SUMMARY
Spine stereotactic body radiotherapy is based on delivering high biologically effective doses to spinal metastases, with the intent to maximize both tumor and pain control. The purpose of this review is to outline the technical details of spine stereotactic body radiotherapy, contrast clinical outcomes to low biologically effective dose conventional palliative radiotherapy, discuss the role of surgery in the era of spine stereotactic body radiotherapy, and summarize the major serious adverse events that patients would otherwise not be at risk of with conventional radiotherapy.
Practice Points.
Spine stereotactic body radiotherapy is an emerging therapy for selected patients with spinal metastases.
Major technological advances have been made in radiation delivery, permitting high-dose radiation with millimeter precision.
The aim of spine stereotactic body radiotherapy for spinal metastases is to improve pain and tumor control over the suboptimal results obtained with conventional radiotherapy.
Spine stereotactic body radiotherapy is increasingly being applied to the postoperative patient and the high rates of gross tumor control are changing the paradigm of surgery towards a more limited decompression and stabilization procedure.
Although serious toxicities have been reported, dosimetric guidelines are emerging for the major organs at risk, which should limit the risk of major complications in the future.
Approximately 100,000 cancer patients develop bone metastases per year in the USA and the most common site is the spine, with approximately 20,000 new cases diagnosed each year [1]. Spinal metastases typically cause a progressive constant dull pain; however, the spine can be rendered mechanically unstable and in this situation the pain is often described as worse with ambulation, bending or lifting, and better when lying down. The most serious complication of spinal metastases is the development of neurologic symptoms due to nerve root or spinal cord compression. Malignant spinal cord compression (MSCC) is a late complication of spine metastases that can be caused by epidural disease, fracture resulting in retropulsion of the posterior vertebral body wall into the spinal canal, or both. Regardless of the etiology, emergency spinal surgery may be required.
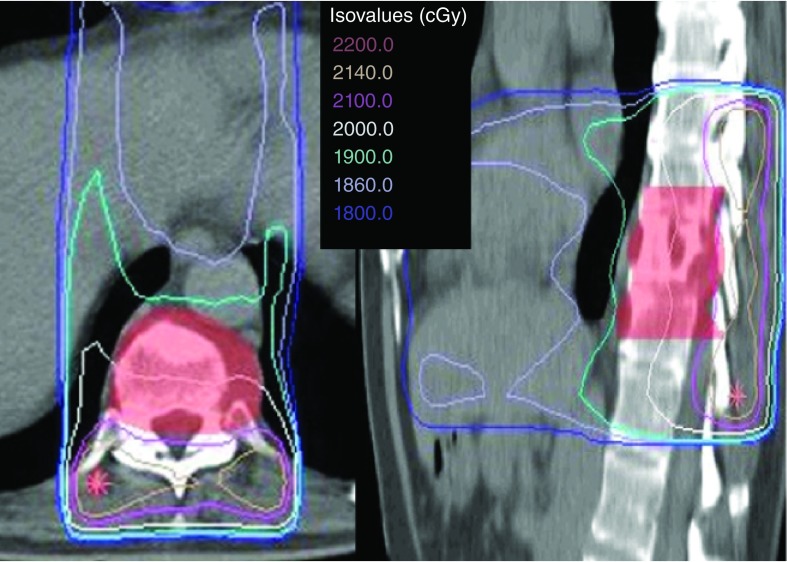
For decades, asymptomatic bony metastases have been treated with systemic therapy, and the predominant treatment of symptomatic spinal metastases has been conventional palliative radiotherapy [2,3]. The standard technique is relatively simple and involves delivering radiation using two opposed beams and, typically, one vertebral level above and below the metastases is included in the target volume (Figure 1). As a result, all of the normal tissues in the beams’ path are also irradiated and, therefore, the radiation dose and number of fractions prescribed are limited to what normal tissues can tolerate, most notably the spinal cord. Typical conventional palliative radiotherapy doses for spinal metastases include 8 Gy in a single fraction, 20 Gy in five fractions and 30 Gy in ten fractions [2]. Several randomized trials and a meta-analysis have concluded that there appears to be a lack of a dose response within this range of low-dose conventional palliative radiation practice [1,2,4,5]. Consequently, low-dose radiation for spinal metastases has been widely adopted, although partial response rates are in the order of approximately 60%, complete pain response rates range from 0 to 14%, with the lowest result coming from a study specifically examining spine metastases as opposed to bony metastases in general [2,4,6,7]. While these results are considered suboptimal, they were the best that could be obtained given the technological limitations of the time.
Figure 1. A conventional radiotherapy treatment plan.
Illustrates the typical practice of including at least one vertebral body above and below the target volume (in red colorwash), and using an anterior and posterior parallel opposed beam arrangement. The target and normal tissues are exposed to the radiation. The patient was treated with 20 Gy in five fractions.
Over the last 10 years, radiation oncology technology has undergone a major revolution with the development of computed tomography (CT)-based 3D treatment planning, CT-MRI fusion, sophisticated immobilization devices, multileaf collimators, intensity-modulated radiotherapy, volumetric modulated arc therapy, on-board image-guidance systems and, most recently, ultra-fast flattening filter-free treatment delivery [8–11]. Ultimately, these technologies have been exploited to develop spine stereotactic body radiotherapy (SBRT), also known as spine stereotactic radiosurgery [10]. Essentially, radiation can now be concentrated within the diseased vertebrae and curved around critical surrounding organs at risk, such as the spinal cord, to safely escalate the dose within the tumor (Figure 2). Doses such as 20–24 Gy in one fraction, 24–27 Gy in two to three fractions and 30–40 Gy in four to five fractions are commonly used in spine SBRT, and represent two- to six-times the radiobiologically equivalent dose previously delivered with conventional palliative radiation [10].
Figure 2. Stereotactic body radiotherapy treatment and response.
(A) T2-weighted axial MRI illustrating C3 metastases in a patient with breast cancer, and a tumor involving the posterior elements and not the vertebral body. (B) The treatment plan isodose distribution of the patient who received 24 Gy in two fractions. (C) Axial T2-weighted MRI illustrating the response 2 months later, in which a drastic shrinkage in tumor size can be observed. This patient also had a complete response to pain.
The Canadian Association of Radiation Oncology (CARO) recently defined SBRT as “the precise delivery of highly conformal and image-guided hypofractionated external beam radiotherapy, delivered in a single or few fraction(s), to an extracranial body target with doses at least biologically equivalent to a radical course when given over a conventionally fractionated (1.8–3.0 Gy/fraction) schedule” [10]. Regarding metastatic disease, this definition reflects the paradigm shift in treatment philosophy such that ‘locally curative doses’, as opposed to ‘locally palliative doses’, are delivered. The purpose of this review is to provide an overview of the technical details for spine SBRT specific to metastatic disease, contrast clinical outcomes and toxicities to conventional radiotherapy, and discuss the challenges we face to move the field forward.
Technological details for spine SBRT delivery
Spine SBRT demands extreme precision in radiotherapy delivery to within 1–2 mm [10]. It is only with recent technical advances in the entire radiotherapy process that this level of technical excellence is now achievable, and has been confirmed by several groups [12–16]. The following section describes the recommended procedures to perform safe spine SBRT, based on the published University of Toronto (ON, Canada) approach [14,17].
▪ Simulation
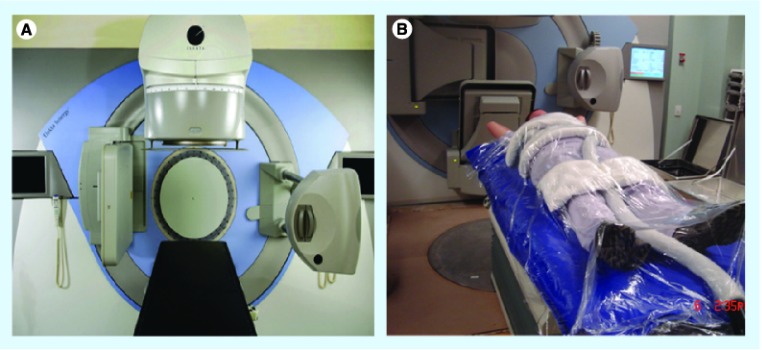
Patients are CT simulated in the supine position in a near-rigid body immobilization device (BodyFIX®, Elekta AB, Stockholm, Sweden) [17]. A comparison of near-rigid immobilization versus a simple evacuated vacuum cushion performed at the University of Toronto, demonstrated that patients are more stable and less likely to move during treatment with the more restrictive immobilization device (Figure 3) [18]. This result confirmed the safety of reducing our margins by 1 mm for technical uncertainties and our current practice of applying a 1.5 mm margin beyond the spinal cord as a planning organ-at-risk volume [17]. Since the steepest dose gradient is adjacent to the spinal cord, a reduction of even 1 mm in margin is of major significance as it can translate to an increase of up to 4 Gy in the tumor tissue adjacent to the spinal cord (Figure 4). For lesions at the fourth thoracic vertebrae and above, a rigid thermoplastic mask that covers the entire head and shoulders is recommended, as the BodyFIX does not immobilize this region [18].
Figure 3. Elekta Synergy® unit curently in use at the University of Toronto and BodyFIX® system for immobilization.
(A) Elekta Synergy® (Elekta AB, Stockholm, Sweden), which is the linear accelerator-based system currently in use at the University of Toronto (ON, USA) [17]. (B) A patient fully immobilized in the BodyFIX® system (Elekta AB).
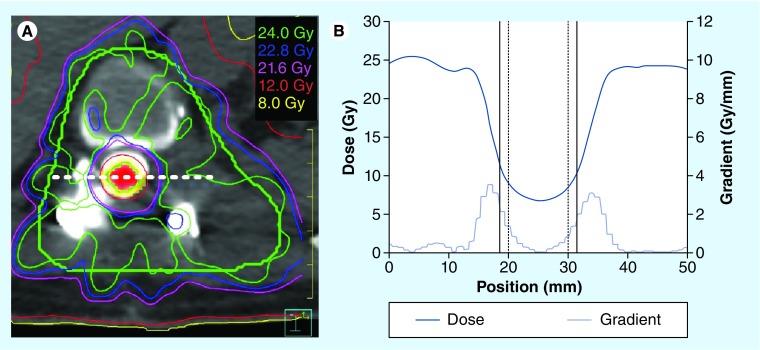
Figure 4. Dose profile through a cross-section of the target volume.
(A) Axial computed tomography slice with the isodoses overlaid for a 24 Gy single-fraction plan. The planning target volume contour is shown in green with the spinal cord shown in red colorwash. The planning organ-at-risk volume for the cord is shown in yellow (represents a margin of 1.5 mm beyond the contoured spinal cord) and is typically restricted to a maximum point dose of 12.4 Gy. (B) The dose profile and dose gradient along the dashed white line in (A) is shown. The location of the cord and the cord planning organ-at-risk volume lateral edges are indicated on (B) by the vertical solid and dashed black lines, respectively.
Thin-slice treatment planning CT scans (1–2.5 mm) are required, as are noncontrast axial T1 and T2 volumetric MRI sequences imaging one vertebrae above and below the target to be fused to the planning CT. MRI is essential for accurate delineation of the target, any soft tissue extension and the spinal cord/thecal sac. A CT myelogram can also be performed to delineate the spinal cord/thecal sac, and is often required in the postoperative patient due to the distortions created by the spinal hardware on the magnetic resonance images. Myelography is not an alternative to MRI, but is complementary for cord delineation, as soft tissues are not visualized sufficiently by CT alone.
▪ Treatment delivery machines
Delivery units can be categorized into two main groups [8,9,11]. Most commonly used are multileaf collimator-based linear accelerator (linac) units, which are fixed in the treatment room, with the patient on a moveable table-top that can move in all three translational axes to position the patient appropriately (Figure 3). One limitation to traditional treatment couches is their inability to rotate; however, new robotic couches are able to adjust the patient in not only the three translational axes, but also in the three rotational axis, to provide the full six degrees of freedom motion. The second type of treatment delivery technology consists of an X-band mini-linac mounted onto a robot that moves the linac itself to compensate for patient motion. This unit is known commercially as the CyberKnife® (Accuray Inc., Sunnyvale, CA, USA) [9,13,19,20].
There are several nuances to all technologies capable of spine SBRT, and the technological aspects have been detailed in several reviews [9,19,21,22]. A treatment planning study comparing the CyberKnife to linac-based multileaf collimator delivery and protons demonstrated that all technologies are able to perform the treatment planning task [19]. However, CyberKnife plans tended to result in the greatest heterogeneity in the dose distribution, while multileaf collimator linac systems resulted in the least heterogeneity, which in turn led to a slight sacrifice in its ability to achieve cord sparing [19]. This study also showed the feasibility of proton therapy to be used for spine SBRT; however, this technology has not yet been clinically applied. Regardless of the treatment delivery device chosen, image guidance is required for the delivery of spine SBRT [14] and is central to all SBRT delivery [10]. Image-guided radiotherapy allows the user to ensure that the patient is adequately positioned prior to the start of treatment, and can also be used during treatment to ensure continued precision [13,14]. It has been shown that without image-guided radiotherapy, the position of the target can drift during treatment and this can have significant dosimetric effects on the actual cord dose delivered [23].
Clinical details for spine SBRT delivery
▪ Volume delineation
An international consortium of expert radiation oncologists and spine surgeons, led by the Memorial Sloan Kettering Cancer Center (MSKCC), developed consensus guidelines to aid target volume delineation [24]. The aim of these guidelines was to help the community avoid common pitfalls in target contouring, such as attempting to only treat the gross tumor volume without an anatomic margin to cover potential areas at risk of microscopic disease spread [25]. It should be noted that these guidelines are based on expert opinion and have not been validated with clinical outcomes, which should be the next step in the group's research agenda.
▪ Dosing
Total dose and fractionation schemes for spine SBRT vary considerably from institution to institution, with little data to support one regimen over another [9]. The most common treatment schemes currently in practice include 16–24 Gy in a single fraction, 24 Gy in two fractions, 24–27 Gy in three fractions and 30–40 Gy in five fractions [9]. Although some institutions have championed high-dose single-fraction SBRT, with doses in the range of 20–24 Gy [26], no prospective controlled data exist to support this practice. Moreover, conflicting data have been reported regarding the risks and benefits of high-dose fractionated spine SBRT [27,28]. A randomized study of single versus fractionated radiosurgery would provide critical new information.
▪ Who to treat
An international group of experts in bone metastases was assembled with the support of the American Society of Therapeutic Radiation Oncology (ASTRO) to compile practice guidelines. Within this document was a section on spine SBRT. The group was only able to provide a summary of inclusion and exclusion criteria to guide patient selection [29]. Table 1 provides a summary of these recommendations, with slight modifications to reflect current practice. Of note, some of the major exclusion criteria included MSCC, cauda equina compression and significant epidural disease. These contraindications reflect the potential need for urgent radiation, as opposed to delaying radiation in order to plan and deliver spine SBRT, which can take up to a week. Furthermore, the epidural space is relatively underdosed to maintain the critical neural structures within tolerance, and epidural progression is known to be the most common pattern of failure [9]. Therefore, until firm toxicity guidelines are established as to how high a dose can be delivered to the spinal cord in these situations, spine SBRT is not indicated for such patients.
Table 1. . Suggested patient inclusion and exclusion criteria for spine stereotactic body radiotherapy based on the American Society of Therapeutic Radiation Oncology evidence-based guidelines.
| Criteria | Inclusion | Exclusion |
|---|---|---|
| Radiographic | Spinal/paraspinal metastatic tumor A maximum of 2–3 contiguous or 3 noncontiguous spinal segments |
Epidural cord or cauda equina compression Spinal instability requiring stabilization Tumor location within 5 mm of the cord or cauda equine (relative contraindication) |
| Patient | Age >18 years KPS ≥40–50 Life expectancy of at least 3 months |
Inability to lie flat and tolerate treatment Contraindication to MRI and/or computed tomography myelogram |
| Tumor | Histologic proof of malignancy Oligometastatic or bone-only metastases |
Radiosensitive histology such as myeloma/lymphoma (relative contraindication) |
| Previous treatment | Previous EBRT Postoperative |
Previous SBRT to same level (relative contraindication) Systemic radionuclide delivery within 30 days prior to SBRT EBRT within 90 days prior to SBRT |
EBRT: External beam radiotherapy; KPS: Karnofsky Performance Score; SBRT: Stereotactic body radiotherapy.
Criteria are based on American Society of Therapeutic Radiation Oncology guidelines [29].
Clinical outcomes
▪ Pain control
Conventional radiotherapy
Several randomized trials evaluating conventional palliative radiotherapy have been conducted to determine the effects of various dose and fractionation schemes on response rates for pain. Selected studies and a landmark meta-analysis reported by Chow et al. are summarized in Table 2 [2]. The Chow meta-analysis found an overall response rate of approximately 60% and a complete response rate of approximately 20% [2]. Retreatment rates were statistically more frequent in the single-fraction 8 Gy arm at 20%, versus 8% in the 30 Gy arm, likely indicating improved durability of pain relief with longer fractionation schemes [2].
Table 2. . Pain control rates with conventional external beam irradiation.
| Study (year) | Dose (Gy)/fractions (n) | Patients (n) | Complete response (%) | Overall response (%) | Ref. |
|---|---|---|---|---|---|
| Bone Pain Trial Working Party (1999) | 8/1 vs 20/5† | 761 | 57 vs 58 | 78 | [51] |
| Dutch Bone Study; Steenland et al. (1999) | 8/1 vs 24/6 | 1157 | 37 vs 33 | 72 vs 69 | [52] |
| Trans-Tasman; Roos et al. (2005) | 8/1 vs 20/5 | 272 | 26 vs 27 | 53 vs 61 | [53] |
| RTOG 97-14; Hartsell et al. (2005) | 8/1 vs 30/10 | 898 | 15 vs 18 | 65 vs 66 | [1] |
| Meta-analyses; Chow et al. (2007) | Varies | 5000 | 23–24 | 58–59 | [2] |
†2% of patients received 30 Gy/10 fractions.
Data taken from [30].
With the intention of creating consistency in reporting pain outcomes following radiation therapy for bone metastases, the International Bone Metastases Consensus Working Party reported recommendations for response criteria in 2002 and recently updated their report in 2012 [30]. A complete response was defined as a pain score of 0 at the treated site with a nonconcomitant increase in analgesic intake. A partial response was defined as reduction in pain by at least 2 points or more at the treated site on a 0–10 scale, without an analgesic increase, or a reduction in analgesic use by 25% or more from baseline without an increase in pain. Critically, when international consensus pain response end points (ICPRE) are used, the same trial can be reinterpreted with significantly inferior outcomes. For example, the Dutch Bone Metastasis Study now reports a 72% overall response rate and a 14% complete response rate with ICPRE, as compared with a 71% overall response rate and a 35% complete response rate in the original publication [5]. Results of those studies that re-evaluated their pain response outcomes according to the ICPRE guidelines appear in Table 3 [1,4,5]. Notably, these studies evaluated pain response rates in bone metastases in general, and did not specifically examine results for spinal metastases. University of Toronto scientists reported the results of a prospective study examining pain response rates in patients with spinal metastases treated using conventional radiotherapy and, using ICPRE, they reported a 0% complete response rate [6]. The results from this series and the randomized trials indicate that there is significant room for improvement.
Table 3. . Pain response in studies using international consensus palliative radiotherapy end point definitions.
| Study (year) | Dose (Gy)/fractions (n) | Patients (n) | Complete response (%) | Overall response (%) | Ref. |
|---|---|---|---|---|---|
| Spanish; Foro Arnalot et al. (2008) | 8/1 vs 30/10 | 78 vs 82 | 13 vs 11 | 65 vs 62 | [4] |
| RTOG 97-14; Hartsell et al. (2005) | 8/1 vs 30/10 | 256 vs 255 | 10 vs 12 | NR | [1] |
| Dutch reanalysis; van der Linden et al. (2004) | 8/1 vs 24/6 | 579 vs 578 | 14 vs 14 | 71 vs 73 | [5] |
NR: Not reported.
Spine SBRT
Pain response rates following spine SBRT have been consistently greater than what one would expect with conventional radiation, and a selected series are summarized in Table 4. In particular, the prospective Phase II MD Anderson Cancer Center (TX, USA) study represents high-quality evidence [31]. In this study, 149 patients and 166 lesions were treated to a total dose of 27–30 Gy in three fractions, and pain control was assessed using the validated Brief Pain Inventory (BPI) assessment tool. With a median follow-up of 15.9 months, investigators concluded a mean reduction of 3.4 points based on the BPI, and 54% of patients were completely pain free 6 months post-SBRT. A concomitant statistically significant decrease in opioid use was also reported. Quality of life outcomes demonstrated improvements in disturbed sleep, drowsiness, sadness, fatigue, distress, lack of appetite, nausea and memory following spine SBRT.
Table 4. . Selected studies reporting clinical outcomes for spine stereotactic body radiotherapy.
| Study (year) | Dose (Gy)/fractions (n) | Patients (n)/targets (n) | Previous radiation treatment (%) | Median follow-up (months) | Local control (%) | Ref. |
|---|---|---|---|---|---|---|
| Amdur et al. (2009) | 15/1 | 21/25 | 57 | 8 | 96 | [54] |
| Gerszten et al. (2007) | 20/1 | 393/500 | 69 | 21 | 90 | [55] |
| Wang et al. (2012) | 27–30/3 | 149/166 | 48 | 15.9 | 72 | [31] |
| Nguyen et al. (2010) | 27/3 (median) | 48/55 | 58 | 13.1 | 82 at 1 year | [32] |
| Sahgal et al. (2009) | 24/3 | 39/60 | 62 | 8.5 | 86.6 | [35] |
| Ahmed et al. (2012) | 24/3 (median) | 66/85 | 26 | 8.2 | 89.2 at 1 year | [56] |
| Yamada et al. (2008) | 24/1 (median) | 93/103 | 0 | 15 | 90 at 1 year | [26] |
High rates of pain response have also been reported in histologies traditionally considered radioresistant. A separate study, also from the MD Anderson Cancer Center, reported on 48 patients treated with spine SBRT for metastatic spinal renal cell carcinoma [32]. Patients were again evaluated using the BPI, and dose fractionation schemes varied from 24–30 Gy in one to five fractions. With a median follow-up of 13.1 months, the investigators found that 48.7% of patients were pain free at 3 months, and by 1 year 52.0% were pain free. Therefore, these data suggest excellent durability of pain relief with spine SBRT. Additional improvements in fatigue, pain, disturbed sleep, drowsiness and distress were also reported.
▪ Radiographic local disease control
Conventional radiotherapy
The largest experience of spinal metastases patients treated with conventional radiotherapy with imaging-based follow-up was recently reported in Japan. Both clinical and radiographic factors predictive for local control were determined from a total of 603 patients [33]. Patients were treated with doses ranging from 8 Gy in a single fraction, 20 Gy in five fractions, 30 Gy in ten fractions to 40 Gy in 20 fractions. Local failure was defined as imaging-based progression or an exacerbation of neurologic symptoms. Tumors were divided into those that were ‘mass type’, meaning tumor extended beyond the vertebral bone, and those that were ‘non-mass type’, meaning that disease was confined to the vertebral bone. A total of 92% of patients were followed for a minimum of 12 months or until death. Local control rates were 91, 79 and 69% at 6, 12 and 24 months, respectively. On multivariate analysis, primary breast cancer, lack of previous chemotherapy and non-mass-type tumors were significant predictors for improved local control. A mass-type tumor was the most significant predictor of local failure with a 1-year local control rate of only 45.7% as compared with 86.3% for non-mass-type tumors. This finding supports, at least for mass-type tumors, the hypothesis that more aggressive treatment may improve outcomes, and supports focussing on techniques such as spine SBRT.
Spine SBRT
Local control rates following spine SBRT have been excellent, with results consistently in the 70–90% range; a summary of selected series is shown in Table 4. The largest published experience is from the University of Pittsburgh (PA, USA) and based on 500 patients treated with single-fraction spine SBRT. The local control rate was 90% [34]. More impressively, these results seem to hold even in traditionally radioresistant histologies. For example, the previously discussed renal cell carcinoma series from the MD Anderson Cancer Center demonstrated local control in 43 out of 55 lesions for a crude rate of control of 79.2% and a 1-year spinal tumor progression-free survival of 82% [32].
One of the major indications for spine SBRT has been failure of previous external beam irradiation, and its role in this scenario has been well studied. It appears that control rates can be equally efficacious for spine SBRT in these cases and in patients with no prior radiation [35]. It is postulated that the high doses inherent to SBRT overcome the acquired radioresistance secondary to the previous radiation course. This is significant because many clinicians do not treat patients with further radiation due to the fear of radiation myelopathy or a belief that further radiation is futile. A recent review summarizes the re-irradiation spine SBRT literature [22].
Role of surgery
The traditional role of surgery for patients with metastatic spinal tumors has been limited to symptomatic MSCC or frank instability in patients with an otherwise good performance status. Spine surgery has been viewed as a major intervention with a high risk for complications [36]. Given that any complication could delay or even negate the opportunity to deliver systemic therapy, surgery has been used sparingly and radiation has become the mainstay of treatment. This position was bolstered when a small randomized study comparing surgery plus radiation versus radiation alone showed no significant benefit in the combined arm, and possibly even worse outcomes [37]. Patients were operated on with a simple laminectomy rather than circumferential decompression with stabilization, which may explain the negative results.
In 2005, Patchell et al. reported the results of a Phase III randomized trial evaluating the role of surgery in patients with symptomatic MSCC [38]. In their study, 101 patients who had radiographic evidence of MSCC, pain or other symptoms, and who were either neurologically intact or if paraplegic were so for less than 48 h, were randomized to decompressive surgery followed by conventional external beam irradiation or to conventional external beam irradiation alone (30 Gy in ten fractions). The primary end point was the ability to walk after treatment. Patients randomized to the surgical arm were more likely to walk after treatment (84 vs 57%; p = 0.001) and retained their ability to walk for a significantly longer duration (122 vs 13 days; p = 0.003). In addition, a small but significant survival benefit (126 vs 100 days; p = 0.033) and substantial reductions in corticosteroid and opioid pain medications were observed. This trial was practice changing, and decompressive surgery should be considered for patients with symptomatic single-level MSCC who are otherwise fit.
Spine SBRT should not be considered an equivalent treatment to surgery when there is clinical evidence of neurological dysfunction, as the most rapid treatment to reverse neurologic compromise from MSCC is surgical. However, spine SBRT may be an alternative in patients who are not surgical candidates, and Ryu et al. recently reported a series of such patients treated with single-fraction SBRT for MSCC. Results are preliminary and limited with respect to sample size and follow-up, but are encouraging [39]. A critical issue in this scenario is the time required for SBRT planning and delivery, which takes 1–2 weeks in most centers, and for patients with MSCC, waiting even 24–48 h could compromise outcomes.
One area where spine SBRT is gaining new ground is in its application to the postoperative patient [9]. Instead of delivering adjuvant conventional palliative radiation, high-dose SBRT can be used. Dosimetrically, postoperative SBRT makes sense, as the cord is decompressed with sufficient clearance to increase dose within the epidural space. Moreover, after putting the patient through major surgery, it is only logical to treat with aggressive radiation to improve local control, as opposed to lower dose conventional radiation. At this time, the evidence is preliminary but encouraging, and a randomized study is required.
Beyond MSCC, surgery is critical to the management of spinal metastases causing mechanical instability. In this regard, radiation is ineffective in palliating mechanical pain. Mechanical pain is regarded as positional, worse with activity, better with recumbency and is associated with a sharp increase in pain intensity with sudden jerking motions. It is ultimately due to the mechanical compromise of the spinal anatomy such that it cannot withstand the axial loading forces. The various surgical interventions to manage the unstable spine are outside of the scope of this article.
Recently, Fisher et al. reported a classification system named the Spinal Instability Neoplastic Score (SINS) [40]. This scoring system is based on both anatomic and pain characteristics, and groups patients into those who are stable, potentially unstable and unstable. Reliability testing has shown excellent results [41]. Clinical validation of SINS based on a clinical end point was lacking until Cunha et al. evaluated SINS as a predictor of SBRT-induced vertebral compression fracture [42]. SBRT-induced vertebral compression fracture and the role of SINS will be discussed in the ‘Vertebral compression fracture’ section of this review.
▪ Using surgery as an adjunct to spine SBRT
The predominant site of local failure following spine SBRT is within the epidural space [9,22]. This is not an unexpected finding, as this area is underdosed relative to the rest of the tumor, in order to respect spinal cord tolerance and minimize the risk of radiation myelopathy [43–45]. As a result, the presence of significant epidural disease has been considered a relative contraindication to spine SBRT [29].
For the patient who does not have symptomatic MSCC, but does have epidural disease, major surgery is typically not indicated, as the potential complications and postoperative pain are difficult to justify. Recent advances in spine surgery, however, have permitted minimally invasive spinal procedures designed to decompress the spinal cord and stabilize without a major incision [46,47]. This is a paradigm shift as the surgical procedure has traditionally involved a major operation with circumferential decompression, instrumentation of at least one to two spinal levels above and below the lesion, and exposure of the spine through a large incision. The University of Toronto recently reported on minimal-access spine surgery, which is based on a tubular retraction system that allows access to the spinal cord for epidural disease resection [46]. The incision required is typically 2-cm long and clearing of the epidural disease can often be achieved on the side of the surgical approach. Bilateral epidural disease may require two separate incisions followed by some form of stabilization. If stabilization is required either due to surgical approach or preoperative SINS assessment, then direct application of cement can be performed at the same time if sufficient bone is available for kyphoplasty or vertebroplasty, or instrumentation can be placed percutaneously. Patients often only require 1–2 days to mobilize from this surgery, and then spine SBRT can proceed without delay [46]. Following minimal-access spine surgery, the median time from surgery to treatment planning was 6.5 days and then 7 days to treatment in the University of Toronto study [46]. This represents an advantage over conventional open surgery, as a delay of 3–4 weeks is required to allow for sufficient wound healing. This novel approach, using less invasive surgery to facilitate spine SBRT, is a major area of research and development.
Complications secondary to spine SBRT
▪ Radiation myelopathy
Spinal cord myelopathy is a rare and devastating complication secondary to overdosing the spinal cord [43,45,48]. It has re-emerged as a direct consequence of spine SBRT. A multi-institutional collaboration compared nine cases of spinal cord myelopathy following spine SBRT with a cohort of 66 controls who did not experience spinal cord injury [43]. Complete dosimetric data based on the thecal sac contour as a surrogate for the true spinal cord contour were obtained. Maximum point doses and doses to larger volumes were compared. The most significant parameter to base constraints upon was the maximum point dose volume. Based on the logistic model, 1– 5% risk profiles for myelopathy were reported for one- to five-fraction SBRT [44]. A similar analysis was performed for re-treatment myelopathy and dose limits have been proposed [43].
▪ Esophageal toxicity
A recent publication from MSKCC examined the risk of esophageal toxicity following single-fraction SBRT [49]. The authors reported on 182 patients and 204 spinal segments, with a median prescription dose of 24 Gy and a median follow-up of 12 months. The incidence of acute and late esophageal toxicities were 15 and 12%, respectively. The overall crude rate of grade 3 or higher late toxicity was 6.8%. Interestingly, the seven cases of grade 4 or higher toxicity were associated with either radiation recall reactions with gemcitabine or doxorubicin chemotherapy, or occurred following procedures involving the esophagus. The authors state that the overall risk of esophageal toxicity is quite low, but could be further lowered by respecting dose constraints of a maximum esophageal dose of 22 Gy, keeping ≤2.5 cm3 of the esophagus from receiving 14 Gy. The study is limited, however, in that the median time to grade 3 or higher toxicity was 11.3 months, and the median follow-up was 12 months. Thus, with time, it is quite possible that the incidence of toxicity might become much higher.
▪ Vertebral compression fracture
Perhaps the most common significant complication following spine SBRT is vertebral compression fracture. The incidence of fracture following conventionally fractionated radiation therapy is not well documented and a risk of approximately 5% is generally quoted [2]. With respect to spine SBRT, the first study to report on this complication was from the MSKCC. The authors evaluated 62 consecutive patients and 71 segments treated with single-fraction SBRT and with detailed radiologic follow-up [50]. Twenty seven (39%) were found to have a new vertebral fracture or progression of a previous fracture. The median time to fracture was 25 months. Patients with lytic lesions, those with more than 40% of the vertebral body involved with tumor, and those with lesions involving T10 vertebral body or below were found to independently predict for risk of compression fractures on multivariate analysis. These results differ somewhat from the second publication on the topic, by the MD Anderson Cancer Center. The authors analyzed 123 treated vertebral bodies in 93 patients, and the median follow-up was 14.9 months [11]. In this series, the rate of new or progressing fractures was 20% and the median time to fracture was 3 months. Risk factors for fracture were found to be age greater than 55 years, pre-existing facture and baseline pain.
The largest experience, and the third series reported, comes from the University of Toronto. The authors examined 167 spinal segments in 90 patients treated with spine SBRT [42]. With a median follow-up of 7.4 months, new fracture or fracture progression was observed in 11% of segments treated, and the mean time to fracture was 3.3 months (median of 2 months; range: 0.5–21.6 months). Factors predictive of fracture on multivariate analysis included spinal alignment, lytic lesions, lung or hepatocellular primary histologies, and dose per fraction of 20 Gy or greater. This last point is of particular interest, as it may explain the very high rate of fracture in the MSKCC study given that their practice was exclusively high-dose single-fraction SBRT. The University of Toronto group also attempted to retrospectively classify patients according to SINS [40] to examine whether the criteria was predictive of fracture. None of the 167 segments examined in the study had a SINS score of unstable. Ninety-five lesions (57%) were considered stable and 72 (43.1%) were considered of indeterminate stability. The rate of fracture in patients with SINS scores of stable and indeterminate stability were 5 and 19%, respectively. On multivariate analysis of the six SINS criteria, two were independently predictive of fracture: lytic lesions and those with misalignment (kyphosis or scoliosis). Therefore, it appears that at least some of the SINS criteria are predictive of potential fracture risk, and further research is required before we can validate the utility of SINS for predicting SBRT-induced vertebral compression fracture.
Conclusion
Spine SBRT is an emerging technique, and preliminary results indicate superior local and pain control as compared with conventional radiotherapy. Ultimately, we await results from randomized studies before we can further confirm the role of spine SBRT in the modern management of patients with spinal metastases. This field is an active area of research and development.
Future perspective
The field of spine SBRT will continue to evolve and become a standard of care for selected patients with spinal metastases. In the re-treatment indication, it will soon be routine practice to refer patients for spine SBRT, as the limitations of further conventional radiation put the patient at a disadvantage for optimal pain and local tumor control. As clinical trials mature, SBRT indications for radiation-naive patients and postoperative patients will be clarified. In particular, as clinical trials develop for treating patients with oligometastatic disease with SBRT, spine SBRT will be adopted as the standard practice in this clinical scenario. We anticipate the technology will also evolve in parallel with the evidence, and ultimately permit more widespread adoption as demand increases. In particular, time spent in treatment planning and treatment delivery will be markedly reduced. In the next 5–10 years, with continued advancements in systemic therapy, we will see a transformation in the application of radiation therapy for metastatic disease, whereby multiple metastatic sites will be treated with SBRT to maximize local control, and advanced cancer becomes more of a chronic disease with long-term survivors.
Footnotes
Financial & competing interests disclosure
A Sahgal has received an honorarium from Medtronic's Kyphoplasty division for past educational seminars. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: ▪ of interest ▪▪ of considerable interest
- 1.Hartsell WF, Scott CB, Bruner DW, et al. Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J. Natl Cancer Inst. 2005;97(11):798–804. doi: 10.1093/jnci/dji139. [DOI] [PubMed] [Google Scholar]
- 2.Chow E, Harris K, Fan G, Tsao M, Sze WM. Palliative radiotherapy trials for bone metastases: a systematic review. J. Clin. Oncol. 2007;25(11):1423–1436. doi: 10.1200/JCO.2006.09.5281. [DOI] [PubMed] [Google Scholar]
- 3.Holt T, Hoskin P, Maranzano E, et al. Malignant epidural spinal cord compression: the role of external beam radiotherapy. Curr. Opin. Support. Palliat. Care. 2012;6(1):103–108. doi: 10.1097/SPC.0b013e32834de701. [DOI] [PubMed] [Google Scholar]
- 4.Foro Arnalot P, Fontanals AV, Galceran JC, et al. Randomized clinical trial with two palliative radiotherapy regimens in painful bone metastases: 30 Gy in 10 fractions compared with 8 Gy in single fraction. Radiother. Oncol. 2008;89(2):150–155. doi: 10.1016/j.radonc.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 5.van der Linden YM, Lok JJ, Steenland E, et al. Single fraction radiotherapy is efficacious: a further analysis of the Dutch Bone Metastasis Study controlling for the influence of retreatment. Int. J. Radiat. Oncol. Biol. Phys. 2004;59(2):528–537. doi: 10.1016/j.ijrobp.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen J, Chow E, Zeng L, et al. Palliative response and functional interference outcomes using the Brief Pain Inventory for spinal bony metastases treated with conventional radiotherapy. Clin. Oncol. 2011;23(7):485–491. doi: 10.1016/j.clon.2011.01.507. [DOI] [PubMed] [Google Scholar]
- 7.Howell DD, James JL, Hartsell WF, et al. Single-fraction radiotherapy versus multifraction radiotherapy for palliation of painful vertebral bone metastases – equivalent efficacy, less toxicity, more convenient: a subset analysis of Radiation Therapy Oncology Group trial 97-14. Cancer. 2012;119(4):888–896. doi: 10.1002/cncr.27616. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪▪ Recent analysis of spine metastases from the original RTOG 97-14 randomized trial evaluating pain relief following conventional radiation.
- 8.Sahgal A, Ma L, Chang E, et al. Advances in technology for intracranial stereotactic radiosurgery. Technol. Canc. Res. Treat. 2009;8(4):271–280. doi: 10.1177/153303460900800404. [DOI] [PubMed] [Google Scholar]
- 9.Sahgal A, Bilsky M, Chang EL, et al. Stereotactic body radiotherapy for spinal metastases: current status, with a focus on its application in the postoperative patient. J. Neurosurg. Spine. 2011;14(2):151–166. doi: 10.3171/2010.9.SPINE091005. [DOI] [PubMed] [Google Scholar]; ▪▪ Describes, in detail, many of the technological and treatment planning issues with respect to spine stereotactic body radiotherapy (SBRT), with a focus on the postoperative indication and the patterns of failure.
- 10.Sahgal A, Roberge D, Schellenberg D, et al. The Canadian Association of Radiation Oncology Scope of Practice Guidelines for Lung, Liver and Spine Stereotactic Body Radiotherapy. Clin. Oncol. 2012;24(9):629–639. doi: 10.1016/j.clon.2012.04.006. [DOI] [PubMed] [Google Scholar]; ▪▪ Practice guidelines that outline key considerations with respect to establishing a spine SBRT program, the training recommendations and quality assurance issues.
- 11.Boehling NS, Chang E, Ma L, Phan N, Yeung R, Sahgal A. Stereotactic radiosurgery for brain metastases: current status and future directions. J. Radiat. Oncol. 2012;1(3):245–253. [Google Scholar]
- 12.Murphy MJ. Intrafraction geometric uncertainties in frameless image-guided radiosurgery. Int. J. Radiat. Oncol. Biol. Phys. 2009;73(5):1364–1368. doi: 10.1016/j.ijrobp.2008.06.1921. [DOI] [PubMed] [Google Scholar]
- 13.Chuang C, Sahgal A, Lee L, et al. Effects of residual target motion for image-tracked spine radiosurgery. Med. Phys. 2007;34(11):4484–4490. doi: 10.1118/1.2790587. [DOI] [PubMed] [Google Scholar]
- 14.Hyde D, Lochray F, Korol R, et al. Spine stereotactic body radiotherapy utilizing cone-beam CT image-guidance with a robotic couch: intrafraction motion analysis accounting for all six degrees of freedom. Int. J. Radiat. Oncol. Biol. Phys. 2012;82(3):e555–e562. doi: 10.1016/j.ijrobp.2011.06.1980. [DOI] [PubMed] [Google Scholar]
- 15.Chang EL, Shiu AS, Lii MF, et al. Phase I clinical evaluation of near-simultaneous computed tomographic image-guided stereotactic body radiotherapy for spinal metastases. Int. J. Radiat. Oncol. Biol. Phys. 2004;59(5):1288–1294. doi: 10.1016/j.ijrobp.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 16.Yenice KM, Lovelock DM, Hunt MA, et al. CT image-guided intensity-modulated therapy for paraspinal tumors using stereotactic immobilization. Int. J. Radiat. Oncol. Biol. Phys. 2003;55(3):583–593. doi: 10.1016/s0360-3016(02)03942-1. [DOI] [PubMed] [Google Scholar]
- 17.Foote M, Letourneau D, Hyde D, et al. Technique for stereotactic body radiotherapy for spinal metastases. J. Clin. Neurosci. 2011;18(2):276–279. doi: 10.1016/j.jocn.2010.04.033. [DOI] [PubMed] [Google Scholar]
- 18.Li W, Sahgal A, Foote M, Millar BA, Jaffray DA, Letourneau D. Impact of immobilization on intrafraction motion for spine stereotactic body radiotherapy using cone beam computed tomography. Int. J. Radiat. Oncol. Biol. Phys. 2012;84(2):520–526. doi: 10.1016/j.ijrobp.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 19.Ma L, Sahgal A, Cozzi L, et al. Apparatus-dependent dosimetric differences in spine stereotactic body radiotherapy. Technol. Cancer Res. Treat. 2010;9(6):563–574. doi: 10.1177/153303461000900604. [DOI] [PubMed] [Google Scholar]
- 20.Adler JR, Jr, Chang SD, Murphy MJ, Doty J, Geis P, Hancock SL. The CyberKnife: a frameless robotic system for radiosurgery. Stereotact. Funct. Neurosurg. 1997;69(1–4 Pt 2):124–128. doi: 10.1159/000099863. [DOI] [PubMed] [Google Scholar]
- 21.Sahgal A, Larson DA, Chang EL. Stereotactic body radiosurgery for spinal metastases: a critical review. Int. J. Radiat. Oncol. Biol. Phys. 2008;71(3):652–665. doi: 10.1016/j.ijrobp.2008.02.060. [DOI] [PubMed] [Google Scholar]
- 22.Masucci GL, Yu E, Ma L, et al. Stereotactic body radiotherapy is an effective treatment in reirradiating spinal metastases: current status and practical considerations for safe practice. Expert Rev. Anticancer Ther. 2011;11(12):1923–1933. doi: 10.1586/era.11.169. [DOI] [PubMed] [Google Scholar]
- 23.Ma L, Sahgal A, Hossain S, et al. Nonrandom intrafraction target motions and general strategy for correction of spine stereotactic body radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2009;75(4):1261–1265. doi: 10.1016/j.ijrobp.2009.04.027. [DOI] [PubMed] [Google Scholar]
- 24.Cox BW, Spratt DE, Lovelock M, et al. International Spine Radiosurgery Consortium consensus guidelines for target volume definition in spinal stereotactic radiosurgery. Int. J. Radiat. Oncol. Biol. Phys. 2012;83(5):e597–e605. doi: 10.1016/j.ijrobp.2012.03.009. [DOI] [PubMed] [Google Scholar]; ▪ Practice guidelines for target volume contouring are presented based on a multi-institutional collaboration. Represents the first report of its kind to guide consistent practice.
- 25.Patel VB, Wegner RE, Heron DE, Flickinger JC, Gerszten P, Burton SA. Comparison of whole versus partial vertebral body stereotactic body radiation therapy for spinal metastases. Technol. Canc. Res. Treat. 2012;11(2):105–115. doi: 10.7785/tcrt.2012.500239. [DOI] [PubMed] [Google Scholar]
- 26.Yamada Y, Bilsky MH, Lovelock DM, et al. High-dose, single-fraction image-guided intensity-modulated radiotherapy for metastatic spinal lesions. Int. J. Radiat. Oncol. Biol. Phys. 2008;71(2):484–490. doi: 10.1016/j.ijrobp.2007.11.046. [DOI] [PubMed] [Google Scholar]
- 27.Jhaveri PM, Teh BS, Paulino AC, et al. A dose-response relationship for time to bone pain resolution after stereotactic body radiotherapy (SBRT) for renal cell carcinoma (RCC) bony metastases. Acta Oncol. 2012;51(5):584–588. doi: 10.3109/0284186X.2011.652741. [DOI] [PubMed] [Google Scholar]
- 28.Heron DE, Rajagopalan MS, Stone B, et al. Single-session and multisession CyberKnife radiosurgery for spine metastases – University of Pittsburgh and Georgetown University experience. J. Neurosurg. Spine. 2012;17(1):11–18. doi: 10.3171/2012.4.SPINE11902. [DOI] [PubMed] [Google Scholar]
- 29.Lutz S, Berk L, Chang E, et al. Palliative radiotherapy for bone metastases: an ASTRO evidence-based guideline. Int. J. Radiat. Oncol. Biol. Phys. 2011;79(4):965–976. doi: 10.1016/j.ijrobp.2010.11.026. [DOI] [PubMed] [Google Scholar]
- 30.Chow E, Hoskin P, Mitera G, et al. Update of the international consensus on palliative radiotherapy end points for future clinical trials in bone metastases. Int. J. Radiat. Oncol. Biol. Phys. 2012;82(5):1730–1737. doi: 10.1016/j.ijrobp.2011.02.008. [DOI] [PubMed] [Google Scholar]; ▪▪ Guidelines that support an international consensus on how to report pain response following radiation and the critical importance of allowing comparability among clinical trials.
- 31.Wang XS, Rhines LD, Shiu AS, et al. Stereotactic body radiation therapy for management of spinal metastases in patients without spinal cord compression: a Phase 1–2 trial. Lancet Oncol. 2012;13(4):395–402. doi: 10.1016/S1470-2045(11)70384-9. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪ Data that represent the highest quality for pain response and local control following spine SBRT in radiation-naive patients.
- 32.Nguyen QN, Shiu AS, Rhines LD, et al. Management of spinal metastases from renal cell carcinoma using stereotactic body radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2010;76(4):1185–1192. doi: 10.1016/j.ijrobp.2009.03.062. [DOI] [PubMed] [Google Scholar]
- 33.Mizumoto M, Harada H, Asakura H, et al. Radiotherapy for patients with metastases to the spinal column: a review of 603 patients at Shizuoka Cancer Center Hospital. Int. J. Radiat. Oncol. Biol. Phys. 2011;79(1):208–213. doi: 10.1016/j.ijrobp.2009.10.056. [DOI] [PubMed] [Google Scholar]
- 34.Gerszten PC, Burton SA, Ozhasoglu C. CyberKnife radiosurgery for spinal neoplasms. Prog. Neurol. Surg. 2007;20:340–358. doi: 10.1159/000100177. [DOI] [PubMed] [Google Scholar]
- 35.Sahgal A, Ames C, Chou D, et al. Stereotactic body radiotherapy is effective salvage therapy for patients with prior radiation of spinal metastases. Int. J. Radiat. Oncol. Biol. Phys. 2009;74(3):723–731. doi: 10.1016/j.ijrobp.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 36.Arrigo RT, Kalanithi P, Cheng I, et al. Predictors of survival after surgical treatment of spinal metastasis. Neurosurgery. 2011;68(3):674–681. doi: 10.1227/NEU.0b013e318207780c. [DOI] [PubMed] [Google Scholar]
- 37.Young RF, Post EM, King GA. Treatment of spinal epidural metastases. Randomized prospective comparison of laminectomy and radiotherapy. J. Neurosurg. 1980;53(6):741–748. doi: 10.3171/jns.1980.53.6.0741. [DOI] [PubMed] [Google Scholar]
- 38.Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366(9486):643–648. doi: 10.1016/S0140-6736(05)66954-1. [DOI] [PubMed] [Google Scholar]
- 39.Ryu S, Rock J, Jain R, et al. Radiosurgical decompression of metastatic epidural compression. Cancer. 2010;116(9):2250–2257. doi: 10.1002/cncr.24993. [DOI] [PubMed] [Google Scholar]
- 40.Fisher CG, Dipaola CP, Ryken TC, et al. A novel classification system for spinal instability in neoplastic disease: an evidence-based approach and expert consensus from the Spine Oncology Study Group. Spine (Phila Pa 1976) 2010;35(22):E1221–E1229. doi: 10.1097/BRS.0b013e3181e16ae2. [DOI] [PubMed] [Google Scholar]
- 41.Fourney DR, Frangou EM, Ryken TC, et al. Spinal instability neoplastic score: an analysis of reliability and validity from the spine oncology study group. J. Clin. Oncol. 2011;29(22):3072–3077. doi: 10.1200/JCO.2010.34.3897. [DOI] [PubMed] [Google Scholar]
- 42.Cunha MV, Al-Omair A, Atenafu EG, et al. Vertebral compression fracture (VCF) after spine stereotactic body radiation therapy (SBRT): analysis of predictive factors. Int. J. Radiat. Oncol. Biol. Phys. 2012;84(3):e343–e349. doi: 10.1016/j.ijrobp.2012.04.034. [DOI] [PubMed] [Google Scholar]; ▪ Provides robust rates of vertebral compression fracture and predictors of this adverse event. The investigators found that high-dose single-fraction SBRT increases the risk of fracture and is probably secondary to osteoradionecrosis.
- 43.Sahgal A, Ma L, Weinberg V, et al. Reirradiation human spinal cord tolerance for stereotactic body radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2012;82(1):107–116. doi: 10.1016/j.ijrobp.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 44.Sahgal A, Weinberg V, Ma L, et al. Probabilities of radiation myelopathy specific to stereotactic body radiation therapy to guide safe practice. Int. J. Radiat. Oncol. Biol. Phys. 2012;85(2):341–347. doi: 10.1016/j.ijrobp.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 45.Sahgal A, Ma L, Gibbs I, et al. Spinal cord tolerance for stereotactic body radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2010;77(2):548–553. doi: 10.1016/j.ijrobp.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 46.Massicotte E, Foote M, Reddy R, Sahgal A. Minimal access spine surgery (MASS) for decompression and stabilization performed as an out-patient procedure for metastatic spinal tumours followed by spine stereotactic body radiotherapy (SBRT): first report of technique and preliminary outcomes. Technol. Canc. Res. Treat. 2012;11(1):15–25. doi: 10.7785/tcrt.2012.500230. [DOI] [PubMed] [Google Scholar]
- 47.Zairi F, Arikat A, Allaoui M, Marinho P, Assaker R. Minimally invasive decompression and stabilization for the management of thoracolumbar spine metastasis. J. Neurosurg. Spine. 2012;17(1):19–23. doi: 10.3171/2012.4.SPINE111108. [DOI] [PubMed] [Google Scholar]
- 48.Sahgal A, Weinberg V, Ma L, et al. Probabilities of radiation myelopathy specific to stereotactic body radiation therapy to guide safe practice. Int. J. Radiat. Oncol. Biol. Phys. 2013;85(2):341–347. doi: 10.1016/j.ijrobp.2012.05.007. [DOI] [PubMed] [Google Scholar]; ▪ Details, for the first time, dose–volume histogram-analyzed spinal cord tolerance guidelines with 1–5% risk estimates to guide safe spine SBRT practice.
- 49.Cox BW, Jackson A, Hunt M, Bilsky M, Yamada Y. Esophageal toxicity from high-dose, single-fraction paraspinal stereotactic radiosurgery. Int. J. Radiat. Oncol. Biol. Phys. 2012;83(5):e661–e667. doi: 10.1016/j.ijrobp.2012.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rose PS, Laufer I, Boland PJ, et al. Risk of fracture after single fraction image-guided intensity-modulated radiation therapy to spinal metastases. J. Clin. Oncol. 2009;27(30):5075–5079. doi: 10.1200/JCO.2008.19.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.8 Gy single fraction radiotherapy for the treatment of metastatic skeletal pain: randomised comparison with a multifraction schedule over 12 months of patient follow-up. Bone Pain Trial Working Party. Radiother. Oncol. 1999;52(2):111–121. [PubMed] [Google Scholar]
- 52.Steenland E, Leer JW, Van Houwelingen H, et al. The effect of a single fraction compared with multiple fractions on painful bone metastases: a global analysis of the Dutch Bone Metastasis Study. Radiother. Oncol. 1999;52(2):101–109. doi: 10.1016/s0167-8140(99)00110-3. [DOI] [PubMed] [Google Scholar]
- 53.Roos DE, Turner SL, O'Brien PC, et al. Randomized trial of 8 Gy in 1 versus 20 Gy in five fractions of radiotherapy for neuropathic pain due to bone metastases (Trans-Tasman Radiation Oncology Group, TROG 96.05) Radiother. Oncol. 2005;75(1):54–63. doi: 10.1016/j.radonc.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 54.Amdur RJ, Bennett J, Olivier K, et al. A Prospective, Phase II study demonstrating the potential value and limitation of radiosurgery for spine metastases. Am. J. Clin. Oncol. 2009;32(5):515–520. doi: 10.1097/COC.0b013e318194f70f. [DOI] [PubMed] [Google Scholar]
- 55.Gerszten PC, Burton SA, Ozhasoglu C, Welch WC. Radiosurgery for spinal metastases: clinical experience in 500 cases from a single institution. Spine (Phila Pa 1976) 2007;32(2):193–199. doi: 10.1097/01.brs.0000251863.76595.a2. [DOI] [PubMed] [Google Scholar]
- 56.Ahmed KA, Stauder MC, Miller RC, et al. Stereotactic body radiation therapy in spinal metastases. Int. J. Radiat. Oncol. Biol. Phys. 2012;82(5):e803–e809. doi: 10.1016/j.ijrobp.2011.11.036. [DOI] [PubMed] [Google Scholar]