including certain laminins and vitronectin101. Collagen-based hydrogels are biodegradable, easily available and versatile. Moreover, they exhibit great tissue compatibility and promote cell attachment and survival when used with CMs. Collagen-based gels can be obtained by either decellularized methods, preserving the original structure of the tissue, or by extraction, in which the obtained product is further conjugated with other materials. However, since collagen is a protein, its structure and stability can be easily compromised when exposed to a high temperature or different kinds of irradiation. For example, they cannot be autoclaved or exposed to low gamma or beta irradiation without altering its molecular structure which may result in the loss of mechanical and enzymatic resistance102. However, despite these drawbacks, the use of collagen-based hydrogels has been broadly studied both in vivo and in vitro. Blackburn et al.54 demonstrated that collagen hydrogels can stimulate the myocardial cytokine profile, promoting angiogenesis and reducing fibrosis and cell death in the injured cardiac tissue of a MI mice model. They also found that the injection of collagen into injured cardiac tissue leads to better cardio-protective effects when it is administrated relatively soon after the onset of ischemia and inflammation. However, while collagen hydrogels are highly biocompatible and widely used for tissue engineering approaches, they have weak mechanical103,104 and electrical properties which are key properties for cardiac tissue engineering that will be further discussed in sections 4.3 and 4.4 respectively. To overcome these limitations, Sun et al.48 tested the addition carbon nanotubes (CNTs) to the collagen matrix. CNTs were chosen due to their ability support electrical conductivity and increase mechanical stiffness105,106. They found that the incorporation of CNTs significantly increased the stiffness of the hydrogels. The stiffness of collagen alone was 13 kilopascals (kPa), whereas collagen with incorporated CNT hydrogels at different concentrations (0.5, 1.5 and 2% w/w) was 21, 24 and 28.8 kPa, (shear modulus of normal and infarcted myocardium is 6 kPa and 18 kPa respectably). In addition, the electrical properties were also increased in the collagen/CNT hydrogels, with the 2% w/w collagen/CNT hydrogel being the most conductive (~600 millisiemens/meter [mS/m]) and the 0.5% w/w collagen/CNT hydrogel being the least conductive (~400 mS/m) of the CNT hydrogels. These values are actually greater than those found in the native myocardium, which can vary from 5 to 160 mS/m107, and consequently such materials being more conductive than the cardiac tissue may be detrimental. Moreover, they also found that the incorporation of CNTs into collagen hydrogels promotes cell alignment and improves cell function when tested in in vitro studies using neonatal rat ventricular myocytes (NRVMs).
3.1.2. Fibrin Polymers
Similar to collagen, fibrin is another natural polymer that has been extensively employed for cardiac cell encapsulation and cardiac tissue engineering efforts108. Fibrin is produced from the rapid polymerization of fibrinogen monomers and the proteolytic enzyme thrombin109. The ratio of these two components can be modulated to vary the gelation rate and mechanical properties of the fibrin scaffold. An advantage of fibrin for use in cardiac tissue engineering is that it is extremely elastic110, enabling for increased deformation that is needed in the heart. In addition, fibrin naturally contains ligands for cell adhesion that will improve survival of transplanted cells108. Moreover, fibrin-based hydrogels present a low inflammatory response and foreign body reaction and can be absorbed during the normal wound healing process. Although fibrin-based gels have great potential for tissue engineering, they present poor mechanical properties and have a tendency to shrink, which could be problematic when injected into the heart tissue108. Despite these problems, fibrin-based hydrogels have been widely used for cardiac tissue engineering approaches111–113. For example, Zhang. et al.111 injected rat adipose derived stem cells (rADSC) in a rat MI model. They found that the LV end diastolic diameter (EDD), the LV end-systolic diameter, the ejection fraction and fraction of shortening were improved in the fibrin + ADSC group compared with controls (fibrin, ADSC and sham PBS). In another study, Christman et al.112 found that the injection of rat skeletal myoblast into the infarct area on a rat MI improved cell transplantation survival and decreased the infarct size when compared with controls (injection of bovine serum albumin (BSA) fibrin gel, and skeletal myoblast in BSA). Ryu et al.113 found that injecting fibrin gel with bone marrow mononuclear cells (BMMNCs) in a rat MI model, induced by cryoinjury, promoted cell survival and enhanced neovascularization when compared with control groups: injection of BMMNCs in media and injection of media. In this regard, the microvessel density and the internal diameter of the microvessels was significantly larger in the fibrin group. In a most recent study, fibrin hydrogels were utilized in MI rat models to promote the controlled delivery of growth factors (GFs) (fibroblast growth factor [FGF]-2 and stromal cell-derived factor [SDF]-1a) as well as tissue inhibitor of metalloproteinases-3 (TIMP3) 57. To stabilize and control the delivery of the GFs, the hydrogels were incorporated into aggregates with a synthetic poly(ethylene argininylaspartate diglyceride) (PEAD) polymer and heparin. The GF/PEAD/heparin aggregates and MMP inhibitor were incorporated together within the fibrin matrix. This investigation found that the controlled release of GFs and MMP inhibitor reduced ventricular dilatation, inflammation, fibrosis, and ECM degradation in the MI rat model. Although this demonstrated positive results for cardiac repair, it is well known that fibrin exhibits weak stiffness114 and slow gelation time115, making it difficult for the matrix to retain encapsulated cells or active molecules upon injection into heart tissue. The use of the PEAD polymer likely helped prolong the benefits of the supplied GFs, while the fast release of the MMP inhibitor helped to prevent cardiac ECM degradation normally caused by MMPs. Therefore, while fibrin alone does not possess characteristics ideal for cardiac tissue engineering, however this polymer can serve as the basis for innovative designs that are more supportive and lead to improved cardiac regeneration.
3.1.3. Decellularized ECM Materials
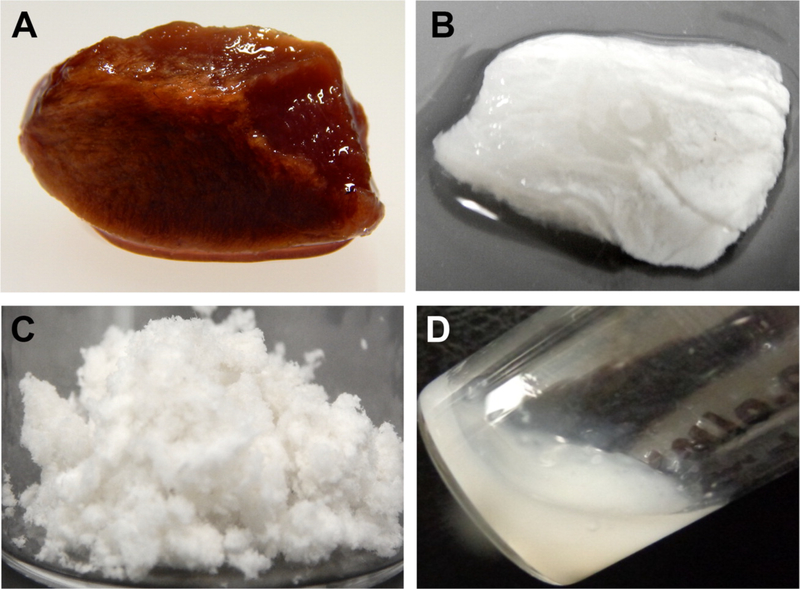
Scaffolds derived from decellularized materials have gained substantial attention in the cardiac engineering field due to their ability to more closely mimic the biophysical and topographical properties of the native ECM45. Most ECM scaffolds are prepared through the decellularization of native tissues, such as the pericardium or myocardium116. In more recent investigations, solubilized ECM has gained increased attention as an injectable hydrogel117. Once the ECM is decellularized, it can be lyophilized, ground into a powder, and the powder then enzymatically digested into a liquid solution49. After exposing this ECM solution to physiological temperature for period of time, it can assemble into a hydrogel. Figure 2 shows the process of decellularization and digestion of porcine cardiac ECM tissue. For example, Seif-Naraghi et al.51 tested a hydrogel derived from porcine myocardium ECM in a MI mini-pig model. Two weeks post-MI, the hydrogel was injected into the LV free wall of the endocardium through a 30-gauge needle. They found that at 3 months post injection, the ejection fraction (EF), which is a measure of cardiac function, was significantly increased in the groups that received the hydrogel injection compared to the control animals (saline injection alone). Moreover, the diastolic volume (EDV) and end systolic volume (ESV) were smaller than those of the control groups, indicating an improvement of the ventricular volumes among the hydrogel groups. In addition, the global wall motion index (a conventional parameter used to estimate and rank the LV function as normal, hypokinetic, akinetic, or dyskinetic118) was scored as normal in the hydrogel groups, while in the control groups it worsened to being scored as hypokinetic. SeifNaraghi et al. also assessed the compatibility of the ECM hydrogel in rats by injecting the material into the LV lumen of the heart. Histological analysis found that the degree of inflammation was minimal and similar to that of controls (which received a saline injection), demonstrating that the ECM hydrogel is well-tolerated for up to at least 112 days post injection. Although decellularized ECM is a scaffold that closely mimics cardiac tissue, the risk of immunogenic response hinders its use in clinical applications119,120. As it stands, there is no decellularizing process to ensure the complete removal of immunogenic proteins that could provoke an immune response in humans, although a number of techniques have been used to fabricate ECM-derived hydrogels with low cellular and DNA content. One such method was published by Ungerleider et al.55, which used detergent treatments and enzymatic digestions to generate ECM hydrogels derived from cardiac and skeletal muscle with low immunogenic content. The treatment involved decellularizing the tissues with a 1% (w/v) sodium dodecyl sulfate solution. The decellularized tissues were treated with isopropyl alcohol to remove lipids, which can inhibit gelation. The tissues were then rinsed with water, lyophilized, and milled with a Wiley mini-Mill, #40 or 60 filter to create a powder. To liquefy the ECM, pepsin treatment was employed, followed by titration of the liquid to pH 7.4. Finally, the material was frozen and lyophilized. They found that this method generated ECM hydrogels with low cellular and DNA content. Moreover, they retained glycosaminoglycans and other ECM proteins. However, they recommended performing all experiments with only one batch of ECM hydrogels to avoid batch-to-batch variability.
Figure 2.
Decellularization and digestion of porcine cardiac ECM tissue. The porcine cardiac tissue was sliced into sections (A) and then decellularized (B). The decellularized tissue was further lyophilized and ground into powder (C), and then enzymatically digested into a liquid at room temperature (D). Reprinted with permission from Jeffords et al.49 Copyright 2017 American Chemical Society
Hydrogels derived from ECMs have been shown to have slow gelation times and rapid degradation, causing a decrease in the retention of encapsulated cells or biomolecules in the target area121; several methods have been tested to reduce the degradation of decellularized, ECM-based hydrogels. In one investigation, Jeffords et al.49 used different amounts of genipin to cross-link hydrogels derived from porcine ECM. In vitro degradation analysis using collagenase showed that genipin addition reduced the hydrogel degradation when compared with non-cross-linked hydrogels. Moreover, the use of genipin reduced the swelling percent of the material from ~7000% to ~4000%, which could be beneficial to avoid heart wall disruption after injection. They also found that the cross-linked ECM hydrogels promoted endothelial differentiation of human mesenchymal stem cells (MSCs), which may be attractive to promote vascularization in the injured cardiac tissue. Other efforts to decrease degradation include adding doxycycline, an MMP inhibitor, into hydrogels derived from porcine ventricular myocardium ECM52. For example, Wassenaar et al.52 found that the addition of doxycycline reduced the material degradation over a period of 2 weeks, without affecting the mechanical and biocompatible properties of the ECM hydrogel when injected into healthy rat myocardium. Efraim et al.53 developed a cross-linked decellularized porcine cardiac ECM, using genipin, and functionalized with chitosan. They found that the material significantly improved cardiac function in a MI rat model after eight weeks post-treatment.
3.1.4. Chitosans
Chitosans are natural polysaccharides derived from chitin122 with considerable biocompatibility, antibacterial, and antifungal properties. Chitosan are easily available as they can be obtained from shellfish and waste from the seafood industry. Chitosan-based hydrogels responds to a variety of external stimuli such as light and temperature and assemble as interconnected-porous structures, which can further aid in cell infiltration. The temperature-responsive chitosan-based hydrogels are attractive because bioactive compounds and/or cells can be easily incorporated into the polymer solution without compromising their activity/viability. Once exposed to temperatures close to body temperature, the polymeric solution becomes a gel within a short period of time, localizing these cells/compounds within the injected area. Moreover, the mechanical properties of these hydrogels can be tuned by controlling the pore sizes and pore orientation of the scaffolds123. The stiffness of porous chitosan-based hydrogels can range from 0.1 to 0.5 MPa, whereas the stiffness of the nonporous chitosan-based hydrogels can range from 5 to 7 MPa123. In addition, chitosan-based hydrogel degradation products are biocompatible and their biodegradability can be controlled124. Because of these properties, chitosans are broadly used to synthetize thermosensitive chitosan-based hydrogels125–127. While chitosans have limited solubility at physiological pH128 and can cause premature metabolism of drugs in the presence of proteolytic enzymes129, chemical modification of chitosans into hydrogels mitigates these drawbacks. Shu et al.56 developed a chitosan chloride-RoY (CSCl-RoY) hydrogel that improves angiogenesis under hypoxia after MI. They functionalized CSCl with RoY, a peptide that binds membrane receptors of vascular endothelial cells under hypoxia and activates cellular pathways related to cell survival and proliferation130. They found that these CSCl-RoY hydrogels not only improved angiogenesis in a MI rat model, but also reduced the infarct size from ~55% to 30%, and improved the MI-induced thinning of the wall by increasing wall thickness (from 550 μm to ~700 μm)131. Regarding cardiac function, both left ventricular fraction shortening (LVFS) and left ventricular ejection fraction (LVEF) were significantly improved with the CSCl-RoY hydrogel when compared with controls (PBS and CSCl injections), demonstrating that the addition of RoY to the CSCL hydrogels improve cardiac function.
Chitosan can be dissolved in weak acidic solutions, such acetic acid, and then be used for in vitro applications in cardiac tissue engineering. In one study, Baei et al.58 used acetic acid to solubilize chitosan and β-glycerophosphate disodium salt (β-GP) dissolved in water to promote hydrogel gelation. The concentrations they used (1.6% w/w for chitosan and 10% w/w for βGP) had previously proven to be cytocompatible and transition to form gels close to body temperature. In addition, they added gold nanoparticles (GNs) to make the polymer conductive (labeling it CS-GN). They assessed the polymer with and without GN in vitro using MSCs and found that the CS-GN hydrogel promoted cell viability, proliferation, and maturation of MSCs into CMs, along with the development of uniform cellular constructs. Immunohistochemistry for early and mature cardiac markers showed enhanced CM differentiation of MSCs within the CS-GN compared to the CS matrix alone. In another investigation, Alimirzaei et al.59 also used acetic acid solutions to solubilize chitosan. They used aqueous acetic acid solution and cell culture media with acetic acid. To promote a gel formation, they change the pH of the solution from acidic to neutral, which allowed the solution to form a gel instantaneously after injection. The hydrogels were tested in vitro using human bone marrow MSC (hBMSCs) and human adipose MSC (hAMSCs). They found that hBMCs survived in the gels for up to 21 days and the degradation products of the hydrogel were not cytotoxic on hAMSCs when evaluated by 3(4,5- dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The results overall demonstrated good cytocompatibility of the material.
3.1.5. Alginate
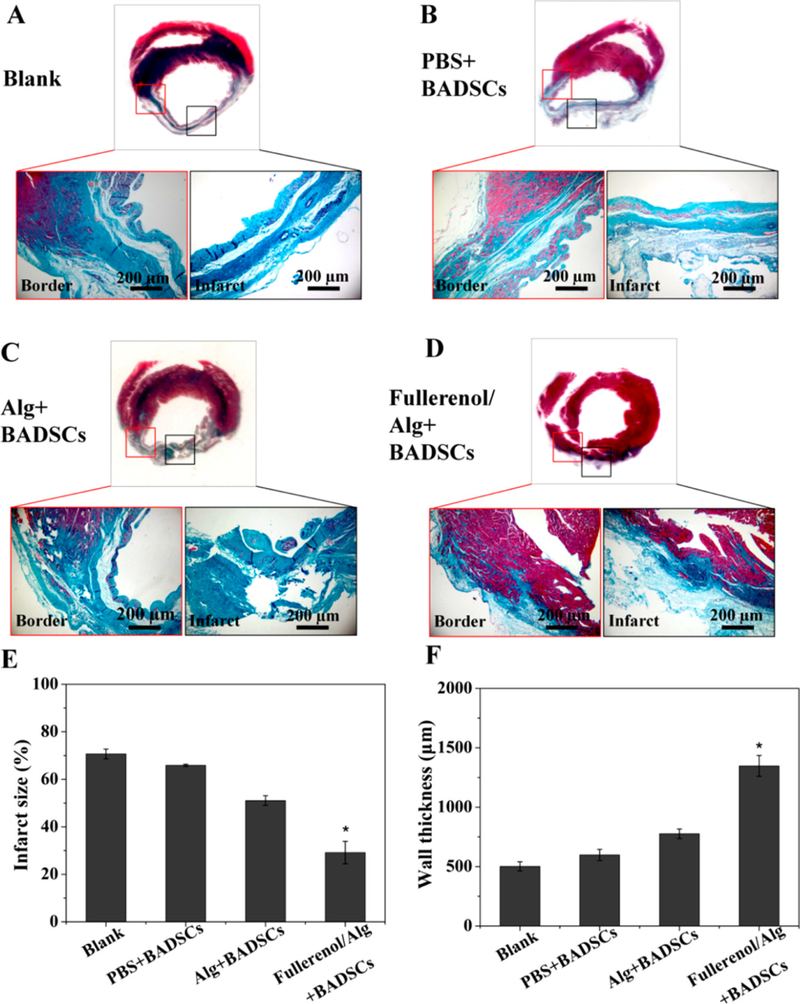
Alginate is a natural polysaccharide isolated from brown seaweed and bacteria. It has excellent biocompatibility, primarily due to its structural resemblance to the native ECM132, and consequently has been widely used for tissue engineering applications133,134. The most common method to prepare alginate-based hydrogels is through the interaction of alginate with divalent cations that facilitate hydrogel crosslinking, but they can be obtained by free radical polymerization and click reactions134. Moreover, alginate is biodegradable, non-antigenic and approved by the US Food and Drug Administration (FDA) for human use. The mechanical and gelation properties of alginate can be easily modified through the conjugation of other materials, immobilization of specific ligands such as peptide and sugar molecules, and crosslinking134. However, alginate also present some drawbacks such as its high hydrophilicity that can negatively impact cell adhesion and proliferation, which can be a limitation for cell delivery therapies50. Moreover, commercial alginate contains a large number of impurities that are responsible for side effects in humans, therefore further purification processes are required for biomedical applications135. In more recent investigations to overcome alginate drawbacks, Hao et al.50 developed a fullerenol-nanoparticle/alginate hydrogel that possesses antioxidant properties, promotes survival of brown adipose-derived stem cells (BADSCs), and improves cardiomyogenic differentiation of BADSCs. They found that the fullerenol/alginate hydrogel can reduce reactive oxygen species (ROS) levels in a MI rat model. Moreover, this injectable hydrogel can improve cell retention and survival of BADSCs, and reduce the infarct size as shown in Figure 3. Most of these protective benefits can be attributed to the fullerenol, which has antioxidant properties that can reduce ROS levels in the injured myocardium50. Moreover, fullerenol nanoparticles can penetrate cell membranes and translocate into organelles that induce the expression of MAPK signaling proteins related to stem cell survival proliferation and cardiomyogenesis50.
Figure 3.
Assessment of in vivo cardiac structure of infarcted hearts after treatment with hydrogel combined with BADSCs. Masson’s trichrome staining images of infarcted hearts for (A) blank group, (B) PBS+BADSCs group, (C) Alg+BADSCs group, and fullerenol/Alg+BADSCs group. Quantitative analysis of (E) infarct size and (F) infarct wall thickness. (*p < 0.05 vs PBS+BADSCs group). Reprinted with permission from Hao et al. 50. Copyright 2017 American Chemical Society
Although alginate has been already used in a pilot clinical trial with positive outcomes, as previously described, a more recent a clinical trial reported by Anker et al.77 showed the injection of an alginate hydrogel into the LV heart muscle of patients with advanced HF to result in an increased mortality rate. These results demonstrate that more advances are required to translate alginate hydrogel-based technologies into additional clinical trials.
3.2. Synthetic Polymers
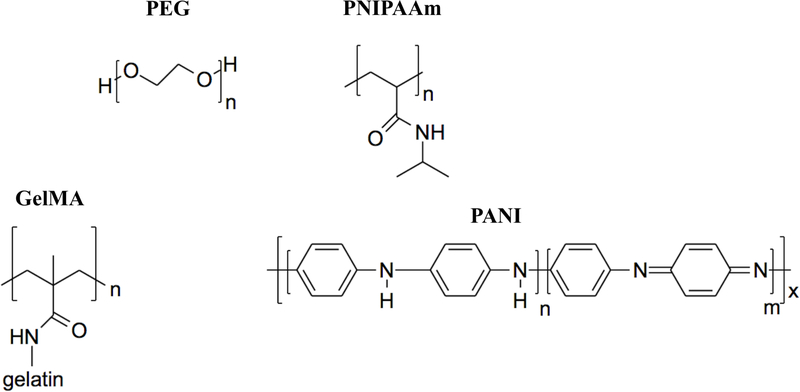
Synthetic polymers have been developed with the aim of eliminating the disadvantageous properties of natural polymers while retaining their desirable ones136–139. Although many advances in cardiac engineering have been made with natural injectable polymers, their variable properties, immunogenic risk, and weak stiffness currently make them unsuitable for clinical applications. Due to this, the focus of many cardiac tissue engineering efforts has turned to utilizing synthetic materials, exploring polymers that come in many variations and that can be more easily manipulated to fit the needs of specific applications. Through changes in the synthesis process, researchers can vary the mechanical strength, porosity, degradation rate, gelation rate, and other polymer properties. Synthetic materials are also more easily controlled to produce a predictable product with less risk for immune rejection upon implantation. In addition, they generally possess less batch-to-batch variation than natural polymers. The disadvantages of synthetic polymers are that they are typically less biocompatible, lack natural cell adhesion sites, and fail to possess the same 3D structure of the complex native ECM140. Current research in this field has focused on developing ways to overcome these disadvantages, as we shall discuss in the subsequent sections. Figure 4 illustrates the chemical structure of some synthetic injectable hydrogels used for cardiac tissue engineering.
Figure 4.
Chemical structure of synthetic materials. PEG: poly(ethylene glycol); PNIPAAm: Poly(N-isopropylacrylamide), GelMA: Gelatin methacryloyl; PANI: poly(aniline).
3.2.1. Polyethylene glycol (PEG)
PEG is a biocompatible synthetic polymer that has been used extensively for tissue engineering approaches141,142. PEG has been quite successful in the tissue engineering area because it is soluble in water and in organic solvents, exhibits low protein adhesion (lending to its nonimmunogenicity), and is nontoxic143. In addition, PEG can be easily tailored to meet the needs of various applications through the conjugation of functional groups to the polymer backbone90. Because PEG is a synthetic polymer, its mechanical properties can be more easily modulated compared to natural polymers. These characteristics have led to injectable PEG hydrogels being broadly used in cardiac regeneration approaches. However, PEG-based hydrogels alone are unable to provide an ideal environment for cell survival, adhesion and growth due to their bioinert nature. To overcome this limitation several investigations have chemically conjugated natural polymers to PEG hydrogels or incorporated bioactive molecules. For example, Chow et al.65 used PEG acrylate copolymerized with PEG dithiol to develop a degradable hydrogel with mechanical properties similar to the normal and infarcted myocardium. They assessed the use of this PEG-based hydrogel as a delivery system of erythropoietin (EPO) and CMs derived from human induced pluripotent stem cell (hiPSC-CMs). EPO was chosen due to its ability to reduce cell apoptosis, improve cardiac remodeling post MI, and have antioxidant properties. Although they found that the PEG-based hydrogel loaded with EPO and hiPSC-CMs improved cardiac function in a MI rat model, they did not observe engrafted hiPSC-CMs within the host tissue. PEG has also been used to modify other polymers due to its long hydrophilic chains being able to prohibit unwanted protein adsorption and improve the biocompatibility of the other materials144. Ciocci et al.63 used PEG-diacrylated (PEGDA) to develop a photo-polymerizable PEGDA-silk fibroin (PEGDA-SF) hydrogel. To improve cell adhesion and increase material porosity, they used protein microspheres. They tested this PEG hydrogel in vitro using MSCs. They found that the hydrogel promoted cell viability and expression of cardiac muscle marker proteins. Although these were promising results, the use of UV to induce gelation is a risk factor for further in vivo applications64, which will be discussed later in this review in section 4.2.2. Light-Induced Crosslinking.
PEG’s ability to act as a stabilizer for natural materials degradation has also been explored in multiple studies with some promising results. For example, Geuss et al.69 crosslinked PEG with fibrin prior the addition of thrombin to obtain a more stable fibrin/PEG-based hydrogel. Fibrin hydrogels normally are biodegraded within 7 days in physiological conditions, limiting their applications for tissue engineering69. Therefore, the hope was to produce a less readily degradable hydrogel that still maintained the biocompatibility of fibrin by incorporating PEG into the hydrogel system. This group assessed the fibrin/PEG-based hydrogels in vitro using HL-1 cells, which are a cardiomyocyte cell line obtained from mouse atrial tumors that possess characteristics of an adult cardiomyocyte phenotype145. Geuss et al. found that their hydrogels supported cell proliferation and expression of cardiac markers. They also compared 2-dimensional (2D) vs 3D cell cultures and found that culture of the cells in these two different formats affected HL-1 cell contractibility; specifically, they surprisingly found that cells growing in 3D did not exhibit spontaneous contraction, whereas cells growing in 2D did.
3.2.2. Poly(N-isopropylacrylamide) (PNIPAAm)
PNIPAAm is a thermosensitive water-soluble homopolymer that has garnered a lot of attention in the biomedical field due to its sharp, reversible solution-to-gelation (sol-to-gel) transition point of 32°C being sufficiently high to enable it to be a solution at room temperature, yet still low enough so that it becomes a gel at body temperature146, making it extremely useful for many biomedical applications147,148. When conjugated with other polymers, PNIPAAm may impart its thermosensitive behavior on otherwise non-thermo-sensitive polymers149–151. Thus, this polymer system may be injected into the treatment site and will conform to the irregularities of the injury site via in situ gelation. PNIPAAm-based polymer systems have been used for cardiac tissue engineering approaches as drug delivery systems, cell scaffolding and transplantation, and in vitro cell culture applications152,153.
Regarding in vitro cell culture applications, PNIPAAm-based hydrogels have been used widely to support co-cultures of cells. Since the cardiac tissue is composed of a variety of cells154, a hydrogel capable of supporting co-cultured cardiac cells is extremely desirable for cardiac tissue engineering. For example, Navaei et al.71 used a 3D PNIPAAM-gelatin based hydrogel to evaluate its potential for co-culturing NRVMs and cardiac fibroblasts (CFs) in vitro. As a control, they used a monoculture of NRVMs cultured in the 3D hydrogel. They found that the co-cultured cells had increased cell interactions, a better cytoskeleton organization, and a more homogeneous beating behavior when compared with controls71. In one of our own investigations, we developed a poly(serinol hexamethylene urea)(PSHU)-PNIPAAm hydrogel functionalized with poly-lysine, which is commonly used to promote cardiomyocyte attachment73. PSHU-PNIPAAm-lysine undergoes sol-to-gel transition close to body temperature. We found that the PSHU-PNIPAAm-lysine can be used as a 3D co-culture in vitro model using NRVMs and CFs. Moreover, PSHU-PNIPAAm promoted survival and function of NRVMs for up to 21 days and controlled the proliferation of CF. Our results demonstrate that these systems could be used as in vitro cardiac tissue models for applications such as drug testing.
Although PNIPAAm-based systems possess several advantages for cardiac tissue engineering, some of these systems are not biodegradable155 and thus they do not readily clear from the body. To mitigate these drawbacks, researchers have developed different methods to improve safe biodegradation of these hydrogel systems. One example is the investigation performed by Fan et al.67, were they developed a degradable poly(NIPAAm-co-2-hydroxyethyl methacrylate (HEMA)-co-acrylate oligolactide (AOLA) hydrogel for the delivery of CTTHWGFTLC (CTT), a peptide that inhibits MMP-267. Post cardiac injury, MMP-2 becomes upregulated, causing an imbalance with the MMP inhibitors and degradation of the cardiac ECM156,157. In this study, they found that incorporation of AOLA and HEMA in poly(NIPAAm) allows the hydrogel to be degraded by creating degradation byproducts with gelation temperatures of ~41°C, causing these byproducts to be in solution form and water soluble at body temperature. In addition, Fan et al. found that this CTT delivery system prevented cardiac ECM from degradation, attenuated cardiac fibrosis, and improved cardiac function when used in a MI rat model. In another study, Xia et al.68 conjugated collagen type I to a biodegradable Poly(NIPAAm)-co-acrylic acid (AAc)-co-2-hydroxylethyl methacrylate (HEMA)-poly(ecaprolactone)(PCL) copolymer to develop a MSC delivery system. They found that HEMAPCL contributed biodegradability to the hydrogel, and that AAc adjusted the lower critical solution temperature (LCST) of the degradation products. Overall, this PNIPAAm-based hydrogel was shown to promote MSC survival, increase neovascularization, and improve heart function when tested in a MI mouse model. Wan et al.72 studied the use of a partially degradable dextran (Dex)PCL-HEMA-PNIPAAm hydrogel as a delivery system of a short-hairpin (sh) RNA of angiotensin converting enzyme (ACE) in a MI rat model. ACE is an important protein that is expressed following cardiac tissue damage and ultimately leads to increased cell death and thus infarct magnitude158. The study by Wan et al. showed that their Dex-PCL-HEMA-PNIPAAm injectable polymer system could effectively deliver a sustained flow of plasmid encoding ACEshRNA following cardiac infarct in a rat MI model. Thirty days following MI and intramyocardial injection of the polymer/ACE-shRNA system, there was a decrease in ACE expression, cell death, infarct size as well as improved cardiac function as compared to bolus injection of free ACE-shRNA72.
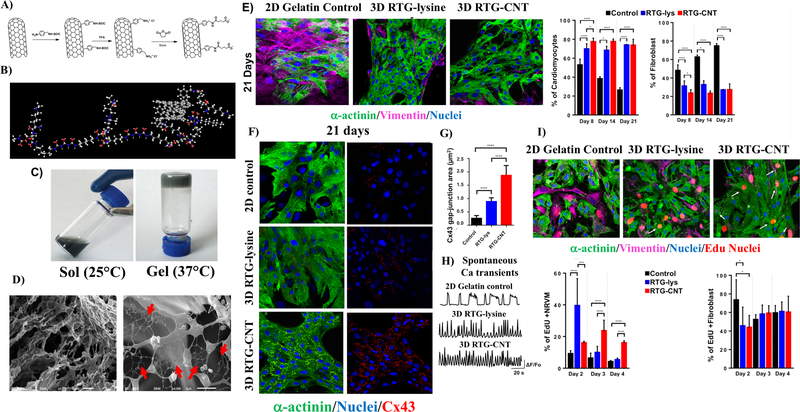
In more recent studies, PNIPAAm has been used in conjunction with CNTs, an attractive combination for cardiac engineering in particular due to the conductive abilities and strong mechanical properties of CNTs159,160. In one such study, Li et al.70 used PNIPAAm’s unique abilities to permit the injection of single-walled carbon nanotubes (SWCNTs) and encapsulated brown adipose-derived stem cells (BASCs) into the myocardium of a rat MI model. They found that the hydrogel showed enhanced cell integration and therapeutic benefit70. Since CNTs are toxic when aggregated, to avoid CNT release and aggregation, CNTs should be chemically conjugated into hydrogels rather than simply mixed into the matrix. To this aim, we chemically incorporated multi-walled carbon nanotubes (MWCNT) in our PSHU-PNIPAAm-lysine hydrogel and further assessed in vitro co-culture of NRVMs and CFs62. Figure 5A illustrates the synthesis of the CNT-COOH and Figure 5B shows the chemical structure of PSHUPNIPAAm-lysine-CNT. We found that the chemical incorporation of CNT-COOH into the polymer did not affect the gelation properties of the materials as shown in Figure 5C. Moreover, the CNTs organized as net inside the polymer pore structure. Figure 5D shows the morphological structure of the PHUS-PNINAAm-lysine-CNT hydrogel. Importantly, the incorporation of CNTs provided electrical cues to the hydrogel and improved its mechanical properties, making the polymer stiffer. Culturing the cells in the 3D CNT hydrogel was also found to promote long-term CM survival and CMs alignment. Moreover, our polymeric matrices, both with and without CNTs, control the proliferation of fibroblast, maintaining their population almost constant for up to 21 days (Figure 5E). Regarding CM function, the area of Cx43 was significantly increased in the CMs cultured in the 3D RTG-CNT hydrogel after 21 days of culture (Figure 5F-G), we believe that this promoted more homogeneous spontaneous Ca+2 transients on the CMs cultured for 21 days in the 3D RTG-CNT polymers compared with controls (Figure 5H). In addition, the CMs growing within the 3D CNT hydrogel also appeared to be proliferative in the initial days of culturing (Figure 5I). This apparent burst of proliferation has also been reported by Martinelli et al.161 in 2D CNT substrate experiments.
Figure 5.
MWCNT-COOH were chemically incorporated in PSHU-PNIPAAm-lysine to develop a RTG-CNT hydrogel. A) incorporation of COOH groups in MWCN and B) chemical structure of the RTG-CNT. C) the RTG-CNT becomes a gel close to body temperature. D) The gel presents a porous mesh with interconnected CNT (red arrows). The RTG-CNT promotes CM long-term survival, function and proliferation: (E) NRVM after 21 days growing in 1) 2D gelatin control, 2) 3D RTG-lysine and 3) 3D-RTG-CNT. (F) Cx43 and α-actinin immunostaining. (G) Quantification of Cx43 gap junction area. (H) Spontaneous calcium transient. I) EdU proliferation assay. Reprinted and modified with permission from Pena et al. 62 Copyright 2017 American Chemical Society.
Lastly, PNIPAAm-based hydrogels have the potential to be used as protein delivery systems. We developed a sulfonated-PSHU-PNIPAAm hydrogel for the delivery of positively charged proteins74. Some examples of positively charged proteins are angiogenic growth factors (GF) such as vascular endothelial growth factor (VEGF)162. We found that the incorporation of sulfonated groups in the hydrogels prolonged the release of positively-charged proteins, without altering their structures, for at least to 70 days in vitro. Moreover, this hydrogel was well tolerated in vitro using C2C12 myoblasts and an in vivo subcutaneous rat injection model. Our results suggest that this sulfonated hydrogel could potentially be used as a control delivery system for the release of supportive GFs (such as angiogenic growth factors) to further improve vascularization in the injured heart.
3.2.3. Aniline-Based Materials
Aniline-based materials have been attractive for cardiac tissue engineering approaches due to their electroactive and antioxidant properties163,164 which are key for cardiac regeneration and will be discussed more details in section 4.4 and section 4.5.1. Several approaches have been used to provide electrical cues to polymeric materials. One of them is the use of electroactive biopolymers based on conductive oligomers such as aniline oligomers165. Some examples of these aniline oligomers are tetraaniline and aniline pentamer. Furthermore, polyaniline and its oligomers present antioxidant properties which can block the effect of reactive oxygen species (ROS) to avoid cellular damage such as, direct damage to membranes and proteins or indirect damage through the activation of pro-apoptotic pathways61.
Some examples of aniline-based hydrogels were reported by Dong et al.60 They developed hydrogels based on a chitosan-graft-aniline tetramer (CS-AT) and di-benzaldehyde-terminated poly(ethylene glycol) (PEG-DA). Polyaniline is intrinsically conductive, and so its incorporation into the polymer backbone enabled the hydrogels to transmit electrical cues. In vitro studies using murine myoblasts and adipose-derived MSC (ADMSCs), marked with CellTraker Green, demonstrated that the cells maintained good levels of viability and proliferation when encapsulated within the hydrogels. Moreover, subcutaneous injections in rats showed good material biocompatibility and ADMSCs retention (marked with cellTraker deep red). Cui et al.61,66 developed antioxidant and conductive carboxyl tetra-aniline (CTA)poly(D,L, lactic acid-co-glycolic acid) (PLGA)-PEG-PLGA-CTA and P(NIPAAm)poly(ethylene glycol methacrylate) (PEGMA)- 2-methylene-1,3-dioxepane (MDO)- methacrylate tetra-aniline (MATA) hydrogels. In vitro live/dead studies using myoblasts in vitro demonstrated that the material is tolerated well by cells in culture, possessing good cytocompatibility. Moreover, subcutaneous injections in a rat model of the acellular material demonstrated acceptable biocompatibility in vivo. Although aniline-based hydrogels intrinsically possess relevant mechanical, conductive, and antioxidant properties, additional studies using cardiomyocytes and cardiac injections in animal models should be undertaken to demonstrate any clinical potential they have for cardiac tissue engineering efforts.
3.2.4. hybrid Gelatin Methacryloyl Hydrogels
Hybrid gelatin methacryloyl (GelMA) hydrogels have been widely used for various tissue engineering approaches due to their high biocompatibility and controlled biodegradability166,167. GelMA can be synthetized by conjugating gelatin with methacrylic anhydride 167. Gelatin is obtained from denatured and partially hydrolyzed native collagen. Due to the denaturalization process, gelatin present low antigenicity but retain the bioactive sequences of collagen to promote cell attachment and matrix metalloproteinase (MMP)-sensitive sites for biodegradation. Although gelatin is highly used in in vitro applications, it is unstable at body temperature and thus, the conjugation of methacrylic anhydride offers a biocompatible hybrid polymer with great potential for tissue engineering. GelMA hydrogels can be tailored by modifying the amount of methacrylic anhydride. This can provide the hydrogels with considerable strength and stiffness167. For example Li et al. 75 developed muscle myofibers using GelMA, PEGDMA (PEG dimethacrylate) and C2C12 myoblast with a variety of stiffness ranging from 12 to 42 kPa. By applying magnetic and non-contact tensile stretch to these GelMA/PEGDMA-myofibers, they were able to induce muscle myofiber formation. The fabrication of these GelMA/PEGDMA-myofibers were performed in a high-throughput manner in which the polymeric/cell solution was injected and exposed to UV light for crosslinking purposes and further squeezed through sieves for fiber formation, obtaining thousands of fibers within seconds. These hydrogel/cell fibers can be further used as templates for the development of fiber-shaped tissues such as blood vessels168.
UV light is commonly used to cross-link GelMA to form hydrogels169. However, UV light can be harmful for the myocardium64, as will be discussed later in this review. To overcome this limitation, Noshadi et al.64 developed cross-linkable GelMa hydrogels that can be induced to cross-link from exposure to visible light. They found that NRVMs cultured on top of the hydrogels were viable and retained their cardiac phenotype for at least 7 days. Additionally, the GelMA hydrogels supported the contractile function of the cells. Moreover, they assessed the injectability and polymerization of the hydrogel in ex vivo tests using adult rat hearts. After injecting the GelMA hydrogel (200 uL total, 10% [w/v] concentration) into the right ventricle of the heart, they applied visible light (for 180 s at a distance of 1 cm) and found that the polymer could easily form a stable gel in this relatively short time period. Furthermore, they assessed the polymer in a MI rat model and found GelMA hydrogel injection to reduce myofibroblast activation, thus reducing fibrosis when compared with control groups (saline injections). In addition, the hydrogel helped in the maintenance of larger blood vessels in the infarcted region. This new approach of using visible light to induce crosslinking makes GelMA hydrogels appealing for such in vivo uses.
3.3. Summary of natural vs synthetic hydrogels
Hydrogels for cardiac tissue engineering applications should have several properties including biocompatibility, degradability, low toxicity, and immunogenicity. However, natural and synthetic materials alone possess only some of these desirable properties. Synthetic hydrogels provide greater control over the mechanical and biochemical properties and they are in general more stable and reproducible than natural hydrogels. However, they are less biocompatible as they lack natural cell adhesion sites. The advantages of natural materials are biocompatibility and biological properties consistent with in vivo features. However, their rapid degradation, long gelation times, poor mechanical properties, insufficient electrical conductivity, and lack of inherent antioxidant properties make them far from ideal for cardiac tissue engineering. Conjugating natural materials with synthetic hydrogels seems to represent the most promising approach to develop materials with controllable mechanical and biochemical properties without compromising biocompatibility and biodegradability. Therefore, hybrid hydrogels that possess the biochemical and biomechanical environment of the native cardiac tissue are needed for successful cardiac tissue engineering approaches.
4. Design of Injectable Hydrogels for Cardiac Tissue Engineering
Ideally, hydrogels for cardiac tissue engineering should be designed in a way that they can be integrated with the host tissue to offer mechanical support to the injured heart, decrease wall stress, compensate for contraction and inhibit pathological remodeling9. Moreover, their biological and physical properties should be designed to mimic those of the cardiac tissue. Engineered hydrogels should be potentially biodegradable with the capability to degrade upon functional regeneration of the injured cardiac tissue. They need to be designed with a stiffness similar to that of cardiac tissue. The gelation time is another important parameter to be considered in the design of injectable hydrogels. Slow gelation times can be problematic as they may increase tissue necrosis by blocking arteries affecting the blood stream. The heart is an electrical conductive system with a continuous electrical network that directs the spontaneous heart cycle. Malfunction of electrical coupling induces arrhythmia and compromises heart function78. Therefore, injectable hydrogels need to be designed with electrical cues to be electrically integrated into the heart and thus, support its electrical function170. Since ROS are increased during cardiac injury, injectable hydrogels with antioxidant properties are appealing to avoid cellular damage due to ROS. Finally, the incorporation of other proteins and cytokines into injectable hydrogels should be also considered as they can offer cardio-protective effects9.
In this section, several material properties that typically belie the success of a given injectable cardiac hydrogel will be explored. Namely this includes the hydrogel’s gelation time, mechanical strength, electrical conductivity, and other biological cues it provides, all features that can significantly impact the formation of functional cardiac tissue. Table 2 and Table 3 show an overview of natural and synthetic material properties reviewed here.
Table 2.
Overview of the material properties of the natural injectable hydrogels used for cardiac tissue engineering.
| Ref. | Material | Mechanical. Properties > 6kPa | Conductive | Gel time | Gel stimuli | Degradable |
|---|---|---|---|---|---|---|
| 48 | CNT mixed in collagen type I hydrogel | Yes | Yes | ~15 min | Temp | Yes |
| 49 | Porcine ECM cross-linked with genipin | No | No | ~ 15 min | Temp | Yes |
| 50 | Fullerenol/ alginate | No | No | 5–10 min | Ca gluconate solution | Yes |
| 53 | Porcine ECM | N/A | No | N/D | Temp | Yes |
| 52 | Porcine ECM with mixed or conjugated doxycycline | No | No | N/D | Temp | Yes |
| 53 | Porcine ECM cross-linked with genipin or chitosan | Yes | No | 3 h | Genipin/temp | Yes |
| 54 | Type I collagen | N/A | No | N/D | Temp | Yes |
| 55 | Decellularized cardiac and skeletal muscle ECM | No | No | 1 h | Temp | Yes |
| 56 | Chitosan chloride-RoY | N/A | No | 8–12 min | Temp | Yes |
| 57 | Fibrin gel with embedded GF and TIMP-3 | N/A | No | N/D | Thrombin | Yes |
| 58 | Chitosan gel with mixed GN | Yes | Yes | Up to 50 min | BGP-Na salt solution | Yes |
| 59 | Chitosan hydrogel | Yes | No | Sec. | pH | Yes |
β-glycerophosphate sodium: BGP-Na; Temperature: temp; N/D: not discussed; seconds: sec; minutes: min; hours: h. carbon nanotubes: CNT; extracellular matrix: ECM; growth factors: GFs; tissue inhibitor of metalloproteinases-3: TIMP-3.
Table 3.
Overview of the material properties of the synthetic injectable hydrogels used for cardiac tissue engineering.
| Ref. | Material | Mechanical properties > 6kPa | Conductive | Gel time | Gel stimuli | Degradable |
|---|---|---|---|---|---|---|
| 60 | CS-AT-di-benzaldehyde- terminated-PEG-DA | Yes | Yes | ~1 min | Temp | Yes |
| 61 | PNIPAM-mPEGMA- MDO-MATA or PN-TA | Yes | Yes | 30 s | Temp | Yes |
| 62 | PSHU-PNIPAAm-co- poly(L-lysine)-CNT | No | Yes | ~30 s | Temp | No |
| 63 | PEGylated silk fibroin functionalized with protein microspheres | N/D | No | 5 min | UV | Yes |
| 64 | GelMA | Yes | No | 20 s | Visible light | N/D |
| 65 | PEG hydrogel EPO | Yes | No | 8 s – 3 min | PEG dithiol | Yes |
| 66 | CTA-PLGA-PEG-PLGA-CTA | Yes | Yes | 15 s – 24 h | Cyclo Dextrin | Yes |
| 67 | PNIPAAm-co-HEMA-co-AOLA with MMP2 inhibitor | Yes | No | 7 s | Temp | Yes |
| 68 | PNIPAAm-AA-HEMA-PCL functionalized with type I collagen | Yes | No | N/D | Temp | Yes |
| 69 | PEGylated Fibrin | No | No | 2 hr | Thrombin | Yes |
| 70 | Single-wall CNT-PNIPAAm | N/D | Yes | N/a | Temp | No |
| 71 | PNIPAAm-gelatin | No | No | ~ 5 min | Temp | N/D |
| 72 | dex-PCL-HEMA-NIPAAm | N/D | No | 60 s | Temp | Yes |
| 73 | PSHU-PNIPAAm-lysine | No | No | ~30 s | Temp | No |
| 74 | Sulfonated PSHU-PNIPAAm | No | No | ~30 s | Temp | No |
| 75 | PEGDMA-GelMA | Yes | No | sec | UV | Yes |
β-glycerophosphate sodium: BGP-Na; Temperature: temp; N/D: not discussed; seconds: sec; minutes: min; hours: h. chitosan-graft-aniline tetramer: CS-AT; polyethylene glycol: PEG; poly(N-isopropyl acrylamide-co-acrylamide): PNIPAM; methylene 1,3-dioxepane: MDO; Nisopropyl acrylamide: NIPAAm; methoxy PEG methacrylate: mPEGMA; methacrylictetraaniline: MATA; carbon nanotube: CNTs; poly(serinol hexamethylene urea)-co-poly(Nisopropylacrylamide): PSHU-PNIPAAm; gelatin methacryloyl: GelMA; erythropoietin: EPO; carboxyl tetra-aniline: CTA; poly(D,L-lactic acid-co-glycolic acid): PLGA; hydroxyethyl methacrylate: HEMA; D,L-lactide oligolactide: AOLA; matrix metalloproteinase-2: MMP2; acrylic acid: AA; poly(ε-caprolactone): PCL; dextran: dex; PEGDMA: PEG-dimethacrylate.
4.1. Design of Gelation Time
Ideally, injectable hydrogels for cardiac tissue engineering efforts should undergo a rapid, tightly-controlled phase transition from a liquid solution (while in a catheter prior to injection) to a gel state (after being injected into the targeted region)38. The sol-to-gel transition should be designed such that it provides optimal cell and/or biomolecule retention, hydrogel deployment, and cellular engraftment. To this end, the gelation kinetics of an injectable hydrogel should maintain the material in solution while still inside of the catheter, and then rapidly – perhaps ideally within seconds -- form a gel after injection into the target tissue171. Natural injectable hydrogels often demonstrate slow sol-to-gel transition rates (15 min to 24 h) as shown in Table 2, which, when injected into vessel-rich cardiac tissue, may increase loss of cells and biomolecules as the semi-liquid gel can be washed away. In addition to concerns over cell and therapeutic molecule loss, slow gelation rates could impede normal blood flow, ultimately leading to tissue necrosis172. Synthetic injectable materials, on the other hand, typically possess much faster gelation times, on the scale of seconds to a few minutes, as shown in Table 3. Since slow gelation time is the main problem of natural hydrogels, here will overview the most recent approaches to improve gelation times in natural hydrogels and possible applications for materials with relatively slow gelation times.
Several investigations have reduced gelation on natural materials by incorporating peptides or organic other compounds. As one example, Shu et al.56 demonstrated that when natural chitosan chloride hydrogels have the addition of RoY, a peptide that can bind 78 kilo Daltons glucoseregulated protein (GRP78) receptors expressed on membrane surface of vascular endothelial cells under hypoxia, their gelation times become significantly reduced, from 17 min to 8–12 min.
Although natural materials do not gel as fast as synthetic materials, they can be used as acellular matrices to cardiac function or as fast release delivery systems in vivo as previously mentioned in section 3.1. For example, Seif-Naraghi 51et al. used porcine myocardial ECM in a Yucatan mini pig MI model. Two weeks post MI, the hydrogel was injected into the infarcted area. They found that after 3 months post injection, the hydrogel improved cardiac function. Similarly, Wassenaar et al.52 tested a hydrogel derived from decellularized porcine ventricular myocardium with matrix metalloproteinase (MMP) inhibitor in vivo using rats to assess degradation and biocompatibility. Efraim et al.53 assessed porcine ECM crosslinked with genipin and/or modified with chitosan and tested them in vivo using a MI rat model. All these investigations were previously discussed in more detail in section 3.1.3 decellularized ECM materials. Awada et al.57 assessed a fibrin hydrogel for fast protein delivery using a MI rat model (more details in section 3.1.2 Fibrin polymers).
4.2. Gelation stimuli
4.2.1. Thermal Stimuli
Several mechanisms have been explored for controlling the sol-to-gel transition of injectable hydrogels used for cardiac tissue engineering with varying results. These approaches include manipulation via light-induced crosslinking, ionic interactions, chemical crosslinking, hydrophobic interactions, and thermal stimuli. Among these, using thermal stimuli is one of the most widely used for both natural and synthetic systems155 as shown in Table 2 and Table 3. Gelation triggered by thermal stimuli in such thermo-sensitive hydrogels is advantageous in that it is potentially less harmful to encapsulated cells, as it does not require UV radiation for crosslinking, which may generate endogenous oxidative damage to DNA173, or other potentially irritating solutions62. Thermo-sensitive hydrogels can be formed by several methods, the first one is the swelling behavior due to a change in their temperature174. Below its lower critical solution temperature (LCST), the hydrogel retains water and swells due to water affinity. Above the LCST, the hydrogel becomes more hydrophobic and the swelling process ends by creating a stable hydrogel9. Another variation of thermo-sensitive materials involves those that gel due to hydrophobic interactions induced by an increase in temperature, without the swelling process73. In these cases, the polymer is more hydrophilic below the LCST and becomes more hydrophobic when the temperature is above the LCST. In those cases, the higher the temperature is, the more hydrophobic the material becomes. Another category for thermosensitive hydrogels is triblock co-polymer. Triblock co-polymers are composed of a hydrophilic-hydrophobic-hydrophilic backbone that undergoes sol-to-gel transition based on micelle formation due to an increase in temperature175. An example of these polymers is a type developed by Park et al.176 that involved an triblock with two terminal hydrophilic blocks composed of poly(ethylene glycol) and an hydrophobic block composed of poly(serinol hexamethylene urethane). This hydrogel undergoes sol-to-gel transition close to body temperature and has been used successfully in several tissue engineering applications177–179.
4.2.2. Light-Induced Crosslinking
Light-inducible crosslinking or photopolymerization is another popular gelation mechanism by which injectable hydrogels undergo the sol-to-gel transition180. Light can promote polymerization of a monomer or the crosslinking of a hydrogel. The main interest of using light to induce a chemical reaction lies in the high initiation rate provided by intense illumination and its fast reaction. Thus, a liquid polymeric solution can polymerize quickly to a hydrogel by simple exposure to UV radiation or other sources of light181. However, most systems are not readily activated by light, and in this case a photoinitiator is required. If the reaction if performed at physiological pH and temperature, the in-situ formation of crosslinked hydrogels may have the ability to encapsulate cells or biomolecules within their 3D structure without highly compromising their viability or function as demonstrated by Li et al.75. However, because using UV radiation to induce crosslinking may affect the integrity of sensitive tissues such as the cardiac tissue, some researchers, such as Noshadi et al.64, have developed visible light cross-linkable gelatin methacryloyl hydrogels that are more compatible with cardiac tissue to overcome these hurdles. In addition, these hydrogels have a gelation time on the order of seconds. These novel hydrogels represent a promising alternative to thermos-sensitive hydrogels with suitable mechanical properties for cardiac tissue engineering.
4.2.3. Michael Addition
The Michael addition reaction is another gelation mechanism that has been used to generate chemically crosslinked injectable hydrogels for cardiac bioengineering efforts90. The Michael addition reaction is characterized by the reaction of a nucleophile (Michael donor) with an activated electrophilic olefin (Michael acceptor) in the presence of a catalyst to an α, β- unsaturated carbonyl to form a Michael adduct182. This reaction can yield highly selective products in an efficient manner under non-toxic reaction conditions. Most specifically, the thiolMichael addition reaction has generated recent interest. In this regard, the thiol-acrylate reaction is one of the most commonly used thiol-Michael reactions. Here the reaction is based on thiols and either acrylates or vinyl sulfones precursors182. An example of this type of hydrogel is the one developed by Chow et al.65 in which they added PEG dithiol to PEG acrylate to form PEG hydrogels. They tested the PEG hydrogels in vivo in a MI model and found that the hydrogels attenuated the pathogenic ventricular remodeling of the heart compared with controls (saline injections).
4.2.4. Ionic Crosslinking
Ionic crosslinking has been used as a common gelation mechanism to obtain alginate hydrogels133,183, which have been used as injectable hydrogels for cardiac bioengineering studies. In these experiments, calcium gluconate or calcium chloride solutions have been used as calcium donors184,185. The ionic crosslinking occurs when sodium alginate is placed in contact with a solution of calcium ions, whereupon the calcium ions replace the negatively charged sodium ions. The positively charged calcium ions then interact with the alginate strands, starting a crosslinking reaction186. Hao et al.50 developed fullerenol/alginate hydrogels using calcium gluconate to induce gelation. They found this hydrogel can reduce reactive oxygen species (ROS) levels in a MI rat model, improve cell retention and survival of BADSCs, and promote angiogenesis.
4.2.5. pH stimuli
Lastly, pH has also been explored as a stimulus to induce hydrogel gelation. Such pHresponsive hydrogels were reported by Alimirzaei et al.59, where a pH sensitive chitosan hydrogel was developed for human adipose MSCs (hADSCs) encapsulation. This hydrogel undergoes sol-to-gel transition when it reaches physiological pH. They dissolved chitosan in either an aqueous acetic solution (1% w/w) or a cell culture media acetic solution (1% w/w). The final pH concentrations of the solutions were between 4.0 and 5.0. To create a gel, they added sodium hydroxide (NaOH) (10 N) to adjust the pH to 6.8 to 6.9. They also developed a coaxial needle to deliver both chitosan solution and NaOH. They found this hydrogel capable of undergoing sol-to-gel transition in seconds.
4.3. Design of Mechanical Strength
Post MI, both cellular loss and changes in the myocardial biomechanical microenvironment can lead to a decrease in cardiac function and strength via wall stress and adverse ventricular remodeling (e.g., ventricular dilation) culminating in fibrosis, hypertrophy and myocardial dysfunction. This maladaptive remodeling starts when the myocardium undergoes irreversible necrosis due to a cascade of effects that disrupt the cell membrane and disorder the structure of the cardiac tissue187,188. Within the first 3 days, an inflammatory response takes place in which leukocytes accumulate in the infarcted site. Then, neutrophils start to infiltrate until they fill the infarcted area. Finally, macrophages are called to the site to phagocytose dead cardiomyocytes. Around day 5, fibroblasts start to proliferate and collagen deposition begins. After 4 weeks, a dense fibrous scar tissue is formed, and the pathological cardiac remodeling is nearly completed. The maturation of the scar tissue formation will further contribute to heart wall dilation to compensate for the lost heart function ending in myocardial dysfunction187,188. Injectable hydrogels should ideally offer relevant mechanical support to the injured myocardium to compensate for the damaging of this tissue. While many injectable materials present with relatively soft mechanical properties that enable easy injection into the damaged myocardium, such as ECM hydrogels, these materials are typically insufficiently robust for providing sustained, continual mechanical support to the injured cardiac tissue, which remains under considerable strain and constant contraction within the active cardiac muscle environment. Two strategies to tune the mechanical strength of hydrogels consist in modifying the degree of crosslinking and altering the polymer concentration; these will be discussed next.
4.3.1. Modulating the Polymer Concentration to Regulate Mechanical Strength
Investigators have found that by varying the amount of polymer dissolved in aqueous solutions, the mechanical strength can be altered, and in this way injectable hydrogels can be developed with mechanical strengths similar to those found in the cardiac tissue environment. For example, Chow et al.65 designed a mechanically-tailored injectable PEG hydrogel. They specifically designed and tested hydrogels containing polymer concentrations of 5, 10, 20, and 30% w/v. The shear modulus for the materials was 0.8, 6.9, 17.2, and 35 kPa, respectively. They found that the 10% and the 20% w/v hydrogels most closely matched the shear modulus of normal (6 kPa) and infarcted (18 kPa) myocardium, respectively. Upon injection of the 10% hydrogels into a MI rat model, they found that, at 10 weeks post injection, pathogenic ventricular remodeling was attenuated in these rats (compared to controls). In another experiment, Fan et al.67 demonstrated that a thermosensitive PNIPAAm-co-HEMA-co-AOLA hydrogel loaded with a MMP-2 inhibitor displayed a stiffness of 35 kPa when produced using a high concentration of PNIPAAm-co-HEMA-co-AOLA (20% w/v). This relatively stiff system efficiently prevented cardiac ECM degradation in a MI rat model and improved cardiac function.
4.3.2. Varying Monomer Composition to Regulate Mechanical Strength
Another way to modify the mechanical properties of hydrogels is to change the chemical composition of the polymer itself by using monomers at varying concentrations. For example, Cui61 et al. developed PNIPAM-mPEGMA-MDO-MATA or PN-TA hydrogels with storage moduli ranging from 1 to 10 kPa. The mechanical properties of these hydrogels were tuned by adjusting the hydrogel concentration in solution and chemically varying the monomer composition termed PN-TA1, PN-TA2 and PN-TA3, as shown in Table 4. The final copolymers were dissolved in phosphate buffer saline solution at 20% (w/v). They found that the PN-TA1 and PN-T3 presented a mechanical stiffness around ~10 kPa and the PN-TA2 around ~1kPa at 37 °C. However, only the PN-TA2 presented a phase transition temperature close to 37 °C, whereas the PN-TA1 and PN-T3 had phase transition temperatures of ~40 °C and ~30 °C, respectively. Upon testing these materials in vitro using murine myoblasts (H9C2 cell line), they found that the myoblasts retained relatively high viability when cultured in 3D using the PN-TA2 hydrogel for at least 7 days. Subcutaneous injection in rats of the acellular hydrogel also demonstrated that the material is well-tolerated, with no apparent abnormalities on the skin nor signs of edema, redness, or tissue necrosis around the implanted hydrogel. Another example of varying the monomer composition to obtain a different stiffness is the investigation performed by Li et al. 75 By changing the mass ratio of PEGDMA and keeping the constant the mass ratio of GelMA (2:1, 3:1 and 4:1) they were able to fabricate fibers with a stiffness of ~12, 23 and 24 kPa for mass ratios of 2:1, 3:1 and 4:1 respectively.
Table 4.
Monomer composition of P(NIPAAm)-PEGMA-MDO-MATA hydrogels61
| Sample | NIPAAm (mol) | MAA (mol) | mPEGMA (mol) | MDO (mol) | TA (mol) |
|---|---|---|---|---|---|
| PN-TA1 | 0.85 | 0.06 | 0.09 | 0.2 | 0.06 |
| PN-TA2 | 0.9 | 0.02 | 0.08 | 0.2 | 0.02 |
| PN-TA3 | 0.9 | 0.04 | 0.06 | 0.2 | 0.04 |
NIPAAm, methacrylic acid (MAA), methoxy(polyethylene glycol) methacrylate (mPEGMA) and tetraaniline (TA)
4.3.3. Using Crosslinking to Regulate Mechanical Strength
A third strategy frequently used by researchers to alter the mechanical strength of injectable hydrogels for cardiac engineering efforts is using various cross-linking agents. For example, Efraim et al.53 synthetized hydrogels using soluble decellularized porcine ECM that was crosslinked using a constant amount of genipin (0.01g), and/or different amounts of chitosan. Chitosan is well-known to offer stability and increase mechanical strengths of collagen gels by crosslinking with collagen in the presence of genipin189. Efraim et al. specifically created decellularized ECM-based gels with either genipin alone, genipin with relatively high levels of chitosan (0.05 g w/w), or genipin with relatively low amounts of chitosan (0.02 g w/w). By modulating these hydrogels using genipin and different amounts of chitosan, the investigators were able to create hydrogels with different mechanical strengths, with the genipin-alone gel measuring at 2 kPa, genipin with high chitosan being 13.6 kPa, and genipin with low chitosan being 36.8 kPa. They further investigated the hydrogels in vitro using hMSCs. For this, tissue culture plates were coated with the different gel and cells seeded on top. They found that the gel with only genipin and the gel with genipin and high chitosan supported the adherence and viability of the cells for at least 28 days. Moreover, the hydrogels without cells were tested in vivo using a MI rat model. Following injection into the MI region, these gels were shown to improve the cardiac function in acute and chronic MI rat models (as measured at 8 weeks post MI). Particularly, the gel with genipin and lower amounts of chitosan best preserved normal heart function and improved the ejection fraction and fractional shorting when compared with the negative control (PBS injection). Moreover, Efraim et al. observed migration of progenitor cells into the scaffolds, indicating the beginning stages of cardiac remodeling.
4.3.4. Other Alternatives to Improve Mechanical Strength
Other alternatives have been explored to improve the mechanical strength of hydrogels. For example, Alimirzaei et al.59 changed the mechanical properties of chitosan hydrogels by dissolving the chitosan in different solutions: (1) aqueous acetic acid solution (WH) (with 1% w/w of acetic acid) and (2) acetic acid solution (1% w/w) prepared in cell culture media (MH). They found that the chitosan dissolved in the WH solution possessed a stiffness of 19.8 kPa and the chitosan dissolved in the MH solution had a lower stiffness, of 10.3 kPa. They further evaluated the hydrogels in vitro by encapsulating hADSCs within the hydrogels. They found that the number of dead cells significantly increased by almost 2-fold in the WH hydrogel when compared with the MH after 21 days of culture. In another investigation, Cui et al.66 modified the mechanical properties of CTA-PLGA-PEG-PLGA-CTA by mixing different concentrations of alpha cyclodextrin (𝑎−CD) in to the hydrogels and increasing the amount of polymer in solution. 𝑎−CD is commonly added to PEG-based hydrogels to lower the amount of polymer in solution needed to form a gel, and thus lowering the viscosity of the polymeric solution190. 𝑎−CD has a strong hydrophobic interaction between hydrophobic polymer blocks, thus facilitating the gelation of the thermos-sensitive polymers (as discussed above in the gelation section)66. They found that the mechanical strength increased from 10 to 65 kPa when the polymer was dissolved in 5 and 10% w/w solutions (with 25% (w/w) of 𝑎−CD remaining constant), respectively. They also found that when the polymer concentration in solution is maintained at 10% while the concentration of 𝑎−CD is increased from 15 to 25%, the mechanical strength increased from 15 to 65 kPa. This demonstrated that either high concentrations of polymer or high concentrations of 𝑎−CD favors increased strength in these hydrogel systems. Although all of these formulations resulted in gels with mechanical properties that may be suitable for use in cardiac tissue engineering applications, only the 15% polymer in solution with 20% 𝑎−CD had acceptable viscosity (0.0008 Pa s). This group further evaluated their hydrogel systems for their ability to support encapsulated rat myoblast (H9C2 cell line). They found that the cells remained viable for up to 5 days. An in vivo subcutaneous injection into rats was also performed, and they found that the hydrogel was well tolerated and almost cleared completely from the injection site after 3 weeks post injection.
4.4. Electrical Conductivity
Native cardiac tissue has unique electrophysiological behavior, involving the transfer of electrical signals that is critically important for proper CM function191. The main components of the heart conductive system are the sinoatrial node (SAN), the internodal pathways, the atrioventricular node (AVN), the bundle of His and the Purkinje fibers. Its function is highly regulated by specialized CMs. The electrical activity of the heart begins when CMs located in the SAN spontaneously generate an action potential (AP) which then propagates to the atrial myocardium, internodal pathways and to the AVN. Finally, the AP is propagated through the bundle of His and Purkinje fibers towards the ventricular myocardium, which then contracts in syncytial manner. At the cellular level, APs are generated by the membrane potential of CMs that follow an initial depolarization from a resting to a threshold potential mediated by ion channels192. Due to the loss of CMs in MI and the further formation of the scar tissue, abnormalities in the electrical signaling of the injured heart are often observed193. The design of a strong and fast conducting hydrogel capable of electromechanical coupling with the myocardium could potentially promote efficient improvement of heart function without causing arrhythmias. Unfortunately, the majority of the injectable materials for cardiac regeneration are electrically insulated106. To this end, some investigators have chemically modified injectable materials by conjugating conductive nanoparticles, such as gold or CNT, or conductive polymers to hydrogel systems, making the materials more suited to support the electrical signaling of CMs194. To this end, our group recently developed a reverse thermal gel (RTG) functionalized with CNTs, specifically a poly(serinol hexamethylene urea) (PSHU)PNIPAAm-lysine-CNT, also referred to here as a RTG-CNT62. CNT present unique mechanical, electrical and thermal features and have been recently explored to improve the electrical features of polymeric materials for cardiac tissue engineering105,106,161,195,196.
NRVMs cultured in our RTG-CNT polymers possessed stronger and more homogeneous spontaneous calcium transients when compared with cells growing in the 3D hydrogel without CNT and in 2D gelatin coated tissue culture plates. The measurement of spontaneous calcium transients is a common method to determine the spontaneous waves of cardiomyocytes cyclic contractions attributed to the oscillatory release and uptake of calcium by the sarcoplasmic reticulum. These measurements are highly correlated with cell-cell coupling, which is maintained by gap junctions. We found that the cells growing in the RTG-CNT presented an increased and more organized localization of gap junctions when compared with controls. These results suggest that conductive materials have the capacity to improve the intracellular communication and function of CMs, which is crucial for cell integration in injured cardiac tissue. Along these lines, Sun et al.70 incorporated SWCNTs into collagen hydrogels, referred as CNT/Col. They found that the incorporation of CNTs improved the electrical properties of these hydrogels. Most importantly, they found that NRVMs growing in the CNT/Col hydrogels showed improved cell alignment – a key feature of myoblasts – with stronger contraction potentials.
Although CNTs present several safety concerns due to potential long-term toxicity, they can be functionalized to improve their biocompatibility. For example, we reported the synthesis of CNT-COOH by diazonium salt arylation reaction, to introduce amino groups, followed by a succinic anhydride reaction to incorporate the COOH groups. This method avoids toxic effects caused by metallic impurities and oxidative debris present in oxidized CNT-COOH62. Moreover, it promotes CNT solubility in aqueous solutions. In addition, several other modifiable factors, such as CNT length and dispersion within the hydrogel matrix, can potentially be used to mitigate toxicity62. However, it may be a long process to translate this technology into clinical trials. One alternative to CNTs is the use of conductive polymers. Dong60 et al. and Cui et al.61,66 developed conductive aniline-based hydrogels that, when grown with murine myoblasts, improved cell viability and proliferation. In addition, these acellular hydrogels showed good in vivo biocompatibility when injected subcutaneously in rats. Another alternative to promote polymer conductivity is the incorporation of GN, which are highly conductive biocompatible biostructures197. Baei et al.58 mixed GN with chitosan hydrogels at different concentrations, 0.5, 1.0, and 1.5 % (w/w). They found that the 1.0% (w/w) concentration had electrical properties closest to the native myocardium (0.13 S m− 1). Moreover, they found that chitosan-GN (with 1% w/w of GN) promoted MSC maturation in vitro.
4.5. Biological Cues
4.5.1. Antioxidant Properties
As previously mentioned, MI occurs when the blood supply of the heart is interrupted. Once the blood flow is restored, it reintroduces oxygen to the myocardium and leads to the generation of reactive oxygen species (ROSs)198. ROSs are molecular ions generated by oxygen reduction during the metabolism of oxygen. They are produced either by the injured myocardium or by inflammatory cells, leading to cellular damage199. This oxidative stress results in a volatile environment and damaged tissue that can limit the therapeutic efficacy of hydrogel-based cellular approaches. To overcome this limitation, a number of studies have proposed incorporating antioxidant compounds and antioxidants materials themselves into the design of injectable hydrogels.
Several materials with antioxidant properties have been identified and explored for their potential protective and regenerative effects in cardiac systems. Polyaniline and its oligomers is one such material that has been reported to have antioxidant properties200. Using this material, Cui et al. designed a variety of injectable hydrogels by conjugating tetra-aniline to Poly(NIPAM)-based hydrogels and PLG-PEG copolymers6166. This group cultured rat myoblasts in the aniline containing polymers and, using a radical scavenging activity assay, found that this material protects cells against high levels of ROS. Along these lines, Hao et al.50 developed an injectable fullerenol/alginate hydrogel to suppress oxidative damage. Fullerenol possesses antioxidant properties due to its electron deficient surfaces, which are able to attract ROS201. Once ROS interacts with fullerenol, an electron transfer takes place that suppresses the oxidant stress caused by ROS. Hao et al. tested this hydrogel in a MI rat model and found that the hydrogel appeared to decrease the levels of ROS in this model.50.
Other alternatives that have been explored for protecting CMs against damaging ROS include incorporating antioxidant agents into hydrogels. For example, Chow et al.65 used PEG hydrogels to deliver erythropoietin (EPO), an antioxidant agent used in clinical trials to reduce cell death and adverse remodeling post MI202. They found that the addition of a relatively small amount of EPO (1 unit/ml) significantly improved the viability of hiPSC-CMs when they were used as an in vitro model cultured under stress-inducing conditions (using doxorubicin).
4.5.2. Degradation
While degradable materials may be desirable, inert, non-degradable materials can also be used for cardiac regeneration203. Matrices that degrade too quickly and may be subsequently cleared away soon after injection can be problematic due to a loss of the protective microenvironment that would otherwise be supportive for the injured cardiac tissue, the encapsulated cells that require time to become established and engrafted, and therapeutic molecules that are locally required for their action. For most natural materials, such as collagen, fibrin, and decellularized ECM, degrade quickly due to the MMP family of proteases204. To promote relatively slow degradation of decellularized porcine ventricular myocardium, Wassenaar et al.52added doxycycline, an MMP inhibitor, into the porcine ECM. This was accomplished using two different methods: chemically cross-linking doxycycline to the hydrogel and mixing doxycycline with the hydrogel matrix during preparation in solution. They tested the degradation of the decellularized myocardium hydrogels both in vitro and in vivo. For in vitro studies, they used a bacterial collagenase (125 U/mL) degradation assay for 24 h. As a control, they used hydrogels exposed to PBS. They found that both hydrogels presented a significantly lower peptide concentration, indicative of inhibition of collagenase degradation when compared with unmodified ECM hydrogels. For the in vivo studies, they labeled the ECM hydrogels with AF568 for visualization and performed an intra-myocardial injection in healthy rat hearts. They found that only the mixed MMP inhibitor was able to reduce in vivo degradation while the crosslinking of the MMP inhibitor had no significant effect to avoid degradation. Another method that has been explored in efforts to decrease material degradation is the use of genipin for cross-linking ECM hydrogels. As mentioned above, decellularized ECM matrix can be lyophilized and ground into powder and then enzymatically digested into a liquid solution. This liquid ECM can then be injected as a hydrogel together with crosslinkers, such as genipin, to reduce degradation of the hydrogel. Jeffords et al.49 demonstrated that while collagenase treatment can degrade non-cross-linked cardiac matrix in vitro within 24 h, upon cross-linking these hydrogels with genipin, they were significantly less degraded after undergoing the same 24 h in vitro collagenase treatment. In another study, Efraim et al.53 used genipin (in combination with chitosan) to decrease degradation – of porcine cardiac ECM in vivo. This group evaluated the stability and degradation of these hydrogels over 8 weeks in a rat MI model. All of these findings offer strategies for the fine-tuning of degrading natural materials.
5. Conclusions and Future Efforts
Injectable hydrogels have demonstrated potential to be used in cardiac regeneration efforts. As explored in this review, natural, synthetic, and hybrid injectable hydrogel systems have been tested both in vitro and in vivo and now the field is moving to develop promising hybrid materials, combining optimal natural and synthetic components. Because the heart is a complex, conductive, and constantly contracting muscle, ideal materials need to be biocompatible, potentially biodegradable, durable, and conductive, and be able to resist the harsh environment created during cardiac injury. Moreover, they must have morphological, mechanical, and functional properties similar to the heart. In addition to the material requirements, bioactive compounds and cardiac cells can be combined with hydrogels to develop an ideal cardiac tissue engineering approach. MMP inhibitors and antioxidant components that overcome the effects of ROS are highly desirable for cardiac regeneration. An ideal material may also integrate a combination of cardiomyocytes and possibly fibroblasts, to compensate for loss of cells and mechanotransduction cues due to cardiac injury, with a supportive vasculature for survival when transplanted.
Despite the progress achieved in injectable hydrogels for cardiac tissue engineering, many challenges need to be addressed before these approaches can be safely translated for human applications. For instance:
The development of a hydrogel that covers all the requirements needed to repair the heart. None of the hydrogels mentioned in this review possess all the characteristics needed to properly mimic cardiac tissue. Therefore, new materials, both synthetic and natural, suitable for injectable cardiac tissue engineering need to be developed.
Hydrogel dose, timing of injection (when to inject the material) and material deployment are other key factors that need further investigation. For this, pre-clinical studies should be performed in large animal models with similar physiological cues to the human heart. These studies need to cover long-term outcomes and should be followed up very carefully.
The design and development of a catheter suitable for the delivery of different hydrogels in a minimally invasive way are still needed. For instance, a catheter that keeps the material cold before deployment would be ideal for temperature sensitive hydrogels. A double channel injection catheter, will be needed for ionic or pH sensitive hydrogels. The mechanism behind the functional problems of hydrogels injections also needs to be investigated. For example, what to do if the material is injected in the wrong location? All this information has very briefly been covered by a few investigations mentioned in this review.
Understanding the mechanism of the injured heart is key for the development of novel technologies. For this, basic science research needs to be applied to fully understand the pathways involved during a heart injury. For instance, during acute MI, factors that reduce myocardial necrosis and augment vascular blood flow would be desired. Therefore, molecular incorporation with the injectable hydrogels based on the mechanism studies would help regenerate the heart tissue.
Acknowledgements.
This study was supported by generous grants of the American Heart Association (AHA) SFRN 16SFRN29580008 (to Dr. Peña), the AHA 17GRNT33661024 and R21 HL124100–01 (to Dr. Park), the European Union grant FP7-PEOPLE2011-IRSES 291834 SarcoSI and U.S. National Institutes of Health grants UL1 RR025780, UL1 TR001082, R01 HL69071, and R01 116906 (to Dr. Mestroni); in part by a Trans-Atlantic Network of Excellence grant from the Leducq Foundation (14-CVD 03).
Abbreviations
- CNT
carbon nanotubes
- NRVMs
neonatal rat ventricular cardiomyocytes
- ECM
extracellular matrix
- ADSCs
adipose-derived stem cells
- MSCs
mesenchymal stem cells
- HUVECs
human umbilical vein endothelial cells
- GFs
growth factors
- TIMP-3
tissue inhibitor of metalloproteinases-3
- GF
Growth factors
- hBMSCs
human bone marrow MSC
- CS-AT
human adipose MSC (hAMSCs). chitosan-graft-aniline tetramer
- PEG
polyethylene glycol
- PNIPAM
poly(N-isopropyl acrylamide-co-acrylamide)
- MDO
methylene 1,3-dioxepane
- NIPAAm
N-isopropyl acrylamide
- mPEGMA
methoxy PEG methacrylate
- MATA
methacrylic-tetraaniline
- CNTs
carbon nanotube
- PSHU-PNIPAAm
poly(serinol hexamethylene urea)co-poly(N-isopropylacrylamide)
- GelMA
gelatin methacryloyl
- EPO
encapsulation erythropoietin
- CTA
carboxyl tetra-aniline
- PLGA
poly(D,L-lactic acid-coglycolic acid)
- HEMA
hydroxyethyl methacrylate
- AOLA
D,L-lactide oligolactide
- MMP2
matrix metalloproteinase-2
- PCL
acrylic acid: AA; poly(ε-caprolactone)
- Dex
Dextran
- NRVMs
neonatal rat ventricular cardiomyocytes
- MSCs
mesenchymal stem cells
- CMs
cardiomyocytes
- iPSC-CMs
induced pluripotent stem cell-derived CMs
- ADSCs
adipose-derived stem cells
- BGP-Na
short-hairpin RNA of angiotensin converting enzyme, ACE-shRNA. βglycerophosphate sodium
- Temp
Temperature
- not discussed
N/D
- sec
Seconds
- min
Minutes
- (TA)
hours: h. methacrylic acid (MAA), methoxy(polyethylene glycol) methacrylate (mPEGMA) and tetraaniline
- (PEGDMA)
PEG dimethacrylate
Biographies
 Brisa Peña received her Ph.D. and her Master’s degree in Chemical, Environmental and Process Engineering from the University of Rovira i Virgili, Spain 2012. She joined the Bioengineering Department at the University of Colorado Denver, Anschutz Medical Campus, as a Postdoctoral Fellow in 2012 and has continued her training in the Cardiology Department since 2014. She is currently a Research Instructor in the Cardiology Department at UC Denver. Her research focuses on the development of novel materials for cardiac tissue engineering with particular interest on polymeric materials, hIPSC-CMs and nanotechnology to mitigate the effects of heart failure.
Brisa Peña received her Ph.D. and her Master’s degree in Chemical, Environmental and Process Engineering from the University of Rovira i Virgili, Spain 2012. She joined the Bioengineering Department at the University of Colorado Denver, Anschutz Medical Campus, as a Postdoctoral Fellow in 2012 and has continued her training in the Cardiology Department since 2014. She is currently a Research Instructor in the Cardiology Department at UC Denver. Her research focuses on the development of novel materials for cardiac tissue engineering with particular interest on polymeric materials, hIPSC-CMs and nanotechnology to mitigate the effects of heart failure.
 Daewon Park is an Assistant Professor in the Bioengineering Department at University of Colorado Denver, Anschutz Medical Campus. He obtained his Ph.D. at the Yonsei University, South Korea 2003. He then did his Postdoctoral training at the MIT (2005), Cornell University (2007), Georgia Tech. (2009) and the University of Pittsburgh (2011). His research involves the development of polymeric biomaterials for drug delivery and tissue engineering. His lab focuses to create biomaterials which meet the key requirements such as harmonization with the host cell environment and creation of a growth-permissive environment using primary cells, cell lines and animal models.
Daewon Park is an Assistant Professor in the Bioengineering Department at University of Colorado Denver, Anschutz Medical Campus. He obtained his Ph.D. at the Yonsei University, South Korea 2003. He then did his Postdoctoral training at the MIT (2005), Cornell University (2007), Georgia Tech. (2009) and the University of Pittsburgh (2011). His research involves the development of polymeric biomaterials for drug delivery and tissue engineering. His lab focuses to create biomaterials which meet the key requirements such as harmonization with the host cell environment and creation of a growth-permissive environment using primary cells, cell lines and animal models.
 Luisa Mestroni, M.D. is a Professor of Medicine at the University of Colorado Denver, Anschutz Medical Campus. Her research’s goal is to discover causes and mechanisms leading to cardiomyopathies and heart failure. With particular interest on genes causing dilated cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy, left ventricular noncompaction and hypertrophic cardiomyopathy. Her team already discovered several cardiomyopathy genes including the lamin A/C, the cardiac sodium channel (SCN5A), the titin gene and the filamin C gene. She is also interested to investigate novel therapeutic treatments for these diseases which are characterized by high mortality and morbidity, including tissue engineering and nanotechnology.
Luisa Mestroni, M.D. is a Professor of Medicine at the University of Colorado Denver, Anschutz Medical Campus. Her research’s goal is to discover causes and mechanisms leading to cardiomyopathies and heart failure. With particular interest on genes causing dilated cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy, left ventricular noncompaction and hypertrophic cardiomyopathy. Her team already discovered several cardiomyopathy genes including the lamin A/C, the cardiac sodium channel (SCN5A), the titin gene and the filamin C gene. She is also interested to investigate novel therapeutic treatments for these diseases which are characterized by high mortality and morbidity, including tissue engineering and nanotechnology.
Bibliography
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, De Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, MacKey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir RW, Neumar K, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfghi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey J. z., Wilkins JT, Wu JHY, Alger HM, Wong SS, Muntner P, Circulation 2017, 135, e146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alraies MC, Eckman PJ, Thorac. Dis 2014, 6, 1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Messer S, Large S, Eur J Cardio-thoracic Surg. 2016, 49, 1. [DOI] [PubMed] [Google Scholar]
- 4.Sun X, Nunes SS, Biomed. Mater 2015, 10, 034005. [DOI] [PubMed] [Google Scholar]
- 5.Minicucci MF, Azevedo PS, Polegato BF, Paiva SAR, Zornoff LAM, Clin. Cardiol 2011, 34, 410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Struthers AD, Heart 2005, 91, ii14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehta LS, Beckie TM, DeVon HA, Grines CL, Krumholz HM, Johnson MN, Lindley KJ, Vaccarino V, Wang TY, Watson KE, Wenger NK, Circulation 2016; 133, 916. [DOI] [PubMed] [Google Scholar]
- 8.Chi JS, Kloner R, a. Heart 2003, 89, 475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sepantafar M, Maheronnaghsh R, Mohammadi H, Rajabi-Zeleti S, Annabi N, Aghdami N, Baharvand H, Biotechnol. Adv 2016, 34, 362. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen MM, Gianneschi NC, Christman KL, Curr. Opin. Biotechnol 2015, 34, 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masci PG, Bogaert J, Cardiovasc. Diagn. Ther 2012, 2, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flachskampf FA, Schmid M, Rost C, Achenbach S, Demaria AN, Daniel WG, Eur. Heart. J 2011, 32, 272. [DOI] [PubMed] [Google Scholar]
- 13.Pazos-López P, Peteiro-Vázquez J, Carcía-Campos A, García-Bueno L, de Torres JPA, Castro-Beiras A, Vasc. Health Risk. Manag 2011, 7, 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braunwald E, JACC Hear. Fail 2013, 1, 1. [DOI] [PubMed] [Google Scholar]
- 15.Sergeeva IA, Christoffels VM, Biochim. Biophys. Acta – Mol. Basis Dis 2013, 1832, 2403. [DOI] [PubMed] [Google Scholar]
- 16.Ikegami R, Shimizu I, Yoshida Y, Minamino T, Circ. J 2018, 82, 10. [DOI] [PubMed] [Google Scholar]
- 17.Inamdar A, Inamdar A, J. Clin. Med 2016, 5, 62. [Google Scholar]
- 18.Hobbs R, Br J Gen. Pract 2010, 60, 611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cowie M. r., Anker SD, Cleland JGF, Felker JGM, Filippatos G, Jaarsma T, Jourdain P, Knight E, Massie B, Ponikowski P, López-Sendón J, ESC Hear. Fail 2014, 1, 110. [DOI] [PubMed] [Google Scholar]
- 20.Voigt J, Sasha John M, Taylor A, Krucoff M, Reynolds MR, Michael Gibson C, Clin. Cardiol 2014, 37, 312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barr ML, Taylor DO, Am. J Transplant 2015, 15, 7.25534539 [Google Scholar]
- 22.Chin C, Miller J, Robbins R, Reitz B, Bernstein D, Pediatr. Transpl 1999, 3, 309. [DOI] [PubMed] [Google Scholar]
- 23.Macdonald P, Verran D, O’Leary M, Cavazzoni E, Dhital K, Transplantation 2015, 99, 1101. [DOI] [PubMed] [Google Scholar]
- 24.Kikuchi K, Poss KD, Annu. Rev. Cell Dev. Biol 2012, 28, 719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Major RJ, Poss KD, Drug Discov. Today Dis. Model 2007, 4, 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Von Harsdorf R, Poole-Wilson PA, Dietz R, Lancet (London, England) 2004, 363, 1306. [DOI] [PubMed] [Google Scholar]
- 27.Prabhu SD, Frangogiannis NG, Circ. Res 2016, 119, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de S. Rebouças J, Santos-Magalhães NS, Formiga FR, Arq. Bras. Cardiol 2016, 271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Korf-Klingebiel M, Reboll MR, Klede S, Brod T, Pich A, Polten F, Napp LC, Bauersachs J, Ganser A, Brinkmann E, Reimann I, Kempf T, Niessen HW, Mizrahi J, Schönfeld HJ, Iglesias A, Bobadilla M, Wang Y, Wollert KC, Nat. Med 2015, 21, 140. [DOI] [PubMed] [Google Scholar]
- 30.Dobaczewski M, Frangogiannis NG, Futur. Cardiol 2008, 4, 347. [DOI] [PubMed] [Google Scholar]
- 31.Finan A, Richard S, Front. Cell Dev. Biol 2015, 3, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amado LC, Saliaris AP, Schuleri KH, St. John M, Xie JS, Cattaneo S, Durand DJ, Fitton T, Kuang JQ, Stewart G, Lehrke S, Baumgartner WW, Martin BJ, Heldman AW, Hare JM, Proc. Natl. Acad. Sci 2005, 102, 11474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garbern JC, Lee RT, Cell Stem Cell 2013, 12, 689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh A, Singh A, Sen D, Stem Cell Res. Ther 2016, 7, 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Truskey GA, F1000Research 2016, 5, 1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jackman CP, Shadrin IY, Carlson AL, Bursac N, Curr. Opin. Chem. Eng 2015, 7, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chiu LLY, Radisic M, Curr. Opin. Chem. Eng 2013, 2, 41. [Google Scholar]
- 38.Hasan A, Khattab A, Islam MA, Hweij KA, Zeitouny J, Waters R, Sayegh M, Hossain MM, Paul A, Adv. Sci 2015, 2, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tous E, Purcell B, Ifkovits JL, Burdick JAJ, Cardiovasc. Transl. Res 2011, 4, 528. [DOI] [PubMed] [Google Scholar]
- 40.Ye L, Zimmermann WH, Garry DJ, Zhang J, Circ. Res 2013, 113, 922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Castells-Sala C, Semino CE, Curr. Opin. Organ. Transplant 2012, 17, 681. [DOI] [PubMed] [Google Scholar]
- 42.Matsuura K, Masuda S, Shimizu T, Anat. Rec 2014, 297, 65. [DOI] [PubMed] [Google Scholar]
- 43.Yamato M, Okano T, Mater. Today 2004, 7, 42. [Google Scholar]
- 44.Ye KY, Black LDJ, Cardiovasc. Transl. Res 2011, 4, 575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen FM, Liu X, Prog. Polym. Sci 2016, 53, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Langer NB, Argenziano M, Methodist. Debakey. Cardiovasc. J 2016, 12, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moorthi A, Tyan YC, Chung TW, Biomater. Sci 2017, 5, 1976. [DOI] [PubMed] [Google Scholar]
- 48.Sun H, Zhou J, Huang Z, Qu L, Lin N, Liang C, Dai R, Tang L, Tian F, Int. J. Nanomedicine 2017, 12, 3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jeffords ME, Wu J, Shah M, Hong Y, Zhang G, ACS Appl. Mater. Interfaces 2015, 7, 11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hao T, Li J, Yao F, Dong D, Wang Y, Yang B, Wang C, ACS Nano 2017, 11, 5474. [DOI] [PubMed] [Google Scholar]
- 51.Seif-Naraghi SB, Singelyn JM, Salvatore MA, Osborn KG, Wang JJ, Sampat U, Kwan OL, Strachan GM, Wong J, Schup-Magoffin PJ, Braden RL, Bartels K, DeQuach JA, Preul M, Kinsey AM, De Maria AN, Dib N, Christman KL, Sci. Transl. Med 2013, 5, 1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wassenaar JW, Braden RL, Osborn KG, Christman KL, Mater. Chem. B 2016, 4, 2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Efraim Y, Sarig H, Cohen Anavy N, Sarig U, de Berardinis E, Chaw SY, Krishnamoorthi M, Kalifa J, Bogireddi H, Duc TV, Kofidis T, Baruch L, Boey FYC, Venkatraman SS, Machluf M, Acta Biomater. 2017, 50, 220. [DOI] [PubMed] [Google Scholar]
- 54.Blackburn NJR, Sofrenovic T, Kuraitis D, Ahmadi A, McNeill B, Deng C, Rayner KJ, Zhong Z, Ruel M, Suuronen EJ, Biomaterials 2015, 39, 182. [DOI] [PubMed] [Google Scholar]
- 55.Ungerleider JL, Johnson TD, Rao N, Christman KL, Methods 2015, 84, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shu Y, Hao T, Yao F, Qian Y, Wang Y, Yang B, Li J, Wang C, ACS Appl. Mater. Interfaces 2015, 7, 6505–6517. [DOI] [PubMed] [Google Scholar]
- 57.Awada HK, Long DW, Wang Z, Hwang MP, Kim K, Wang Y, Biomaterials 2017, 125, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baei P, Jalili-Firoozinezhad S, Rajabi-Zeleti S, Tafazzoli-Shadpour M, Baharvand H, Aghdami N, Mater. Sci. Eng. C 2016, 63, 131. [DOI] [PubMed] [Google Scholar]
- 59.Alimirzaei F, Vasheghani-Farahani E, Ghiaseddin A, Soleimani M, Pouri1 Zeinab Naja -Gharavi, J. Tissue Sci. Eng 2017, 8, 1. [Google Scholar]
- 60.Dong R, Zhao X, Guo B, Ma PX, ACS Appl. Mater. Interfaces 2016, 8, 17138. [DOI] [PubMed] [Google Scholar]
- 61.Cui H, Liu Y, Cheng Y, Zhang Z, Zhang P, Chen X, Wei Y, Biomacromolecules 2014, 15, 1115. [DOI] [PubMed] [Google Scholar]
- 62.Peña B, Bosi S, Aguado BA, Borin D, Farnsworth NL, Dobrinskikh E, Rowland TJ, Martinelli V, Jeong M, Taylor MRG, Long CS, Shandas R, Sbaizero O, Prato M, Anseth KS, Park D, Mestroni L, ACS Appl. Mater. Interfaces 2017, 9, 31645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ciocci M, Cacciotti I, Seliktar D, Melino S, Int. J. Biol. Macromol 2017, 108, 960. [DOI] [PubMed] [Google Scholar]
- 64.Noshadi I, Hong S, Sullivan KE, Shirzaei Sani E, Portillo-Lara R, Tamayol A, Shin SR, Gao AE, Stoppel WL, Black III LD, Khademhosseini A, Annabi N, Biomater. Sci 2017, 2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chow A, Stuckey DJ, Kidher E, Rocco M, Jabbour RJ, Mansfield CA, Darzi A, Harding SE, Stevens MM, Athanasiou T, Stem Cell Reports 2017, 9, 1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cui H, Cui L, Zhang P, Huang Y, Wei Y, Chen X, Macromol. Biosci 2014, 14, 440. [DOI] [PubMed] [Google Scholar]
- 67.Fan Z, Fu M, Xu Z, Zhang B, Li Z, Li H, Zhou X, Liu X, Duan Y, Lin PH, Duann P, Xie X, Ma J, Liu Z, Guan J, Biomacromolecules 2017, 18, 2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xia Y, Zhu K, Lai H, Lang M, Xiao Y, Lian S, Guo C, Wang C, Exp. Biol. Med 2015, 240, 593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Geuss LR, Allen ACB, Ramamoorthy D, Suggs LJ, Biotechnol. Bioeng 2015, 112, 1446. [DOI] [PubMed] [Google Scholar]
- 70.Li X, Zhou J, Liu Z, S Chen JL, Sun H, Li J, Lin Q, Yang B, Duan C, Xing M, Wang C, Biomaterials 2014, 35, 5679. [DOI] [PubMed] [Google Scholar]
- 71.Navaei A, Truong D, Heffernan J, Cutts J, Brafman D, Sirianni RW, Vernon B, Nikkhah M, Acta Biomater. 2016, 32, 10. [DOI] [PubMed] [Google Scholar]
- 72.Wan WG, Jiang XJ, Li XY, Zhang C, Yi X, Ren S, Zhang XZ, J Biomed. Mater. Res. - Part A 2014, 102, 3452. [DOI] [PubMed] [Google Scholar]
- 73.Peña B, Martinelli V, Jeong M, Bosi S, Lapasin R, Taylor MRG, Long CS, Shandas R, Park D, Mestroni L, Biomacromolecules 2016, 17, 1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Peña B, Shandas R, Park D, J Biomed. Mater. Res. - Part A 2015, 103, 2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li Y, Poon CT, Li M, Lu TJ, Pingguan-Murphy B, Xu F, Adv. Funct. Mater 2015, 25, 5999. [Google Scholar]
- 76.Frey N, Linke A, Süselbeck T, Müller-Ehmsen J, Vermeersch P, Schoors D, Rosenberg M, Bea F, Tuvia S, Leor J, Circ. Cardiovasc. Interv 2014, 7, 806. [DOI] [PubMed] [Google Scholar]
- 77.Anker SD, Coats AJS, Cristian G, Dragomir D, Pusineri E, Piredda M, Bettari L, Dowling R, Volterrani M, Kirwan BA, Filippatos G, Mas JL, Danchin N, Solomon SD, Lee RJ, Ahmann F, Hinson A, Sabbah HN, Mann DL, Eur. Heart J 2015, 36, 2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li Z, Guan J, Polymers (Basel) 2011, 3, 740. [Google Scholar]
- 79.Camci-Unal G, Annabi N, Dokmeci MR, Liao R, Khademhosseini A, NPG Asia Mater. 2014, 6, e99–12. [Google Scholar]
- 80.Khan S, Ullah A, Ullah K, Rehman NU, Des. Monomers Polym 2016, 19, 456. [Google Scholar]
- 81.Hastings CL, Roche ET, Ruiz-Hernandez E, Schenke-Layland K, Walsh CJ, Duffy GP, Adv. Drug Deliv. Rev 2015, 84, 85. [DOI] [PubMed] [Google Scholar]
- 82.Burdick JA, Mauck RL, Gerecht S, Cell Stem Cell 2016, 18, 13. [DOI] [PubMed] [Google Scholar]
- 83.Wang H, Zhou J, Liu Z, Wang C, J Cell Mol. Med 2010, 14, 1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu Z, Chen G, Zhang J, Hua Y, Li J, Liu B, Huang A, Li H, Chen M, Ou C, Sci. Rep 2017, 7, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhou J, Chen J, Sun H, Qiu X, Mou Y, Liu Z, Zhao Y, Li X, Han Y, Duan C, Tang R, Wang C, Zhong W, Liu J, Luo Y, Mengqiu Xing M, Wang C, C. Sci. Rep 2014, 4, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Weinberger F, Mannhardt I, Eschenhagen T, Circ. Res 2017, 120, 1487. [DOI] [PubMed] [Google Scholar]
- 87.Tibbitt MW, Anseth KS, Biotechnol. Bioeng 2009, 103, 655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Anderson JM, Rodriguez A, Chang DT, Semin. Immunol 2008, 20, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Barbarash L, Kudryavtsev I, Rutkovskaya N, Golovkin A, Mediat. inflamation 2016, 2016, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhu J, Marchant RE, Expert. Rev. Med. Devices 2011, 8, 607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Faust T, Nat. Chem 2015, 7, 681. [Google Scholar]
- 92.Radhakrishnan J, Krishnan UM, Sethuraman S, Biotechnol. Adv 2014, 32, 449. [DOI] [PubMed] [Google Scholar]
- 93.Hoare TR, Kohane DS, Polymer (Guildf) 2008, 49, 1993. [Google Scholar]
- 94.Jawad H, Lyon AR, Harding SE, Ali NN, Boccaccini AR, Br. Med. Bull 2008, 87, 31. [DOI] [PubMed] [Google Scholar]
- 95.O’Brien FJ, Mater. Today 2011, 14, 88. [Google Scholar]
- 96.Dhandayuthapani B, Yoshida Y, Maekawa T, Kumar DS, Int. J Polym. Sci 2011, 2011, 19. [Google Scholar]
- 97.Ahmed EM, J Adv. Res 2015, 6, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ahearne M, Interface Focus 2014, 4, 20130038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ige OO, Umoru LE, Aribo S, ISRN Mater. Sci 2012, 2012, 1. [Google Scholar]
- 100.Asti A, Gioglio L, Int. J Artif. Organs 2014, 37, 187. [DOI] [PubMed] [Google Scholar]
- 101.Li RK, Jia ZQ, Weisel RD, Mickle DA, Choi A, Yau TM, Circulation 1999, 100, II63. [DOI] [PubMed] [Google Scholar]
- 102.Parenteau-Bareil R, Gauvin R, Berthod F, Materials (Basel) 2010, 3, 1863. [Google Scholar]
- 103.Hu K, Shi H, Zhu J, Deng D, Zhou G, Zhang W, Cao Y, Liu W, Biomed. Microdevices 2010, 12, 627. [DOI] [PubMed] [Google Scholar]
- 104.Brigham MD, Bick A, Lo E, Bendali A, Burdick JA, Khademhosseini A, Tissue Eng. Part A 2009, 15, 1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Martinelli V, Cellot G, Fabbro A, Bosi S, Mestroni L, Ballerini L, Front. Physiol 2013, 4, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shin SR, Jung SM, Zalabany M, Kim K, Zorlutuna P, Kim SB, Nikkhah M, Khabiry M, Azize M, Kong J, Wan KT, Palacios T, Dokmeci MR, Bae H, Tang X, Khademhosseini A, ACS Nano 2013, 7, 2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Roberts-Thomson KC, Kistler PM, Sanders P, Morton JB, Haqqani HM, Stevenson I, Vohra JK, Sparks PB, Kalman JM, Hear Rhythm. 2009, 6, 587. [DOI] [PubMed] [Google Scholar]
- 108.Li Y, Meng H, Liu Y, Lee BP, Sci. World J 2015, 2015,1. [Google Scholar]
- 109.Janmey PA, Winer JP, Weisel JW, J R Soc. Interface 2009, 6, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Brown AEX, Litvinov RI, Discher DE, Purohit PK, Weisel JW, Science 2009, 325, 741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang X, Wang H, Ma X, Adila A, Wang B, Liu F, Chen B, Wang C, Ma Y, Exp. Biol. Med 2010, 235, 1505. [DOI] [PubMed] [Google Scholar]
- 112.Christman KL, Vardanian AJ, Fang Q, Sievers RE, Fok HH, Lee Am RJJ. Coll. Cardiol 2004, 44, 654. [DOI] [PubMed] [Google Scholar]
- 113.Ryu JH, Kim IK, Cho SW, Cho MC, Hwang KK, Piao H, Piao S, Lim SH, Hong YS, Choi CY, Yoo KJ, Kim BS, Biomaterials 2005, 26, 319. [DOI] [PubMed] [Google Scholar]
- 114.Brougham CM, Levingstone TJ, Jockenhoevel S, Flanagan TC, O’Brien FJ, Acta Biomater. 2015, 26, 205. [DOI] [PubMed] [Google Scholar]
- 115.Zhang X, Xu B, Puperi DS, Wu Y, West JL, Grande-Allen KJ, Long Term Eff J. Med. Implants 2015, 25, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Flynn LE, Biomaterials 2010, 31, 4715. [DOI] [PubMed] [Google Scholar]
- 117.Wua J, Ding Q, Dutta A, Wang Y, Huang YH, Wenga H, Tang L, Hong Y, Acta Biomater. 2015, 16, 49. [DOI] [PubMed] [Google Scholar]
- 118.Eek C, Grenne B, Brunvand H, Aakhus S, Endresen K, Hol PK, Smith HJ, Smiseth OA, Edvardsen T, Skulstad H, Circ. Cardiovasc. Imaging 2010, 3, 187. [DOI] [PubMed] [Google Scholar]
- 119.Sawkins MJ, Bowen W, Dhadda P, Markides H, Sidney LE, Taylor AJ, Rose FRAJ, Badylak SF, Shakesheff KM, White L, J. Acta Biomater 2013, 9, 7865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cheng CW, Solorio LD, Alsberg E, Biotechnol. Adv 2014, 32, 462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Moroni F, Mirabella T, Am. J Stem Cells 2014, 3, 1. [PMC free article] [PubMed] [Google Scholar]
- 122.Yang G, Sau C, Lai W, Cichon J, Li W, Int. J Adv. Res 2016, 4, 411. [Google Scholar]
- 123.Kim IY, Seo SJ, Moon HS, Yoo MK, Park IY, Kim BC, Cho CS, Biotechnol. Adv 2008, 26, 1. [DOI] [PubMed] [Google Scholar]
- 124.Pang Y, Qin A, Lin X, Yang L, Wang Q, Wang Z, Shan Z, Li S, Wang J, Fan S, Hu Q, Oncotarget 2017, 8, 35583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liu L, Gao Q, Lu X, Zhou H, Asian J Pharm. Sci 2016, 11, 673. [Google Scholar]
- 126.Liu M, Zeng X, Ma C, Yi H, Ali Z, Mou X, Li S, Deng Y, He N, Bone Res. 2017, 5,1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ji C, Khademhosseini A, Dehghani F, Biomaterials 2011, 32, 9719. [DOI] [PubMed] [Google Scholar]
- 128.Croisier F, Jérôme C, Eur. Polym. J 2013, 49, 780. [Google Scholar]
- 129.Elgadir MA, Uddin MS, Ferdosh S, Adam A, Chowdhury AJK, Sarker MZI, J Food Drug Anal. 2015, 23, 619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hardy b., Battler A, Weiss C, Kudasi O, Raiter A, Biochem. Pharmacol 2008, 75, 891. [DOI] [PubMed] [Google Scholar]
- 131.McAdams R, McPherson R, Dabestani N, Gleason C, Juul S, Comp. Med 2010, 60, 357. [PMC free article] [PubMed] [Google Scholar]
- 132.Rinaudo M, Tip. 2014, 17, 92. [Google Scholar]
- 133.Lee KY, Mooney DJ, Prog. Polym. Sci 2012, 37, 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sun J, Tan H, Materials (Basel) 2013, 6, 1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Qi Y, Lu L, Zhou C, Luo B 3rd Int. Conf. Bioinforma. Biomed Eng iCBBE 2009. 2009, No. 51207042, 1–4. [Google Scholar]
- 136.Varilly P, Chandler D, 2014, 57, 490. [Google Scholar]
- 137.Gunatillake PA, Adhikari R, Gadegaard N, Eur. Cells. Mater 2003, 5, 1. [DOI] [PubMed] [Google Scholar]
- 138.Ozdil D, Aydin HM, J Chem. Technol. Biotechnol 2014, 89, 1793. [Google Scholar]
- 139.Place ES, George JH, Williams CK, Stevens MM, Chem. Soc. Rev 2009, 38, 1139. [DOI] [PubMed] [Google Scholar]
- 140.Do A, Khorsand B, Geary SM, Salem AK, Adv. Heal. Mater 2015, 4, 1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tan h., Marra KG, Materials (Basel) 2010, 3, 1746. [Google Scholar]
- 142.Zhu J, Biomaterials 2010, 31, 4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lin CC, Anseth KS, Pharm. Res 2009, 26, 631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Slaughter BV, Fisher OZ, Adv Mater 2009, 21, 3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Claycomb WC, Lanson NA, Stallworth BS, Egeland DB, Delcarpio JB, Bahinski A, Izzo NJ, Proc. Natl. Acad. Sci. U S A 1998, 95, 2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Schmaljohann D, Adv. Drug Deliv. Rev 2006, 58, 1655. [DOI] [PubMed] [Google Scholar]
- 147.Wu J, Zeng F, Huang XP, Chung JCY, Konecny F, Weisel RD, Li RK, Biomaterials 2011, 32, 579. [DOI] [PubMed] [Google Scholar]
- 148.Kim EH, Joo MK, Bahk KH, Park MH, Chi B, Lee YM, Jeong B, Biomacromolecules 2009, 10 2476. [DOI] [PubMed] [Google Scholar]
- 149.Gandhi A, Paul A, Sen SO, Sen KK, Asian J Pharm. Sci 2015, 10, 99. [Google Scholar]
- 150.Le PN, Pham DC, Nguyen DH, Tran NQ, Dimitrov V, Ivanov P, Xuan CN, Nguyen HN, Nguyen CK, Adv. Nat. Sci. Nanosci. Nanotechnol 2017, 8, 1. [Google Scholar]
- 151.Zhu Z, Li Y, Xu H, Peng X, Chen YN, Shang C, Zhang Q, Liu J, Wang H, ACS Appl. Mater. Interfaces 2016, 8, 15637. [DOI] [PubMed] [Google Scholar]
- 152.Nakayama M, Okano T, Miyazaki T, Kohori F, Sakai K, Yokoyama M, J Control. Release 2006, 115, 46. [DOI] [PubMed] [Google Scholar]
- 153.Coughlan DC, Quilty FP, Corrigan OI, J Control. Release 2004, 98, 97. [DOI] [PubMed] [Google Scholar]
- 154.Pinto AR, Ilinykh A, Ivey MJ, Kuwabara JT, D’antoni ML, Debuque R, Chandran A, Wang L, Arora K, Rosenthal NA, Tallquist MD, Circ Res 2016, 118, 400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Klouda L, Eur. J Pharm. Biopharm 2015, 97, 338. [DOI] [PubMed] [Google Scholar]
- 156.Kandasamy AD, Chow AK, Ali MAM, Schulz R, Cardiovasc. Res 2010, 85, 413. [DOI] [PubMed] [Google Scholar]
- 157.Liu P, Sun M, Sader S, Can. J Cardiol 2006, 22, 25B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sweitzer NK, Circulation 2003, 108, 16e. [DOI] [PubMed] [Google Scholar]
- 159.Meincke O, Kaempfer D, Weickmann H, Friedrich C, Vathauer M, Warth H, Polymer (Guildf) 2004, 45, 739. [Google Scholar]
- 160.Koerner H, Price G, Pearce NA, Alexander M, Vaia RA, Nat. Mater 2004, 3, 115. [DOI] [PubMed] [Google Scholar]
- 161.Martinelli V, Cellot G, Toma FM, Long CS, Caldwell JH, Zentilin L, Giacca M, Turco A, Prato M, Ballerini L, Mestroni L, Nano Lett. 2012, 12, 1831. [DOI] [PubMed] [Google Scholar]
- 162.Niu G, Chen X, Curr. Drug Targets 2010, 11, 1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Balint R, Cassidy NJ, Cartmell SH, Acta Biomater. 2014, 10, 2341. [DOI] [PubMed] [Google Scholar]
- 164.Ravichandran R, Sundarrajan S, Venugopal JR, Mukherjee S, Ramakrishna S, Soc JR. Interface 2010, 7, S559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zarrintaj P, Bakhshandeh B, Rezaeian I, Heshmatian B, Ganjali MR, Sci. Rep 2017, 7, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yue K, Trujillo-de Santiago G, Alvarez MM, Tamayol A, Annabi N, Khademhosseini A, Biomaterials 2015, 73, 254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Klotz BJ, Gawlitta D, Rosenberg AJWP, Malda J, Melchels FPW, Trends. Biotechnol 2016, 34, 394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Onoe H, Okitsu T, Itou A, Kato-Negishi M, Gojo R, Kiriya D, Sato K, Miura S, Iwanaga S, Kuribayashi-Shigetomi K, Matsunaga YT, Shimoyama Y, Takeuchi S, Nat. Mater 2013, 12, 584. [DOI] [PubMed] [Google Scholar]
- 169.Shirahama H, Lee BH, Tan LP, Cho NJ, Sci. Rep 2016, 6, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hirt MN, Hansen A, Eschenhagen T, Circ. Res 2014, 114, 354. [DOI] [PubMed] [Google Scholar]
- 171.Hong LTA, Kim YM, Park HH, Hwang DH, Cui Y, Lee EM, Yahn S, Lee JK, Song SC, Kim BG, Nat. Commun 2017, 8, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Li Z, Guo X, Matsushita S, Guan J, Biomaterials 2011, 32, 3220. [DOI] [PubMed] [Google Scholar]
- 173.Rastogi RP, Kumar Richa, A., Tyagi MB, Sinha RP, J Nucleic Acids 2010, 2010, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kushwaha S, Rai A, Saxena P, Int. J Pharm. Investig 2012, 2, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Tsitsilianis C, Soft. Matter 2010, 6, 2372. [Google Scholar]
- 176.Park D, Weinman CJ, Finlay JA, Fletcher BR, Paik MY, Sundaram HS, Dimitriou MD, Sohn KE, Callow ME, Callow JA, Handlin DL, Willis CL, Fischer DA, Kramer EJ, Ober CK, Langmuir 2010, 26, 9772. [DOI] [PubMed] [Google Scholar]
- 177.Park D, Shah A, Rauck BM, Friberg TR, Wang Y, Macromol. Biosci 2013, 13, 464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Rauck BM, Friberg TR, Medina Mendez CA, Park D, Shah V, Bilonick RA, Wang Y, Investig. Ophthalmol. Vis. Sci 2013, 55, 469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ritfeld GJ, Rauck BM, Novosat TL, Park D, Patel P, Roos RAC, Wang Y, Oudega M, Biomaterials 2014, 35, 1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Li J, Mooney DJ, Nat. Rev. Mater 2016, 1, 16071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Decker C, Polym. Int 2002, 51, 1141. [Google Scholar]
- 182.Nair DP, Podgórski M, Chatani S, Gong T, Xi W, Fenoli CR, Bowman CN, Chem. Mater 2014, 26, 724. [Google Scholar]
- 183.Higham AK, Bonino CA, Raghavan SR, Khan SA, Soft. Matter 2014, 10, 4990. [DOI] [PubMed] [Google Scholar]
- 184.Galateanu B, Dimonie D, Vasile E, Nae S, Cimpean A, Costache M, BMC Biotechnol. 2012, 12, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Nokhodchi A, Tailor A, Farmaco. 2004, 59, 999. [DOI] [PubMed] [Google Scholar]
- 186.Russo R, Malinconico M, Santagata G, Biomacromolecules 2007, 8, 3193. [DOI] [PubMed] [Google Scholar]
- 187.Konstam MA, Kramer DG, Patel AR, Maron MS, Udelson JE, JACC Cardiovasc. Imaging 2011, 4, 98. [DOI] [PubMed] [Google Scholar]
- 188.French BA, Kramer CM, Drug Discov. Today Dis. Mech 2007, 4, 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Muzzarelli RAA, El Mehtedi M, Bottegoni C, Aquili A, Gigante A, Mar. Drugs 2015, 13, 7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wang J, Williamson GS, Yang H, Colloids Surfaces B Biointerfaces 2018, 165, 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Tandon N, Cannizzaro C, Chao PHG, Maidhof R, Marsano A, Au HTH, Radisic M, Vunjak-Novakovic G, Nat. Protoc 2009, 4, 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Monteiro LM, Vasques-Nóvoa F, Ferreira L, Pinto-do-Ó P, Nascimento DS, npj Regen. Med 2017, 2, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ongstad EL, Gourdie RG, Semin. Cell Dev. Biol 2016, 58, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Huang G, Li F, Zhao X, Ma Y, Li Y, Lin M, Jin G, Lu TJ, Genin GM, Xu F, Chem. Rev 2017, 117, 12764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Martinelli V, Cellot G, Toma FM, Long CS, Caldwell JH, Zentilin L, Giacca M, Turco A, Prato M, Ballerini L, Mestroni L, ACS Nano 2013, 7, 5746. [DOI] [PubMed] [Google Scholar]
- 196.Wei J, Hu Y, Liang Y, Kong B, Zhang J, Song J, Bao Q, Simon GP, Jiang SP, Wang H, Adv. Funct. Mater 2015, 25, 5768. [Google Scholar]
- 197.Wu Y, Li Y, Liu P, Gardner S, Ong BS, Chem. Mater 2006, 18, 4627. [Google Scholar]
- 198.Braunersreuther V, Jaquet V, Curr. Pharm. Biotechnol 2012, 13, 97. [DOI] [PubMed] [Google Scholar]
- 199.Frangogiannis NG, Smith CW, Entman ML, Cardiovasc. Res 2002, 53, 31. [DOI] [PubMed] [Google Scholar]
- 200.Banerjee S, Saikia JP, Kumar A, Konwar BK, Nanotechnology 2010, 21. [DOI] [PubMed] [Google Scholar]; Banerjee S, Saikia JP, Kumar A, Konwar BK, Nanotechnology 2010, 21, 045101. [DOI] [PubMed] [Google Scholar]
- 201.Rašović I, Mater. Sci. Technol. (United Kingdom) 2017, 33, 777. [Google Scholar]
- 202.Melloni C, Rao SV, Povsic TJ, Melton L, Kim RJ, Kilaru R, Patel MR, Talan M, Ferrucci L, Longo DL, Lakatta EG, Najjar SS, Harrington RA, Am. Heart J 2010, 160, 795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Radisic M, Christman KL, Mayo Clin. Proc 2013, 88, 884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Jabłońska-Trypuć A, Matejczyk M, Rosochacki SJ, Enzyme Inhib. Med. Chem 2016, 31, 177. [DOI] [PubMed] [Google Scholar]