Abstract
Background:
Nonalcoholic fatty liver disease (NAFLD) is one of the most common chronic liver diseases with a high prevalence in the general population. The association between NAFLD and cardiovascular disease has been well addressed in previous studies. However, whether NAFLD is associated with carotid artery disease in a community-based Chinese population remained unclear. The aim of this study was to investigate the association between NAFLD and carotid artery disease.
Methods:
A total of 2612 participants (1091 men and 1521 women) aged 40 years and older from Jidong of Tangshan city (China) were selected for this study. NAFLD was diagnosed by abdominal ultrasonography. The presence of carotid stenosis or plaque was evaluated by carotid artery ultrasonography. Logistic regression was used to analyze the association between NAFLD and carotid artery disease.
Results:
Participants with NAFLD have a higher prevalence of carotid stenosis (12.9% vs. 4.6%) and carotid plaque (21.9% vs. 15.0%) than those without NAFLD. After adjusting for age, gender, smoking status, income, physical activity, diabetes, hypertension, triglyceride, waist-hip ratio, and high-density lipoprotein, NAFLD is significantly associated with carotid stenosis (odds ratio [OR]: 2.06, 95% confidence interval [CI]: 1.45–2.91), but the association between NAFLD and carotid plaque is not statistically significant (OR: 1.10, 95% CI: 0.8–1.40).
Conclusion:
A significant association between NAFLD and carotid stenosis is found in a Chinese population.
Keywords: Association, Carotid Artery Disease, Carotid Stenosis, Nonalcoholic Fatty Liver Disease
摘要
背景:
非酒精性脂肪性肝(NAFLD)是一种在一般人群中患病率较高的常见慢性肝病。以往的研究已经证明了非酒精性脂肪 肝与心血管疾病之间存在关联。然而,在中国的社区人群中非酒精性脂肪肝是否与颈动脉疾病存在关联仍不清楚。本研究的 目的就是探讨非酒精性脂肪肝与颈动脉疾病之间的关联。
方法:
本研究共纳入了2612名来自唐山市冀东地区,年龄在40岁及以上的受试者(1091名男性和1521名女性)。 非酒精性脂 肪肝的确诊主要是通过腹部超声检查。通过颈动脉超声检查来判断颈动脉狭窄或斑块的存在。我们还采用逻辑回归的方法来 分析非酒精性脂肪肝与颈动脉疾病之间的关联。
结果:
患有非酒精性脂肪肝的受试者中颈动脉狭窄患病率(12.9%vs 4.6%)和颈动脉斑块患病率(21.9%vs 15.0%)均高于 未患有非酒精性脂肪肝的受试者。在调整年龄,性别,吸烟状况,收入,体力活动,糖尿病,高血压,甘油三酯,腰臀比和 高密度脂蛋白等因素后,我们发现非酒精性脂肪肝与颈动脉狭窄显著相关(OR: 2.06, 95% CI: 1.45-2.91),但非酒精性脂肪肝与 颈动脉斑块之间的关联却无统计学意义(OR: 1.10, 95% CI: 0.86-1.40)。
结论:
在中国人群中,非酒精性脂肪肝与颈动脉狭窄之间存在显著关联。
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) has become a major public health concern in developing countries, and it emerges as the most common chronic liver disease around the world with a prevalence of 20–30% in the general population.[1] The clinical condition with histological features of NAFLD is ranging from simple steatosis to steatohepatitis and cirrhosis. NAFLD is also recognized as the hepatic manifestation of metabolic syndrome which includes obesity, type 2 diabetes mellitus, dyslipidemia, and hypertension. The close association between NAFLD and metabolic syndrome has been demonstrated previously.[2] Indeed, NAFLD is considered to be another component of the metabolic syndrome which is a key mediator for the relationship of NAFLD and cardiovascular disease. A strong association between NAFLD and metabolic syndrome has stimulated more attention to its putative role in the occurrence and development of cardiovascular disease.[3]
Carotid atherosclerosis, a common carotid artery disease, is recognized as one of the major cardiovascular diseases. Recently, NAFLD was suspected to be associated with an increased risk of cardiovascular disease, including exacerbating carotid atherosclerosis and coronary artery disease (CAD).[4] NAFLD patients might be at a heightened risk to suffer from cardiovascular disease, especially carotid artery disease.[5] The presence of carotid plaque indicated that a clinical model of early atherosclerosis was an indicator of increased risk of cardiovascular disease.[6,7] In addition, carotid stenosis was an important risk factor for transient ischemic attacks and strokes which was correlated well with cardiovascular disease.[8] We use carotid stenosis and carotid plaque measured by carotid ultrasound as good surrogate markers of carotid artery disease. Therefore, the aim of this study was to investigate the association between NAFLD and carotid artery disease.
METHODS
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Jidong Oilfield Inc., Medical Centers. Informed written consent was obtained from all participants before their enrollment in this study.
Study design and population
From July 2013 to August 2014, all residents aged ≥18 years from Jidong community were invited to participate in this study at the time of their regular annual physical examination performed at the Jidong Oilfield Hospital. Almost 9078 residents completed a standard questionnaire, underwent physical examinations and laboratory assessments, and provided informed consent at recruitment.[9] The Jidong community is located in Caofeidian District which is in the south of Tangshan city and near the Bohai Sea. The detail information about the research has been described in the past.[10,11] Among these resident population, those aged ≥40 years, without missing data on ultrasonography, who signed informed consent were selected as participants. Furthermore, they should meet the following criteria: (1) no history of cancer, stroke, atrial fibrillation, heart failure, or myocardial infarction; (2) without excessive alcohol consumption (men: ≥20 g/day and women: ≥10 g/day for more than a year); (3) absence of a history of positive HBsAg; and (4) with complete information.
Measurement of nonalcoholic fatty liver disease
Liver ultrasonography was performed in participants aged ≥40 years using a high-resolution B-mode topographic ultrasound system with a 3.5 MHz probe (ACUSON X300, Siemens, Germany) to assess the prevalence of NAFLD. Compared to histology, ultrasonography had a sensitivity of 85% and a specificity of 94% in detecting fatty liver disease.[12] According to conventional criteria, fatty liver disease was diagnosed through characteristic echo patterns, such as diffusely increased liver near-field ultrasound echo (bright liver); liver echo was greater than kidney and vascular blurring and the gradual attenuation of far-field ultrasound echo.[13] In addition, abdominal ultrasonography scanning was examined by well-trained sonographers who were unaware of the clinical presentation and laboratory findings of participants during the whole ultrasonic examination.
Assessment of carotid stenosis and carotid plaque
For the evaluation of the prevalence of carotid stenosis, all participants (≥40 years) underwent bilateral carotid duplex sonography in a supine position by expert operators who were blinded to the goal of the study, clinical data, and laboratory findings. According to the Society of Radiologists in Ultrasound Consensus Conference, we graded the severity of carotid stenosis.[14] The categories were classified as normal (no stenosis) and carotid stenosis (<50% stenosis; ≥50% stenosis or occlusion). In the light of the established ultrasound criteria, (1) normal (no presence of stenosis) was defined that internal carotid artery (ICA) peak systolic velocity (PSV) was less than 125 cm/s and no plaque or intimal thickening was visible; (2) <50% stenosis was defined that ICA PSV was less than 125 cm/s but plaque or intimal thickening was visible; and (3) ≥50% stenosis or occlusion was considered when ICA PSV was greater than 125 cm/s and plaque was visible, or there was no detectable patent lumen on gray-scale ultrasonography and no flow on spectral, power, and color Doppler ultrasonography.[10,15,16] Moreover, the higher value (left or right) was considered for analysis if bilateral stenosis was present.
To assess the complexity and stability of carotid plaque, ultrasound examination (Philips iU22 ultrasound system, Philips Medical Systems, Bothell, WA, USA) was also operated by well-trained and certified sonographers, and the results of the examination were reviewed by two independent operators. Carotid plaque was defined as a focal structure encroaching into the arterial lumen of at least 0.5 mm or 50% of the surrounding intima-media thickness (IMT) value, or demonstrated as a thickness of 1.5 mm from the intima-lumen interface to the media–adventitia interface.[17] In this study, we classified carotid plaque as normal (without plaque), stable plaque (plaques had a uniform texture and present a smooth and regular surface and plaques with high-level or homogeneous echoes), and unstable plaque (plaques with incomplete fibrous cap or ulcerated plaques and plaques with low-level or heterogeneous echoes) according to different stabilities.[18] Both longitudinal and transverse images of bilateral carotid arteries were obtained to extensively evaluate plaques while differences between their evaluations needed to be resolved by consensus.
Assessment of potential covariates
The information of demographic (age, sex, income, physical activities, and smoking status) and clinical characteristics (waist-hip ratio (WHR), hypertension, and diabetes) was collected using standardized questionnaires.[9] Biochemical variables containing some indexes such as triglyceride (TG), total cholesterol (TC), high-density lipoprotein-cholesterol (HDL-C), and low-density lipoprotein-cholesterol (LDL-C) were measured using standard methods at the Central Laboratory of Jidong Oilfield Hospital.[10]
According to the response of smoking status, participants were divided into three categories, never (<100 cigarettes in entire life), past, and current smoker. Education level was also classified into three categories: primary school or below, middle or high school, and college or above. The classification of physical activity was based on the following three kinds of circumstances: inactive, moderately active, and active. Simultaneously, WHR was calculated as waist circumference (cm) divided by the hip circumference (cm) which was used as measure of abdominal obesity. Hypertension was defined as a self-report history of hypertension, using antihypertensive medication or systolic blood pressure ≥140 mmHg and diastolic blood pressure ≥90 mmHg.[10] The definition of diabetes was fasting blood glucose ≥7.0 mmol/L, current treatment with insulin, oral hypoglycemic agents, or a history of diabetes mellitus.[11,19,20]
Statistical analyses
Considering that the prevalence of NAFLD and carotid stenosis is 25% and 12%, respectively, at two-sided α = 0.05, power = 0.80, and odds ratio (OR) = 1.50, the sample size can be assumed to be 2360 (PASS11, NCSS, LLC 329 North 1000 East, Kaysville, Utah 84037, USA).[21] The normal distribution of continuous variables was tested by one-sample Kolmogorov-Smirnov test. Continuous variables underlying normal distribution were presented as mean ± standard deviation and compared using t-test or analysis of variance (ANOVA), and otherwise presented as median (interquartile range) and compared by corresponding nonparametric methods. The frequencies and percentages were used to describe categorical variables, and the Chi-squared test was applied to compare among groups. Logistic regression was used to calculate ORs and 95% confidence intervals (CIs) and to determine the association between NAFLD and carotid stenosis or plaque. After adjusting for age, gender, smoking status, income, physical activity, diabetes, hypertension, WHR, TG, and HDL-C, the association between different severity of carotid stenosis and NAFLD, as well as the relationship between carotid plaque with different stability and NAFLD were also investigated. The association of NAFLD and carotid stenosis or plaque was also examined in stratification of age and gender analysis, respectively.
Statistical analyses were performed using the SAS version 9.4 (SAS Institute, Cary, North Carolina, USA). All statistical tests were two-sided, and P < 0.05 was considered statistically significant.
RESULTS
Baseline characteristics of study participants
From the initial sample of 9078 participants, 3396 residents aged ≥40 years and with complete data on ultrasonography examination were selected as study samples. Among the 3396 participants, 784 participants were excluded for the following reasons: 168 participants with history of myocardial infarction, heart failure, stroke, atrial fibrillation, and cancer; 369 participants with excessive alcohol consumption; 131 participants with history of positive HBsAg; and 116 participants without complete information. Finally, 2612 participants were included in the final analysis.
Demographic data, clinical characteristics, and biochemical variables of all participants were presented in Table 1. Among the total 2612 participants, the mean age was 53.6 ± 8.6 years and 41.8% (n = 1091) were men. The 1375 participants with NAFLD consisted of 707 (64.8%) males and 668 (43.9%) females. Totally 342 (24.9%) participants with NAFLD were current smokers. Biochemical parameters including WHR, TG and TC were higher in participants with NAFLD than those without NAFLD. In addition, higher prevalence of diabetes and hypertension was also found in the group of NAFLD. Participants with NAFLD had a higher prevalence of carotid stenosis compared to those without NAFLD (<50% stenosis: 17% vs. 11.2%; ≥50% stenosis: 13.0% vs. 4.6%). A higher prevalence of unstable plaque (19.4% vs. 12.8%) was also demonstrated in participants with NAFLD than those without NAFLD in this study.
Table 1.
Baseline characteristics of participants enrolled in this study
| Characteristics | Total (n = 2612) | Non-NAFLD (n = 1237, 47.4%) | NAFLD (n = 1375, 52.6%) | Statistics | P |
|---|---|---|---|---|---|
| Age (mean ± SD), years | 53.6 ± 8.6 | 52.5 ± 8.6 | 54.6 ± 8.3 | −6.13* | <0.01 |
| Male, n (%) | 1091 (41.8) | 384 (31.0) | 707 (51.4) | 111.15† | <0.01 |
| Education level, n (%) | |||||
| Primary school or low | 207 (7.9) | 88 (7.1) | 119 (8.7) | 7.88† | 0.02 |
| Middle or high school | 1510 (57.8) | 693 (56.0) | 817 (59.4) | ||
| College or above | 895 (34.3) | 456 (36.9) | 439 (31.9) | ||
| Income per month, n (%) | |||||
| < RMB 3000 Yuan | 1354 (51.8) | 625 (50.5) | 729 (53.0) | 1.75† | 0.42 |
| RMB 3000–5000 Yuan | 1112 (42.6) | 543 (43.9) | 569 (41.4) | ||
| > RMB 5000 Yuan | 146 (5.6) | 69 (5.6) | 77 (5.6) | ||
| Physical activity, n (%) | |||||
| Inactive | 819 (31.4) | 385 (31.1) | 434 (31.6) | 0.70† | 0.71 |
| Moderately active | 189 (7.2) | 95 (7.7) | 94 (6.8) | ||
| Active | 1604 (61.4) | 757 (61.2) | 847 (61.6) | ||
| Smoking, n (%) | |||||
| Never | 1984 (76.0) | 1029 (83.2) | 955 (69.4) | 68.72† | <0.01 |
| Current | 520 (19.9) | 178 (14.4) | 342 (24.9) | ||
| Past | 108 (4.1) | 30 (2.4) | 78 (5.7) | ||
| Diabetes, n (%) | 290 (11.1) | 74 (6.0) | 216 (15.7) | 62.42† | <0.01 |
| Hypertension, n (%) | 1134 (42.4) | 353 (28.5) | 781 (56.8) | 211.74† | <0.01 |
| WHR (mean ± SD) | 0.9 ± 0.1 | 0.8 ± 0.1 | 0.9 ± 0.1 | −20.25* | <0.01 |
| TG (median [IQR]), mmol/L | 1.3 (0.3, 21.8) | 1.1 (0.3, 15.6) | 1.7 (0.4, 21.8) | −21.06‡ | <0.01 |
| TC (mean ± SD), mmol/L | 4.7 ± 0.9 | 4.6 ± 0.8 | 4.8 ± 1.0 | −7.56* | <0.01 |
| HDL-C (mean ± SD), mmol/L | 1.2 ± 0.3 | 1.3 ± 0.3 | 1.1 ± 0.2 | 16.47* | <0.01 |
| LDL-C (mean ± SD), mmol/L | 2.6 ± 0.6 | 2.5 ± 0.6 | 2.8 ± 0.6 | −9.95* | <0.01 |
| CIMT, n (%) | 673 (25.8) | 261 (21.1) | 412 (30.0) | 26.75† | <0.01 |
| Carotid stenosis, n (%) | |||||
| Normal | 2005 (76.8) | 1042 (84.2) | 963 (70.0) | 83.13† | <0.01 |
| <50% stenosis | 372 (14.2) | 138 (11.2) | 234 (17.0) | ||
| ≥50% stenosis | 235 (9.0) | 57 (4.6) | 178 (13.0) | ||
| Carotid plaque, n (%) | |||||
| Normal | 2125 (81.4) | 1051 (85.0) | 1074 (78.1) | 21.20† | <0.01 |
| Stable plaque | 61 (2.3) | 27 (2.2) | 34 (2.5) | ||
| Unstable plaque | 426 (16.3) | 159 (12.8) | 267 (19.4) |
*: t values; †: χ2 values; ‡: Z values. NAFLD: Nonalcoholic fatty liver disease; WHR: Waist-hip ratio; TG: Triglyceride; TC: Total cholesterol; HDL-C: High-density lipoprotein-cholesterol; LDL-C: Low-density lipoprotein-cholesterol; CIMT: Carotid intima-media thickness; SD: Standard deviation; IQR: Interquartile range.
Association between carotid stenosis and nonalcoholic fatty liver disease
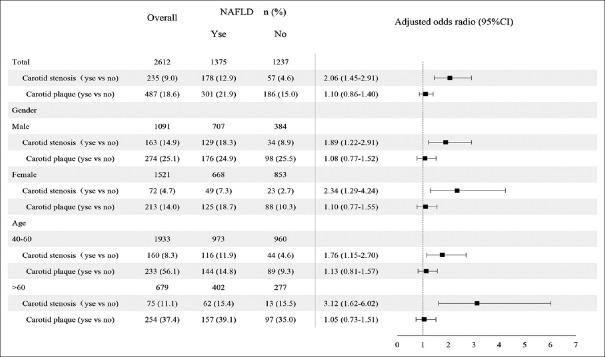
Among the 1375 participants with NAFLD, 12.9% (178/1375) met the diagnostic criteria for carotid stenosis. After adjusting for age, gender, education level, income, physical activity, smoking status, diabetes, hypertension, WHR, TG, and HDL-C, the association between NAFLD and carotid stenosis was statistically significant (OR: 2.06, 95% CI: 1.45–2.91). In age- and gender-stratified analysis, the association between NAFLD and carotid stenosis was positively significant among different groups (female: OR: 2.34, 95% CI: 1.29–4.24; male: OR: 1.89, 95% CI: 1.22–2.91; 40–60 years: OR: 1.76, 95% CI: 1.15–2.70; >60 years: OR: 3.12, 95% CI: 1.62–6.02) [Figure 1]. A positive association was observed between carotid stenosis (≥50% stenosis) and NAFLD according to the classification of the severity of carotid stenosis with normal as reference group (OR: 2.06, 95% CI: 1.45–2.93, P < 0.01) [Table 2].
Figure 1.
The prevalence of carotid stenosis or plaque in overall participants and participants with NAFLD or without NAFLD. OR with 95% CI of NALFD for carotid stenosis or plaque in total, different gender, and age categories. Covariates for total included age, gender, smoking status, income, physical activity, diabetes, hypertension, WHR, TG, and HDL-C. Covariates for different genders included age, smoking status, income, physical activity, diabetes, hypertension, WHR, TG, and HDL-C. Covariates for different ages included gender, smoking status, income, physical activity, diabetes, hypertension, WHR, TG, and HDL-C. NAFLD: Nonalcoholic fatty liver disease; OR: Odds ratio; CI: Confidence interval; WHR: Waist-hip ratio; TG: Triglyceride; HDL-C: High-density lipoprotein-cholesterol.
Table 2.
Association between different severity of carotid stenosis and NAFLD stratified by gender and age
| Items | Total* | Gender† | Age‡ | ||
|---|---|---|---|---|---|
| Male | Female | 40–60 years | >60 years | ||
| <50% stenosis | |||||
| Non-NAFLD | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| NAFLD | 1.10 (0.85–1.43) | 0.97 (0.67–1.41) | 1.23 (0.86–1.77) | 1.28 (0.92–1.76) | 0.84 (0.54–1.32) |
| P | 0.45 | 0.87 | 0.26 | 0.14 | 0.12 |
| ≥50% stenosis | |||||
| Non-NAFLD | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| NAFLD | 2.06 (1.45–2.93) | 1.85 (1.19–2.87) | 2.39 (1.32–4.34) | 1.80 (1.17–2.75) | 2.99 (1.54–5.82) |
| P | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
Data were presented by ORs (95% CIs). *Total adjusted for age, gender, smoking status, income, physical activity, diabetes, hypertension, WHR, TG, and HDL-C; †Gender subgroup adjusted for age, smoking status, income, physical activity, diabetes, hypertension, WHR, TG, and HDL-C; ‡Age subgroup adjusted for gender, smoking status, income, physical activity, diabetes, hypertension, WHR, TG, and HDL-C. NAFLD: Nonalcoholic fatty liver disease; WHR: Waist-hip ratio; TG: Triglyceride; HDL-C: High-density lipoprotein-cholesterol; CIs: Confidence intervals; ORs: Odds ratios.
Relationship between carotid plaque and nonalcoholic fatty liver disease
NAFLD was not significantly associated with carotid plaque in the whole participants (OR: 1.10, 95% CI: 0.8–1.40) after adjusting for age, gender, education level, income, physical activity, smoking status, diabetes, hypertension, WHR, TG, and HDL-C [Figure 1]. The results also showed that there was no significant correlation between carotid plaque and NAFLD after the stratification of age and gender. Moreover, the carotid plaque was classified according to different stability. The association between unstable plaque or stable plaque and NAFLD was still not statistically significant (unstable plaque: OR: 1.13, 95% CI: 0.87–1.35; stable plaque: OR: 0.94, 95% CI: 0.53–1.67) after adjustment for potential confounders in this study [Table 3].
Table 3.
Association between carotid plaque of different stability and NAFLD stratified by gender and age
| Items | Total* | Gender† | Age‡ | ||
|---|---|---|---|---|---|
| Male | Female | 40–60 years | >60 years | ||
| Stable plaque | |||||
| Non-NAFLD | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| NAFLD | 0.94 (0.53–1.67) | 1.38 (0.61–3.15) | 0.62 (0.27–1.47) | 1.16 (0.45–2.98) | 0.77 (0.36–1.66) |
| P | 0.83 | 0.44 | 0.28 | 0.76 | 0.51 |
| Unstable plaque | |||||
| Non-NAFLD | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| NAFLD | 1.13 (0.87–1.35) | 1.05 (0.73–1.50) | 1.20 (0.83–1.74) | 1.13 (0.80–1.60) | 1.11 (0.76–1.62) |
| P | 0.36 | 0.79 | 0.34 | 0.48 | 0.59 |
Data were presented by ORs (95% CIs). *Total adjusted for age, gender, smoking status, income, physical activity, diabetes, hypertension, WHR, TG, and HDL-C; †Gender subgroup adjusted for age, smoking status, income, physical activity, diabetes, hypertension, WHR, TG, and HDL-C; ‡Age subgroup adjusted for gender, smoking status, income, physical activity, diabetes, hypertension, WHR, TG, and HDL-C. NAFLD: Nonalcoholic fatty liver disease; WHR: Waist-hip ratio; TG: Triglyceride; HDL-C: High-density lipoprotein-cholesterol; CIs: Confidence intervals; ORs: Odds ratios.
DISCUSSION
In the present study, we focused on the correlation between NAFLD and carotid artery disease. The primary outcome measure was that the association between NAFLD and carotid stenosis was independently significant, while the association of carotid plaque was not independently significant in this community-based population. This study attempted to explore the association between NAFLD and carotid artery disease (assessed by carotid stenosis and carotid plaque) in China.
A lot of evidence indicated that NAFLD was related to cardiovascular disease.[22,23] Sookoian and Pirola[2] performed a systematic review and described that NAFLD was associated with the presence of carotid plaque and endothelial dysfunction which was a reliable marker of subclinical atherosclerosis. Volzke et al.[24] suggested that carotid plaques were more frequently in NAFLD patients in comparison with normal including 3212 participants. In this study, we did not observe a significant association between NAFLD and carotid plaque. Consistent with our results, a study in middle-aged and elderly Chinese showed that the association between NAFLD and carotid plaque was not statistically significant.[25] However, in a study of 14,445 adults, NAFLD was discovered to be associated with an increased risk of carotid plaque diagnosed by ultrasound.[26] The inconsistent association between NAFLD and carotid plaque might be caused by the ethnic differences, region differences, and the difference in methodology of defining NAFLD. Therefore, the association needed further validation in multiple ethnic and different region populations. Earlier studies also demonstrated the presence of carotid plaque increased with age and gender difference. Accordingly, we stratified by age and gender to analyze the different stability of carotid plaque with NAFLD although the results were not statistically significant. The inconsistence might be possibly ascribed to the less sensitive to ultrasound than to biopsy during identifying NAFLD. NAFLD was considered as a marker of metabolic disorders which could exaggerate the effects on the development of atherosclerosis.[27] However, the biological mechanism of NAFLD promoting the development of athero sclerosis (measured through carotid plaque) was still unclear.
Similarly, same to carotid plaques, carotid stenosis was also used as a surrogate marker to represent the carotid artery disease in this study. Compared with inconsistent results regarding the association between carotid plaque and NAFLD, those between carotid stenosis and NAFLD are consistent. Several studies demonstrated a positive association between NAFLD and cardiovascular-related disease (assessed by carotid IMT or carotid plaque).[3,28] Sinn et al.[29] ever reported that men with NAFLD had a higher risk of carotid stenosis in modest alcohol drinkers. An independently significant correlation of participants with NAFLD and carotid artery disease (assessed by carotid artery stenosis) was detected in the present study, and a positive association between carotid stenosis (≥50% stenosis) and NAFLD was found in the stratified analysis. In addition, NAFLD was assumed to be the hepatic manifestation of metabolic syndrome. However, a study reported that metabolic syndrome was not associated with carotid stenosis (≥50% stenosis) in patients with a recent diagnosis of CAD.[30] The relatively small sample size (168 patients) in that study might partly account for this inconsistency. The results in this study also showed that NAFLD was an independent risk factor for carotid stenosis in different gender and age groups. This gender-specific association might be caused by the effects of estrogens which protected females from cardiovascular disease. Furthermore, age was another important risk factor of the association between carotid stenosis and NAFLD. A previous study demonstrated a higher prevalence of carotid stenosis or NAFLD with the age increased, and the relationship between NAFLD and carotid stenosis among older participants was statistically significant, that was similar to our results.[31]
Furthermore, the association between CAD and NAFLD had been examined in some studies, which detected a higher prevalence of CAD in patients with NAFLD (80.4% vs. 60.7%).[32,33,34] Possible reasons for these inconsistent results might be that patients underwent coronarography before detecting NAFLD. These studies also confirmed that CAD was positively associated with NAFLD (OR: 3.31), which revealed a significant association between NAFLD and cardiovascular disease. Therefore, the findings in the study of the positive association between NAFLD and carotid artery disease (assessed by carotid stenosis) might, to some extent, explain the higher risk of cerebrovascular disease among people with NAFLD, which might add to the available evidence and effects on risk prediction of patients.
According to some recent epidemiological studies, the prevalence of NAFLD in China was higher than the estimates in Western countries while it was increasing and had reached epidemic proportions.[35] In the present study, participants with NAFLD had a higher prevalence of carotid stenosis and carotid plaque which was different from those without NAFLD. Carotid stenosis was the serious stage of the development of carotid atherosclerosis, and the relationship between carotid plaque and carotid atherosclerosis was closely linked. Although the mechanism linking NAFLD and cardiovascular events was elusive, some studies still posed that the mechanism of the association between atherosclerosis and NAFLD might be a complex progress involving an interaction among insulin resistance, an inflammatory status and oxidative stress, and appeared to be important in both early and later stages of the atherosclerotic progress.[36] A systemic inflammatory status with pro-inflammatory and atherogenic molecules might play an important role in the relationship between NAFLD and cardiovascular disease.[37] These studies provided bases for further exploration of the underlying mechanisms between NAFLD and cardiovascular disease. Furthermore, common genetic variants were another factors influencing the risk of cardiovascular disease.[38] Apart from the main risk factors of age and gender, obesity and a list of metabolic-related problems also played an important role in the presence of NAFLD. These factors could predict the risk of carotid stenosis or carotid plaque and forecast some cardiovascular-related diseases in NAFLD patients. Therefore, carotid stenosis or plaque might be the hub of NAFLD and cardiovascular disease.
We acknowledged several limitations in this present study. First, the study could not evaluate the temporal natural of the relationship between NAFLD and carotid stenosis or plaque and also could not draw a causal inference of them because of the cross-sectional design. Second, some participants were absent for the ultrasound examination, which might lead to the selection bias and restrict the generalization of the findings. Moreover, the diagnosis of NAFLD was based on ultrasonography which had less sensitivity compared to liver biopsy and could cause a bias for the prevalence of NAFLD. Third, we excluded the participants who had history of excessive alcohol consumption and were positive for HBsAg; however, other types of liver diseases, such as hepatitis C and liver cirrhosis, were not taken into account and might confound the association between NAFLD and carotid plaque. Finally, since the whole participants were just from the Jidong community of Tangshan city, they could not be regarded as representative of the Chinese population.
In conclusion, our data suggest that NAFLD is associated with carotid stenosis but not with carotid plaque. Compared to non-NAFLD individuals, participants with NAFLD have a higher risk of carotid stenosis, particularly for women and older participants. NAFLD might be a predicator of early carotid atherosclerosis which is assessed by carotid stenosis or carotid plaque as surrogate markers.
Financial support and sponsorship
This study was supported by grants from the National Natural Science Foundation of China (No. 81670294, No. 81202279, and No. 81473057) and the National Social Science Foundation of China (No. 17BGL184).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yuan-Yuan Ji
REFERENCES
- 1.Fitzgerald MA. Nonalcoholic fatty liver disease. Nurse Pract. 2007;32:24–5. doi: 10.1097/00006205-200702000-00002. doi: 10.1097/00006205-200702000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Sookoian S, Pirola CJ. Non-alcoholic fatty liver disease is strongly associated with carotid atherosclerosis: A systematic review. J Hepatol. 2008;49:600–7. doi: 10.1016/j.jhep.2008.06.012. doi: 10.1016/j.jhep.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 3.Kang JH, Cho KI, Kim SM, Lee JY, Kim JJ, Goo JJ, et al. Relationship between nonalcoholic fatty liver disease and carotid artery atherosclerosis beyond metabolic disorders in non-diabetic patients. J Cardiovasc Ultrasound. 2012;20:126–33. doi: 10.4250/jcu.2012.20.3.126. doi: 10.4250/jcu.2012.20.3.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Misra VL, Khashab M, Chalasani N. Nonalcoholic fatty liver disease and cardiovascular risk. Curr Gastroenterol Rep. 2009;11:50–5. doi: 10.1007/s11894-009-0008-4. doi: 10.1007/s11894-009-0008-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363:1341–50. doi: 10.1056/NEJMra0912063. doi: 10.1056/NEJMra0912063. [DOI] [PubMed] [Google Scholar]
- 6.Kozakova M, Palombo C, Eng MP, Dekker J, Flyvbjerg A, Mitrakou A, et al. Fatty liver index, gamma-glutamyltransferase, and early carotid plaques. Hepatology. 2012;55:1406–15. doi: 10.1002/hep.25555. doi: 10.1002/hep.25555. [DOI] [PubMed] [Google Scholar]
- 7.Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: A consensus statement from the American Society of Echocardiography carotid intima-media thickness task force. Endorsed by the society for vascular medicine. J Am Soc Echocardiogr. 2008;21:93–111. doi: 10.1016/j.echo.2007.11.011. doi: 10.1016/j.echo.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 8.Roh YN, Woo SY, Kim N, Kim S, Kim YW, Kim DI, et al. Prevalence of asymptomatic carotid stenosis in Korea based on health screening population. J Korean Med Sci. 2011;26:1173–7. doi: 10.3346/jkms.2011.26.9.1173. doi: 10.3346/jkms.2011.26.9.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Ge S, Yan Y, Wang A, Zhao Z, Yu X, et al. China suboptimal health cohort study: Rationale, design and baseline characteristics. J Transl Med. 2016;14:291. doi: 10.1186/s12967-016-1046-y. doi: 10.1186/s12967-016-1046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hao Z, Zhang Y, Li Y, Zhao J, Zhou Y, Qiu J, et al. The association between ideal cardiovascular health metrics and extracranial carotid artery stenosis in a Northern Chinese population: A Cross-sectional study. Sci Rep. 2016;6:31720. doi: 10.1038/srep31720. doi: 10.1038/srep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu R, Hou F, Wang X, Zhou Y, Sun K, Wang Y, et al. Nonalcoholic fatty liver disease and coronary artery calcification in a Northern Chinese population: A Cross sectional study. Sci Rep. 2017;7:9933. doi: 10.1038/s41598-017-09851-5. doi: 10.1038/s41598-017-09851-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hernaez R, Lazo M, Bonekamp S, Kamel I, Brancati FL, Guallar E, et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: A meta-analysis. Hepatology. 2011;54:1082–90. doi: 10.1002/hep.24452. doi: 10.1002/hep.24452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan JG, Jia JD, Li YM, Wang BY, Lu LG, Shi JP, et al. Guidelines for the diagnosis and management of nonalcoholic fatty liver disease: Update 2010: (published in Chinese on Chinese journal of hepatology 2010; 18:163-166) J Dig Dis. 2011;12:38–44. doi: 10.1111/j.1751-2980.2010.00476.x. doi: 10.1111/j.1751-2980.2010.00476.x. [DOI] [PubMed] [Google Scholar]
- 14.Grant EG, Benson CB, Moneta GL, Alexandrov AV, Baker JD, Bluth EI, et al. Carotid artery stenosis: Gray-scale and Doppler US diagnosis – Society of radiologists in ultrasound consensus conference. Radiology. 2003;229:340–6. doi: 10.1148/radiol.2292030516. doi: 10.1148/radiol.2292030516. [DOI] [PubMed] [Google Scholar]
- 15.Wolff T, Guirguis-Blake J, Miller T, Gillespie M, Harris R. Screening for carotid artery stenosis: An update of the evidence for the U.S. Preventive services task force. Ann Intern Med. 2007;147:860–70. doi: 10.7326/0003-4819-147-12-200712180-00006. doi: 10.7326/0003-4819-147-12-200712180-00006. [DOI] [PubMed] [Google Scholar]
- 16.Huang X, Wang A, Liu X, Chen S, Zhu Y, Liu Y, et al. Association between high sensitivity C-reactive protein and prevalence of asymptomatic carotid artery stenosis. Atherosclerosis. 2016;246:44–9. doi: 10.1016/j.atherosclerosis.2015.12.024. doi: 10.1016/j.atherosclerosis.2015.12.024. [DOI] [PubMed] [Google Scholar]
- 17.Wang A, Wu L, Liu X, Su Z, Luo Y, Chen S, et al. The prevalence of carotid plaque with different stability and its association with metabolic syndrome in China: The asymptomatic polyvascular abnormalities community study. Medicine (Baltimore) 2016;95:e4619. doi: 10.1097/MD.0000000000004619. doi: 10.1097/md.0000000000004619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sztajzel R. Ultrasonographic assessment of the morphological characteristics of the carotid plaque. Swiss Med Wkly. 2005;135:635–43. doi: 10.4414/smw.2005.11038. doi: 2005/43/smw-11038. [DOI] [PubMed] [Google Scholar]
- 19.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. The seventh report of the Joint National Committee on Prevention, detection, evaluation, and treatment of high blood pressure: The JNC 7 report. JAMA. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 20.Pearson TA, Palaniappan LP, Artinian NT, Carnethon MR, Criqui MH, Daniels SR, et al. American Heart Association Guide for Improving Cardiovascular Health at the Community Level, 2013 update: A scientific statement for public health practitioners, healthcare providers, and health policy makers. Circulation. 2013;127:1730–53. doi: 10.1161/CIR.0b013e31828f8a94. doi: 10.1161/CIR.0b013e31828f8a94. [DOI] [PubMed] [Google Scholar]
- 21.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M, et al. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 22.Perazzo H, Poynard T, Dufour JF. The interactions of nonalcoholic fatty liver disease and cardiovascular diseases. Clin Liver Dis. 2014;18:233–48. doi: 10.1016/j.cld.2013.09.014. doi: 10.1016/j.cld.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 23.Hamaguchi M, Kojima T, Takeda N, Nagata C, Takeda J, Sarui H, et al. Nonalcoholic fatty liver disease is a novel predictor of cardiovascular disease. World J Gastroenterol. 2007;13:1579–84. doi: 10.3748/wjg.v13.i10.1579. doi: 10.3748/wjg.v13.i10.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Volzke H, Robinson DM, Kleine V, Deutscher R, Hoffmann W, Ludemann J, et al. Hepatic steatosis is associated with an increased risk of carotid atherosclerosis. World J Gastroenterol. 2005;11:1848–53. doi: 10.3748/wjg.v11.i12.1848. doi: 10.3748/wjg.v11.i12.1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang Y, Bi Y, Xu M, Ma Z, Xu Y, Wang T, et al. Nonalcoholic fatty liver disease is associated with atherosclerosis in middle-aged and elderly Chinese. Arterioscler Thromb Vasc Biol. 2012;32:2321–6. doi: 10.1161/ATVBAHA.112.252957. doi: 10.1161/atvbaha.112.252957. [DOI] [PubMed] [Google Scholar]
- 26.Madan SA, John F, Pyrsopoulos N, Pitchumoni CS. Nonalcoholic fatty liver disease and carotid artery atherosclerosis in children and adults: A meta-analysis. Eur J Gastroenterol Hepatol. 2015;27:1237–48. doi: 10.1097/MEG.0000000000000429. doi: 10.1097/meg.0000000000000429. [DOI] [PubMed] [Google Scholar]
- 27.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the national cholesterol education program (NCEP) Expert panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult treatment panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 28.Fracanzani AL, Burdick L, Raselli S, Pedotti P, Grigore L, Santorelli G, et al. Carotid artery intima-media thickness in nonalcoholic fatty liver disease. Am J Med. 2008;121:72–8. doi: 10.1016/j.amjmed.2007.08.041. doi: 10.1016/j.amjmed.2007.08.041. [DOI] [PubMed] [Google Scholar]
- 29.Sinn DH, Gwak GY, Cho J, Son HJ, Paik YH, Choi MS, et al. Modest alcohol consumption and carotid plaques or carotid artery stenosis in men with non-alcoholic fatty liver disease. Atherosclerosis. 2014;234:270–5. doi: 10.1016/j.atherosclerosis.2014.03.001. doi: 10.1016/j.atherosclerosis.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 30.Ambrosetti M, Pedretti RF. Does metabolic syndrome predict silent carotid stenosis in coronary patients? Intern Emerg Med. 2008;3:81–2. doi: 10.1007/s11739-008-0103-9. doi: 10.1007/s11739-008-0103-9. [DOI] [PubMed] [Google Scholar]
- 31.de Weerd M, Greving JP, de Jong AW, Buskens E, Bots ML. Prevalence of asymptomatic carotid artery stenosis according to age and sex: Systematic review and metaregression analysis. Stroke. 2009;40:1105–13. doi: 10.1161/STROKEAHA.108.532218. doi: 10.1161/strokeaha.108.532218. [DOI] [PubMed] [Google Scholar]
- 32.Açikel M, Sunay S, Koplay M, Gündoğdu F, Karakelleoğlu S. Evaluation of ultrasonographic fatty liver and severity of coronary atherosclerosis, and obesity in patients undergoing coronary angiography. Anadolu Kardiyol Derg. 2009;9:273–9. [PubMed] [Google Scholar]
- 33.Wong VW, Wong GL, Yip GW, Lo AO, Limquiaco J, Chu WC, et al. Coronary artery disease and cardiovascular outcomes in patients with non-alcoholic fatty liver disease. Gut. 2011;60:1721–7. doi: 10.1136/gut.2011.242016. doi: 10.1136/gut.2011.242016. [DOI] [PubMed] [Google Scholar]
- 34.Arslan U, Kocaoğlu I, Balcı M, Duyuler S, Korkmaz A. The association between impaired collateral circulation and non-alcoholic fatty liver in patients with severe coronary artery disease. J Cardiol. 2012;60:210–4. doi: 10.1016/j.jjcc.2012.05.003. doi: 10.1016/j.jjcc.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 35.Lu ZY, Shao Z, Li YL, Wulasihan M, Chen XH. Prevalence of and risk factors for non-alcoholic fatty liver disease in a Chinese population: An 8-year follow-up study. World J Gastroenterol. 2016;22:3663–9. doi: 10.3748/wjg.v22.i13.3663. doi: 10.3748/wjg.v22.i13.3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kotronen A, Juurinen L, Tiikkainen M, Vehkavaara S, Yki-Järvinen H. Increased liver fat, impaired insulin clearance, and hepatic and adipose tissue insulin resistance in type 2 diabetes. Gastroenterology. 2008;135:122–30. doi: 10.1053/j.gastro.2008.03.021. doi: 10.1053/j.gastro.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 37.Dowman JK, Tomlinson JW, Newsome PN. Pathogenesis of non-alcoholic fatty liver disease. QJM. 2010;103:71–83. doi: 10.1093/qjmed/hcp158. doi: 10.1093/qjmed/hcp158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Speliotes EK, Yerges-Armstrong LM, Wu J, Hernaez R, Kim LJ, Palmer CD, et al. Genome-wide association analysis identifies variants associated with nonalcoholic fatty liver disease that have distinct effects on metabolic traits. PLoS Genet. 2011;7:e1001324. doi: 10.1371/journal.pgen.1001324. doi: 10.1371/journal.pgen.1001324. [DOI] [PMC free article] [PubMed] [Google Scholar]