SUMMARY
Aims:
Type 2 diabetes mellitus (DM2) affects 10% of the population, but little is known about how DM2 and antidiabetic medication impact glioblastoma (GBM) patients.
Patients & methods:
We retrospectively reviewed GBM patients with DM2 seen at a single institution from 1998 to 2010.
Results:
Of 988 GBMs, 124 (12.6%) were affected by DM2. Thirty-four developed DM2 after steroid use and 89 had pre-existing DM2. Median overall survival among diabetic GBMs was 10 months compared with 13 months among nondiabetics. Only 15% of diabetic patients achieved sustained steroid taper. Sixty-seven (54%) were managed with a single antidiabetic medication and, within this monotherapy group, Karnofsky Performance Score, resection status, steroid dependency and metformin use were the most important predictors of survival on multivariate analysis.
Conclusion:
The prevalence of DM2 among GBMs is similar to that of the general population. A more aggressive approach to steroid tapering and the choice of antidiabetic drug may improve survival within this patient population.
Practice Points.
Hyperglycemia is associated with reduced survival in patients with glioblastoma (GBM).
This retrospective analysis assessed overall survival based on steroid dependency and antidiabetic medication in diabetic GBM patients.
Out of all GBM patients, 12.6% are diabetic (72% with pre-existing Type 2 diabetes mellitus and 28% with steroid-induced Type 2 diabetes mellitus).
Only 15% of diabetic patients had been tapered from steroids.
Diabetics had a reduced survival (10 months) compared with their nondiabetic counterparts (13.4 months).
Steroid dependency was associated with poor outcome.
Patients receiving metformin had an improved median survival compared with all other antidiabetic medications.
Patients on sulfonylureas had worse outcomes.
Age, Karnofsky Performance Score, extent of resection and use of adjuvant treatment, metformin as well as sulfonylurea, were identified as predictors of survival by univariate analysis.
Steroids should be tapered whenever possible and diabetes controlled more rigorously.
There is a potential survival benefit from the use of metformin, while sulfonylureas may be associated with a poor outcome.
As our evolving understanding of tumor biology translates into improved survival times for patients with glioblastoma, physicians will need to be more attentive to diabetic management, particularly when steroids are administered. Including an endocrinologist or nurse educator on a multidisciplinary team caring for diabetic patients with glioblastoma would improve glycemic control, limit complications and, potentially, extend survival. The role of antidiabetic medication, in particular metformin, merits further evaluation in a prospective clinical trial.
Background
Glioblastoma (GBM) is a rare but lethal brain tumor and, despite recent advances in treatment, median survival is 14 months [1,2]. By contrast, Type 2 diabetes mellitus (DM2) is among the most common chronic illnesses worldwide and affects nearly 10% of the population of the USA [3]. Studies have demonstrated that cancer patients with pre-existing diabetes are at increased risk for long-term all-cause mortality compared with nondiabetic patients. Little is known about the impact of diabetes on patients with GBM. In addition, glucocorticoids are routinely used to control peritumoral edema, placing GBM patients at increased risk for hyperglycemia, but strict glycemic control is often considered less of a priority.
Retrospective studies have demonstrated an association between hyperglycemia and survival in patients with newly diagnosed GBM, suggesting that more aggressive use of antidiabetic therapy (ADT) may be warranted [4,5]. Interpretation of these observations is confounded by the greater comorbidity and reduced life expectancy associated with DM2, as well as the possibility that hyperglycemia may be a marker for steroid dependency, which is typically seen with more aggressive tumors. However, it is also possible that diabetics respond less effectively to certain cancer therapies [6,7]. There is mounting evidence to suggest that the metabolic dysregulation associated with DM2 may actually promote tumor growth – a hypothesis that appears to be well supported by epidemiologic studies linking DM2 and cancer risk [8–16]. Moreover, hyperinsulinemia leads to increased activation of insulin receptor and IGF-1 receptor signaling, promoting mitogenic effects through PI3K pathway activation [10,17–19].
As research yields further evidence of the overlap between aberrant cellular signaling pathways and mechanisms of metabolic control, there has been a growing interest in the role of ADT on the risk of cancer and its progression. Several observational studies and preclinical data have supported a role for thiazolidinediones (PPAR-γ agonists) and the biguanide metformin in the treatment of cancer patients [20–22]. Conversely, a small number of retrospective studies have found a higher risk of cancer and poorer overall survival (OS) among individuals treated with sulfonylureas [23–27]. To address the impact of these therapies in gliomas, we retrospectively reviewed diabetic GBM patients at a single institution. Our primary objective was to investigate the influence of DM2 and ADT on median OS in GBM patients. We compared clinical outcomes across classes of ADT, with the goal of identifying one agent that might be associated with prolonged survival.
Patients & methods
▪ Study methods
Patients with histologically confirmed GBM and DM2 seen at our institution (Memorial Sloan-Kettering Cancer Center, NY, USA) from 1998 to 2010 were identified. The year 1998 was chosen due to US FDA approval of the first PPAR-γ agonist. Diabetic patients were defined as those requiring pharmacological treatment of hyperglycemia beyond a period of 4 weeks. By this definition, patients who were temporarily placed on an insulin sliding scale, while hospitalized patients were excluded. Type 1 diabetics and those managed by diet alone were also excluded because they did not receive ADT (metformin, PPAR-γ agonists or sulfonylureas) as part of their treatment.
Data were collected by reviewing electronic medical records and included the following elements: the date of cancer diagnosis defined as the date of the surgical procedure that provided pathological disease confirmation; age and Karnofsky Performance Score (KPS) at diagnosis; the number and class of antidiabetic agents used; the extent of tumor resection; steroid use and dependency; treatment with chemotherapy and/or radiation; and date of death or last follow-up.
To approximate glycemic control, HbA1c was noted if available. In addition, a median glucose value was calculated for each patient based on all values, inpatient and outpatient, obtained from the date of diagnosis onwards. The number of glucose values varied with a median of 52 values per patient and a range from two to 210. Extent of tumor resection was classified into three categories: biopsy alone; gross total resection, which eliminated all contrast-enhancing tumor as determined by comparison of pre- and post-operative MRIs; and subtotal resection, which encompassed all other cases. Steroid dependency was defined as an inability to be weaned from steroids. Patients whose steroid doses were tapered off only to be raised again were classified as steroid dependent.
The primary end point assessed in this study was OS, defined as the duration between date of diagnosis, and death or last follow-up. Patients whose date of death was not recorded were censored at the date of their last follow-up. To identify predictors of OS, we analyzed potentially relevant demographic, clinical and treatment variables for their impact on survival time. This retrospective study was approved by the institutional review board.
▪ Statistical analyses
Relevant patient characteristics were compared using Fisher's exact test or Student's t-test where appropriate. Kaplan–Meier distributions were estimated to assess survival, and curves were compared for significance using the log rank test. To limit confounders and hone in on the effect of ADT, we limited the additional analysis to patients treated with a single antidiabetic agent. Survival was tested against four hypothesized predictors and, therefore, a Bonferroni-adjusted significance level of 0.025 (α = 0.1/4) was calculated in order to account for the increased possibility of a type 1 error. The relationships of risk factors to OS (e.g., age, gender, baseline KPS, DM2 status, HbA1c, median glucose levels, steroid dependence, extent of resection, treatment with chemotherapy, insulin use, PPAR-γ use, metformin use and sulfonylurea use) were analyzed using simple logistic regression models. Multivariate Cox regression analysis was used to analyze those predictors of survival that were univariately significant at α < 0.1. All variables, except KPS, age, median glucose and HbA1c, were dichotomized in the analysis. Statistical analyses were performed using STATA (TX, USA) statistical software (version 12.0).
Results
▪ Patient characteristics
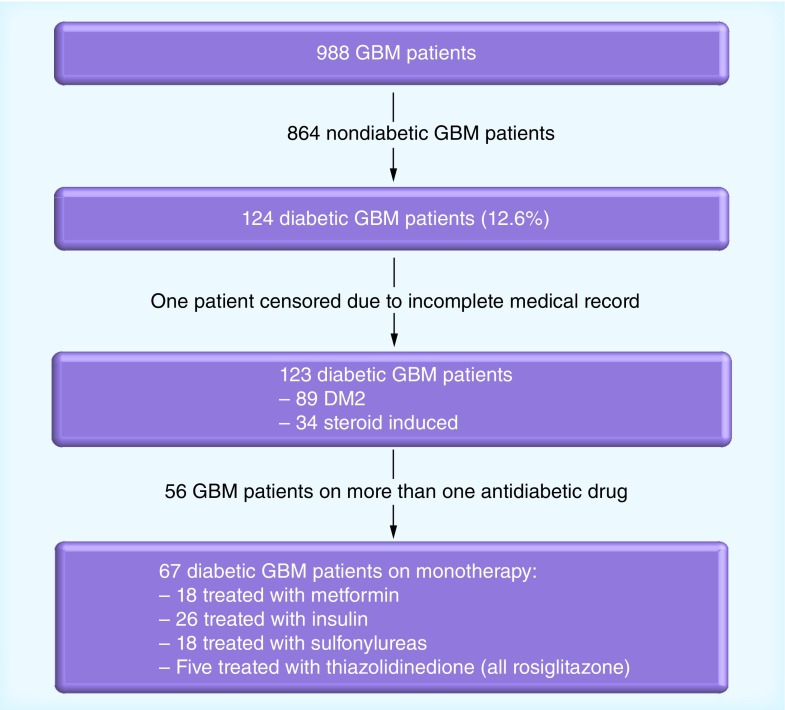
Between 1st January 1998 and 31st December 2010, 988 GBM patients were seen and treated at our institution (Memorial Sloan-Kettering Cancer Center); 124 met criteria for DM2 (12.6%). One patient had an incomplete medical record and was censored (Figure 1). Among the remaining 123 patients, 89 (72%) had a pre-existing diagnosis of DM2 prior to diagnosis of GBM. The remaining 34 patients (28%) developed diabetes in the setting of steroid use after identification of their tumors. For the purpose of this study, we classified these patients as steroid-induced diabetics, although we could not be certain that they would not have developed insulin resistance independently of dexamethasone exposure.
Figure 1. Patient flow chart.
DM2: Type 2 diabetes mellitus; GBM: Glioblastoma.
Patient characteristics are summarized in Table 1. Median age for the entire cohort was 66 years (range: 29–90 years); median baseline KPS was 80 years (range: 40–100 years). A substantial minority (29 out of 123 patients; 24%) underwent biopsy alone, while the remainder (76%) had either a gross total (23 out of 123; 19%) or subtotal (71 out of 123; 57%) resection. Three patients (2%) were treated with chemotherapy alone and 16 (13%) received no additional therapy after surgery due to either performance status or patient preference. In the study group, 85% of the DM2 patients received radiation; 62% of the DM2 patients received radiation in combination with chemotherapy. Compared with the nondiabetic GBM population, there was no significant difference (combination therapy was received by 561 out of 864 patients; 65%).
Table 1. . Patient characteristics.
| Charactersistics | Diabetic patients | Combination therapy | Monotherapy | p-value | Metformin | p-value | Sulfonylurea | p-value | Insulin | p-value | PPAR-γ | p-value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients (n) | 123 | 56 | 67 | – | 18 | – | 18 | – | 26 | – | 5 | – |
| Median age; years (range) | 66 (29–90) | 65 (29–90) | 68 (39–84) | 0.3 | 63 (39–79) | 0.2 | 71.5 (59–84) | 0.005 | 67.5 (40–79) | 0.15 | 71 (50–77) | 0.8 |
| Median KPS (range) | 80 (40–100) | 80 (40–90) | 70 (40–100) | 0.04 | 70 (50–100) | 0.3 | 60 (40–90) | 0.2 | 70 (40–100) | 0.9 | 80 (50–90) | 0.7 |
| Men; n (%) | 86 (70) | 41 (73) | 45 (67) | 0.5 | 13 (72) | 0.6 | 13 (72) | 0.6 | 12 (46) | – | 0 | 0.2† |
| Women; n (%) | 37 (30) | 15 (27) | 22 (33) | – | 5 (28) | – | 5 (28) | – | 14 (53) | 0.07 | 5 (100) | 0.3† |
| DM2; n (%) | 89 (72) | 41 (73) | 48 (72) | 0.9 | 14 (78) | 0.5 | 15 (83) | 0.2 | 14 (54) | 0.01 | 5 (100) | – |
| Steroid use: Data available (n) ▪ Dependent; n (%) ▪ Weaned; n (%) ▪ Data missing; n (%) |
120 102 (83) 18 (15) 3 (2) |
56 49 (87.5) 7 (12.5) 0 |
64 53 (79) 11 (16) 3 (5) |
0.5 |
17 14 (78) 3 (17) 1 (6) |
1.0† |
18 16 (89) 2 (11) 0 |
0.7† |
24 19 (73) 5 (19) 2 (8) |
0.7† |
5 4 (80) 1 (20) 0 |
1.0† |
| Gross total resection; n (%) | 23 (19) | 15 (27) | 8 (12) | 0.04 | 4 (22) | 0.2† | 1 (6) | 0.4† | 2 (8) | 0.5† | 1 (20) | 0.5† |
| Subtotal resection; n (%) | 71 (58) | 31 (55) | 40 (60) | 0.6 | 8 (44) | 0.13 | 13 (72) | 0.2 | 17 (65) | 0.5 | 2 (40) | 0.4† |
| Biopsy only; n (%) | 29 (23) | 10 (18) | 19 (28) | 0.2 | 6 (33) | 0.6 | 4 (22) | 0.6† | 7 (27) | 0.8 | 2 (40) | 0.6† |
| Chemotherapy; n (%) | 79 (64) | 38 (68) | 41 (61) | 0.4 | 13 (72) | 0.4† | 10 (56) | 0.6 | 16 (62) | 0.9 | 2 (40) | 0.4† |
| Radiation; n (%) | 104 (85) | 50 (89) | 54 (81) | 0.2 | 16 (89) | 0.5† | 15 (83) | 0.7 | 20 (77) | 0.6 | 3 (60) | 0.2† |
| Chemoradiation; n (%) | 75 (61) | 37 (66) | 38 (57) | 0.3 | 13 (72) | 0.2† | 9 (50) | 0.5 | 15 (58) | 0.9 | 1 (20) | 0.2† |
| HbA1c: ▪ Data available (n) ▪ Median ▪ Range ▪ Mean |
68 7.65 5.4–13.6 7.88 |
34 7.4 5.4–11 7.8 |
34 7.95 5.5–13.6 8 |
0.6 |
10 7.7 5.5–8.5 7.25 |
0.15 |
8 8.2 5.6–13.6 8.3 |
0.6 |
16 7.8 5.9–12.9 8.3 |
0.4 |
0 – – – |
– |
| Median glucose (mg/dl): ▪ Data available (n) ▪ Median ▪ Range ▪ Mean |
119 198.5 98.5–321.1 196 |
54 204 98.5–321.5 203 |
65 190 111–295 191 |
0.09 |
17 182 111–255.5 178 |
0.1 |
17 193.5 134–268 198 |
0.3 |
26 189 132–295 195 |
0.5 |
5 188 170–205.5 188.5 |
0.9 |
†Fisher's exact test; all other comparisons used the Student's t-test.
DM2: Type 2 diabetes mellitus; KPS: Karnofsky Performance Score.
Fifty-six patients (46%) required the use of more than one antidiabetic agent and 67 patients (54%) were managed with a single drug. Of these, 26 patients received insulin, 18 a sulfonylurea, 18 metformin and five a PPAR-γ inhibitor (Figure 1). Patients in the combination group had a better median KPS (80 vs 70; p = 0.04) and were more likely to have undergone gross total resection (27 vs 12%; p = 0.04) compared with those treated with a single ADT; no other significant differences were observed in the distribution of patients according to sex, age, median glucose or HbA1c. Data on steroid use were available for 120 patients. Of these, only 18 patients (15%) were able to be weaned off steroids; the majority (83%) remained steroid dependent.
Maximum HbA1c values were available for 68 patients (56%) and glucose measurements were available for 100 (97%). Although incomplete, these data nonetheless reveal a pattern of poor glycemic control. Mean HbA1c was 7.9 (range: 5.4–13.6) and mean glucose was 196 mg/dl (range: 98.5–321.5 mg/dl). The addition of a second or third agent did not produce any statistically significant differences in HbA1c or median glucose. Within the monotherapy group, there was a trend towards better glycemic control among patients treated with metformin (mean HbA1c: 7.25) compared with other single agents (mean HbA1c: 8.3; p = 0.15). Conversely, patients on a sulfonylurea tended to have less well-controlled diabetes (mean HbA1c: 8.3 vs 7.9; p = 0.6) (Table 1).
▪ Survival
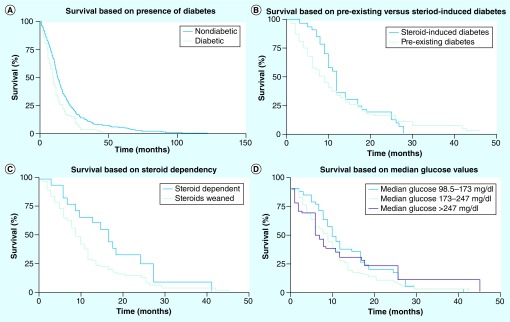
Median OS among diabetics (n = 123) was 10 months (95% CI: 8–12) compared with 13.4 months (95% CI: 12.7–14.4) among nondiabetics (n = 864; log rank = 0.0000) (Figure 2A). Of the 123 patients, 112 (91%) had died at the time of analysis. Of those, all had died as a consequence of tumor progression. While there was a slight trend towards improved survival in patients with steroid-induced diabetes (n = 34) when compared with those with pre-existing DM2 (n = 89), this did not meet statistical significance (log rank = 0.5) (Figure 2B). A strong relationship between survival and steroid dependency was noted. Patients who remained steroid dependent (n = 102) did poorly with a median OS of 9 months (95% CI: 8–11) compared with 17 months (95% CI: 9–25) among those who were weaned off steroids (log rank = 0.05) (Figure 2C). No statistically significant correlation was found between survival and median glucose values (log rank = 0.36). Patients with the best glycemic control whose median glucose ranged from 98.5 to 173 mg/dl (n = 33) had a median OS of 11 months (95% CI: 9–17); those with values from 174 to 247 mg/dl (n = 76) had a median overall survival of 9 months (95% CI: 7–12); and those in the highest tertile whose median glucose was >247 mg/dl (n = 14) had a median overall survival of 8 months (95% CI: 2–26) (Figure 2D).
Figure 2. Survival of diabetic glioblastoma patients.
(A) Comparison of overall survival between diabetic (n = 123; 10 months; 95% CI: 8–12) and nondiabetic (n = 864; 13.4 months; 95% CI: 12.7–14.4) glioblastoma patients (log rank = 0.0000). (B) Comparison of overall survival between patients who had a diagnosis of diabetes at baseline (n = 89) and those who developed diabetes in the setting of steroid use for treatment of glioblastoma (n = 34; log rank = 0.5). (C) Comparison of overall survival between diabetic patients who were able to be tapered off steroids (n = 18; 17 months; 95% CI: 9–25) and those who remained steroid dependent (n = 102; 9 months; 95% CI: 8–11; log rank = 0.05). (D) Comparison of overall survival based on median glucose values divided into tertiles: median glucose 98.5–173 mg/dl (n = 33; 11 months; 95% CI: 9–17); 174–247 mg/dl (n = 76; 9 months; 95% CI: 7–12); and >247 mg/dl (n = 14; 8 months; 95% CI: 2–26; log rank = 0.36).
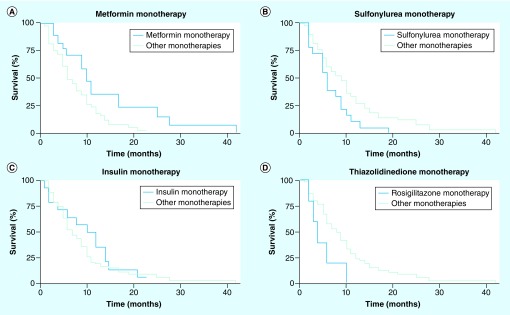
Multidrug regimens were complex and changeable over time, making it difficult to determine how any one agent impacted survival. In order to provide a clean comparison across ADTs, we elected to limit further analysis to the 67 patients on monotherapy. Among these, there was a clear survival benefit with the use of metformin. Patients treated with this drug (n = 18) had a median OS of 10 months (95% CI: 5–17) compared with 6 months (95% CI: 5–9) for all other monotherapies (log rank = 0.02) (Figure 3A). Patients treated with sulfonylureas (n = 18) had worse outcomes. Median OS was only 6 months (95% CI: 3–9) compared with 9 months in other monotherapy patients (log rank = 0.03) (Figure 3B). A 3-month survival advantage (10 vs 7 months) was seen among those who received insulin, but this was not statistically significant (log rank = 0.5) (Figure 3C). Patients on rosiglitazone had the lowest median OS of 4 months (95% CI: 2–∞) compared with 9 months among those on other monotherapies, but only five patients received this drug, limiting the statistical power (Figure 3D). To confirm these observations in a more homogeneous patient population, we focused on only those diabetic GBM patients who had received surgery, radiation and chemotherapy in our ADT monotherapy cohort (n = 38). Median OS in this subpopulation was 10 months. Patients treated with metformin (n = 13) had a median OS of 14 months compared with patients treated with other ADTs (n = 25) who lived for a median of 8 months. We, again, observed a poor median OS of 8 months associated with sulfonylureas (n = 9).
Figure 3. Overall survival among diabetic patients treated with a single-agent antidiabetic drug.
Patients had (A) improved survival with metformin (n = 18; 10 months; 95% CI: 5–17) compared with all other monotherapies (n = 49; 6 months; 95% CI: 5–9; log rank = 0.02); (B) reduced survival with sulfonylureas (n = 18) compared with all other monotherapies (n = 49; log rank = 0.03); (C) improved survival with insulin (n = 14) compared with all other monotherapies (n = 53; log rank = 0.5); and (D) reduced survival with rosiglitazone (n = 5) compared with all other monotherapies (n = 62; log rank = 0.05).
According to univariate regression analysis, previously established prognostic factors, including age, KPS, resection and use of adjuvant treatment, retained significance as predictors of survival. Metformin and sulfonylurea use also had an impact on OS. Patients who received the former experienced a 50% risk reduction (p = 0.03) for death, while those who received the latter had a higher risk for death with a hazard ratio of 1.77 (p = 0.05). On multivariate analysis, KPS, resection status, steroid dependence and metformin use retained significance to the α < 0.1 level. Sulfonylurea use was discounted as a predictor (Table 2). Adjuvant therapy did not reach significance on multivariate analysis. To ensure that changes in tumor-directed therapy, in particular the use of temozolomide, did not influence survival in the patients treated with a single ADT, median survival in those patients diagnosed between 1998 and 2005 (n = 40) were compared with those diagnosed between 2006 and 2008 (n = 27). There was no significant difference in median survival based on the time of diagnosis (1998–2005 group: 9 months; and 2006–2009 group: 6 months; p = 0.1948).
Table 2. . Multivariable regression analysis among 67 patients treated with a single antidiabetic therapy.
| Variable | n | Unadjusted HR | p-value | Adjusted HR | p-value |
|---|---|---|---|---|---|
| Age | – | 1.04 | 0.01 | 1.00 | 0.95 |
| Male gender | 45 | 1.15 | 0.6 | – | – |
| KPS | – | 0.98 | 0.002 | 0.98 | 0.02 |
| DM2 | 48 | 1.25 | 0.4 | – | – |
| PPAR-γ | 5 | 2.36 | 0.07 | 1.79 | 0.3 |
| Metformin | 18 | 0.51 | 0.03 | 0.51 | 0.09 |
| Insulin | 26 | 0.82 | 0.5 | – | – |
| Sulfonylurea | 18 | 1.77 | 0.05 | 1.45 | 0.3 |
| HbA1c | – | 0.88 | 0.2 | – | – |
| Median glucose | – | 1.00 | 0.4 | – | – |
| Weaned off steroids | 11 | 0.48 | 0.06 | 0.32 | 0.04 |
| Gross total resection | 8 | 0.75 | 0.5 | – | – |
| Subtotal resection | 40 | 0.75 | 0.3 | – | – |
| Biopsy | 19 | 1.78 | 0.04 | 3.23 | 0.002 |
| Chemotherapy | 41 | 0.34 | 0.000 | 1.59 | 0.6 |
| Radiation | 54 | 0.19 | 0.000 | 0.45 | 0.09 |
| Chemotherapy plus radiation therapy | 38 | 0.33 | 0.000 | 0.3 | 0.18 |
DM2: Type 2 diabetes mellitus; HR: Hazard ratio; KPS: Karnofsky Performance Score.
Discussion
Limited data exist on the prevalence of DM2 among patients with GBM. Earlier studies reported prevalence as low as 3–6%, while more recent work identified DM2 in up to 16% of GBM patients [28–31]. The 124 diabetic patients we identified accounted for 12.6% of our institution's (Memorial Sloan-Kettering Cancer Center) GBM population from 1998 to 2010, a figure that is consistent with prior studies, as well as the prevalence of diabetes among adults in the USA aged 20 years and older [3]. In the face of demographic pressures, lifestyle changes and growing obesity rates, these numbers are projected to rise and physicians will increasingly encounter metabolic disease in their GBM patients. In the field of neuro-oncology, DM2 poses a particular challenge as successfully tapering steroids might not always be feasible. In prior studies [4,5], as well as in our cohort, increased hyperglycemia was associated with poorer outcome. Hyperglycemia, however, can be managed more aggressively, and the current study suggests that ADT choice can have a significant influence on survival, with up to 4 months difference between ADT groups. While acknowledging the biases inherent in a retrospective study, this is nonetheless a marked difference, particularly in GBM where median OS is only 14–20 months [32]. We did not observe a difference in survival between diabetic GBM patients diagnosed between 1998 and 2005 compared with between 2006 and 2008. This was unexpected due to the establishment of a new standard of care in 2005 [1]. The small overall sample size, as well as the smaller-sized 2006–2009 cohort, might have contributed to the nonsignificant survival difference. Furthermore, some of the patients diagnosed before 2005 were treated with temozolomide, which would also contribute to the nonsignificant survival difference between the two patient cohorts.
Recent work, both in vitro and in vivo, has demonstrated that metformin inhibits cancer cell growth and may work synergistically with other agents to exert an anti-tumor effect [17,23,33–39]. Moreover, epidemiologic data in colorectal, breast and lung cancers have suggested that the use of metformin may be associated with a reduced risk of cancer in patients with DM2 [6,25,40–42]. The mechanism for these findings is still under investigation, but it is thought that metformin may influence cancer cells either through indirect insulin-mediated effects or by direct interaction with key oncogenic signaling pathways. To date, much of the data supporting a role for metformin in the treatment of cancer has been observational, but prospective trials are currently underway to explore the potential for this drug in the adjuvant setting. While the largest of these is a multicenter Phase III trial for early-stage breast cancer, smaller studies are also accruing patients with pancreatic, endometrial and prostate cancers. A Phase I factorial study investigating several potentially anti-tumorigenic agents, including metformin, is also currently underway in brain tumor patients [101,102]. While further clinical research will ultimately determine whether this drug has a role in cancer treatment, our study is the first to indicate a potential benefit in GBM patients with both pre-existing and steroid-induced DM2.
Given that steroid dependency often correlates with tumor burden, it is likely that the 8-month survival benefit we found among patients weaned from dexamethasone was, in part, a reflection of less aggressive disease. However, other data have shown that steroid-induced hyperglycemia has a negative prognostic influence, irrespective of tumor size [43]. Moreover, exacerbation of DM2 is only one of the well-identified side effects produced by chronic steroid use, any one of which could impact mortality. Although our study lacked the power to unravel potential interactions between disease burden, blood glucose levels and dexamethasone use, it is clear that minimizing the latter remains one of the simplest methods to improve glycemic control and potentially improve OS. This was supported by our multivariate analysis that identified steroid independence as a strong predictor of improved survival (hazard ratio: 0.32; p = 0.04). Notably, only 15% of our 123 diabetic patients fell into this category. Although it is possible that the patients who required continuous steroid use had more symptomatic disease, it is also possible that aggressive attempts to wean were simply not a priority. Treating physicians, rationalizing that patients would not be alive to face the long-term consequences of diabetes, might have elected to err on the side of symptomatic relief. A similar approach to glucose management might account for the high median glucose and elevated HbA1c values we found, raising the question of whether more aggressive measures to control hyperglycemia might improve patient survival. Further study in a prospective fashion with a standardized approach to glucose and HbA1c measurements would be required to unravel these issues.
In addition to the challenges inherent in studying any rare tumor, there are several limitations to our study. First, details regarding medication use were obtained from a review of medical and pharmacy records, and it is possible that patients may have been taking additional medications prescribed by other physicians. Second, while limiting our regression analysis to the 67 patients on monotherapy allowed for a cleaner comparison across agents, it also limited the study's power. For example, it is interesting to speculate whether the survival disadvantage observed with both sulfonylureas and rosiglitazone would have achieved significance in a larger sample size. Third, due to its retrospective nature, relevant patient information, including the rationale for the choice of ADT, was unavailable. It is possible that metformin was given only to patients with less brittle diabetes whose hyperglycemia was well controlled at baseline. One might then argue that it was improved glycemic control, rather than any inherent antineoplastic property, that provided the observed survival benefit. However, while median glucose was measurably lower among patients treated with metformin, this did not meet statistical significance (Table 1). Notably, neither HbA1c nor median glucose achieved significance as predictors of survival on regression analysis, while metformin use did. Of course, these measurements were, by necessity, crude, but the best available option in a retrospective setting.
Conclusion & future perspective
Despite the limitations of a retrospective analysis, our study makes a strong argument for steroid tapering whenever possible and aggressive use of ADT in patients with GBM. Moreover, it is the first to suggest a potential survival benefit with the use of metformin among diabetic GBM patients. These observations, the drug's well-known and limited side effect profile, as well as its widespread availability, makes metformin an intriguing subject for additional study. Prospective trials are warranted to formally evaluate the use of this agent and more fully elucidate the complex interactions between ADT, hyperglycemia and survival.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: ▪ of interest ▪▪ of considerable interest
- 1.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised Phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]; ▪ Landmark paper that established the current standard of care – a combination of temozolomide and radiation – in the treatment of glioblastoma.
- 2.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J. Clin. Oncol. 2009;27(28):4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 3.CDC. National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States, 2011. US Department of Health and Human Services; CDC, GA, USA: 2011. [Google Scholar]
- 4.Derr RL, Ye X, Islas MU, Desideri S, Saudek CD, Grossman SA. Association between hyperglycemia and survival in patients with newly diagnosed glioblastoma. J. Clin. Oncol. 2009;27(7):1082–1086. doi: 10.1200/JCO.2008.19.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪▪ First study to identify hyperglycemia as a negative predictor of survival in patients with glioblastoma.
- 5.McGirt MJ, Chaichana KL, Gathinji M, et al. Persistent outpatient hyperglycemia is independently associated with decreased survival after primary resection of malignant brain astrocytomas. Neurosurgery. 2008;63(2):286–291. doi: 10.1227/01.NEU.0000315282.61035.48. [DOI] [PubMed] [Google Scholar]; ▪▪ Identified survival differences based on postoperative glucose values in a large cohort of high-grade glioma patients following craniotomy.
- 6.Currie CJ, Poole CD, Jenkins-Jones S, Gale EA, Johnson JA, Morgan CL. Mortality after incident cancer in people with and without Type 2 diabetes: impact of metformin on survival. Diabetes Care. 2012;35(2):299–304. doi: 10.2337/dc11-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪ Confirmed that cancer patients with Type 2 diabetes have worse outcomes compared with their nondiabetic counterparts. It also noted a survival benefit with the use of metformin.
- 7.Underwood JM, Townsend JS, Stewart SL, et al. Surveillance of demographic characteristics and health behaviors among adult cancer survivors – behavioral risk factor surveillance system, United States, 2009. MMWR Surveill. Summ. 2012;61(1):1–23. [PubMed] [Google Scholar]
- 8.Barone BB, Yeh HC, Snyder CF, et al. Postoperative mortality in cancer patients with preexisting diabetes: systematic review and meta-analysis. Diabetes Care. 2010;33(4):931–939. doi: 10.2337/dc09-1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barone BB, Yeh HC, Snyder CF, et al. Long term all-cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta-analysis. JAMA. 2008;300(23):2754–2764. doi: 10.1001/jama.2008.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. Diabetes Care. 2010;33(7):1674–1685. doi: 10.2337/dc10-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Willett WC. Diabetes mellitus and risk of prostate cancer (United States) Cancer Causes Control. 1998;9(1):3–9. doi: 10.1023/a:1008822917449. [DOI] [PubMed] [Google Scholar]
- 12.Hu FB, Giovannucci E. Re: prospective study of adult onset diabetes mellitus (Type 2) and risk of colorectal cancer in women. J. Natl Cancer Inst. 1999;91(15):1334A–1334A. doi: 10.1093/jnci/91.15.1334a. [DOI] [PubMed] [Google Scholar]
- 13.Hu FB, Manson JE, Liu S, et al. Prospective study of adult onset diabetes mellitus (type 2) and risk of colorectal cancer in women. J. Natl Cancer Inst. 1999;91(6):542–547. doi: 10.1093/jnci/91.6.542. [DOI] [PubMed] [Google Scholar]
- 14.Wu AH, Yu MC, Tseng CC, Stanczyk FZ, Pike MC. Diabetes and risk of breast cancer in Asian–American women. Carcinogenesis. 2007;28(7):1561–1566. doi: 10.1093/carcin/bgm081. [DOI] [PubMed] [Google Scholar]
- 15.Yang X, Ko GT, So WY, et al. Associations of hyperglycemia and insulin usage with the risk of cancer in Type 2 diabetes: the Hong Kong diabetes registry. Diabetes. 2010;59(5):1254–1260. doi: 10.2337/db09-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang YX, Hennessy S, Lewis JD. Insulin therapy and colorectal cancer risk among Type 2 diabetes mellitus patients. Gastroenterology. 2004;127(4):1044–1050. doi: 10.1053/j.gastro.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 17.Gallagher EJ, LeRoith D. Diabetes, cancer, and metformin: connections of metabolism and cell proliferation. Ann. NY Acad. Sci. 2011;1243:54–68. doi: 10.1111/j.1749-6632.2011.06285.x. [DOI] [PubMed] [Google Scholar]
- 18.Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat. Rev. Drug Discov. 2005;4(12):988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 19.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zander T, Kraus JA, Grommes C, et al. Induction of apoptosis in human and rat glioma by agonists of the nuclear receptor PPAR-gamma. J. Neurochem. 2002;81(5):1052–1060. doi: 10.1046/j.1471-4159.2002.00899.x. [DOI] [PubMed] [Google Scholar]
- 21.He X, Esteva FJ, Ensor J, Hortobagyi GN, Lee MH, Yeung SC. Metformin and thiazolidinediones are associated with improved breast cancer-specific survival of diabetic women with HER2+ breast cancer. Ann. Oncol. 2011;23(7):1771–1780. doi: 10.1093/annonc/mdr534. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪ Demonstrates improved outcomes among diabetic HER2+ breast cancer treated with either metformin or thiazolidinediones.
- 22.Grommes C, Landreth GE, Heneka MT. Antineoplastic effects of peroxisome proliferator-activated receptor gamma agonists. Lancet Oncol. 2004;5(7):419–429. doi: 10.1016/S1470-2045(04)01509-8. [DOI] [PubMed] [Google Scholar]
- 23.Ruiter R, Visser LE, van Herk-Sukel MP, et al. Lower risk of cancer in patients on metformin in comparison with those on sulfonylurea derivatives: results from a large population-based follow-up study. Diabetes Care. 2012;35(1):119–124. doi: 10.2337/dc11-0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Monami M, Lamanna C, Balzi D, Marchionni N, Mannucci E. Sulphonylureas and cancer: a case–control study. Acta Diabetol. 2009;46(4):279–284. doi: 10.1007/s00592-008-0083-2. [DOI] [PubMed] [Google Scholar]
- 25.Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in Type 2 diabetes. Diabetologia. 2009;52(9):1766–1777. doi: 10.1007/s00125-009-1440-6. [DOI] [PubMed] [Google Scholar]
- 26.Bowker SL, Majumdar SR, Veugelers P, Johnson JA. Increased cancer-related mortality for patients with Type 2 diabetes who use sulfonylureas or insulin: Response to Farooki and Schneider. Diabetes Care. 2006;29(8):1990–1991. doi: 10.2337/dc06-0997. [DOI] [PubMed] [Google Scholar]
- 27.Bowker SL, Majumdar SR, Veugelers P, Johnson JA. Increased cancer-related mortality for patients with Type 2 diabetes who use sulfonylureas or insulin. Diabetes Care. 2006;29(2):254–258. doi: 10.2337/diacare.29.02.06.dc05-1558. [DOI] [PubMed] [Google Scholar]
- 28.Brenner AV, Linet MS, Fine HA, et al. History of allergies and autoimmune diseases and risk of brain tumors in adults. Int. J. Cancer. 2002;99(2):252–259. doi: 10.1002/ijc.10320. [DOI] [PubMed] [Google Scholar]
- 29.Schwartzbaum J, Jonsson F, Ahlbom A, et al. Prior hospitalization for epilepsy, diabetes, and stroke and subsequent glioma and meningioma risk. Cancer Epidemiol. Biomarkers Prev. 2005;14(3):643–650. doi: 10.1158/1055-9965.EPI-04-0119. [DOI] [PubMed] [Google Scholar]
- 30.Grommes C, Conway DS, Alshekhlee A, Barnholtz-Sloan JS. Inverse association of PPAR-gamma agonists use and high grade glioma development. J. Neurooncol. 2010;100(2):233–239. doi: 10.1007/s11060-010-0185-x. [DOI] [PubMed] [Google Scholar]
- 31.Schlehofer B, Blettner M, Preston-Martin S, et al. Role of medical history in brain tumour development. Results from the international adult brain tumour study. Int. J. Cancer. 1999;82(2):155–160. doi: 10.1002/(sici)1097-0215(19990719)82:2<155::aid-ijc1>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 32.Lai A, Tran A, Nghiemphu PL, et al. Phase II study of bevacizumab plus temozolomide during and after radiation therapy for patients with newly diagnosed glioblastoma multiforme. J. Clin. Oncol. 2011;29(34):142–148. doi: 10.1200/JCO.2010.30.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buzzai M, Jones RG, Amaravadi RK, et al. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res. 2007;67(14):6745–6752. doi: 10.1158/0008-5472.CAN-06-4447. [DOI] [PubMed] [Google Scholar]
- 34.Cheong JH, Park ES, Liang J, et al. Dual inhibition of tumor energy pathway by 2-deoxyglucose and metformin is effective against a broad spectrum of preclinical cancer models. Mol. Cancer Ther. 2011;10(12):2350–2362. doi: 10.1158/1535-7163.MCT-11-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chong CR, Chabner BA. Mysterious metformin. Oncologist. 2009;14(12):1178–1181. doi: 10.1634/theoncologist.2009-0286. [DOI] [PubMed] [Google Scholar]
- 36.Garcia A, Tisman G. Metformin, B(12), and enhanced breast cancer response to chemotherapy. J. Clin. Oncol. 2010;28(2):e19. doi: 10.1200/JCO.2009.25.7857. author reply e20. [DOI] [PubMed] [Google Scholar]
- 37.Hirsch HA, Iliopoulos D, Tsichlis PN, Struhl K. Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Res. 2009;69(19):7507–7511. doi: 10.1158/0008-5472.CAN-09-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiralerspong S, Palla SL, Giordano SH, et al. Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J. Clin. Oncol. 2009;27(20):3297–3302. doi: 10.1200/JCO.2009.19.6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tan BX, Yao WX, Ge J, et al. Prognostic influence of metformin as first-line chemotherapy for advanced nonsmall cell lung cancer in patients with Type 2 diabetes. Cancer. 2011;117(22):5103–5111. doi: 10.1002/cncr.26151. [DOI] [PubMed] [Google Scholar]
- 40.Bodmer M, Meier C, Krahenbuhl S, Jick SS, Meier CR. Long-term metformin use is associated with decreased risk of breast cancer. Diabetes Care. 2010;33(6):1304–1308. doi: 10.2337/dc09-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, Evans JM. New users of metformin are at low risk of incident cancer: a cohort study among people with Type 2 diabetes. Diabetes Care. 2009;32(9):1620–1625. doi: 10.2337/dc08-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monami M, Colombi C, Balzi D, et al. Metformin and cancer occurrence in insulin-treated Type 2 diabetic patients. Diabetes Care. 2011;34(1):129–131. doi: 10.2337/dc10-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chaichana KL, McGirt MJ, Woodworth GF, et al. Persistent outpatient hyperglycemia is independently associated with survival, recurrence and malignant degeneration following surgery for hemispheric low grade gliomas. Neurol. Res. 2010;32(4):442–448. doi: 10.1179/174313209X431101. [DOI] [PubMed] [Google Scholar]; ▪▪ Correlates reduced survival, greater tumor burden and malignant transformation with postoperative hyperglycemia in a large cohort of patients with low-grade gliomas.
▪ Websites
- 101.http://clinicaltrials.gov/ct2/show/NCT01430351?term=NCT01430351&rank=1 ClinicalTrials.gov. Phase I Factorial Trial of Temozolomide, Memantine, Mefloquine, and Metformin for Post-Radiation Therapy (RT) Glioblastoma Multiforme (GBM)
- 102.http://clinicaltrials.gov/ct2/show/NCT01101438?term=NCT01101438&rank=1 ClinicalTrials.gov. A Phase III Randomized Trial of Metformin vs Placebo in Early Stage Breast Cancer.