Abstract
Background:
Shensong Yangxin Capsule (SSYX), traditional Chinese medicine, has been used to treat arrhythmias, angina, cardiac remodeling, cardiac fibrosis, and so on, but its effect on cardiac energy metabolism is still not clear. The objective of this study was to investigate the effects of SSYX on myocardium energy metabolism in angiotensin (Ang) II-induced cardiac hypertrophy.
Methods:
We used 2 μl (10−6 mol/L) AngII to treat neonatal rat cardiomyocytes (NRCMs) for 48 h. Myocardial α-actinin staining showed that the myocardial cell volume increased. Expression of the cardiac hypertrophic marker-brain natriuretic peptide (BNP) messenger RNA (mRNA) also increased by real-time polymerase chain reaction (PCR). Therefore, it can be assumed that the model of hypertrophic cardiomyocytes was successfully constructed. Then, NRCMs were treated with 1 μl of different concentrations of SSYX (0.25, 0.5, and 1.0 μg/ml) for another 24 h. To explore the time-depend effect of SSYX on energy metabolism, 0.5 μg/ml SSYX was added into cells for 0, 6, 12, 24, and 48 h. Mitochondria was assessed by MitoTracker staining and confocal microscopy. mRNA and protein expression of mitochondrial biogenesis-related genes – Peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α), energy balance key factor – adenosine monophosphate-activated protein kinase (AMPK), fatty acids oxidation factor – carnitine palmitoyltransferase-1 (CPT-1), and glucose oxidation factor – glucose transporter- 4 (GLUT-4) were measured by PCR and Western blotting analysis.
Results:
With the increase in the concentration of SSYX (from 0.25 to 1.0 μg/ml), an increased mitochondrial density in AngII-induced cardiomyocytes was found compared to that of those treated with AngII only (0.25 μg/ml, 18.3300 ± 0.8895 vs. 24.4900 ± 0.9041, t = 10.240, P < 0.0001; 0.5 μg/ml, 18.3300 ± 0.8895 vs. 25.9800 ± 0.8187, t = 12.710, P < 0.0001; and 1.0 μg/ml, 18.3300 ± 0.8895 vs. 24.2900 ± 1.3120, t = 9.902, P < 0.0001; n = 5 per dosage group). SSYX also increased the mRNA and protein expression of PGC-1α (0.25 μg/ml, 0.8892 ± 0.0848 vs. 1.0970 ± 0.0994, t = 4.319, P = 0.0013; 0.5 μg/ml, 0.8892 ± 0.0848 vs. 1.2330 ± 0.0564, t = 7.150, P < 0.0001; and 1.0 μg/ml, 0.8892 ± 0.0848 vs. 1.1640 ± 0.0755, t = 5.720, P < 0.0001; n = 5 per dosage group), AMPK (0.25 μg/ml, 0.8872 ± 0.0779 vs. 1.1500 ± 0.0507, t = 7.239, P < 0.0001; 0.5 μg/ml, 0.8872 ± 0.0779 vs. 1.2280 ± 0.0623, t = 9.379, P < 0.0001; and 1.0 μg/ml, 0.8872 ± 0.0779 vs. 1.3020 ± 0.0450, t = 11.400, P < 0.0001; n = 5 per dosage group), CPT-1 (1.0 μg/ml, 0.7348 ± 0.0594 vs. 0.9880 ± 0.0851, t = 4.994, P = 0.0007, n = 5), and GLUT-4 (0.5 μg/ml, 1.5640 ± 0.0599 vs. 1.7720 ± 0.0660, t = 3.783, P = 0.0117; 1.0 μg/ml, 1.5640 ± 0.0599 vs. 2.0490 ± 0.1280, t = 8.808, P < 0.0001; n = 5 per dosage group). The effect became more obvious with the increasing concentration of SSYX. When 0.5 μg/ml SSYX was added into cells for 0, 6, 12, 24, and 48 h, the expression of AMPK (6 h, 14.6100 ± 0.6205 vs. 16.5200 ± 0.7450, t = 3.456, P = 0.0250; 12 h, 14.6100 ± 0.6205 vs. 18.3200 ± 0.9965, t = 6.720, P < 0.0001; 24 h, 14.6100 ± 0.6205 vs. 21.8800 ± 0.8208, t = 13.160, P < 0.0001; and 48 h, 14.6100 ± 0.6205 vs. 23.7400 ± 1.0970, t = 16.530, P < 0.0001; n = 5 per dosage group), PGC-1α (12 h, 11.4700 ± 0.7252 vs. 16.9000 ± 1.0150, t = 7.910, P < 0.0001; 24 h, 11.4700 ± 0.7252 vs. 20.8800 ± 1.2340, t = 13.710, P < 0.0001; and 48 h, 11.4700 ± 0.7252 vs. 22.0300 ± 1.4180, t = 15.390; n = 5 per dosage group), CPT-1 (24 h, 15.1600 ± 1.0960 vs. 18.5800 ± 0.9049, t = 6.048, P < 0.0001, n = 5), and GLUT-4 (6 h, 10.2100 ± 0.9485 vs. 12.9700 ± 0.8221, t = 4.763, P = 0.0012; 12 h, 10.2100 ± 0.9485 vs. 16.9100 ± 0.8481, t = 11.590, P < 0.0001; 24 h, 10.2100 ± 0.9485 vs. 19.0900 ± 0.9797, t = 15.360, P < 0.0001; and 48 h, 10.2100 ± 0.9485 vs. 14.1900 ± 0.9611, t = 6.877, P < 0.0001; n = 5 per dosage group) mRNA and protein increased gradually with the prolongation of drug action time.
Conclusions:
SSYX could increase myocardial energy metabolism in AngII-induced cardiac hypertrophy. Therefore, SSYX might be considered to be an alternative therapeutic remedy for myocardial hypertrophy.
Keywords: AMP-Activated Protein Kinase, Cardiac Hypertrophy, Energy Metabolism, Peroxisome Proliferator-Activated Receptor Gamma Coactivator-1 Alpha, Shensong Yangxin Capsule
摘要
背景:
作为中国的传统中药,参松养心胶囊(Shensong Yangxin Capsule, SSYX)可以用来治疗心律失常、心绞痛、心肌重 构、心肌纤维化等等,但是它对心脏能量代谢的影响还尚不明确,本研究的目的主要是探究参松养心胶囊在血管紧张度 (Angiotensin)II诱导的心肌肥大过程中对心肌细胞能量代谢的影响。
方法:
我们用2 μl (10-6mol/L) Ang(Angiotensin) II诱导Sprague-Dawley (SD)大鼠的乳鼠心肌细胞(Neonatal rat cardiomyocytes ,NRCMs)48小时,用心肌细胞的a-actinin染色观察细胞体积大小,用实时定量聚合酶链反应(Real-time Polymerase chain Reaction , PCR)检测心肌肥厚标记物BNP(Brain natriuretic peptide)mRNA表达以判断心肌细胞肥大模型 构建是否成功。然后, 用1 μl浓度梯度为0.25, 0.5, and 1.0 μg/ml的参松养心胶囊溶液处理细胞24小时。同时,为了探究参松 养心胶囊对心肌细胞能量代谢影响的时间效应,我们用0.5 μg/ml参松养心胶囊溶液分别作用细胞0,6,12,24和48小时。用 线粒体Mito-Red Tracker探针标记心肌细胞中的线粒体并用共聚焦显微镜观察线粒体密度,用PCR检测线粒体生物起源相关因 子- PGC-1α(peroxisome proliferator-activated receptor coactivator-1 alpha),能量平衡关键因子- AMPK (AMP-activated protein kinase),脂肪酸代谢因子- CPT-1(carnitine acyltransferase enzyme-1),葡萄糖氧化关键因子- GLUT-4(glucose transporter protein 4)的mRNA表达。
结果:
随着参松养心胶囊溶液药物浓度的增加,SSYX处理组的线粒体密度逐渐增多(0.25 μg/ml, 18.3300 ± 0.8895 vs. 24.4900 ± 0.9041, t = 10.240, P < 0.0001; 0.5 μg/ml, 18.3300 ± 0.8895 vs. 25.9800 ± 0.8187, t = 12.710, P < 0.0001; 1.0 μg/ml, 18.3300 ± 0.8895 vs. 24.2900 ± 1.3120, t = 9.902, P < 0.0001; 每组 n均等于5);而且,SSYX也可以促进AngII诱导心肌细胞肥大过程中能量 代谢相关因子- PGC-1α (0.25 μg/ml, 0.8892 ± 0.0848 vs. 1.0970 ± 0.0994, t = 4.319, P = 0.0013; 0.5 μg/ml, 0.8892 ± 0.0848 vs. 1.2330 ± 0.0564, t = 7.150, P < 0.0001; 1.0 μg/ml, 0.8892 ± 0.0848 vs. 1.1640 ± 0.0755, t = 5.720, P < 0.0001; 每组 n均等于5), AMPK (0.25 μg/ml, 0.8872 ± 0.0779 vs. 1.1500 ± 0.0507, t = 7.239,P < 0.0001; 0.5 μg/ml, 0.8872 ± 0.0779 vs. 1.228 ± 0.0623, t = 9.379,P < 0.0001; 1.0 μg/ml, 0.8872 ± 0.0779 vs. 1.3020 ± 0.0450, t = 11.400; ,P < 0.0001; n均等于5), CPT-1 (1.0 μg/ml, 0.7348 ± 0.0594 vs. 0.9880 ± 0.0851, t = 4.994, P = 0.0007, n = 5) 和GLUT-4 (0.5 μg/ml, 1.5640 ± 0.0599vs. 1.7720 ± 0.0660, t = 3.783, P = 0.0117; 1.0 μg/ml, 1.5640 ± 0.0599 vs. 2.0490 ± 0.1280, t = 8.808, P < 0.0001; 每组 n均等于5) 的mRNA和蛋白质表达,并且随着SSYX药物浓度的增加,这 种作用效果越发显著;当用0.5 μg/ml相同剂量的参松养心胶囊溶液分别处理细胞0,6,12,24和48小时后,AMPK (6 h, 14.6100 ± 0.6205 vs. 16.5200 ± 0.7450, t = 3.456, P = 0.0250; 12 h, 14.6100 ± 0.6205 vs. 18.3200 ± 0.9965, t = 6.720, P < 0.0001; 24 h, 14.6100 ± 0.6205 vs. 21.8800 ± 0.8208, t = 13.160, P < 0.0001; 48 h, 14.6100 ± 0.6205 vs. 23.7400 ± 1.0970, t = 16.530, P < 0.0001; 每组 n均 等于5), PGC-1α (12 h, 11.4700 ± 0.7252 vs. 16.9000 ± 1.0150, t = 7.910; 24 h, 11.4700 ± 0.7252 vs. 20.8800 ± 1.234, t = 13.710, P < 0.0001; 48 h, 11.4700 ± 0.7252 vs. 22.0300 ± 1.4180, t = 15.390, P < 0.0001; n均等于5), CPT-1 (24 h, 15.1600 ± 1.0960 vs. 18.5800 ± 0.9049, t = 6.048, P < 0.0001, n = 5), GLUT-4 (6 h, 10.2100 ± 0.9485 vs. 12.9700 ± 0.8221, t = 4.763, P = 0.0012; 12 h, 10.2100 ± 0.9485 vs. 16.9100 ± 0.8481, t = 11.590, P < 0.0001; 24 h, 10.2100 ± 0.9485 vs. 19.0900 ± 0.9797, t = 15.360, P < 0.0001; 48 h, 10.2100 ± 0.9485 vs. 14.1900 ± 0.9611, t = 6.877, P < 0.0001; 每组 n均等于5)的mRNA和蛋白质表达均随着时间的延长而逐渐增多。
结论:
参松养心胶囊可以促进血管紧张度II诱导的心肌肥大过程中心肌细胞的能量代谢,这可能成为治疗心肌肥大的新的治 疗方法。
INTRODUCTION
It is well known that myocardial hypertrophy is often accompanied by cardiac energy metabolism disorders and that myocardial metabolic disorders aggravate the pathological progression of cardiac hypertrophy to heart failure. Energy metabolism includes mitochondrial metabolism, carbohydrate metabolism, and lipid metabolism. Mitochondria are essential and critical regulators of oxidative phosphorylation.[1,2,3] Peroxisome proliferator-activated receptor gamma coactivator-1α (PGC-1α), a transcription coactivator of nuclear receptors and master regulator of metabolism, plays an important role in cardiac metabolism regulation. Previous study[4] had demonstrated that PGC-1α played an important role in the development of pathological cardiac hypertrophy, metabolic disorders, and reactivation of the fetal genetic program on animals models of chronic heart failure. PGC1-α was also involved in mitochondrial biogenesis, which was vital for cell survival. Experimental evidence[5] supported the roles of mitochondrial dysfunction and oxidative stress as determinants of neuronal death as well as endogenous protective mechanisms after stroke. Accumulating evidence suggested that activation of 5’AMP-activated protein kinase (AMPK) during myocardial ischemia increased both glucose uptake and glycolysis as well as fatty acid oxidation (FAO) during reperfusion. Gain-of-function mutations of AMPK in cardiac muscle might also be causally related to the development of hypertrophic cardiomyopathies.[6] Mitochondrial FAO is an important energy provider for cardiac work, and changes in cardiac substrate preference are associated with different heart diseases. Carnitine palmitoyltransferase-1 (CPT-1) is thought to be the rate-limiting enzyme in FAO and is inhibited by malonyl-CoA.[7] Insulin-regulated glucose transporter-4 (GLUT-4) is immunolocalized in rat cardiac muscle under conditions of basal and stimulates glucose uptake, which are achieved by fasting and a combined exercise/insulin stimulus, respectively.[8] Mitochondrial dysfunction has been demonstrated to contribute to heart disease and its clinical manifestations, and damaged mitochondrial homeostasis might lead to heart failure over time.[9] Consequently, exploration of a new therapeutic strategy to improve mitochondrial function and energy metabolism may have great clinical significance.
The Shensong Yangxin Capsule (SSYX) was designed and carefully formulated in accordance with the rules of traditional Chinese medicine (TCM) and includes Panax ginseng, Salvia miltiorrhiza, Nardostachys jatamansi, and so on.[10] According to TCMs, SSYX has long been used in China as a traditional Chinese remedy to treat a variety of cardiac diseases, especially to treat cardiac arrhythmias and various arrhythmias in coronary heart disease and chronic heart failure,[6,11,12,13] angina in coronary heart disease,[14,15] cardiac remodeling, cardiac fibrosis, regulation of lipid levels, and so on. The above studies have revealed the positive role of SSYX in cardiac energy metabolism, providing the experimental basis for our research. SSYX might block multiple ion channels,[16] which might change the action potential duration and contribute to its antiarrhythmic effects.[17,18] SSYX suppressed transforming growth factor-β1/Smad signaling to inhibit fibrosis in diabetic cardiomyopathy and improved cardiac function.[19] SSYX could block the Smad signaling pathway to attenuate cardiac remodeling after myocardial infarction.[11] However, the influence of SSYX on myocardium energy metabolism remains unclear. In this study, we studied the regulatory effects of different SSYX drug concentrations on myocardial energy metabolism during AngII-induced cardiac hypertrophy using neonatal rat cardiomyocytes (NRCMs). We particularly focused on the mitochondrial density, glucose metabolism, fatty acid metabolism, and deciphering the underlying molecular mechanisms. Our results might provide a significant therapeutic strategy for ameliorating mitochondrial function through the use of SSYX.
METHODS
Cell culture and treatment
All experiments involving animals were conducted in accordance with the 8th Edition of the Guide for the Care and Use of Laboratory Animals (Guide NRC, 2011) published by the US National Institutes of Health and were approved by the Animal Care and Use Committee of Renmin Hospital at Wuhan University (Production license: SCXK 2015–0018). All animals used in this study were postnatal days 1–3 Sprague-Dawley (SD) rats, which were supplied by the Experiment Animal Centre of Wuhan University. We also followed the methods reported in the literature;[20] NRCMs were isolated from the hearts of SD rat pups on postnatal days 1–3 and were cultured to <98% purity.[21] Briefly, SD rats were sacrificed by swift decapitation, and the hearts were excised and minced immediately. Heart tissues were digested in a solution containing 0.08% collagenase Type II and 0.125% trypsin, and the supernatant was collected. The process was repeated 7–10 times. Then, cardiomyocytes were enriched via differential attachment, plated, and cultured in a culture dish with DMEM/F12 medium (Gibco, Grand Island, NY, USA) containing 15% fetal bovine serum (Gibco, Grand Island, NY, USA), 1% penicillin/streptomycin, and 0.1 mmol/L BrdU (Sigma-Aldrich, St. Louis, MO, USA).[22,23] After culturing for 48 h, the culture medium was replaced with complete medium for 12 h. Then, cardiomyocytes were randomly assigned into five groups and subsequently subjected to different treatments. These groups were as follows: control, 10−6 mol/L angiotensin (Ang) II (Sigma-Aldrich, St. Louis, MO, USA), 10−6 mol/L AngII + SSYX (0.25 μg/ml), 10−6 mol/L AngII + SSYX (0.5 μg/ml), and 10−6 mol/L AngII + SSYX (1.0 μg/ml). SSYX, provided by Shijiazhuang Yiling Pharmaceutical Co., Ltd. (Shijiazhuang, Hebei, China), was diluted in culture media to various concentrations (0.25, 0.5, and 1.0 μg/ml).
Real-time polymerase chain reaction
Real-time polymerase chain reaction (PCR) was performed on a StepOne TM Real-Time PCR Instrument (Life technologies, ShangHai, China). Each sample was used for 3 replicates using a SYBR® Premix Ex Taq™ Kit (TaKaRa). The primers were prepared by Wuhan Jin Kairui Bioengineering Co., Ltd. For synthesis, the following primers were used: β-actin forward: 5’-CACGATGGAGGGGCCGGACTCATC-3’; reverse: 5’-TAAAGACCTCTATGCCAACACAGT-3’; the gene information of the other factors is shown in [Table 1].
Table 1.
Gene information for some factors
| Gene | Species | Forward | Reverse |
|---|---|---|---|
| BNP | Rat | 5‘- AGCCAGTCTCCAGAACAATCC -3’ | 5‘- CGGTCTATCTTCTGCCCAAA -3’ |
| Capase-3 | Rat | 5‘- TGGAATGTCAGCTCGCAATG -3’ | 5‘- CAGGTCCGTTCGTTCCAAAA -3’ |
| PGC-1α | Rat | 5‘-GGAGCAATAAAGCAAAGCGCA-3’ | 5‘- GTGTGAGGAGGGTCATCGTT-3’ |
| AMPK | Rat | 5’-ACA GAA GCC AAA TCA GGG ACT-3’ | 5’-CAC GGA TGA GGT AAG AGA GAC T-3’ |
| CPT-1 | Rat | 5’-CCA GGC AAA GAG ACA GAC TTG-3’ | 5’-GCCAAACCTTGAAGAAGCGA-3’ |
| GLUT-4 | Rat | 5‘-GTATGTTGCGGATGCTATGG -3’ | 5‘-CCTCTGGTTTCAGGCACTCT -3’ |
BNP: Brain natriuretic peptide; Capase-3: Cysteiny aspartate specific proteinase-3; PGC-1α: Peroxisome proliferator-activated receptor coactivator 1 alpha; AMPK: AMP-activated protein kinase; CPT-1: Carnitine acyltransferase enzyme-1; GLUT-4: Glucose transporter protein-4.
Western blotting analysis
TBS buffer solution was used to moisten the adherent cells 2–3 times, and the last time, the residual liquid was removed. A suitable volume of total protein extraction reagent (with proteinase inhibitor added for a few minutes) was added for 3–5 min to the culture plate/bottle. The plate/bottle was shaken repeatedly during this period to ensure that the reagent fully contacted the cells. The cells and reagents were scraped with cell scrapers and then collected in 1.5 ml centrifuge tubes. The sample was placed on an ice bath 30 min, and a pipette was used repeatedly to ensure that the cells were completely lysed. Centrifuged for 5 minutes at 4°C and 13000×g with the following primary antibodies: AMPKα1 (1:200 dilution, sc-130394, Santa Cruz Biotechnology), p-AMPK (1:1000 dilution, YT639-FIW, Baiao Bolai, BeiJing), PGC-1α (1:500 dilution, sc-518025, Santa Cruz Biotechnology), CPT-1 (1:200 dilution, sc-514555, Santa Cruz Biotechnology), and GLUT-4 (1:1000 dilution, sc-53566, Santa Cruz Biotechnology). The supernatant was collected and was considered the total protein solution. A BCA protein (Beyotime, Nanjing, China) concentration assay kit was used to determine the concentration of the sample protein. The protein concentration was also determined by SDS-PAGE electrophoresis. The film was scanned and archived, and the AlphaEaseFC software (Alpha Innotech, California, USA) processing system was used to analyze the optical density of the target band.
Alpha-actinin staining detects myocardial cell size
The NRCM cell suspension was added to a cover glass and cultured in an incubator with a CO2 concentration of 5% at 37°C for 2 h. Then, 2 ml of the cell culture solution was added and incubated for approximately 6 h. Cells were then fixed with 4% polyoxymethylene for 30 min. To prevent the flow of antibodies, the creeping piece was dried slightly, and then, a uniform position in the middle cell of the cover glass was drawn with a histochemical pen, adding 50–100 μl of working fluid to break the membrane, followed by incubation for 10 min at room temperature. A 3% hydrogen peroxide solution was added to the ring and was incubated for 20 min at room temperature. The glass was washed with PBS (pH 7.4) 3 times and placed on a shaking table. After drying, the cells were covered with 5% bovine serum albumin (BSA). The cells were laid in a wet box at 4°C overnight. The glass was washed in PBS (pH 7.4) 3 times on a shaking table and washed for 5 min each time. After dripping, the glass was incubated for 50 min at room temperature with two anti-covering cells from a kit. Each slice was placed in 50–100 μl of DAPI dye and incubated at room temperature for 5 min. A proper amount of anti-fluorescence quenching agent was added to the cells, and the cover glass slide was sealed and observed under a fluorescence microscope.
MitoTracker staining and confocal microscopy
NRCMs were plated in chamber slides (BD Bioscience, Sparks, MD, USA) at a density of 1 × 106 cells/well and treated with or without SSYX for 48 h. Cells were then stained with MitoTracker® Red CMXRos (Yisheng Biological Technology, Shanghai, China) at a concentration of 300 nmol/L diluted in prewarmed culture media for 30 min. MitoTracker® Red CMXRos contains a weak thiol-reactive chloromethyl functional group labeled with mitochondria that can label mitochondria in cardiomyocytes and is used to observe the number of mitochondria in cells. Then, cells were fixed in 4% formaldehyde in prewarmed culture media and stained with DAPI staining solution (Beyotime, China) to mark cell nuclei. A total of 20 fields/well (×400) were taken by a confocal microscope (Carl Zeiss, Thuringia, Germany). MitoTracker® Red CMXRos make mitochondria to emit a red fluorescence under a confocal microscope. Therefore, we were able to judge the number and density of mitochondria based on the amount of red fluorescence. The cell size and fluorescence intensity were measured by ImageJ (National Institutes of Health, NIH, USA) and Zeiss software (Carl Zeiss Jena, Germany), respectively.
Statistical analysis
Statistical analyses and all calculations were performed using GraphPad Prism version 5.0 statistical software (GraphPad Software, San Diego, CA, USA). All experimental data were expressed as the mean ± standard deviation. One-way analysis of variance (ANOVA; three or more groups) was used for intergroup comparison. A value of P < 0.05 was considered statistically significant.
RESULTS
Shensong Yangxin Capsule attenuates the myocardial hypertrophy and myocardial apoptosis induced by Angiotensin II in vivo
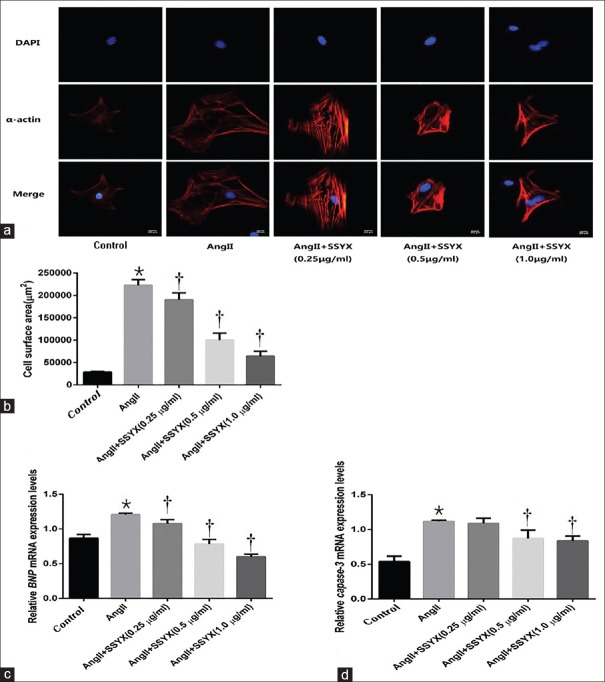
To evaluate the effect of the SSYX on the myocardial hypertrophy and apoptosis induced by AngII in vivo, NRCMs were cultured for 48 h, 10−6 mol/L AngII was added to the experimental group for 24 h, and α-actinin staining was used to observe myocardial hypertrophy in each group [Figure 1a and 1b]. PCR was used to detect the mRNA expression of the cardiomyocyte hypertrophy marker – brain natriuretic peptide (BNP) [Figure 1c] and cardiomyocyte apoptosis factor marker – Capase-3 [Figure 1d]. Comparing with the control group, cell volume in AngII group (28,369 ± 1783 μm2 vs. 222,400 ± 12,602 μm2, t = 24.380, P < 0.0001, n = 5) increased. Comparing with the control group, the expression of BNP and Capase-3 mRNA in AngII group (0.8654 ± 0.0542 vs. 1.2060 ± 0.0206, t = 10.730, P < 0.0001; 0.5400 ± 0.0758 vs. 1.1170 ± 0.0160, t = 11.740, P < 0.0001, n = 5 per dosage group) increased. The cardiac hypertrophy model was successfully constructed. Then, the cells were treated with 0.25, 0.5, and 1.0 μg/ml SSYX for 12 h. Comparing with the AngII group, cell volume in 0.25 μg/ml SSYX (222,400 ± 12,602 μm2 vs. 190,000 ± 15,811 μm2, t = 4.070, P = 0.0024, n = 5), 0.5 μg/ml SSYX (222,400 ± 12,602 μm2 vs. 100,000 ± 15,811 μm2, t = 15.380, P < 0.0001, n = 5), and 1.0 μg/ml SSYX (22,2400 ± 12,602 μm2 vs. 64,000 ± 11,402 μm2, t = 19.900, P < 0.0001, n = 5) groups decreased. Comparing with the AngII group, the expression of BNP and Capase-3 mRNA in 0.25 μg/ml SSYX (1.2060 ± 0.0206 vs. 1.0770 ± 0.0581, t = 4.072, P = 0.0024; 1.1170 ± 0.0160 vs. 1.0880 ± 0.0739, t = 0.5942, P > 0.9999; n = 5, respectively), 0.5 μg/ml SSYX (1.2060 ± 0.0206 vs. 0.7814 ± 0.0684, t = 13.370, P < 0.0001; 1.1170 ± 0.0160 vs. 0.8724 ± 0.1181, t = 4.977, P = 0.0007; n = 5, respectively), and 1.0 μg/ml SSYX (1.2060 ± 0.0206 vs. 0.5992 ± 0.0348, t = 19.110, P < 0.0001; 1.1170 ± 0.0160 vs. 0.8364 ± 0.0692, t = 5.710, P = 0.0001; n = 5, respectively) decreased. Studies have shown that only when the concentration of SSYX drug reached 0.5 μg/ml could reduce the expression of Capase-3 mRNA and resist the apoptosis of cardiomyocytes induced by AngII. However, low concentrations of SSYX (0.25 μg/ml) have been able to reduce the expression of BNP mRNA and alleviate AngII-induced cardiomyocyte hypertrophy, and its effect was positively related to the drug concentration.
Figure 1.
Myocardial hypertrophy and apoptosis in the control, AngII, SSYX (0.25, 0.5, and 1.0 μg/ml drug concentration) groups. (a) Representative immunofluorescent (α-actinin) images of NRCMs in each group. Bar = 200 μm. Red staining denotes α-actinin-stained cardiomyocytes. Blue staining denotes DAPI-stained myocardial cell nuclei. (b) Summarized data of surface area of myocardial cells in each group. (c) Relative expression levels of BNP mRNA in each group. (d) Relative expression levels of Capase-3 mRNA in each group. *P < 0.0001 versus the control group; †P < 0.05 versus the AngII group. The data are expressed as the mean ± SD (n = 5, samples were collected from five different passages). The symbols “*, †” indicate that the value of cell volume and BNP and Capase-3 mRNA expression significantly differed from different groups. BNP: Brain natriuretic peptide; Capase-3: Cysteiny aspartate specific proteinase-3; mRNA: Messenger RNA; Ang: Angiotensin; SSYX: Shensong Yangxin Capsule; NRCMs: Neonatal rat cardiomyocytes; SD: Standard deviation.
Shensong Yangxin Capsule protects the mitochondrial density in the process of Angiotensin II-induced cardiac hypertrophy
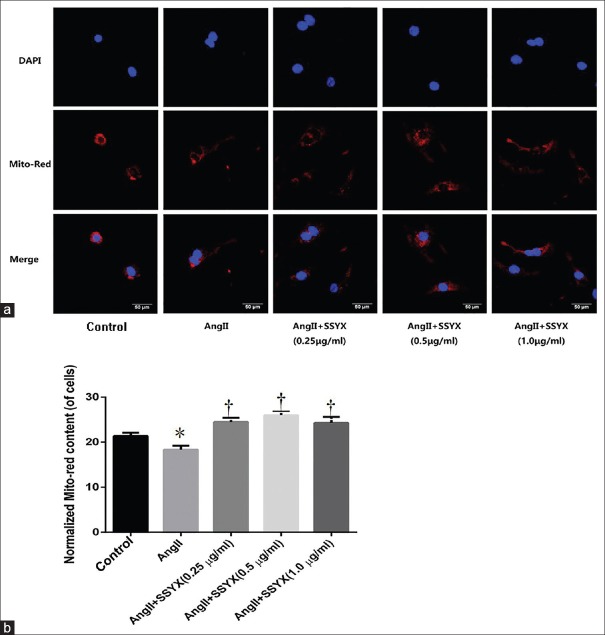
To evaluate the protective effect of SSYX on mitochondria, AngII-induced cells were treated with SSYX at different concentrations of 0.25, 0.5, and 1.0 μg/ml for 12 h. Then, 300 μmol/L preheated mitochondrial MitoTracker Red (40741ES50, Yisheng Biological Technology Co., Ltd. Shanghai) was added to the cell plates for 30 min, and the cells were observed with a confocal microscope [Figure 2a]. We found that the number of mitochondria decreased after adding 10−6 mol/L AngII compared with the control group (21.3700 ± 0.7222 vs. 18.3300 ± 0.8895, t = 5.045, P = 0.0002, n = 5; Normalized MitoTracker Red content, Figure 2b). However, when different concentrations of SSYX were added to AngII-induced cells, the number of mitochondria in cardiomyocytes began to increase. When the different concentrations (0.25 μg/ml, 0.5 μg/ml, and 1.0 μg/ml) of SSYX were compared, it was interesting to note that the number of mitochondria increased with increasing concentrations of SSYX (0.25 μg/ml,18.3300 ± 0.8895 vs. 24.4900 ± 0.9041, t = 10.240, P < 0.0001;0.5 μg/ml,18.3300 ± 0.8895 vs. 25.9800 ± 0.8187, t = 12.710, P < 0.0001; and 1.0 μg/ml,18.3300 ± 0.8895 vs. 24.2900 ± 1.3120, t = 9.902, P < 0.0001; n = 5 per dosage group). Therefore, we concluded that SSYX had a protective effect on the mitochondrial density in AngII-induced cardiac hypertrophy. This effect became more obvious as the concentration of SSYX increased.
Figure 2.
Mitochondrial density in AngII-induced cardiomyocyte hypertrophy 12 h after SSYX treatment with different drug concentrations. (a) Representative fluorescence microscope image of cardiomyocytes costained with mitochondrial Mito-Red Tracker and myocardial cell nuclei DAPI staining at 12 h after treatment with 0.25, 0.5, and 1.0 μg/ml SSYX. Scale bars = 50 μm. Red staining denotes Mito-Red Tracker-stained mitochondria. Blue staining denotes DAPI-stained myocardial cell nuclei. (b) Normalized MitoTracker Red content. *P = 0.0002 versus control group; †P < 0.0001 versus AngII group. The data were expressed as the mean ± SD (n = 5, samples were collected from five different passages), the symbols “*, †” indicate that the value of Mito-Red Tracker content significantly differed from different groups. Ang: Angiotensin; SSYX: Shensong Yangxin Capsule; SD: Standard deviation.
Shensong Yangxin Capsule regulates mitochondrial biogenesis and energy balance with peroxisome proliferator-activated receptor gamma coactivator-1 alpha and AMP-activated protein kinase activation
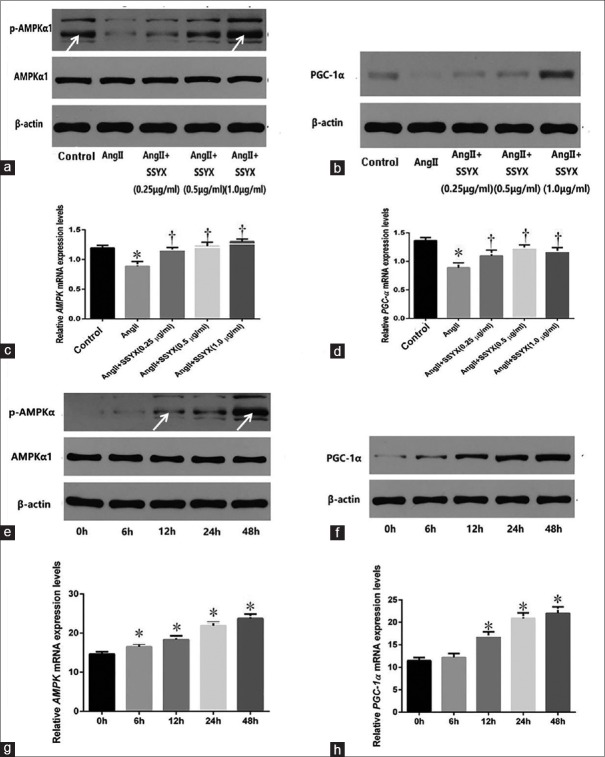
To explore the effects of SSYX on mitochondrial biogenesis and energy balance, we examined the mRNA and protein expression of the mitochondrial biological origin and energy balance key factors – PGC-1α and AMPK – after treating cells with the above concentrations of SSYX (0.25 μg/ml, 0.5 μg/ml, and 1.0 μg/ml) [Figure 3a–3d]. PGC-1α and AMPK mRNA were detected by PCR. PGC-1α, p-AMPK, and AMPK protein expressions were detected by Western blotting analysis. As shown in Figure 3c, the expression of AMPK mRNA decreased in AngII group compared with the control group (1.1940 ± 0.0447 vs. 0.8872 ± 0.0779, t = 8.450, P < 0.0001, n = 5), while it increased in 0.25 μg/ml SSYX (0.8872 ± 0.0779 vs. 1.1500 ± 0.0507, t = 7.239, P < 0.0001, n = 5), 0.5 μg/ml SSYX (0.8872 ± 0.0779 vs. 1.2280 ± 0.0623, t = 9.379, P < 0.0001, n = 5), and 1.0 μg/ml SSYX (0.8872 ± 0.0779 vs. 1.3020 ± 0.0450, t = 11.400, P < 0.0001, n = 5) groups compared with AngII group. The expression of PGC-1α mRNA decreased in AngII group compared with the control group (1.3640 ± 0.0545 vs. 0.8892 ± 0.0848, t = 9.865, P < 0.0001, n = 5), while it increased in 0.25 μg/ml SSYX (0.8892 ± 0.0848 vs. 1.0970 ± 0.0994 t = 4.319, P = 0.0013, n = 5), 0.5 μg/ml SSYX (0.8892 ± 0.0848 vs. 1.2330 ± 0.0564, t = 7.150, P < 0.0001, n = 5), and 1.0 μg/ml SSYX (0.8892 ± 0.0848 vs. 1.1640 ± 0.0755, t = 5.720, P < 0.0001, n = 5) groups compared with AngII group. To explore time-depend effect of SSYX on mitochondrial biogenesis and energy balance, 0.5 μg/ml SSYX was added into cells for 0, 6, 12, 24, and 48 h [Figure 3e–3h]. The expression of AMPK and PGC-1α mRNA presented an upward trend in different hours (AMPK: 6 h, 14.6100 ± 0.6205 vs. 16.5200 ± 0.7450, t = 3.456, P = 0.0250; 12 h, 14.6100 ± 0.6205 vs. 18.3200 ± 0.9965, t = 6.720, P < 0.0001; 24 h, 14.6100 ± 0.6205 vs. 21.8800 ± 0.8208, t = 13.160, P < 0.0001; and 48 h, 14.6100 ± 0.6205 vs. 23.7400 ± 1.097, t = 16.530, P < 0.0001; n = 5 per dosage group;PGC-1α: 12 h, 11.4700 ± 0.7252 vs 16.9000 ± 1.0150, t = 7.910, P < 0.0001; 24 h, 11.4700 ± 0.7252 vs. 20.8800 ± 1.2340, t = 13.710, P < 0.0001; and 48 h, 11.4700 ± 0.7252 vs. 22.0300 ± 1.4180, t = 15.390, P < 0.0001; n = 5 per dosage group). In summary, SSYX increased the mRNA and protein expression of AMPK and PGC-1α in a dose-dependent manner. Compared with different hours (from 0 to 48 h), the effect was becoming more and more remarkable. Therefore, SSYX could improve mitochondrial biogenesis and energy balance in cardiac hypertrophy.
Figure 3.
Expression of PGC-1α and AMPK mRNAs and protein in AngII-induced cardiac hypertrophy. (a) Expression of p-AMPK and AMPK protein in the control, AngII, and SSYX (0.25, 0.5, and 1.0 μg/ml) groups. (b) Expression of PGC-1α protein in each group. (c) Relative expression levels of AMPK mRNA in groups. (d) Relative expression levels of PGC-1α mRNA in groups. (e) Expression of p-AMPK and AMPK protein treated with 0.5 μg/ml SSYX for 0, 6, 12, 24, and 48 h. (f) Expression of PGC-1α protein in groups. (g) Relative expression levels of AMPK mRNA in groups; (h) relative expression levels of PGC-1α mRNA in groups. *P < 0.0001 versus control group; †P < 0.05 versus AngII group; *P < 0.0001 versus 0 h; the data are expressed as the mean ± SD (n = 5, samples were collected from five different passages). The symbols “*, †” indicate that the value of PGC-1α and AMPK mRNA expression significantly differed from those of different groups and different durations. p-AMPK: Activity AMP-activated protein kinase; AMPK: AMP-activated protein kinase; PGC-1α: Peroxisome proliferator-activated receptor coactivator 1 alpha; mRNA: Messenger RNA; Ang: Angiotensin; SD: Standard deviation; SSYX: Shensong Yangxin Capsule.
Shensong Yangxin Capsule regulates fatty acids and glucose oxidation during the process of cardiac hypertrophy
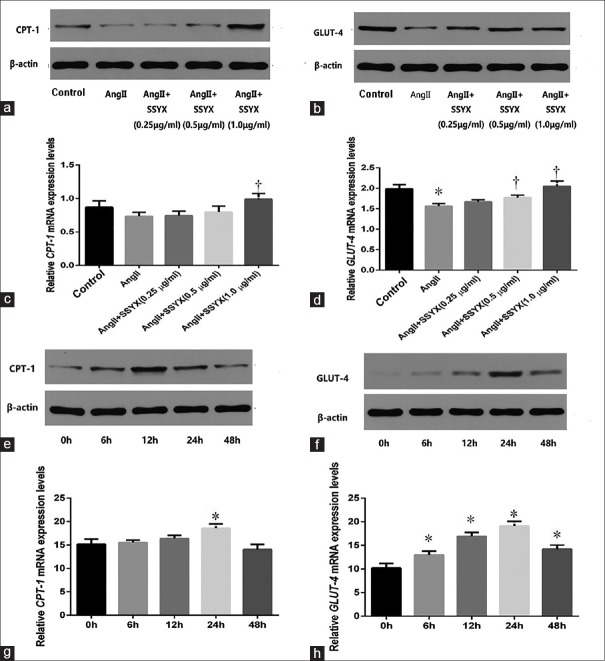
To explore the effects of SSYX on fatty acids and glucose oxidation, we examined the mRNA and protein expression of the fatty acid and glucose oxidation key factors – CPT-1 and GLUT-4 – after treating cells with 0.25 μg/ml, 0.5 μg/ml, and 1.0 μg/ml SSYX [Figure 4a–4d]. CPT-1 and GLUT-4 mRNA were detected by PCR. CPT-1 and GLUT-4 protein expression were detected by Western blotting analysis. As shown in Figure 4c, adding AngII into cardiomyocytes, the decrease of CPT-1 mRNA expression was not statistically significant compared with the control group. During the process of AngII-induced cardiac hypertrophy, the increase of CPT-1 mRNA expression in 0.25 and 0.5 μg/ml SSYX group was not statistically significant compared with AngII group. Studies have shown that lipid metabolism was only affected when the concentration of SSYX reached 1.0 μg/ml (0.7348 ± 0.0594 vs. 0.9880 ± 0.0851, t = 4.994, P = 0.0007, n = 5), 0.25, and 0.5 μg/ml SSYX had less effect on lipid metabolism. Different from fatty acid metabolism, in the process of AngII-induced cardiomyocyte hypertrophy, the expression of GLUT-4 mRNA was decreased and glucose metabolism was weakened. When 0.25 μg/ml SSYX was used to treat cardiac hypertrophy, the effect on glucose metabolism was small, and the increase of GLUT-4 mRNA expression was not statistically significant compared with AngII group. Only when the concentration of SSYX reached 0.5 μg/ml, its effect on glucose metabolism would appear. At this time, the expression of GLUT-4 mRNA in the 0.5 μg/ml SSYX group (1.5640 ± 0.0599 vs. 1.7720 ± 0.0660, t = 3.783, P = 0.0117, n = 5) and 1.0 μg/ml SSYX group (1.5640 ± 0.0599 vs. 2.0490 ± 0.1280, t = 8.808, P < 0.0001, n = 5) increased. SSYX increased mRNA and protein expression of CPT-1 and GLUT-4 with the increasing drug concentration. To explore time-depend effect of SSYX on fatty acids and glucose oxidation, 0.5 μg/ml SSYX was added into cells for 0, 6, 12, 24, and 48 h [Figure 4e–4h]. The results showed that the effect of 0.5 μg/ml SSYX on the fatty acid metabolism of cardiomyocytes had no obvious regularity with time, only when the action time reached 24 h, and the expression of CPT-1 mRNA was statistically significant (15.1600 ± 1.0960 vs. 18.5800 ± 0.9049, t = 6.048, P < 0.0001, n = 5). The effect of 0.5 μg/ml SSYX on the glucose metabolism of cardiomyocytes became more and more significant with time, and the expression of GLUT-4 mRNA was measured at 0, 6 (10.2100 ± 0.9485 vs. 12.9700 ± 0.8221, t = 4.763, P = 0.0012, n = 5), 12 (10.2100 ± 0.9485 vs. 16.9100 ± 0.8481, t = 11.590, P < 0.0001, n = 5), 24 (10.2100 ± 0.9485 vs. 19.0900 ± 0.9797, t = 15.360, P < 0.0001, n = 5), and 48 (10.2100 ± 0.9485 vs. 14.1900 ± 0.9611, t = 6.877, P < 0.0001, n = 5) h. Compared with different hours (from 0 to 48 h), the effect was becoming more and more remarkable, but when the action time reached 48 h, both CPT-1 and GLUT-4 mRNA and protein expression decreased, which suggested that glucose metabolism and fatty acid metabolism were both weakened at this time. In conclusion, SSYX can improve fatty acid metabolism and glucose metabolism in cardiac hypertrophy and it needs to reach the appropriate dose and time.
Figure 4.
Expression of CPT-1 and GLUT-4 mRNAs and protein in AngII-induced cardiac hypertrophy. (a) Expression of CPT-1 protein in the control, AngII, and SSYX (0.25, 0.5, and 1.0 μg/ml) groups. (b) Expression of GLUT-4 protein in each group. (c) Relative expression levels of CPT-1 mRNA in each group. (d) Relative expression levels of GLUT-4 mRNA in groups. (e) Expression of CPT-1 protein treated with 0.5 μg/ml SSYX for 0, 6, 12, 24, and 48 h. (f) Expression of GLUT-4 protein in each group. (g) Relative expression levels of CPT-1 mRNA in each group. (h) Relative expression levels of GLUT-4 mRNA in each group. *P < 0.0001 versus control group; †P < 0.05 versus AngII group; *P < 0.05 versus 0 h; the data are expressed as the mean ± SD (n = 5, samples were collected from five different passages). The symbols “*, †” indicate that the value of CPT-1 and GLUT-4 mRNA expression significantly differed from different groups and durations. CPT-1: Carnitine acyltransferase enzyme-1; GLUT-4: Glucose transporter protein-4; mRNA: Messenger RNA; Ang: Angiotensin; SSYX: Shensong Yangxin Capsule; SD: Standard deviation.
DISCUSSION
This study revealed that SSYX not only can reduce the cardiomyocyte hypertrophy and apoptosis induced by AngII but also can protect the mitochondrial density in hypertrophic cardiomyocytes in a dose-dependent manner (from 0.25 to 1.0 μg/ml). In addition, SSYX improved energy metabolism by activating the expression of related factors in AngII-induced cardiac hypertrophy, such as mitochondrial biogenesis factor – PGC-1α, energy balance factor – AMPK, fatty acid metabolism factor – CPT-1, and glucose metabolism factor – GLUT-4. Moreover, these effects need appropriate drug action time and drug concentration. We found that only when the concentration of SSYX reached 1.0 μg/ml, lipid metabolism was affected, and 0.25 and 0.5 μg/ml SSYX had less effect on it. Unlike fatty acid metabolism, when the concentration of SSYX reached 0.5 μg/ml, the expression of GLUT-4 mRNA was increased and its effect on glucose metabolism had appeared. SSYX not only increased mRNA and protein expression of CPT-1 and GLUT-4, but the effect increased with the increasing drug concentration. In order to observe the best action time, we chose the same concentration of SSYX (0.5 μg/ml) for different time (from 0 to 48 h); the effect on mitochondrial biogenesis and energy balance was becoming more and more remarkable. However, when the action time reached 48 h, both CPT-1 and GLUT-4 mRNA and protein expression decreased, which suggests that glucose and fatty acid metabolism were both weakened at this time. It was gratifying that our findings were not only consistent with our expectations but also consistent with previous experiments.
Mitochondrial biogenesis is regulated by a complex regulatory system. Peroxisome proliferator-activated receptor coactivator 1 alpha (PGC-1α) is a critical regulator that participates in mitochondrial biogenesis and oxidative metabolism in the heart.[24,25] Some reports have shown that the mitochondrial function is increased by the activation of PGC-1α.[26] AMP-activated protein kinase (AMPK) is a key modulator of lipid and glucose metabolism and energy balance. When the ATP levels are reduced, AMPK is rapidly activated and becomes involved in cell energy regulation.[27] The current study confirmed that SSYX activated AMPK and upregulated the expression of PGC-1α compared with the control group. Myocardial remodeling can reduce the oxygen level in myocardial tissue and affects substrate oxidation, thus resulting in a reduction of the oxidative phosphorylation efficiency.[2] In this experiment, SSYX increased the expression levels of carnitine acyltransferase enzyme-1 (CPT-1) and glucose transporter protein-4 (GLUT-4) in cardiac hypertrophy, which were key factors of fatty acid and glucose oxidation, respectively. These studies illustrated that SSYX promoted the oxidation of fatty acids and glucose and protected cardiac myocytes from energy deficiency.
However, the experiments still have many limitations. This study did not deeply investigate the specific mechanism SSYX regulated the mitochondria, glucose metabolism, and fatty acid metabolism. We did not conduct animal experiments to verify our results. Although SSYX at different drug concentrations was used in this study, due to the limited number of experimental groups, the optimal concentration of SSYX for regulating myocardial energy metabolism was not explored. In addition, SSYX itself is a compound preparation of TCM, so we did not study which specific component of SSYX regulates energy metabolism. These experiments will be the next stage of our research. We are fully aware that many issues still need to be resolved, but this study is still significant.
We proposed and demonstrated for the first time that SSYX regulated myocardial energy metabolism. SSYX not only reduced AngII-induced cardiac hypertrophy and cardiomyocyte apoptosis but also regulated the myocardial mitochondrial density and energy metabolism in cardiac hypertrophy. The mitochondrion is the main organelle of oxidative phosphorylation. Mitochondrial density is relatively reduced during cardiac hypertrophy, so oxidative phosphorylation of the myocardium produces insufficient energy to meet the needs of hypertrophic cardiomyocytes, which leads to myocardial dysfunction and aggravates myocardial injury. We demonstrated that SSYX increased the myocardial mitochondrial density while cardiac hypertrophy, which implied that SSYX increased the capacity of the oxidative phosphorylation of cells during myocardial hypertrophy, improved myocardial function and slow or even reversed the progression of cardiac hypertrophy to heart failure. These results provided evidence of a new treatment for cardiac hypertrophy and heart failure.
In summary, SSYX can increase myocardial energy metabolism in AngII-induced cardiac hypertrophy. SSYX can be considered to be an alternative therapeutic remedy for myocardial hypertrophy.
Financial support and sponsorship
This study was supported by a grant from the National Natural Science Foundation of China (No. 81670363).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Peng Lyu
REFERENCES
- 1.Warda M, Kim HK, Kim N, Ko KS, Rhee BD, Han J, et al. A matter of life, death and diseases: Mitochondria from a proteomic perspective. Expert Rev Proteomics. 2013;10:97–111. doi: 10.1586/epr.12.69. doi: 10.1586/epr.12.69. [DOI] [PubMed] [Google Scholar]
- 2.Jiao P, Ma J, Feng B, Zhang H, Diehl JA, Chin YE, et al. FFA-induced adipocyte inflammation and insulin resistance: Involvement of ER stress and IKKβ pathways. Obesity (Silver Spring) 2011;19:483–91. doi: 10.1038/oby.2010.200. doi: 10.1038/oby.2010.200. [DOI] [PubMed] [Google Scholar]
- 3.Park KS, Wiederkehr A, Wollheim CB. Defective mitochondrial function and motility due to mitofusin 1 overexpression in insulin secreting cells. Korean J Physiol Pharmacol. 2012;16:71–7. doi: 10.4196/kjpp.2012.16.1.71. doi: 10.4196/kjpp.2012.16.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lauritzen KH, Kleppa L, Aronsen JM, Eide L, Carlsen H, Haugen ØP, et al. Impaired dynamics and function of mitochondria caused by mtDNA toxicity leads to heart failure. Am J Physiol Heart Circ Physiol. 2015;309:H434–49. doi: 10.1152/ajpheart.00253.2014. doi: 10.1152/ajpheart.00253.2014. [DOI] [PubMed] [Google Scholar]
- 5.Kulikova TG, Stepanova OV, Voronova AD, Valikhov MP, Sirotkin VN, Zhirov IV, et al. Pathological remodeling of the myocardium in chronic heart failure: Role of PGC-1α. Bull Exp Biol Med. 2018;164:794–7. doi: 10.1007/s10517-018-4082-1. doi: 10.1007/s10517-018-4082-1. [DOI] [PubMed] [Google Scholar]
- 6.Chen SD, Yang DI, Lin TK, Shaw FZ, Liou CW, Chuang YC, et al. Roles of oxidative stress, apoptosis, PGC-1α and mitochondrial biogenesis in cerebral ischemia. Int J Mol Sci. 2011;12:7199–215. doi: 10.3390/ijms12107199. doi: 10.3390/ijms12107199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sambandam N, Lopaschuk GD. AMP-activated protein kinase (AMPK) control of fatty acid and glucose metabolism in the ischemic heart. Prog Lipid Res. 2003;42:238–56. doi: 10.1016/s0163-7827(02)00065-6. doi: 10.1016/S0163-7827(02)00065-6. [DOI] [PubMed] [Google Scholar]
- 8.van Weeghel M, Abdurrachim D, Nederlof R, Argmann CA, Houtkooper RH, Hagen J, et al. Increased cardiac fatty acid oxidation in a mouse model with decreased malonyl-coA sensitivity of CPT1B. Cardiovasc Res. 2018;114:1324–34. doi: 10.1093/cvr/cvy089. doi: 10.1093/cvr/cvy089. [DOI] [PubMed] [Google Scholar]
- 9.Slot JW, Geuze HJ, Gigengack S, James DE, Lienhard GE. Translocation of the glucose transporter GLUT4 in cardiac myocytes of the rat. Proc Natl Acad Sci U S A. 1991;88:7815–9. doi: 10.1073/pnas.88.17.7815. doi: 10.1073/pnas.88.17.7815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang XG, Jia JM, Ye-Shuang LI. Simultaneous determination of eight bioactive constituents in Shensong Yangxin capsule by UPLC. Chin Herbal Med. 2013;5:212–6. doi: 10.3969/j.issn.1674-6348.2013.03.006. [Google Scholar]
- 11.Zhou Y, Yang Z, Huang M, Hospital DQ. Clinical observation and safety analysis of amiodarone combined with Shensong Yangxin capsule in the treatment of coronary heart disease with arrhythmia. Med Innov China. 2018;15:130–3. doi: 10.3969/j.issn.1674-4985.2018.05.035. [Google Scholar]
- 12.Hou WQ. Department P. Clinical study of the combination of Shensong Yangxin capsule with amiodarone in the treatment of chronic heart failure with ventricular arrhythmia. Chin J Ration Drug Use. 2018;15:44–6. doi: 10.3969/j.issn.2096-3327.2018.01.014. [Google Scholar]
- 13.Gao Z, Jiang S. Discussion on the effect and value of Shensong Yangxin capsule in the treatment of coronary heart disease with arrhythmia. Chin Community Doctors. 2018;34:112–3. doi: 10.3969/j.issn.1007-614x.2018.7.69. [Google Scholar]
- 14.Xiao X. Curative effect of paroxetine combined with Shensong Yangxin capsule on senile coronary heart disease with depression and effect on oxidant/antioxidant stress balance and quality of life. Mod J Integr Tradit Chin West Med. 2017;26:481–4. doi: 10.3969/j.issn.1008-8849.2017.05.008. [Google Scholar]
- 15.Yuzhen LI, Shu Q, Cardiology DO. Clinical effects of Shensong Yangxin capsule for coronary heart disease and stable angina and influence on blood lipid. China Health Stand Manag. 2018;4:90–1. doi: 10.3969/j.issn.1674-9316.2018.04.049. [Google Scholar]
- 16.Jiang XG, Jia JM, Shuang LY. Simultaneous determination of eight bioactive constituents in Shensong Yangxin capsule by UPLC. Chin Her Med. 2013;5:212–6. doi: 10.3969/j.issn.1674-6348.2013.03.006. [Google Scholar]
- 17.Sun LP, Li N, Wu YL, Pu JL. Effects of Shensong Yangxin capsule on pacemaker channels encoded by human HCN4 gene. Chin Med J. 2010;123:3148–50. doi: 10.3760/cma.j.issn.0366-6999.2010.21.036. [PubMed] [Google Scholar]
- 18.Zou JG, Zhang J, Jia ZH, Cao KJ. Evaluation of the traditional Chinese medicine Shensongyangxin capsule on treating premature ventricular contractions: A randomized, double-blind, controlled multicenter trial. Chin Med J. 2011;124:76–83. doi: 10.3760/cma.j.issn.0366-6999.2011.1.015. [PubMed] [Google Scholar]
- 19.Xiang J, Zhao JB, Wang Y, Hua XF, Huang H, Lei YH. Effect of Shensong Yangxin capsule on cardiac remodelling of myocardial infarction mouse model. Her Med. 2016;6:588–92. doi: 10.3760/cma.j.issn.0366-6999.2011.1.015. [Google Scholar]
- 20.Wang S, Cheng M, Hu Z, Hu S, Zou Q, Lai X, et al. Angiotensin II facilitates matrix metalloproteinase-9-mediated myosin light chain kinase degradation in pressure overload-induced cardiac hypertrophy. Cell Physiol Biochem. 2017;44:2281–95. doi: 10.1159/000486066. doi: 10.1159/000486066. [DOI] [PubMed] [Google Scholar]
- 21.Li HL, Liu C, de Couto G, Ouzounian M, Sun M, Wang AB, et al. Curcumin prevents and reverses murine cardiac hypertrophy. J Clin Invest. 2008;118:879–93. doi: 10.1172/JCI32865. doi: 10.1172/JCI32865. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Cui J, Chen S, Zhang C, Meng F, Wu W, Hu R, et al. Inhibition of MMP-9 by a selective gelatinase inhibitor protects neurovasculature from embolic focal cerebral ischemia. Mol Neurodegener. 2012;7:21. doi: 10.1186/1750-1326-7-21. doi: 10.1186/1750-1326-7- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonfil RD, Sabbota A, Nabha S, Bernardo MM, Dong Z, Meng H, et al. Inhibition of human prostate cancer growth, osteolysis and angiogenesis in a bone metastasis model by a novel mechanism-based selective gelatinase inhibitor. Int J Cancer. 2010;118:2721–6. doi: 10.1002/ijc.21645. doi: 10.1002/ijc.21645. [DOI] [PubMed] [Google Scholar]
- 24.Fang WJ, Wang CJ, He Y, Zhou YL, Peng XD, Liu SK, et al. Resveratrol alleviates diabetic cardiomyopathy in rats by improving mitochondrial function through PGC-1α deacetylation. Acta Pharmacol Sin. 2018;39:59–73. doi: 10.1038/aps.2017.50. doi: 10.1038/aps.2017.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeninga EH, Schoonjans K, Auwerx J. Reversible acetylation of PGC-1: Connecting energy sensors and effectors to guarantee metabolic flexibility. Oncogene. 2010;29:4617–24. doi: 10.1038/onc.2010.206. doi: 10.1038/onc.2010.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang Y, Zhang H, Zhao Y, Wang Y, Wang W, He Y, et al. Telomere dysfunction disturbs macrophage mitochondrial metabolism and the NLRP3 inflammasome through the PGC-1α/TNFAIP3 axis. Cell Rep. 2018;22:3493–506. doi: 10.1016/j.celrep.2018.02.071. doi: 10.1016/j.celrep.2018.02.071. [DOI] [PubMed] [Google Scholar]
- 27.Tan B, Li X, Yin Y, Wu Z, Liu C, Tekwe CD, et al. Regulatory roles for L-arginine in reducing white adipose tissue. Front Biosci (Landmark Ed) 2012;17:2237–46. doi: 10.2741/4047. doi: 10.2741/4047. [DOI] [PMC free article] [PubMed] [Google Scholar]