SUMMARY
High-grade gliomas are the most common type of primary brain tumor and are among the most lethal types of human cancer. Most patients with a high-grade glioma have glioblastoma multiforme (GBM), the most malignant glioma subtype that is associated with a very aggressive disease course and short overall survival. Standard treatment of newly diagnosed GBM involves surgery followed by chemoradiation with temozolomide. However, despite this extensive treatment the mean overall survival is still only 14.6 months and more effective treatments are urgently needed. Although different types of GBMs are indistinguishable by histopathology, novel molecular pathological techniques allow discrimination between the four main GBM subtypes. Targeting the aberrations in the molecular pathways underlying these subtypes is a promising strategy to improve therapy. In this article, we will discuss the potential avenues and pitfalls of molecularly targeted therapies for the treatment of GBM.
Practice Points.
Glioblastoma multiforme (GBM) is characterized by a collection of mutated signaling pathways.
Three core pathways are affected in a substantial fraction of patients.
Therapies directly targeting just a single mutated pathway are unlikely to be successful.
Instead, the combination of targeted therapeutics should be explored.
Owing to its invasive character, GBM is a disease that affects the whole brain.
Consequently, therapeutics against GBM must cross the blood–brain barrier (BBB) to also reach the more remote areas of the brain containing tumor cells.
Most targeted agents have been designed for other cancers rather than for GBM, and many of them will not meet the requirement of sufficient BBB penetration.
Candidate agents that are not or are very weak substrates of ABC transporters have an advantage with regard to BBB penetration.
Alternatively, inhibitors of ABC transporters may be used to enhance the BBB penetration of substrate drugs.
High-grade gliomas are the most common type of primary brain tumors and are among the most lethal types of human cancer [1]. High-grade gliomas are classified by the WHO in grade III as: anaplastic astrocytoma, anaplastic oligodendroglioma and anaplastic oligoastrocytoma; and grade IV: gliosarcoma and glioblastoma multiforme (GBM) [2]. Unfortunately, the majority of high-grade glioma patients are diagnosed with GBM, the most malignant subtype that is associated with a very aggressive course of disease and less than 3 months overall survival if left untreated [2]. Standard treatment of newly diagnosed GBM includes surgery followed by radiotherapy (30 × 2 Gy) plus temozolomide (75 mg/m2; daily) for 6 weeks and maintenance temozolomide therapy (150–200 × 5 mg/m2 per 28 days) for 6 months. However, despite this extensive treatment the mean overall survival is still only 14.6 months and more effective treatments are, thus, urgently needed [3].
In contrast to conventional chemotherapies that work by interfering with DNA synthesis or cell metabolism, targeted therapies work by inhibition of the deregulated cell signaling pathways in cancer cells by small molecules or antibodies. The underlying concept is that these signaling pathways are more critical for survival and growth of cancer cells than for normal cells. Consequently, targeted therapy holds the promise of being effective with less toxicity than conventional chemotherapies. Despite emerging success in some other tumor types, for example, imatinib for chronic myelogeneous leukemia [4] or vemurafenib in melanoma [5], the development of molecularly targeted therapy for gliomas appears to be much more challenging. Two small-molecule inhibitors of the EGF receptor (EGFR) tyrosine kinase that received regulatory approval for the treatment of lung cancer, erlotinib (Tarceva®, Genentech, Inc., CA, USA) and gefitinib (Iressa®, AstraZeneca, DE, USA), have been extensively evaluated for GBM treatment. The expectations were high since EGFR overexpression and mutations are common in GBMs. The results of the first Phase I studies with erlotinib were exciting [6,7] and a Phase II, single institution study demonstrated that treatment with erlotinib plus temozolomide before and after radiation significantly increased median survival of GBM patients to 19.3 months compared with 14.1 months in historical controls [8]. However, the results of subsequent clinical trials with EGFR inhibitors were all disappointing [9–14]. In particular, a randomized controlled Phase II study carried out by the European Organisation for Research and Treatment of Cancer (EORTC) demonstrated no clear benefit in progressive GBM patients treated with erlotinib compared with a control group receiving temozolomide or carmustine [10].
The failures of targeted therapy in the treatment of GBM are not limited to EGFR inhibitors. Inhibitors of mTOR have also been regarded as promising candidates for treating GBM, as the frequently deregulated PI3K–AKT–mTOR signaling pathway is considered to be a key mediator of GBM cell survival and growth. Rapamycin (sirolimus) and its analogs (rapalogs) temsirolimus (CCI-779) and everolimus (RAD001) are three mTOR inhibitors that have undergone extensive clinical evaluation for their therapeutic effect in GBMs [15–22]. Similar to the EGFR inhibitors, most trials with mTOR inhibitors as a single agent in GBM have failed to show any significant therapeutic benefit.
Despite these disappointing results, important lessons have been learned from translational studies with these agents. This article will focus on the recent development of targeted therapies on the core mutated pathways of GBM. Moreover, several major putative resistance mechanisms of GBM to the earlier studied targeted therapies will also be discussed.
Genetic alterations & classification of GBMs
The majority of patients with GBMs suffer from primary (or de novo) GBMs. In comparison with secondary GBMs, which evolve from low-graded gliomas, primary GBMs usually develop without pre-existing precursor lesions. Primary and secondary GBMs are histopathologically indistinguishable and are characterized by a high proliferative index, serpentine pseudopallisading necrosis and microvascular proliferation. However, primary and secondary GBMs are associated with differences in age of onset, clinical history, median survival and genetic changes (Tables 1 & 2).
Table 1. . Classic classification of glioblastoma multiforme.
| Subtype | Incidence (%) | Origin | Alterations (%) | Clinical history | Median overall survival (months)† |
|---|---|---|---|---|---|
| Primary or de novo GBM | 95% | No recognizable precursor lesions |
LOH 10q (70) EGFR amplification (36) P16INK4a deletion (31) TP53 mutation (28) PTEN mutation (25) |
<3 months (68%) <6 months (84%) |
4.7 |
| Secondary GBM | 5% | Developed from diffuse astrocytoma or anaplastic astrocytoma |
LOH 10q (63) EGFR amplification (8) P16INK4a deletion (19) TP53 mutation (65) PTEN mutation (4) |
Low-grade astrocytoma origin: 5.1 years; anaplastic astrocytoma: 1.9 years | 7.8 |
The classic classification includes primary and secondary GBM.
†Median overall survival without treatment.
GBM: Glioblastoma multiforme.
Table 2. . The Cancer Genome Atlas genomic classification of glioblastoma multiforme.
| Subtype | Biomarker | Signature | Major alterations | Treatment response |
|---|---|---|---|---|
| Classical | Neuroembryonic stem cell | Astrocytic | EGFR, CDKN2A/2B and PTEN | Good |
| Mesenchymal | Mesenchymal markers | Astroglial | NF1, PTEN, CHI3L1 and MET | Modest |
| Proneural | Oligodendrocytic development genes | Oligodendrocytic | TP53, PDGFRA or PI3KCA/PIK3RI, IDH1 and PTEN | Poor or no response |
| Neural | Neural markers | Neuronal and astrocytic | EGFR | Marginal |
The Cancer Genome Atlas classification reveals four clinically relevant subtypes based on the genomic profiles of glioblastoma multiforme and their correlations with biomarker expression, cellular lineages and response to standard aggressive chemoradiation therapy.
Data taken from [28].
Primary and secondary GBMs develop as a result of multiple genetic alterations that differ in the two types of GBM. Secondary GBM is more frequently a result of an early mutation in P53, whereas primary GBM more frequently harbors mutations in EGFR deletions within the CDKN2 locus that codes for p14Arf, p16Ink4a and p15Ink4b, and a homozygous loss of chromosome 10q23, which contains the PTEN gene. Overall, loss of chromosome 10q, EGFR amplification and deletion of p16Ink4a have been demonstrated to be the most frequent genetic alterations in primary GBM [1,23–26].
▪ The Cancer Genome Atlas project in GBMs
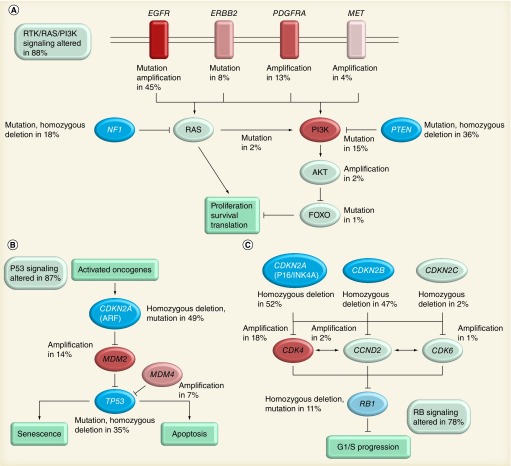
By implementation of large-scale multi-dimensional analytic platforms, a comprehensive characterization of the molecular basis of malignant gliomas recently became available. The Cancer Genome Atlas (TCGA) is a project that aims to catalog genetic mutations responsible for cancer. In 2008, TCGA published the results of their first cancer project on the analysis of genomic abnormalities in human GBM (mostly primary GBM) [27]. This work not only confirmed the common genetic aberrations reported previously, but also provided new insight into the roles of some known tumor-related genes, such as ERBB2/HER2, NF1 and P53, and also uncovered new gene mutations. More importantly, it provided a network view of the pathways altered in the development of GBMs, which can be instructive for future therapeutic decisions and facilitate the search for more efficacious targeted therapies. As shown in Figure 1, frequent genetic alterations of GBM occur in three core pathways; RTK/RAS/PI3K signaling, and P53 and Rb tumor suppressor pathways were mapped based on genetic analyses of 206 GBM samples.
Figure 1. The Cancer Genome Atlas of glioblastoma multiforme.
Primary sequence alterations and significant copy number changes for components of the (A) RTK/RAS/PI3K, (B) P53 and (C) Rb signaling pathways are shown. Red indicates activating genetic alterations, with frequently altered genes showing deeper shades of red. Conversely, blue indicates inactivating alterations, with darker shades of blue corresponding to a higher percentage of alteration. For each altered component of a particular pathway, the nature of the alteration and the percentage of tumors affected are indicated. Boxes contain the final percentages of glioblastoma multiformes with alterations in at least one known component gene of the designated pathway.
Reprinted with permission from [27] © Macmillan Publishers Ltd (2008).
Another important finding based on the TCGA is the molecular classification of GBM [28]. Four GBM subtypes: proneural; neural; classical; and mesenchymal subtypes, described in this study showed strong correlations with GBM cells of origin, clinical characteristics and response to standard chemoradiation therapy. For example, the proneural subtype was associated with younger age and IDH1 and P53 mutations with a trend toward longer survival for these patients. Intriguingly, however, patients with proneural subtype GBM did not have an improved survival when receiving aggressive treatment. On the contrary, patient's with the classical subtype GBM, usually harboring EGFR amplification and homozygous deletion of CDKN2 and PTEN, demonstrated the greatest benefit from standard treatment among all subtypes (Tables 1 & 2). Given the fact that each subtype harbors specific aberrations in molecular pathways, one may expect that targeting these pathways by specific inhibitors may provide new avenues for developing improved therapies.
Blood–brain barrier & drug efflux transporters
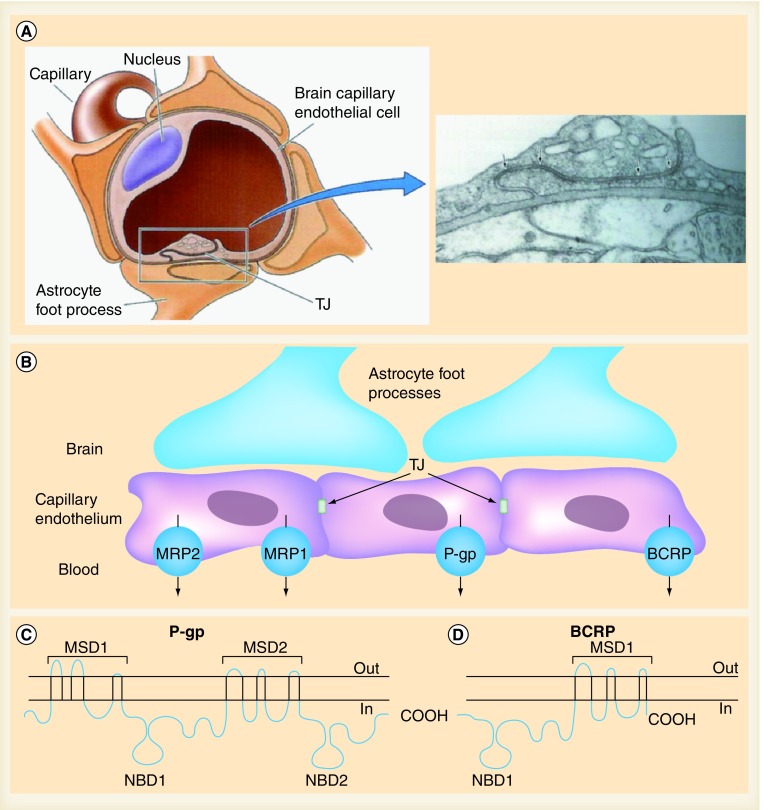
The brain is often referred to as a pharmacological sanctuary site since most drugs are unable to cross the blood–brain barrier (BBB) [29–31]. The BBB represents one of the major challenges to the efficacy of chemotherapy against GBMs. The BBB is formed by endothelial cells that are closely linked by tight junctions, disabling the paracellular movement of substances. Moreover, in contrast to most endothelial cells in the rest of the body, endothelial cells in the BBB lack fenestra and have low endocytic activity. Consequently, entry of substances into the brain can only occur by transcellular passage through the endothelium. Moreover, the pericytes and astrocytes intimately surrounding the endothelial cells form a secondary lipid layer, which further enforces the impermeability of the BBB [32]. Entry of essential nutrients (e.g., glucose) is strictly regulated by a range of uptake transporters. Other substances can only enter the brain by passive diffusion across the BBB, and the ability to do so is determined by a series of molecular parameters such as sufficient lipid solubility (octanol:water partition coefficient), molecular weight, degree of ionization, plasma protein binding and tissue binding. Nonetheless, even compounds that have molecular characteristics in favor of passive diffusion demonstrate much lower brain penetration than expected due to the activity of drug efflux transporters [31].
ABC drug transporters expressed at the BBB have well-known roles in the restriction of therapeutic agents into the brain [33]. Of all the efflux transporters present in the BBB, two transporters are mainly responsible for the efflux of anticancer agents back into the blood capillaries. These proteins are ABCB1 and ABCG2 (Figure 2B).
Figure 2. The blood–brain barrier.
(A & B) Blood–brain barrier and ABC drug efflux transporters at the blood–brain barrier. (C & D) Secondary structures of P-gp and BCRP, respectively.
MSD: Membrane-spanning domain; NBD: Nucleotide-binding domain; TJ: Tight junction.
Right-hand panel in (A) reproduced with permission from [110] © Oxford University Press (1991).
Left-hand panel in (A) and (B) adapted with permission from [111].
(C & D) Adapted with permission from [35].
▪ ABCB1
ABCB1 (also called P-gp or MDR1) is a 170-kDa membrane-associated protein expressed at high levels in normal human tissues, including the brain capillaries (Figure 2C). It was first discovered by its ability to confer multidrug resistance in cultured tumor cells [34]. ABCB1 is a highly promiscuous transporter, which recognizes an amazing range of drugs. Like all members of the ABC transporter superfamily, energy for the active transport of compounds is provided by hydrolysis of ATP at the nucleotide binding domains [31,35].
In addition to affecting cellular drug accumulation in tumor cells, ABC drug efflux transporters also actively affect the drug disposition by its expression at various barrier sites (BBB, intestinal epithelium and blood–testis barrier) [36–40]. ABCB1 was the first drug efflux transporter showing a remarkable impact on the brain delivery of substrate agents. Mice have two genes that are equivalent to ABCB1, namely Abcb1a and Abcb1b of which Abcb1a is the subtype that is expressed in the BBB. Abcb1a-deficient mice demonstrate a dramatic sensitivity to the neurotoxic pesticide ivermectin and to the cytotoxic drug vinblastine [41]. The role of ABCB1/Abcb1a in limiting drug brain penetration has been extended to a plethora of agents, including many novel targeted agents.
▪ ABCG2
ABCG2 (murine subtype Abcg2), also known as BCRP, is a 72-kDa ABC transporter. Similar to ABCB1, it plays an important role in drug disposition and distribution in the body (Figure 2D). ABCG2 is expressed in many tissues of the body, including the apical side of the intestinal lumen, the bile canaliculus in liver hepatocytes and the capillaries of the BBB. In addition, ABCG2 transports a broad range of endogenous and exogenous compounds [31,35]. However, pharmacokinetic studies using Abcg2-knockout mice showed little effect on the brain penetration of drugs, with a few exceptions. This is most likely due to most drugs being substrates of both ABC transporters and the fact that the accumulation of these substances by the brain is limited by Abcb1, which is still present in Abcg2-knockout mice. The absence of both Abcb1 and Abcg2, however, results in a profound increase in brain uptake compared with the absence of each transporter alone [42]. Due to the extremely broad substrate specificities of these two transporters, the concerted action of ABCB1 and ABCG2 is not restricted to only a few drugs, but represents a common mechanism to limit the brain entry of many drugs and, thus, potentially confers resistance to brain tumor chemotherapies (Table 3).
Table 3. . Impact of Abcb1 and Abcg2 on the brain penetration of targeted agents as demonstrated in Abcb1- and/or Abcg2-deficient mice.
| Agent | Target protein(s) | Brain penetration limited by Abcb1? | Brain penetration limited by Abcg2? | Ref. |
|---|---|---|---|---|
| Sirolimus | mTOR | Yes | No | [Lin et al., Unpublished data] |
| Palomid 529 | mTOR | No | No | [112] |
| Erlotinib | EGFR | Yes | Yes | [47,113] |
| Gefitinib | EGFR | Yes | Yes | [49] |
| Sunitinib | VEGFR-2 and -3, c-Kit, FLT3 and PDGFR | Yes | Yes | [50] |
| Cediranib | VEGFR | Yes | Yes | [114] |
| Axitinib | VEGFR | Yes | Yes | [115] |
| Sorafenib | c-Kit, PDGFR and Raf | Yes | Yes | [102] |
| Dasatinib | BCR–ABL, c-Kit, PDGFR, SRC | Yes | Yes | [52] |
| Vemurafenib | B-RafV600E | Yes | Yes | [116,117] |
| Dabrafenib | B-RafV600E | Yes | Yes | [118] |
| Imatinib | BCR–ABL, c-Kit, PDGFR | Yes | Yes | [53,105] |
| Lapatinib | HER2 (ERBB2), EGFR | Yes | Yes | [54] |
| GDC-0941 | PI3K | Yes | Yes | [69] |
| Tandutinib | c-Kit, FLT3 and PDGFRβ | Yes | Yes | [119] |
EGFR: EGF receptor; PDGFR: PDGF receptor; VEGFR: VEGF receptor.
Targeting EGFR & lessons learned from erlotinib trials in GBM
The EGFR (ERBB1) is a member of the ERBB family of transmembrane RTKs and binds to at least six different ligands, including EGF and TGF-α. After binding a ligand, dimerization of EGFR takes place and the complex is activated and recruits PI3K. This activates the PI3K–AKT–mTOR pathway, transducing a proliferation signal to the cell. In tumor cells, EGFR amplification is often present as small fragments of extrachromosomal DNA (double minutes) and is often associated with structural mutations in the EGFR gene, of which several variants have been identified. EGFRvIII (i.e., ΔEGFR) is the most commonly occurring mutation in GBMs derived by a nonrandom 801 bp in-frame deletion of exons 2–7, and codes for a truncated and constitutively activated protein [43–45]. Overall, EGFRvIII expression in the presence of EGFR amplification plays an important role in enhanced tumorigenicity and indicates a poor survival prognosis in GBM patients [46].
Although EGFR amplification and mutation is considered to be an important factor, none of the currently tested EGFR inhibitors have shown any clinical efficacy against GBM. The contrast between the more successful application of EGFR inhibitors in other types of cancer such as lung cancer and failure of EGFR inhibitors in GBM have been extensively studied. These studies suggest that the lack of response to EGFR inhibitors in GBM is multifactorial. A first issue is that it is not clear whether glioma cells will be exposed to therapeutic levels of erlotinib (i.e., can a therapeutic level of erlotinib be reached in the glioma tissue?). Erlotinib is a substrate of both ABCB1 and ABCG2, and the two drug efflux transporters together resulted in a sevenfold reduction of brain:plasma ratio in wild-type compared with Abcb1- and Abcg2-knockout mice [47,48]. Thus, the limited BBB penetration of erlotinib caused by ABCB1 and ABCG2 may be at least partly responsible for the resistance of GBM to erlotinib treatment. Unfortunately, the fact is that ABCB1 and ABCG2 have a long list of overlapping substrates, including most RTK inhibitors, such as gefitinib [49], sunitinib [50], dasatinib [51,52], imatinib [53] and lapatinib [54], and the brain penetration of these compounds is also markedly restricted by these two transporters (Table 3).
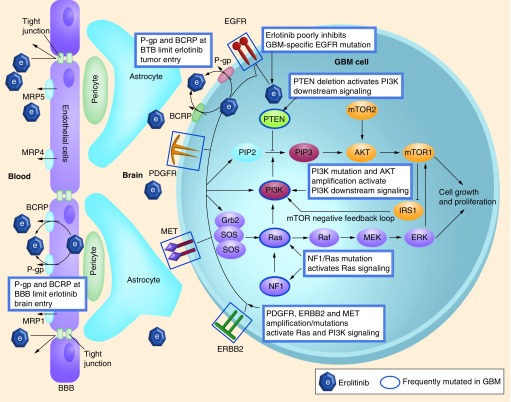
A second issue is that deregulated components downstream of EGFR could abolish the effects of EGFR inhibition. For example, Mellinghoff et al. reported that PTEN loss in GBM cells would cause resistance to erlotinib [55]. However, this is not the only reason, since the randomized EORTC study also found tumors with expression of PTEN, and EGFR and/or EGFRvIII that responded poorly [10]. There was only a weak relationship between the levels of phosphorylated AKT and the response to erlotinib. As we know, PTEN is not the only key factor controlling the signaling downstream of EGFR. In addition, PI3K mutation and AKT amplification can lead to activation of the PI3K pathway. Furthermore, there is active crosstalk between the PI3K and RAS pathways [56,57], and activation of the RAS–RAF–MEK–ERK pathway is common in GBM [58]. This pathway activation can be caused by a mutation or deletion of NF1 or (more rarely) by mutation of RAS. In addition, mutation and amplification of other parallel RTKs, such as ERBB2, PDGFR and c-MET, could also activate signaling via the PI3K–mTOR and RAS pathway, thereby conferring resistance to EGFR inhibition [59]. Last but not least, another explanation for the disappointing clinical activity of erlotinib in GBM versus lung cancer was delivered by a recent study by Vivanco et al. [60]. Vivanco et al. demonstrated that distinct types of EGFR mutations in lung cancer and GBM responded differently to EGFR inhibitors. Importantly, they also found that in lung cancer, the first-generation EGFR inhibitor erlotinib effectively inhibits EGFRs carrying mutations in the kinase domain, whereas it performs very poorly against EGFRs with mutations or deletions in the extracellular domain as in GBM [60]. The putative resistance of GBM to erlotinib caused by drug efflux transporters and/or intrinsic molecular mechanisms are demonstrated in Figure 3.
Figure 3. Putative mechanisms of glioblastoma multiforme resistance to erlotinib treatment.
Erlotinib acts on the EGFR causing downstream signaling of the PI3K–mTOR and Ras–MEK–ERK pathways. However, the glioblastoma multiforme-specific mutation EGFRvIII is less susceptible to erlotinib, and redundant receptor tyrosine kinases (PDGFR, ERBB2 and MET) may also cause downstream signaling. Moreover, the activity of the drug transporters (ABCB1 and ABCG2) located at the BBB and in the tumor cell may cause insufficient entry of erlotinib to elicit target inhibition.
BBB: Blood–brain barrier; BTB: Blood–tumor barrier; EGFR: EGF receptor; GBM: Glioblastoma multiforme; PDGFR: PDGF receptor.
Targeting the PI3K–AKT–mTOR pathway
The PI3K–AKT–mTOR pathway, activated by extracellular survival signaling factors via RTKs, is a major cell signaling pathway involved in regulating a variety of cellular processes, including cell proliferation, survival, growth, glucose metabolism and protein synthesis [61]. The most frequent alteration responsible for the deregulation of this pathway in GBM is the loss of PTEN (36%). In addition, mutation of the PI3KCA (15%) gene, and occasionally AKT amplification (2%) or FOXO mutation (1%), also contribute to the activation of downstream signaling (Figures 1 & 4) [27,62,63]. Constitutive PI3K–AKT–mTOR pathway activation is a hallmark of GBM.
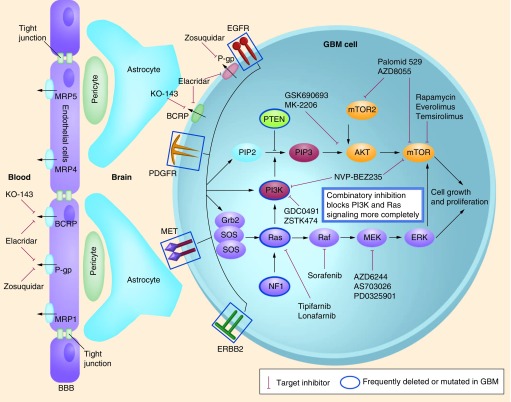
Figure 4. Core pathways involved in glioblastoma multiforme.
RTK–PI3K–AKT–mTOR and RTK–RAS–RAF–MEK–ERK signaling pathways and putative inhibitors.
BBB: Blood–brain barrier; EGFR: EGF receptor; GBM: Glioblastoma multiforme; PDGFR: PDGF receptor.
The class IA PI3K is a heterodimer composed of an 85-kDa regulatory subunit (P85α) and a 110-kDa catalytic subunit (P110α). Once RTK recruits PI3K to the cellular membrane, the PI3K subunit converts inactive PIP2 into active PIP3, which then recruits AKT to the membrane together with PDK1. Furthermore, PTEN counteracts PI3K by converting PIP3 back into PIP2, functioning as a tumor suppressor. Unlike other components of cellular pathways with multiple protein family members, there is no PTEN-related protein present in the cells that can compensate for its loss. Therefore, it is not surprising that the loss of PTEN function plays a pivotal role in tumorigenesis [64].
▪ PI3K inhibitors
Due to the high mutation rates of PTEN and PI3KCA (the gene that encodes the catalytic subunit P110α of PI3K), and the importance of this pathway in GBM, PI3K, and especially its subunit P110α, provides an attractive drug target. The first generation of PI3K inhibitors (LY294002 and wortmannin) showed in vivo antitumor efficacy, but were associated with poor stability or solubility, undesirable toxicities and crossover inhibition of other lipid and protein kinases [65,66]. Therefore, clinical trials with these compounds have not been initiated. Since the crystal structure of PI3K was elucidated, the development of new PI3K inhibitors has been accelerated. More selective PI3K inhibitors have been developed, with promising antitumor efficacy and low toxicity in preclinical research. For example, GDC-0941 is a potent and selective ATP-competitive PI3K inhibitor. It inhibits the PI3K P110α subunit with an inhibitory concentration at 50% inhibition (<10 nM) and inhibits the phosphorylation of AKT with an inhibitory concentration at 50% inhibition (28 nM) [67]. GDC-0941 treatment has led to an increase in apoptosis and inhibition of growth in a subset of xenograft tumor cell lines [68]. In vivo antitumor activity with daily oral dosing at 150 mg/kg of GDC-0941 achieved 98% growth inhibition in subcutaneous U87MG xenografts [65,68]. Unfortunately, GDC-0941 is also a substrate of both ABCB1 and ABCG2. After intravenous or oral administration, the GDC-0941 brain-to-plasma ratio in Abcb1- and Abcg2- knockout mice was approximately 30-fold higher than in wild-type mice. The PI3K pathway was markedly inhibited as evidenced by 60% suppression of the phosphorylated AKT in the brains of Abcb1- and Abcg2- knockout mice, whereas no inhibition was detected in the brains of wild-type mice [69]. Therefore, the potential efficacy of GDC-0941 as a targeted agent for treatment of GBM is limited due to ABCB1 and ABCG2.
AKT is a serine/threonine protein kinase that can be activated by phosphorylation at the threonine-308 by PDK1, or serine-473 by mTORC2. The mechanism by which the latter phosphorylation occurs is not fully understood; however, recent work suggests that activation of mTORC2 kinase activity is induced by EGFRvIII in GBM cells, and that abnormal mTOR2 signaling can promote GBM growth and survival [70]. When phosphorylated, AKT in turn phosphorylates a variety of downstream effector proteins, of which mTORC1 is one of the most important ones. There are very few trials with AKT inhibitors in GBM. The planned clinical trial with MK-2206 has been canceled by Merck (NJ, USA) due to a reprioritization within their oncology program [201].
▪ mTORC1 & mTORC2 inhibitors
mTORC1 is regarded as a central regulator of cell growth and has a critical role in tumor development. Via two major downstream targets, S6K (p70 S6 kinases) and 4EBP1, mTORC1 triggers the synthesis of proteins involved in cell survival, growth and proliferation [71–73]. Mutations of the mTOR gene are rare in GBM, but frequently deregulated upstream signaling drives mTORC1 activation. Inhibition of mTORC1 by rapamycin or other rapalogs has shown efficacy in a subset of cancers [74,75]. However, rapamycin and other rapalogs only inhibit mTORC1 and not mTORC2 [76]. This can lead to activation of AKT via an mTORC2-driven positive-feedback loop [74,77,78]. The novel generation of mTOR inhibitors are multitargeting agents, which are capable of inhibiting dual targets in the PI3K pathway or even more targets, to more completely block the feedback loop activation caused by inhibition of mTORC1. Dual mTORC1 and mTORC2 inhibitors that disrupt downstream signaling of mTORC1, and at the same time inhibit AKT activation by blocking mTORC2 activity, are interesting candidates for evaluation of treatment efficacy in GBM. AZD8055, a dual mTORC1 and mTORC2 inhibitor, is a highly potent, ATP-competitive and specific mTOR kinase inhibitor. In vivo, AZD8055 demonstrated potent single-agent antitumor activity against a range of subcutaneous xenografts, including U87 malignant glioma [79]. AZD8055 is currently being evaluated in a clinical trial in adults with recurrent glioma [202]. To date, no data have been presented in orthotopic brain tumor models, or to assess whether AZD8055 is able to cross the BBB. Similar to AZD8055, Palomid 529 is another dual mTORC1/mTORC2 inhibitor that markedly reduces the phosphorylation of AKT (S473-Akt) signaling by inhibition of both mTORC1 and mTORC2 activity. In vivo studies demonstrated that Palomid 529 reduced angiogenesis, vascular permeability and tumor growth [80]. Moreover, Palomid 529 was shown to enhance the antiproliferative effect of radiotherapy in GBM in an orthotopic model [81], as well as in prostate tumor models [82]. Another way to interrupt the mTORC2–PI3K positive-feedback loop is by combined inhibition of mTORC1 and PI3K. Particularly, the imidazo(4,5-c)quinoline derivative, NVP-BEZ235, selectively inhibits both PI3K and mTOR kinase activity by binding the ATP-binding cleft of these enzymes, thus, resulting in G1 arrest and autophagy in tumor cells. It displayed remarkable antitumor activity in U87MG GBM xenograft models with a dose-dependent effect, and it could further enhance the efficacy of temozolomide [83]. Further studies using U87 intracranial xenograft models also confirmed the antitumor potency of NVP-BEZ235 in the treatment of GBM [84]. NVP-BEZ235 has not been tested clinically against glioma, most likely because the company (Novartis, Basel, Switzerland) has prioritized NVP-BKM120 for development in treating glioma. BKM120 is a pan-class 1 PI3K inhibitor, but has no inhibitory activity against mTOR [85]. This compound is assumed to penetrate the BBB [86].
Targeting the RAS–RAF–MEK–ERK pathway
The MAPK pathway is activated in the majority of GBMs through various mechanisms, such as via EGFR mutation or amplification (45%), PDGFR amplification (13%) or deletion of NF1 (18%) (Figures 1 & 4) [27]. Upon activation, the growth factor receptors generate binding sites for adaptor proteins, such as GRB2, containing a SH2 domain. Next, GRB2 recruits SOS to the membrane, which, in turn, activates RAS through the replacement of inactive GDP with active GTP. As a result, RAS is able to recruit RAF kinases (A-RAF, B-RAF and C-RAF) to the plasma membrane, where they are activated. RAF is able to phosphorylate and thereby activate MEK1 and MEK2, which, in turn, activate ERK1 and ERK2. Activation of ERK leads to activation of a variety of nuclear and cytoplasmic substrates associated with gene regulation, cell cycle progression, differentiation and cell division [27,87,88]. Due to its important role in cell proliferation and survival, the MAPK pathway is frequently altered in a variety of tumors. K-RAS, one of the three RAS genes, is often mutated in leukemia, colon cancer, pancreatic cancer and lung cancer. Although human GBMs rarely show RAS mutations (2%), almost all malignant human gliomas show elevated levels of activated RAS as a result of other upstream molecular alterations.
▪ MEK inhibitors
Inhibition of MEK is an effective strategy to prevent the subsequent downstream signaling of the RAS pathway, and consequently induces tumor regression and/or stasis. A recent study by See et al. demonstrated that PD0325901 and AZD6244, as single agents, suppressed the growth of NF1-deficient and MEK inhibitor-sensitive glioma cells both in vitro and in vivo [89]. Their findings indicate that a subset of NF1-deficient GBMs may be responsive to MEK inhibitors. Moreover, they found that NF1-deficient glioma cells that are intrinsically resistant to MEK inhibition were sensitized by the addition of the dual PI3K/mTOR inhibitor PI-103. Many commonly used MEK inhibitors are benzohydroxamate derivatives, sharing many similarities in chemical structure. These inhibitors result in MEK-specific inhibition by binding to the hydrophobic pocket, adjacent to the ATP binding site of the MEK protein, which keeps the kinase in a catalytically inactive state. This allosteric mechanism contributes to the high selectivity for MEK without affecting other protein kinases that have structurally similar ATP-binding pockets. Therefore, MEK inhibitors are usually highly specific and non-ATP-competitive inhibitors. PD-0325901 was the first clinically tested MEK inhibitor. In vivo results demonstrated that PD-0325901 potently inhibits growth of human tumor xenografts bearing activating mutations of B-Raf, concomitant with suppression of ERK1/2 phosphorylation [90]. Interestingly, during Phase I and II clinical trials in advanced cancers, antitumor activity was seen when treated with 4–30 mg twice-daily doses of PD-0325901 [91,92]. However, beside the more common side effects like rash, diarrhea and fatigue, the drug also caused ocular and CNS toxicities at doses above 15 mg, and Pfizer (NY, USA) has suspended its further evaluation. Notably, a similar ocular toxicity has been observed with the MEK1 inhibitor AZD6244 (selumetinib), albeit to a lesser extent than PD-0325901. Whether these CNS toxicities are a direct consequence of MEK inhibition in the brain or caused by off-target drug effects is still unclear; both are possible regarding the structural similarities of the MEK inhibitors tested so far. Clearly, these CNS toxicities suggest that MEK inhibitors, such as PD-0325901, are able to enter the CNS, which would qualify these as candidates for testing in GBM. However, MEK inhibitors are predominantly evaluated against non-CNS tumors and the selection of novel candidates is narrowed to those having a low BBB permeability to avoid CNS toxicities. It should be noted that this strategy holds the risk that a complete class of targeted agents may become useless for treating GBM. The central role of an activated RAS pathway in GBM argues in favor of using MEK inhibitors, although it is obvious that finding the optimal dose level will be a challenging task.
Rb pathway & CDK inhibiton
Deregulation of the G1/S checkpoint is very common in GBM. Cyclin-dependent kinases (CDKs) are serine/threonine protein kinases whose activity depends on binding and activation by cyclin partners, and they are required for cell cycle progression. CDK4 and CDK6, which are both under control of P16INK4a and P15INK4b, bind to cyclin D and phosphorylate Rb, causing subsequent release of the transcription factor E2F and synthesis of proteins that are needed in the S phase. The most common alteration of the Rb pathway in GBM (52% of cases) is a homozygous deletion of parts of the CDKN2 locus that code for P16INK4a and P15INK4b. Other alterations include amplification and overexpression of CDK4 (15–20%) and homozygous deletion/mutation of the RB1 gene (∼10%) (Figure 1 & 5). Deletion of CDKN2A (or amplification of CDK4), CDKN2B and CDKN2C leads to loss of cell cycle control and increased cell proliferation. Codeletion of CDKN2A and CDKN2C serves as a strong predictor of sensitivity to a selective inhibitor of CDK4/6 [93]. Amplification of CDK6 and individual D-type cyclins, and homozygous deletion of CDKN2C encoding P18INK4c are less common [27].
Figure 5. Core pathways involved in glioblastoma multiforme.
The Rb pathway and a listing of some example drugs that have been developed to inhibit these pathways.
▪ CDK inhibitor PD-0332991
CDK4 is a logical target, taking into consideration that the loss of CDKN2A/B or amplification of CDK4 is a frequent event in GBM. PD-0332991 is an orally bioavailable CDK inhibitor, which selectively inhibits CDK4 and CDK6. Antiproliferative activity has been demonstrated in luminal breast cancer, myeloma and GBM cell lines [94,95]. As expected, RB1-deficient tumors were resistant to PD-0332991. Michaud et al. demonstrated that PD-0332991 was effective in suppressing the growth of intracranial U87MG tumors, including those that recurred after initial therapy with temozolomide [94]. The combination of PD-0332991 and radiation therapy resulted in significantly increased survival compared with either therapy alone. Based on these results, it was argued that this compound can efficiently cross the BBB [94]. It should be noted, however, that the BBB in U87MG tumors is very leaky [96].
Two completed Phase I trials showed that PD-0332991 is generally well tolerated and neutropenia was the sole significant toxicity at maximum tolerated dose (125 mg once daily) [97,98]. A Phase II clinical study to test PD-0332991 in patients with recurrent Rb-positive GBM is currently ongoing [203].
Future strategies for targeted therapy
▪ Combined inhibition of multiple pathways
As outlined above, at least three core signaling pathways (RAS–RAF–MEK–ERK, PI3K–AKT–mTOR and CDKN2–CDK4/6–RB1) are jointly activated in the majority of GBMs through different mechanisms, and targeting just one of these components may be insufficient to achieve a meaningful effect on tumor progression. In addition, crosstalk between different molecules of two or more pathways increases the plasticity of tumor-survival signaling and reduces oncogene addiction [56].
As depicted in Figure 3, inhibition of EGFR will not be able to suppress the activation of PI3K and RAS pathways in case other oncogenic alterations in parallel (e.g., other RTKs) and/or downstream components (e.g., PI3K activation) have occurred. Similarly, as shown with mTOR inhibitors, treatment with an inhibitor of a single pathway may also not sufficiently block parallel signaling pathways to reach a significant antiproliferative effect. For example, Di Nicolantonio et al. have shown that a number of human cancer cell lines carrying alterations in the PI3K pathway responded to everolimus, but only when there was no concomitant KRAS mutation [99].
Although several studies with PI3K and RAS inhibitors, given as a single agent, have demonstrated promising tumor growth inhibitory potencies by in vitro or in vivo models using established GBM cell lines, such as U87-MG, it should be taken into account that these GBM cells have been cultured for many generations. When grown in vivo they form homogeneous noninvasive lesions with a relative stable genome, unlike the highly heterogeneous GBMs that are typically found in patients. This discrepancy may be a plausible explanation for their poor predictive value on the usefulness of these agents against GBM in the clinic.
The considerations above argue in favor of targeting multiple pathways simultaneously, by analogy with the polypharmacy commonly applied in antiretroviral therapy. Ideally, this would include targeting all three core signaling pathways simultaneously. Although it will be challenging to design combination therapies that result in sufficient inhibition of these three core pathways simultaneously with acceptable toxicities, this concept would have the intrinsic potential to be beneficial for a substantial fraction of GBM patients. To date, just a few studies on combinations of targeted agents have been reported. Clinical trials combining EGFR and mTOR inhibitors reported considerable toxicities and the potential of drug–drug interactions, highlighting some of the issues that may be encountered [100,101]. However, whereas cytotoxic drugs in oncology are traditionally dosed at the maximum tolerated dose level, this ‘more equals better’ strategy is most likely suboptimal for targeted agents. Taking into consideration the basic principles of pharmacokinetic–pharmacodynamic relationships, the optimal dose should be determined by verifying target inhibition, since higher dose levels may not contribute to improved efficacy, but may increase toxicities due to off-target effects. Implementing methods to verify target inhibition in tumor tissue will be crucial to the further development of combination therapy with targeted agents, not just in gliomas but in all cancers.
▪ Targeted therapy combined with drug efflux transporters inhibitors
The important roles of ABCB1 and ABCG2 in drug resistance, and in limiting the brain penetration of therapeutic drugs, are well established. However, surprisingly little attention has been paid to this fact when designing clinical trials with targeted agents in GBM. Erlotinib, lapatinib and most other newly developed kinase inhibitors are substrates of ABCB1 and/or ABCG2 and, as a consequence, their usefulness in the treatment of GBM growth may be compromised by an inadequate brain penetration. The reality is that most targeted agents are initially developed for the treatment of major tumor types, such as lung and breast cancer, in which good BBB penetration is irrelevant or considered undesirable (e.g., MEK inhibitors). Consequently, however, agents from this panel that are being considered for further evaluation in GBM may not be the best BBB-permeable drugs.
Elacridar (GF120918) and tariquidar are both dual ABCB1 and ABCG2 inhibitors that were developed in the 1990s to improve the treatment of ABCB1-mediated multidrug resistant tumors. Due to the lack of success in this area, this concept is not currently receiving much attention. These same agents, however, have the potential to enhance the brain penetration of targeted therapies by blocking the efflux of drugs by these two transporters at the BBB, and perhaps also at the blood–tumor barrier. Coadministration of elacridar with a number of anticancer drugs has been proven to be an effective strategy to enhance the brain accumulation of these drugs, including a range of potentially effective targeted therapeutics [49–52,102–109]. Therefore, the use of elacridar might represent a feasible strategy to improve the brain entry of potentially effective targeted therapeutics for GBMs.
Conclusion & future perspective
The TCGA project and other collaborative research efforts have revealed how the oncogenetic processes of GBM are driven by multiple deregulated core signaling pathways and will provide new avenues for more effective targeted therapies in the treatment of GBM. Because the crosstalk between these molecular pathways fuels the plasticity of these processes, targeting a single, prevalent target that promotes and dominates GBM proliferation will, at best, provide only very short-lived effects. Consequently, the next generation of targeted therapies should focus on multitargeting agents or combinations of single-targeting agents against these core pathways.
Importantly, when selecting the most appropriate candidates of targeted therapeutics, the brain penetration of such candidates and, in particular, their interactions with the drug efflux transporters ABCB1 and ABCG2 should be taken into consideration. No matter how potent an agent is in inhibiting or activating its target, it has to reach that target at a therapeutic level, which is more difficult to achieve in the brain than in other tissues. Ideally, substances should be designed to have a low affinity for drug efflux transporters. Alternatively, coadminstration of targeted agents together with inhibitors of these drug efflux transporters (e.g., elacridar) may be helpful and should also be considered.
The progress that has been made in the treatment of GBM during recent years has been very modest. Therapies that are based on targeting core signaling pathways underlying the processes of malignant transformation is an emerging therapeutic strategy that holds great potential and receives a lot of attention. However, if we continue testing such agents against GBM, one-by-one and without considering whether the candidate drugs are able to cross the BBB sufficiently, it is likely that again little progress will have been made in 5–10 years from now.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: ▪▪ of considerable interest
- 1.Furnari FB, Fenton T, Bachoo RM, et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21(21):2683–2710. doi: 10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- 2.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK. WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 4.O'Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N. Engl. J. Med. 2003;348(11):994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 5.Sosman JA, Kim KB, Schuchter L, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N. Engl. J. Med. 2012;366(8):707–714. doi: 10.1056/NEJMoa1112302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prados MD, Lamborn KR, Chang S, et al. Phase 1 study of erlotinib HCl alone and combined with temozolomide in patients with stable or recurrent malignant glioma. Neuro. Oncol. 2006;8(1):67–78. doi: 10.1215/S1522851705000451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krishnan S, Brown PD, Ballman KV, et al. Phase I trial of erlotinib with radiation therapy in patients with glioblastoma multiforme: results of North Central Cancer Treatment Group protocol N0177. Int. J. Radiat. Oncol. Biol. Phys. 2006;65(4):1192–1199. doi: 10.1016/j.ijrobp.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 8.Prados MD, Chang SM, Butowski N, et al. Phase II study of erlotinib plus temozolomide during and after radiation therapy in patients with newly diagnosed glioblastoma multiforme or gliosarcoma. J. Clin. Oncol. 2009;27(4):579–584. doi: 10.1200/JCO.2008.18.9639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown PD, Krishnan S, Sarkaria JN, et al. Phase I/II trial of erlotinib and temozolomide with radiation therapy in the treatment of newly diagnosed glioblastoma multiforme: North Central Cancer Treatment Group Study N0177. J. Clin. Oncol. 2008;26(34):5603–5609. doi: 10.1200/JCO.2008.18.0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van den Bent MJ, Brandes AA, Rampling R, et al. Randomized Phase II trial of erlotinib versus temozolomide or carmustine in recurrent glioblastoma: EORTC brain tumor group study 26034. J. Clin. Oncol. 2009;27(8):1268–1274. doi: 10.1200/JCO.2008.17.5984. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪▪ Although the EGF receptor is a clearly defined target (commonly overexpressed and/or mutated) in glioblastoma multiformes (GBMs), a potent EGF receptor inhibitor (erlotinib) given as a single agent lacks efficacy in GBM patients.
- 11.Peereboom DM, Shepard DR, Ahluwalia MS, et al. Phase II trial of erlotinib with temozolomide and radiation in patients with newly diagnosed glioblastoma multiforme. J. Neurooncol. 2010;98(1):93–99. doi: 10.1007/s11060-009-0067-2. [DOI] [PubMed] [Google Scholar]
- 12.Brandes AA, Franceschi E, Tosoni A, Hegi ME, Stupp R. Epidermal growth factor receptor inhibitors in neuro-oncology: hopes and disappointments. Clin. Cancer Res. 2008;14(4):957–960. doi: 10.1158/1078-0432.CCR-07-1810. [DOI] [PubMed] [Google Scholar]
- 13.Franceschi E, Cavallo G, Lonardi S, et al. Gefitinib in patients with progressive high-grade gliomas: a multicentre Phase II study by Gruppo Italiano Cooperativo di Neuro-Oncologia (GICNO) Br. J. Cancer. 2007;96(7):1047–1051. doi: 10.1038/sj.bjc.6603669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rich JN, Reardon DA, Peery T, et al. Phase II trial of gefitinib in recurrent glioblastoma. J. Clin. Oncol. 2004;22(1):133–142. doi: 10.1200/JCO.2004.08.110. [DOI] [PubMed] [Google Scholar]
- 15.Chang SM, Wen P, Cloughesy T, et al. Phase II study of CCI-779 in patients with recurrent glioblastoma multiforme. Invest. New Drugs. 2005;23(4):357–361. doi: 10.1007/s10637-005-1444-0. [DOI] [PubMed] [Google Scholar]
- 16.Galanis E, Buckner JC, Maurer MJ, et al. Phase II trial of temsirolimus (CCI-779) in recurrent glioblastoma multiforme: a North Central Cancer Treatment Group Study. J. Clin. Oncol. 2005;23(23):5294–5304. doi: 10.1200/JCO.2005.23.622. [DOI] [PubMed] [Google Scholar]
- 17.Chang SM, Kuhn J, Wen P, et al. Phase I/pharmacokinetic study of CCI-779 in patients with recurrent malignant glioma on enzyme-inducing antiepileptic drugs. Invest. New Drugs. 2004;22(4):427–435. doi: 10.1023/B:DRUG.0000036685.72140.03. [DOI] [PubMed] [Google Scholar]
- 18.Mason WP, Macneil M, Kavan P, et al. A Phase I study of temozolomide and everolimus (RAD001) in patients with newly diagnosed and progressive glioblastoma either receiving or not receiving enzyme-inducing anticonvulsants: an NCIC CTG study. Invest. New Drugs. 2012;30(6):2344–2351. doi: 10.1007/s10637-011-9775-5. [DOI] [PubMed] [Google Scholar]
- 19.Sarkaria JN, Galanis E, Wu W, et al. North Central Cancer Treatment Group Phase I trial N057K of everolimus (RAD001) and temozolomide in combination with radiation therapy in patients with newly diagnosed glioblastoma multiforme. Int. J. Radiat. Oncol. Biol. Phys. 2011;81(2):468–475. doi: 10.1016/j.ijrobp.2010.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hainsworth JD, Shih KC, Shepard GC, Tillinghast GW, Brinker BT, Spigel DR. Phase II study of concurrent radiation therapy, temozolomide, and bevacizumab followed by bevacizumab/everolimus as first-line treatment for patients with glioblastoma. Clin. Adv. Hematol. Oncol. 2012;10(4):240–246. [PubMed] [Google Scholar]
- 21.Reardon DA, Desjardins A, Vredenburgh JJ, et al. Phase 2 trial of erlotinib plus sirolimus in adults with recurrent glioblastoma. J. Neurooncol. 2010;96(2):219–230. doi: 10.1007/s11060-009-9950-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cloughesy TF, Yoshimoto K, Nghiemphu P, et al. Antitumor activity of rapamycin in a Phase I trial for patients with recurrent PTEN-deficient glioblastoma. PLoS Med. 2008;5(1):e8. doi: 10.1371/journal.pmed.0050008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohgaki H, Dessen P, Jourde B, et al. Genetic pathways to glioblastoma: a population-based study. Cancer Res. 2004;64(19):6892–6899. doi: 10.1158/0008-5472.CAN-04-1337. [DOI] [PubMed] [Google Scholar]
- 24.Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321(5897):1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lang FF, Miller DC, Koslow M, Newcomb EW. Pathways leading to glioblastoma multiforme: a molecular analysis of genetic alterations in 65 astrocytic tumors. J. Neurosurg. 1994;81(3):427–436. doi: 10.3171/jns.1994.81.3.0427. [DOI] [PubMed] [Google Scholar]
- 26.von Deimling A, von Ammon K, Schoenfeld D, Wiestler OD, Seizinger BR, Louis DN. Subsets of glioblastoma multiforme defined by molecular genetic analysis. Brain Pathol. 1993;3(1):19–26. doi: 10.1111/j.1750-3639.1993.tb00721.x. [DOI] [PubMed] [Google Scholar]
- 27.Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪▪ Provides a comprehensive network view of the pathways altered in the development of GBM, and provides information that will be important for the implementation of targeted agents against GBM.
- 28.Verhaak RG, Hoadley KA, Purdom E, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17(1):98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agarwal S, Sane R, Oberoi R, Ohlfest JR, Elmquist WF. Delivery of molecularly targeted therapy to malignant glioma, a disease of the whole brain. Expert Rev. Mol. Med. 2011;13:e17. doi: 10.1017/S1462399411001888. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪▪ Discusses the challenge of glioma as a disease of the whole brain and emphasizes the need to deliver drugs efficiently across the blood–brain barrier in order to reach both the central core of the tumor and the invasive glioma cells.
- 30.Muldoon LL, Soussain C, Jahnke K, et al. Chemotherapy delivery issues in central nervous system malignancy: a reality check. J. Clin. Oncol. 2007;25(16):2295–2305. doi: 10.1200/JCO.2006.09.9861. [DOI] [PubMed] [Google Scholar]
- 31.de Vries NA, Beijnen JH, Boogerd W, van Tellingen O. Blood–brain barrier and chemotherapeutic treatment of brain tumors. Expert Rev. Neurother. 2006;6(8):1199–1209. doi: 10.1586/14737175.6.8.1199. [DOI] [PubMed] [Google Scholar]
- 32.Urquhart BL, Kim RB. Blood–brain barrier transporters and response to CNS-active drugs. Eur. J. Clin. Pharmacol. 2009;65(11):1063–1070. doi: 10.1007/s00228-009-0714-8. [DOI] [PubMed] [Google Scholar]
- 33.Deeken JF, Loscher W. The blood–brain barrier and cancer: transporters, treatment, and trojan horses. Clin. Cancer Res. 2007;13(6):1663–1674. doi: 10.1158/1078-0432.CCR-06-2854. [DOI] [PubMed] [Google Scholar]
- 34.Juliano RL, Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim. Biophys. Acta. 1976;455(1):152–162. doi: 10.1016/0005-2736(76)90160-7. [DOI] [PubMed] [Google Scholar]
- 35.Leslie EM, Deeley RG, Cole SP. Multidrug resistance proteins: role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol. Appl. Pharmacol. 2005;204(3):216–237. doi: 10.1016/j.taap.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 36.Dietrich CG, Geier A, Oude Elferink RP. ABC of oral bioavailability: transporters as gatekeepers in the gut. Gut. 2003;52(12):1788–1795. doi: 10.1136/gut.52.12.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Szakacs G, Varadi A, Ozvegy-Laczka C, Sarkadi B. The role of ABC transporters in drug absorption, distribution, metabolism, excretion and toxicity (ADME-Tox) Drug Discov. Today. 2008;13(9–10):379–393. doi: 10.1016/j.drudis.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 38.Ayrton A, Morgan P. Role of transport proteins in drug absorption, distribution and excretion. Xenobiotica. 2001;31(8–9):469–497. doi: 10.1080/00498250110060969. [DOI] [PubMed] [Google Scholar]
- 39.Schinkel AH, Jonker JW. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: an overview. Adv. Drug Deliv. Rev. 2003;55(1):3–29. doi: 10.1016/s0169-409x(02)00169-2. [DOI] [PubMed] [Google Scholar]
- 40.Enokizono J, Kusuhara H, Ose A, Schinkel AH, Sugiyama Y. Quantitative investigation of the role of breast cancer resistance protein (Bcrp/Abcg2) in limiting brain and testis penetration of xenobiotic compounds. Drug Metab. Dispos. 2008;36(6):995–1002. doi: 10.1124/dmd.107.019257. [DOI] [PubMed] [Google Scholar]
- 41.Schinkel AH, Smit JJ, van Tellingen O, et al. Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood–brain barrier and to increased sensitivity to drugs. Cell. 1994;77(4):491–502. doi: 10.1016/0092-8674(94)90212-7. [DOI] [PubMed] [Google Scholar]; ▪▪ First report demonstrating that the drug efflux transporter ABCB1 has a remarkable impact on the brain delivery of substrate agents.
- 42.de Vries NA, Zhao J, Kroon E, Buckle T, Beijnen JH, van Tellingen O. P-glycoprotein and breast cancer resistance protein: two dominant transporters working together in limiting the brain penetration of topotecan. Clin. Cancer Res. 2007;13(21):6440–6449. doi: 10.1158/1078-0432.CCR-07-1335. [DOI] [PubMed] [Google Scholar]; ▪▪ The first in vivo demonstration that ABCG2 is also an important efflux transporter at the blood–brain barrier. ABCG2 together with ABCB1 cooperatively limit the brain penetration of common substrate agents and since many tyrosine kinase inhibitors are dual substrates, the cooperative action of ABCB1 and ABCG2 has a considerable impact on their brain penetration.
- 43.Aldape KD, Ballman K, Furth A, et al. Immunohistochemical detection of EGFRvIII in high malignancy grade astrocytomas and evaluation of prognostic significance. J. Neuropathol. Exp. Neurol. 2004;63(7):700–707. doi: 10.1093/jnen/63.7.700. [DOI] [PubMed] [Google Scholar]
- 44.Frederick L, Wang XY, Eley G, James CD. Diversity and frequency of epidermal growth factor receptor mutations in human glioblastomas. Cancer Res. 2000;60(5):1383–1387. [PubMed] [Google Scholar]
- 45.Wong AJ, Ruppert JM, Bigner SH, et al. Structural alterations of the epidermal growth factor receptor gene in human gliomas. Proc. Natl Acad. Sci. USA. 1992;89(7):2965–2969. doi: 10.1073/pnas.89.7.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shinojima N, Tada K, Shiraishi S, et al. Prognostic value of epidermal growth factor receptor in patients with glioblastoma multiforme. Cancer Res. 2003;63(20):6962–6970. [PubMed] [Google Scholar]
- 47.Kodaira H, Kusuhara H, Ushiki J, Fuse E, Sugiyama Y. Kinetic analysis of the cooperation of P-glycoprotein (P-gp/Abcb1) and breast cancer resistance protein (Bcrp/Abcg2) in limiting the brain and testis penetration of erlotinib, flavopiridol, and mitoxantrone. J. Pharmacol. Exp. Ther. 2010;333(3):788–796. doi: 10.1124/jpet.109.162321. [DOI] [PubMed] [Google Scholar]
- 48.de Vries NA, Buckle T, Zhao J, Beijnen JH, Schellens JH, van Tellingen O. Restricted brain penetration of the tyrosine kinase inhibitor erlotinib due to the drug transporters P-gp and BCRP. Invest. New Drugs. 2012;30(2):443–449. doi: 10.1007/s10637-010-9569-1. [DOI] [PubMed] [Google Scholar]
- 49.Agarwal S, Sane R, Gallardo JL, Ohlfest JR, Elmquist WF. Distribution of gefitinib to the brain is limited by P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2)-mediated active efflux. J. Pharmacol. Exp. Ther. 2010;334(1):147–155. doi: 10.1124/jpet.110.167601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang SC, Lagas JS, Lankheet NA, et al. Brain accumulation of sunitinib is restricted by P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) and can be enhanced by oral elacridar and sunitinib coadministration. Int. J. Cancer. 2012;130(1):223–233. doi: 10.1002/ijc.26000. [DOI] [PubMed] [Google Scholar]
- 51.Chen Y, Agarwal S, Shaik NM, Chen C, Yang Z, Elmquist WF. P-glycoprotein and breast cancer resistance protein influence brain distribution of dasatinib. J. Pharmacol. Exp. Ther. 2009;330(3):956–963. doi: 10.1124/jpet.109.154781. [DOI] [PubMed] [Google Scholar]
- 52.Lagas JS, van Waterschoot RA, van Tilburg VA, et al. Brain accumulation of dasatinib is restricted by P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) and can be enhanced by elacridar treatment. Clin. Cancer Res. 2009;15(7):2344–2351. doi: 10.1158/1078-0432.CCR-08-2253. [DOI] [PubMed] [Google Scholar]
- 53.Zhou L, Schmidt K, Nelson FR, Zelesky V, Troutman MD, Feng B. The effect of breast cancer resistance protein and P-glycoprotein on the brain penetration of flavopiridol, imatinib mesylate (Gleevec), prazosin, and 2-methoxy-3-(4-[2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)phenyl)propanoic acid (PF-407288) in mice. Drug Metab. Dispos. 2009;37(5):946–955. doi: 10.1124/dmd.108.024489. [DOI] [PubMed] [Google Scholar]
- 54.Polli JW, Olson KL, Chism JP, et al. An unexpected synergist role of P-glycoprotein and breast cancer resistance protein on the central nervous system penetration of the tyrosine kinase inhibitor lapatinib (N-{3-chloro-4-[(3-fluorobenzyl)oxy]phenyl}-6-[5-({[2-(methylsulfonyl)ethyl]amino }methyl)-2-furyl]-4-quinazolinamine; GW572016) Drug Metab. Dispos. 2009;37(2):439–442. doi: 10.1124/dmd.108.024646. [DOI] [PubMed] [Google Scholar]
- 55.Mellinghoff IK, Wang MY, Vivanco I, et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N. Engl. J. Med. 2005;353(19):2012–2024. doi: 10.1056/NEJMoa051918. [DOI] [PubMed] [Google Scholar]
- 56.Mendoza MC, Er EE, Blenis J. The Ras–ERK and PI3K–mTOR pathways: cross-talk and compensation. Trends Biochem. Sci. 2011;36(6):320–328. doi: 10.1016/j.tibs.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.To MD, Perez-Losada J, Mao JH, Balmain A. Crosstalk between Pten and Ras signaling pathways in tumor development. Cell Cycle. 2005;4(9):1185–1188. doi: 10.4161/cc.4.9.2039. [DOI] [PubMed] [Google Scholar]
- 58.Jeuken J, van den Broecke C, Gijsen S, Boots-Sprenger S, Wesseling P. RAS/RAF pathway activation in gliomas: the result of copy number gains rather than activating mutations. Acta Neuropathol. 2007;114(2):121–133. doi: 10.1007/s00401-007-0239-0. [DOI] [PubMed] [Google Scholar]
- 59.Stommel JM, Kimmelman AC, Ying H, et al. Coactivation of receptor tyrosine kinases affects the response of tumor cells to targeted therapies. Science. 2007;318(5848):287–290. doi: 10.1126/science.1142946. [DOI] [PubMed] [Google Scholar]
- 60.Vivanco I, Robins HI, Rohle D, et al. Differential sensitivity of glioma- versus lung cancer-specific EGFR mutations to EGFR kinase inhibitors. Cancer Discov. 2012;2(5):458–471. doi: 10.1158/2159-8290.CD-11-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cheng CK, Fan QW, Weiss WA. PI3K signaling in glioma – animal models and therapeutic challenges. Brain Pathol. 2009;19(1):112–120. doi: 10.1111/j.1750-3639.2008.00233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat. Rev. Cancer. 2002;2(7):489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 63.Courtney KD, Corcoran RB, Engelman JA. The PI3K pathway as drug target in human cancer. J. Clin. Oncol. 2010;28(6):1075–1083. doi: 10.1200/JCO.2009.25.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bader AG, Kang S, Zhao L, Vogt PK. Oncogenic PI3K deregulates transcription and translation. Nat. Rev. Cancer. 2005;5(12):921–929. doi: 10.1038/nrc1753. [DOI] [PubMed] [Google Scholar]
- 65.Yap TA, Garrett MD, Walton MI, Raynaud F, de Bono JS, Workman P. Targeting the PI3K–AKT–mTOR pathway: progress, pitfalls, and promises. Curr. Opin. Pharmacol. 2008;8(4):393–412. doi: 10.1016/j.coph.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 66.Carnero A, Blanco-Aparicio C, Renner O, Link W, Leal JF. The PTEN/PI3K/AKT signalling pathway in cancer, therapeutic implications. Curr. Cancer Drug Targets. 2008;8(3):187–198. doi: 10.2174/156800908784293659. [DOI] [PubMed] [Google Scholar]
- 67.Folkes AJ, Ahmadi K, Alderton WK, et al. The identification of 2-(1H-indazol-4-yl)-6-(4-methanesulfonyl-piperazin-1-ylmethyl)-4-morpholin-4-yl-t hieno[3,2-d]pyrimidine (GDC-0941) as a potent, selective, orally bioavailable inhibitor of class I PI3 kinase for the treatment of cancer. J. Med. Chem. 2008;51(18):5522–5532. doi: 10.1021/jm800295d. [DOI] [PubMed] [Google Scholar]
- 68.Raynaud FI, Eccles SA, Patel S, et al. Biological properties of potent inhibitors of class I phosphatidylinositide 3-kinases: from PI-103 through PI-540, PI-620 to the oral agent GDC-0941. Mol. Cancer Ther. 2009;8(7):1725–1738. doi: 10.1158/1535-7163.MCT-08-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Salphati L, Lee LB, Pang J, Plise EG, Zhang X. Role of P-glycoprotein and breast cancer resistance protein-1 in the brain penetration and brain pharmacodynamic activity of the novel phosphatidylinositol 3-kinase inhibitor GDC-0941. Drug Metab. Dispos. 2010;38(9):1422–1426. doi: 10.1124/dmd.110.034256. [DOI] [PubMed] [Google Scholar]
- 70.Tanaka K, Babic I, Nathanson D, et al. Oncogenic EGFR signaling activates an mTORC2-NF-kappaB pathway that promotes chemotherapy resistance. Cancer Discov. 2011;1(6):524–538. doi: 10.1158/2159-8290.CD-11-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shor B, Gibbons JJ, Abraham RT, Yu K. Targeting mTOR globally in cancer: thinking beyond rapamycin. Cell Cycle. 2009;8(23):3831–3837. doi: 10.4161/cc.8.23.10070. [DOI] [PubMed] [Google Scholar]
- 72.Samuels Y, Wang Z, Bardelli A, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304(5670):554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 73.Petroulakis E, Mamane Y, Le Bacquer O, Shahbazian D, Sonenberg N. mTOR signaling: implications for cancer and anticancer therapy. Br. J. Cancer. 2006;94(2):195–199. doi: 10.1038/sj.bjc.6602902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abraham RT, Gibbons JJ. The mammalian target of rapamycin signaling pathway: twists and turns in the road to cancer therapy. Clin. Cancer Res. 2007;13(11):3109–3114. doi: 10.1158/1078-0432.CCR-06-2798. [DOI] [PubMed] [Google Scholar]
- 75.Easton JB, Houghton PJ. mTOR and cancer therapy. Oncogene. 2006;25(48):6436–6446. doi: 10.1038/sj.onc.1209886. [DOI] [PubMed] [Google Scholar]
- 76.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307(5712):1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 77.Phung TL, Eyiah-Mensah G, O'Donnell RK, et al. Endothelial Akt signaling is rate-limiting for rapamycin inhibition of mouse mammary tumor progression. Cancer Res. 2007;67(11):5070–5075. doi: 10.1158/0008-5472.CAN-06-3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.O'Reilly KE, Rojo F, She QB, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66(3):1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chresta CM, Davies BR, Hickson I, et al. AZD8055 is a potent, selective, and orally bioavailable ATP-competitive mammalian target of rapamycin kinase inhibitor with in vitro and in vivo antitumor activity. Cancer Res. 2010;70(1):288–298. doi: 10.1158/0008-5472.CAN-09-1751. [DOI] [PubMed] [Google Scholar]
- 80.Xue Q, Hopkins B, Perruzzi C, Udayakumar D, Sherris D, Benjamin LE. Palomid 529, a novel small-molecule drug, is a TORC1/TORC2 inhibitor that reduces tumor growth, tumor angiogenesis, and vascular permeability. Cancer Res. 2008;68(22):9551–9557. doi: 10.1158/0008-5472.CAN-08-2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cerna D, Carter D, Flaherty S, Cal L, Sherris D, Yoo SS. Palomid 529, a PI3K/Akt/mTOR dual TORC1/2 inhibitor, is a radiosensitizer with effect in both subcutaneous and orthotopic U251 glioblastoma tumor xenograft models [abstract] Cancer Res. 2010;70(Suppl. 8):2506. [Google Scholar]
- 82.Diaz R, Nguewa PA, Diaz-Gonzalez JA, et al. The novel Akt inhibitor Palomid 529 (P529) enhances the effect of radiotherapy in prostate cancer. Br. J. Cancer. 2009;100(6):932–940. doi: 10.1038/sj.bjc.6604938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Maira SM, Stauffer F, Brueggen J, et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol. Cancer Ther. 2008;7(7):1851–1863. doi: 10.1158/1535-7163.MCT-08-0017. [DOI] [PubMed] [Google Scholar]
- 84.Liu TJ, Koul D, LaFortune T, et al. NVP-BEZ235, a novel dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor, elicits multifaceted antitumor activities in human gliomas. Mol. Cancer Ther. 2009;8(8):2204–2210. doi: 10.1158/1535-7163.MCT-09-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bendell JC, Rodon J, Burris HA, et al. Phase I, dose-escalation study of BKM120, an oral pan-Class I PI3K inhibitor, in patients with advanced solid tumors. J. Clin. Oncol. 2012;30(3):282–290. doi: 10.1200/JCO.2011.36.1360. [DOI] [PubMed] [Google Scholar]
- 86.Wen PY, Lee EQ, Reardon DA, Ligon KL, Alfred Yung WK. Current clinical development of PI3K pathway inhibitors in glioblastoma. Neuro. Oncol. 2012;14(7):819–829. doi: 10.1093/neuonc/nos117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rodriguez-Viciana P, Tetsu O, Oda K, Okada J, Rauen K, McCormick F. Cancer targets in the Ras pathway. Cold Spring Harb. Symp. Quant. Biol. 2005;70:461–467. doi: 10.1101/sqb.2005.70.044. [DOI] [PubMed] [Google Scholar]
- 88.Peyssonnaux C, Eychene A. The Raf/MEK/ERK pathway: new concepts of activation. Biol. Cell. 2001;93(1–2):53–62. doi: 10.1016/s0248-4900(01)01125-x. [DOI] [PubMed] [Google Scholar]
- 89.See WL, Tan IL, Mukherjee J, Nicolaides T, Pieper RO. Sensitivity of glioblastomas to clinically available MEK inhibitors is defined by neurofibromin 1 deficiency. Cancer Res. 2012;72(13):3350–3359. doi: 10.1158/0008-5472.CAN-12-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Solit DB, Garraway LA, Pratilas CA, et al. BRAF mutation predicts sensitivity to MEK inhibition. Nature. 2006;439(7074):358–362. doi: 10.1038/nature04304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Haura EB, Ricart AD, Larson TG, et al. A Phase II study of PD-0325901, an oral MEK inhibitor, in previously treated patients with advanced non-small cell lung cancer. Clin. Cancer Res. 2010;16(8):2450–2457. doi: 10.1158/1078-0432.CCR-09-1920. [DOI] [PubMed] [Google Scholar]
- 92.LoRusso PM, Krishnamurthi SS, Rinehart JJ, et al. Phase I pharmacokinetic and pharmacodynamic study of the oral MAPK/ERK kinase inhibitor PD-0325901 in patients with advanced cancers. Clin. Cancer Res. 2010;16(6):1924–1937. doi: 10.1158/1078-0432.CCR-09-1883. [DOI] [PubMed] [Google Scholar]
- 93.Wiedemeyer WR, Dunn IF, Quayle SN, et al. Pattern of retinoblastoma pathway inactivation dictates response to CDK4/6 inhibition in GBM. Proc. Natl Acad. Sci. USA. 2010;107(25):11501–11506. doi: 10.1073/pnas.1001613107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Michaud K, Solomon DA, Oermann E, et al. Pharmacologic inhibition of cyclin-dependent kinases 4 and 6 arrests the growth of glioblastoma multiforme intracranial xenografts. Cancer Res. 2010;70(8):3228–3238. doi: 10.1158/0008-5472.CAN-09-4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Konecny GE, Winterhoff B, Kolarova T, et al. Expression of p16 and retinoblastoma determines response to CDK4/6 inhibition in ovarian cancer. Clin. Cancer Res. 2011;17(6):1591–1602. doi: 10.1158/1078-0432.CCR-10-2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kemper EM, Leenders W, Kusters B, et al. Development of luciferase tagged brain tumour models in mice for chemotherapy intervention studies. Eur. J. Cancer. 2006;42(18):3294–3303. doi: 10.1016/j.ejca.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 97.Schwartz GK, LoRusso PM, Dickson MA, et al. Phase I study of PD 0332991, a cyclin-dependent kinase inhibitor, administered in 3-week cycles (schedule 2/1) Br. J. Cancer. 2011;104(12):1862–1868. doi: 10.1038/bjc.2011.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Flaherty KT, LoRusso PM, Demichele A, et al. Phase I, dose-escalation trial of the oral cyclin-dependent kinase 4/6 inhibitor PD 0332991, administered using a 21-day schedule in patients with advanced cancer. Clin. Cancer Res. 2012;18(2):568–576. doi: 10.1158/1078-0432.CCR-11-0509. [DOI] [PubMed] [Google Scholar]
- 99.Di Nicolantonio F, Arena S, Tabernero J, et al. Deregulation of the PI3K and KRAS signaling pathways in human cancer cells determines their response to everolimus. J. Clin. Invest. 2010;120(8):2858–2866. doi: 10.1172/JCI37539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nghiemphu PL, Lai A, Green RM, Reardon DA, Cloughesy T. A dose escalation trial for the combination of erlotinib and sirolimus for recurrent malignant gliomas. J. Neurooncol. 2012;110(2):245–250. doi: 10.1007/s11060-012-0960-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Reardon DA, Cloughesy T, Rich J, et al. Pharmacokinetic drug interaction between AEE788 and RAD001 causing thrombocytopenia in patients with glioblastoma. Cancer Chemother. Pharmacol. 2012;69(1):281–287. doi: 10.1007/s00280-011-1754-1. [DOI] [PubMed] [Google Scholar]
- 102.Lagas JS, van Waterschoot RA, Sparidans RW, Wagenaar E, Beijnen JH, Schinkel AH. Breast cancer resistance protein and P-glycoprotein limit sorafenib brain accumulation. Mol. Cancer Ther. 2010;9(2):319–326. doi: 10.1158/1535-7163.MCT-09-0663. [DOI] [PubMed] [Google Scholar]
- 103.Bihorel S, Camenisch G, Lemaire M, Scherrmann JM. Modulation of the brain distribution of imatinib and its metabolites in mice by valspodar, zosuquidar and elacridar. Pharm. Res. 2007;24(9):1720–1728. doi: 10.1007/s11095-007-9278-4. [DOI] [PubMed] [Google Scholar]
- 104.Sane R, Agarwal S, Elmquist WF. Brain distribution and bioavailability of elacridar after different routes of administration in the mouse. Drug Metab. Dispos. 2012;40(8):1612–1619. doi: 10.1124/dmd.112.045930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Oostendorp RL, Buckle T, Beijnen JH, van Tellingen O, Schellens JH. The effect of P-gp (Mdr1a/1b), BCRP (Bcrp1) and P-gp/BCRP inhibitors on the in vivo absorption, distribution, metabolism and excretion of imatinib. Invest. New Drugs. 2009;27(1):31–40. doi: 10.1007/s10637-008-9138-z. [DOI] [PubMed] [Google Scholar]
- 106.Minocha M, Khurana V, Qin B, Pal D, Mitra AK. Co-administration strategy to enhance brain accumulation of vandetanib by modulating P-glycoprotein (P-gp/Abcb1) and breast cancer resistance protein (Bcrp1/Abcg2) mediated efflux with m-TOR inhibitors. Int. J. Pharm. 2012;434(1–2):306–314. doi: 10.1016/j.ijpharm.2012.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lee YJ, Kusuhara H, Jonker JW, Schinkel AH, Sugiyama Y. Investigation of efflux transport of dehydroepiandrosterone sulfate and mitoxantrone at the mouse blood-brain barrier: a minor role of breast cancer resistance protein. J. Pharmacol. Exp. Ther. 2005;312(1):44–52. doi: 10.1124/jpet.104.073320. [DOI] [PubMed] [Google Scholar]
- 108.Breedveld P, Pluim D, Cipriani G, et al. The effect of Bcrp1 (Abcg2) on the in vivo pharmacokinetics and brain penetration of imatinib mesylate (Gleevec): implications for the use of breast cancer resistance protein and P-glycoprotein inhibitors to enable the brain penetration of imatinib in patients. Cancer Res. 2005;65(7):2577–2582. doi: 10.1158/0008-5472.CAN-04-2416. [DOI] [PubMed] [Google Scholar]
- 109.Agarwal S, Mittapalli RK, Zellmer DM, et al. Active efflux of dasatinib from the brain limits efficacy against murine glioblastoma: broad implications for the clinical use of molecularly-targeted agents. Mol. Cancer Ther. 2012;11(10):2183–2192. doi: 10.1158/1535-7163.MCT-12-0552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Peters A, Palay SL, deF Webster H. Fine Structure of the Nervous System: Neurons and Their Supporting Cells (3rd Edition) Oxford University Press; Oxford, UK: 1991. p. 347. [Google Scholar]
- 111.Purves D. In: Neuroscience. Purves D, Augustine GJ, Fitzpatrick D, et al., editors. Sinauer Associates; MA, USA: 2008. [Google Scholar]
- 112.Lin F, Buil L, Sherris D, Beijnen JH, van Tellingen O. Dual mTORC1 and mTORC2 inhibitor Palomid 529 penetrates the blood–brain barrier without restriction by ABCB1 and ABCG2. Int. J. Cancer. 2013 doi: 10.1002/ijc.28126. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 113.Marchetti S, de Vries NA, Buckle T, et al. Effect of the ATP-binding cassette drug transporters ABCB1, ABCG2, and ABCC2 on erlotinib hydrochloride (Tarceva) disposition in in vitro and in vivo pharmacokinetic studies employing Bcrp1-/-/Mdr1a/1b-/- (triple-knockout) and wild-type mice. Mol. Cancer Ther. 2008;7(8):2280–2287. doi: 10.1158/1535-7163.MCT-07-2250. [DOI] [PubMed] [Google Scholar]
- 114.Wang T, Agarwal S, Elmquist WF. Brain distribution of cediranib is limited by active efflux at the blood–brain barrier. J. Pharmacol. Exp. Ther. 2012;341(2):386–395. doi: 10.1124/jpet.111.190488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Poller B, Iusuf D, Sparidans RW, Wagenaar E, Beijnen JH, Schinkel AH. Differential impact of P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) on axitinib brain accumulation and oral plasma pharmacokinetics. Drug Metab. Dispos. 2011;39(5):729–735. doi: 10.1124/dmd.110.037317. [DOI] [PubMed] [Google Scholar]
- 116.Durmus S, Sparidans RW, Wagenaar E, Beijnen JH, Schinkel AH. Oral availability and brain penetration of the B-RAFV600E inhibitor vemurafenib can be enhanced by the P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) inhibitor elacridar. Mol. Pharm. 2012;9(11):3236–3245. doi: 10.1021/mp3003144. [DOI] [PubMed] [Google Scholar]
- 117.Mittapalli RK, Vaidhyanathan S, Sane R, Elmquist WF. Impact of P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) on the brain distribution of a novel BRAF inhibitor: vemurafenib (PLX4032) J. Pharmacol. Exp. Ther. 2012;342(1):33–40. doi: 10.1124/jpet.112.192195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mittapalli RK, Vaidhyanathan S, Dudek AZ, Elmquist WF. Mechanisms limiting distribution of the threonine-protein kinase B-RaFV600E inhibitor dabrafenib to the brain: implications for the treatment of melanoma brain metastases. J. Pharmacol. Exp. Ther. 2013;344(3):655–664. doi: 10.1124/jpet.112.201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yang JJ, Milton MN, Yu S, et al. P-glycoprotein and breast cancer resistance protein affect disposition of tandutinib, a tyrosine kinase inhibitor. Drug Metab. Lett. 2010;4(4):201–212. doi: 10.2174/187231210792928279. [DOI] [PubMed] [Google Scholar]
▪ Websites
- 201.http://clinicaltrials.gov/show/NCT01249105 ClinicalTrials.gov. Identifier: NCT01249105.
- 202.http://clinicaltrials.gov/ct2/show/NCT01316809 ClinicalTrials.gov. Identifier: NCT01316809.
- 203.http://clinicaltrials.gov/show/NCT01227434 ClinicalTrials.gov. Identifier: NCT01227434.