Abstract
Aim:
The aim is to study the effect of glycemic level in Type 2 diabetes and cardiovascular risk factors on periodontal health.
Materials and Method:
Type 2 diabetic and nondiabetic patients in the age group of 35–80 years (n = 1700) were recruited for the study. Periodontal examination included as follows: Probing depth, clinical attachment level (CAL), gingival recession, and bleeding on probing. Periodontitis was diagnosed based on the CAL levels and diabetes was diagnosed based on glycated hemoglobin (HbA1c) levels. Body mass index, total cholesterol, triglyceride, and low-density lipoprotein (LDL) were assessed for cardiovascular risk. Patients were characterized into two groups as follows: diabetic (n = 1235) and nondiabetic (n = 465). Sociodemographic variables included were: age, sex, obesity, smoking, duration of diabetes, and periodontitis were assessed. SPSS version 20.0.1.0 was used for all the statistical assessments.
Conclusion:
HbA1c and lipid levels were statistically significant with the severity of periodontitis (odds ratio [OR] [95% confidence interval [CI]: HbA1c 1.34 [1.019–1.21]; Total cholesterol 1.01 [1.03–1.42]; triglycerides 1.01 [1.01–1.14]; LDL 1.028 [1.08–1.71]). Smoking and obesity were also found to be significantly associated with the presence of periodontitis [OR (95% CI): smoking 1.35 (1.10–1.67); obesity 1.23 (1.73–2.05)]. The study concluded that uncontrolled HbA1c levels and elevated cardiovascular risk factors significantly increase the severity of periodontitis in Type 2 diabetes mellitus.
Keywords: Body mass index, diabetes mellitus, glycemic level, periodontitis, triglyceride
INTRODUCTION
Periodontitis is an inflammatory disease associated with oral biofilm dysbiosis and unresolved inflammation leading to the destruction of tooth-supporting structures and ultimately tooth loss. The global burden of severe periodontitis measured using disability-adjusted life-years increased by 57.3% worldwide between 1990 and 2010, in the context of a 20.8% increase in the global burden of all oral conditions, which was attributed mainly to population growth and aging.[1] Generalized severe periodontitis contributes to the systemic inflammatory burden that impacts overall health and control of conditions such as diabetes mellitus and atherosclerotic cardiovascular disease.[2,3,4] Diabetes mellitus is a highly prevalent metabolic disorder, constituting a serious public-health burden. A predicted 300 million people are going to suffer from diabetes by 2025.[5] The severity of periodontal disease has been seen to be more pronounced in patients with type 2 diabetes patients than in patients with Type 1 diabetes.[6]
Prediabetes and diabetes, two chronic conditions characterized by altered endocrine and metabolic pathways in glucose utilization resulting in sustained hyperglycemia, affect 12.3% (29 millions) of U.S. population ≥20 years of age.[7] India has one of the highest incidence rates for diabetes with more than 62 million suffering from diabetes and a projected increase to 80 million by 2030.[8] Periodontitis elevates the systemic inflammation leading to a state of insulin resistance precipitating into hyperglycemia and advanced glycation end product accumulation.[9] Hyperglycemia is known to favor pro-inflammatory priming of periodontal tissues thus contributing to the earlier onset of gingivitis, the initial and reversible stage of periodontitis, in patients with diabetes.[10,11] Persistent hyperglycemic state and advanced glycation end product besides causing the macrovascular and microvascular complications of diabetes also result in the impaired gingival fibroblast synthesis, defective phagocytic activity of mononuclear, and polymorphonuclear cells.[9] This results in the loss of periodontal fibers and supporting alveolar bone.[12] These findings led to consider periodontitis as the sixth complication of diabetes mellitus.[13] Importantly, the relationship between diabetes and periodontitis is bidirectional.[14] Central to these relationships seems to be unresolved systemic inflammation diagnosed by high sensitivity C-reactive protein measurements and white blood cell counts.[15] Overweight and obesity as defined by a body mass index (BMI) ≥25 and ≥30, respectively, are estimated to affect 36% of U.S. adults.[16] Studies carried out over the past two decades indicate a positive association between BMI ≥25 and periodontitis; although, the magnitude of this association has varied in different settings.
Several studies have revealed the association between diabetes and periodontitis/cardiovascular disease and periodontitis.[17,18,19,20,21,22] However, not much has been published on the association of the glycated hemoglobin (HbA1c) levels, cardiovascular risk and severity of periodontal disease. We hypothesized that HbA1c level is associated with severity of periodontitis. The secondary outcome of the study was to further understand the association of glycemic control levels, cardiovascular risk factors with periodontitis.
METHODS
Patients and study design
This study was conducted in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards CTRI registration (CTRI/2014/06/002748) and ethics approval was obtained from the Institutional Ethical Committee at Max Super Specialty Hospital, New Delhi, India. Two thousand and twenty-nine patients were screened between December 2016–June 2017. Patients underwent comprehensive examination for metabolic parameters which comprised HbA1c, fasting glucose (F-Glucose), cholesterol, triglycerides, and low-density lipoproteins (LDL). Inclusion criteria for diabetes were: history of diabetes, and/or antidiabetic treatment including diet modification and HbA1c ≥6.5.[23] Nondiabetics were screened based on the previous history of diabetes and HbA1c <6.5. The final recruited sample size was 1700 (Type 2 diabetics and nondiabetics) average age group of 30–80 years including both males and females. The co-investigator assigned the patients into two groups: diabetic group (n = 1235) and nondiabetic group (n = 465). Diabetic group was further categorized based on the glycemic levels, i.e., poor control diabetes (HbA1c >7) and good control diabetes (HbA1c <7). The periodontal examination was done by the coinvestigator for the presence/absence of periodontitis in both groups. The periodontal assessment included the presence of more than 14 natural teeth with no periodontal treatment in the past 6 months. Diagnosis for chronic periodontitis was made according to the American Academy of Periodontology classification.[24] Informed written consent was obtained from all the participants included in the study. The demographic assessment was done for: age, gender, cigarette smoking years, family history of diabetes, family history of periodontitis, the frequency of tooth brushing, duration of diabetes, and obesity. The current study conforms to the STROBE guidelines.
Glycemic level examination
Twelve hour fasting venous blood samples were collected from the patients for measuring the glycemic levels: F-Glucose and HbA1c. HBA1c was measured using ion exchange high-performance liquid chromatography (Bio-Rad USA, Variant-2 Turbo) F-Glucose was measured by Hexokinase method (DXC-800, Beckmancoulter, USA). Nondiabetics were defined as (HbA1c ≤5.7%, at risk HBA1c ≤6.4) and diabetic when HBA1c ≥6.5%.[23] Patients were considered to have well-controlled diabetes if HbA1c <7% and poor-controlled if HbA1c >7%.[25]
Clinical periodontal examination
Periodontal disease progression clinical variables as recorded, i.e., probing depth (PD), bleeding on probing (BOP), gingival recession (GR) and clinical attachment level (CAL), on 6 sites/tooth. CAL was measured from the cement-enamel junction (CEJ) to the base of the sulcus as composite measurement of recession (distance from free gingival margin to CEJ) and PD (distance from free gingival margin to the base of the sulcus). Patients were divided into three categories based on the CAL level as follows: mild periodontitis (CAL ≥2 mm), moderate (CAL ≥3 mm), severe (CAL >5 mm), and no periodontitis if there is no loss of attachment.[24]
Assessment of obesity
Asian Indian obesity and overweight criteria are defined by BMI and were calculated by dividing weight in kilograms by the square of height in meters (kg/m2) (*Normal BMI: 18.0–22.9 kg/m2, Overweight: 23.0–24.9 kg/m2, Obesity: >25).[26]
Assessment of lipid profiles
Serum cholesterol and LDL was analyzed with the cholesterol oxidase, esterase, and peroxidase method (DXC-800 Beckmancoulter, USA). Serum triglycerides were assessed with enzymatic with Glycerol Blank (DXC-800, Beckmancoulter–USA).
Statistical analysis
Periodontal health was defined when there is no loss of periodontal attachment. Patients were divided into three categories based on CALs: mild periodontitis (CAL ≥2 mm), moderate periodontitis (CAL ≥3 mm), and severe periodontitis (CAL ≥5 mm).[24] This categorization was done to facilitate the interpretation and the range of variation of the variables.[27] The difference in mean age, BMI, years of smoking, and other quantitative measurements were tested for significance by one-way ANOVA followed by Tukey test for all pairwise comparisons. Prevalence and severity of periodontitis were compared using Chi-square test. Multivariate regression analyses were used to find the association between glycemic levels and periodontitis after adjusting confounding factors. Outliers were excluded from the analysis based on the residual analysis. For all statistical analysis, data was entered into a Microsoft Excel spreadsheet and analyzed using IBM SPSS Statistics 20.0.1 and Graphpad Prisms version 5. For all the statistical tests, a valueof P < 0.05 was considered to indicate statistical significance.
RESULTS
The study comprised 56% of males (n = 896) and 45% of females (n = 704). Mean age group of 50 years. Table 1 lists the demographic parameters and information by tertiles of mean CAL as categorized according to the severity of periodontal disease. The mean percentage of the severity of periodontitis was: 15% (mild), 28.5% (moderate), and 34.2% (severe). Mean periodontal clinical measures for PD, CAL, and GR were 3.21 ± 3.73, 3.83 ± 0.67, and 1.9 ± 0.41. Mean BOP was 37.87% ± 16.12%, respectively.
Table 1.
Demographic variables of study subjects by tertiles of mean periodontal disease
Glycemic metabolic levels (glycated hemoglobin, fasting sugar, cholesterol, triglycerides, low-density lipoprotein)
The mean levels of HbA1c in diabetic group were 7.4% ± 1.04% and the nondiabetic was 5.7% ± 0.10%. Mean fasting sugar levels in diabetic group was 8.34 ± 1.04 mmol/l and control group was 5.06 ± 1.21 mmol/l. The mean cholesterol levels in diabetic and nondiabetic groups were as follows: 8.12 ± 1.01 mmol/l and 6.74 ± 1.13 mmol/l, respectively. Mean triglyceride levels in the diabetic and nondiabetic group were: 7.31 ± 1.71 mmol/l and 5.50 ± 1.41 mmol/l, respectively. Mean LDL levels in the diabetic and nondiabetic group were: 6.97 ± 1.72 mmol/l and 4.61 ± 1.87 mmol/l, respectively. Patients with increased HbA1c levels (HbA1c >7) had elevated cholesterol (P = 0.003), triglycerides (P = 0.002), LDL (P = 0.003) [Table 2]. Mean HbA1c level in poor controlled diabetics, well controlled, and nondiabetics was 8.5 ± 2.56, 6.37 ± 0.20 and 5.31 ± 0.10, respectively. Glycemic control levels (HbA1c >7, P = 0.001) HbA1c <7 P = 0.001 and HbA1c <5.6 P = 0.003) were found to be associated with severity of periodontitis [Table 3]. In the multivariable analysis, after adjusting for other covariates, a significant relationship was found between diabetes and periodontitis. Triglyceride and LDL also showed an increase in the odds of having periodontitis [Table 4]. Patients with increased BMI (a marker for obesity) were found to be associated with periodontitis (P = 0.001).
Table 2.
Metabolic parameters (glycated hemoglobin, fasting glucose, cholesterol, triglycerides, low-density lipoprotein), and periodontal disease severity: Glycated hemoglobin >6.5%

Table 3.
Glycated hemoglobin control levels and its association with periodontitis

Table 4.
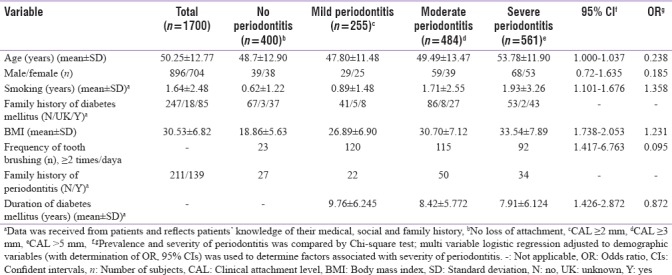
Odds ratio estimates of factors on the presence of periodontitis
Nonobese people were found to be 0.44 times less likely to have periodontitis [Table 4]. One year of smoking showed an increase of 1.36 times in the odds to have periodontitis [Table 5]. A proportional odds model when applied onto the severity of periodontitis revealed that diabetes does not affect the severity of periodontitis. However, smoking and obesity both can increase its severity. High-blood cholesterol, LDL, and triglyceride were also found to be associated with increased severity of periodontitis [Table 5].
Table 5.
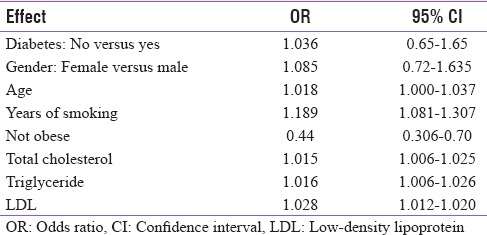
Odds ratio estimates of factors on the severity of periodontitis
When replacing binary variable “diabetes” with HbA1c in the model, results revealed that one unit increase in HbA1c could lead to 1.34 times more likelihood of having periodontitis [Tables 6 and 7]. In correlation analysis, HbA1c measurements were found to be significantly associated with the severity of periodontitis [Figure 1].
Table 6.
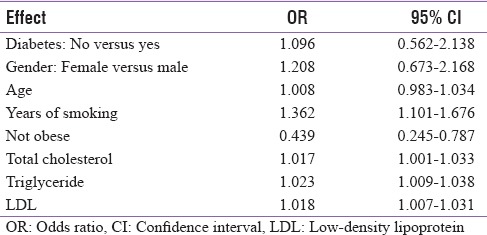
Effect of glycated hemoglobin on the presence of periodontitis
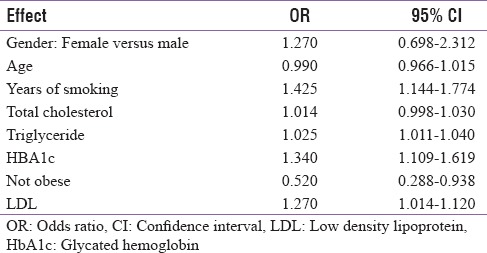
Table 7.
Effect of glycated hemoglobin on severity of periodontitis
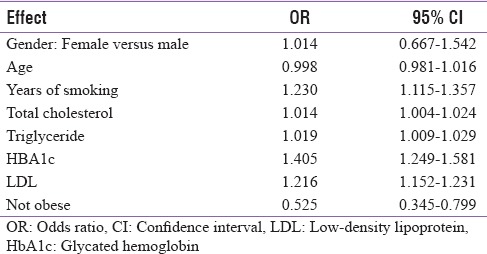
Figure 1.
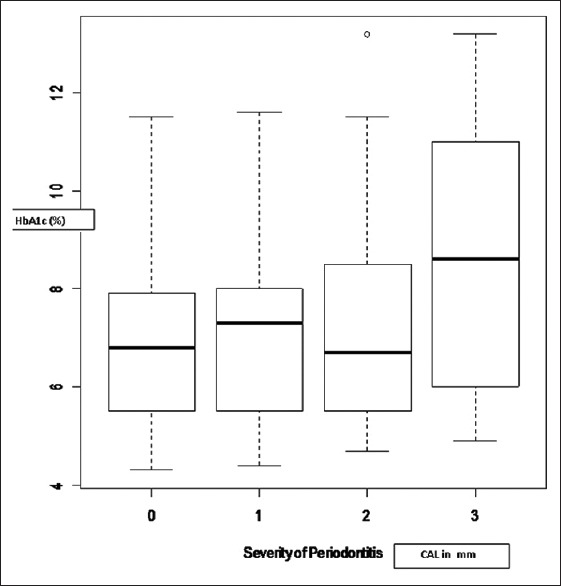
Effect of glycated haemoglobin on severity of periodontitis
DISCUSSION
This large dataset study in the Indian population highlighted an important finding that poor periodontal health is found in diabetics with poor glycemic controls (HbA1c ≥ 7) and high cardiovascular risk levels. This is in agreement with the previously published study.[28] The probable reasons cited for the failing periodontal health in poor glycemic control individuals are the monocyte-macrophage and periodontal bacteria interaction. The ulcerated pocket lining in chronic periodontitis gives a favorable environment to the bacteria and their byproducts that alleviate the systemic inflammation leading to stimulation of the proinflammatory cytokines (C-reactive proteins, tumor necrosis factor-alpha, interleukin-6). This, in turn, may lead to insulin resistance and hyperglycemia.[29,30] In this study, the cutout level of HbA1c defining diabetes was kept at 7.0% to classify the good control versus uncontrolled diabetics.[31] Several studies have used HbA1c of 8% or 9% at the cutout level.[29,32] Importance of keeping the cutout level low as close to 7.0% gives an added benefit of decreasing the adverse periodontal outcomes.[30] Percentage of poorly controlled, well controlled, and nondiabetics was assessed as 50%, 22%, and 27%, respectively [Table 3] Overall periodontitis prevalence as found in our study among diabetics and nondiabetics was 72% and 27%, respectively.
Further, the study revealed that with increased severity of periodontitis, the patients with poorly-controlled diabetes (HbA1c ≥7) also had elevated lipid levels (LDL, total cholesterol, and triglyceride). The effect of lipid levels on the severity of periodontal inflammation is in agreement with the published study.[32] The odds ratio estimates showed increased severity of periodontitis with obesity, smoking, and lipid levels. Results are in concurrence to the similar studies.[33,34,35,36] Recent meta-analysis and meta-regression suggested that periodontitis is associated with elevation of LDL and triglyceride concentrations. This analysis supported the important finding that periodontal disease affects the lipid metabolic control levels.[37]
The third NHANES survey reported that individuals ≥45 years of age suffering from severe periodontitis were 2.3 times more likely to have metabolic syndrome compared with unaffected individuals (95% confidence interval: 1.13–4.47).[38] Chronic periodontitis is a low-grade inflammatory condition characterized by increased levels of the circulating cytokines.[39,40] The underlying mechanisms for the association between obesity and periodontal disease are not well known. Obesity may act as a factor responsible for the severity of periodontitis through immune modulation. Obesity triggers inflammation with increased proinflammatory markers and adipokines (adiponectin, leptin, and oromucosoids).[41,42] These obesity markers have been found to be associated with metabolic conditions in diabetes and atherosclerosis.[40] Meta-analysis has reported associations between BMI and periodontitis. The study is in consensus with a recent study evaluating the severity of periodontitis in morbidly obese patients which concluded that disease severity was associated with raised inflammatory markers.[42] Overweight individuals have double the incidence of periodontitis, and obese have triple the incidence of periodontitis.[42,43,44,45,46]
A key finding of this study suggests that in addition to the preponderance of periodontitis (72%) with Type 2 diabetes mellitus (T2DM), those who continue to have poor/uncontrolled glycemic level have increased the risk of severe periodontitis and not merely the presence of diabetes alone. In other words, it can be interpreted that a well-controlled diabetic will not have any significantly higher risk of developing periodontal disease and in anyway contribute to its severity when compared to the nondiabetic.
CONCLUSION
Risk of worsening of periodontal disease is associated with HbA1c in T2DM. Results support the clinical relevance of identifying patients with poor glycemic control and periodontitis and harness the individualized integrative approaches for the management of metabolic conditions and periodontitis by endocrinologists and periodontists. At the level of clinical practice, this concept would possibly reinforce the need for development and implementation of guidelines to screen prediabetes and diabetes in dental and medical primary care settings.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors would like to thank Dr. Abhay Indrayan for statistical analysis.
REFERENCES
- 1.Marcenes W, Kassebaum NJ, Bernabé E, Flaxman A, Naghavi M, Lopez A, et al. Global burden of oral conditions in 1990-2010: A systematic analysis. J Dent Res. 2013;92:592–7. doi: 10.1177/0022034513490168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tonetti MS. Periodontitis and risk for atherosclerosis: An update on intervention trials. J Clin Periodontol. 2009;36(Suppl 10):15–9. doi: 10.1111/j.1600-051X.2009.01417.x. [DOI] [PubMed] [Google Scholar]
- 3.Tonetti MS, Van Dyke TE Working group 1 of the joint EFP/AAP workshop. Periodontitis and atherosclerotic cardiovascular disease: Consensus report of the joint EFP/AAP workshop on periodontitis and systemic diseases. J Clin Periodontol. 2013;40(Suppl 14):S24–9. doi: 10.1111/jcpe.12089. [DOI] [PubMed] [Google Scholar]
- 4.Chapple IL, Genco R Working group 2 of joint EFP/AAP workshop. Diabetes and periodontal diseases: Consensus report of the joint EFP/AAP workshop on periodontitis and systemic diseases. J Clin Periodontol. 2013;40(Suppl 14):S106–12. doi: 10.1111/jcpe.12077. [DOI] [PubMed] [Google Scholar]
- 5.Green A, Christian Hirsch N, Pramming SK. The changing world demography of type 2 diabetes. Diabetes Metab Res Rev. 2003;19:3–7. doi: 10.1002/dmrr.340. [DOI] [PubMed] [Google Scholar]
- 6.Pranckeviciene A, Siudikiene J, Ostrauskas R, Machiulskiene V. Severity of periodontal disease in adult patients with diabetes mellitus in relation to the type of diabetes. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2014;158:117–23. doi: 10.5507/bp.2013.098. [DOI] [PubMed] [Google Scholar]
- 7.Atlanta, GA: US Department of Health and Human Services; 2014. Centres for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States. [Google Scholar]
- 8.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Kiran M, Arpak N, Unsal E, Erdoğan MF. The effect of improved periodontal health on metabolic control in type 2 diabetes mellitus. J Clin Periodontol. 2005;32:266–72. doi: 10.1111/j.1600-051X.2005.00658.x. [DOI] [PubMed] [Google Scholar]
- 10.Salvi GE, Franco LM, Braun TM, Lee A, Rutger Persson GS, Lang NP, et al. Proinflammatory biomarkers during experimental gingivitis in patients with type 1 diabetes mellitus: A proof-of-concept study. J Clin Periodontol. 2009;37:9–16. doi: 10.1111/j.1600-051X.2009.01500.x. [DOI] [PubMed] [Google Scholar]
- 11.Sima C, Rhourida K, Van Dyke TE, Gyurko R. Type 1 diabetes predisposes to enhanced gingival leukocyte margination and macromolecule extravasation in vivo . J Periodontal Res. 2010;45:748–56. doi: 10.1111/j.1600-0765.2010.01295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janket SJ, Jones JA, Meurman JH, Baird AE, Van Dyke TE. Oral infection, hyperglycemia, and endothelial dysfunction. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:173–9. doi: 10.1016/j.tripleo.2007.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Löe H. Periodontal disease. The sixth complication of diabetes mellitus. Diabetes Care. 1993;16:329–34. [PubMed] [Google Scholar]
- 14.Sima C, Glogauer M. Diabetes mellitus and periodontal diseases. Curr Diab Rep. 2013;13:445–52. doi: 10.1007/s11892-013-0367-y. [DOI] [PubMed] [Google Scholar]
- 15.Demmer RT, Squillaro A, Papapanou PN, Rosenbaum M, Friedewald WT, Jacobs DR, Jr, et al. Periodontal infection, systemic inflammation, and insulin resistance: Results from the continuous national health and nutrition examination survey (NHANES) 1999-2004. Diabetes Care. 2012;35:2235–42. doi: 10.2337/dc12-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307:491–7. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 17.Taylor GW. Bidirectional interrelationships between diabetes and periodontal diseases: An epidemiologic perspective. Ann Periodontol. 2001;6:99–112. doi: 10.1902/annals.2001.6.1.99. [DOI] [PubMed] [Google Scholar]
- 18.Saito T, Shimazaki Y, Kiyohara Y, Kato I, Kubo M, Iida M, et al. The severity of periodontal disease is associated with the development of glucose intolerance in non-diabetics: The Hisayama study. J Dent Res. 2004;83:485–90. doi: 10.1177/154405910408300610. [DOI] [PubMed] [Google Scholar]
- 19.Khader YS, Dauod AS, El-Qaderi SS, Alkafajei A, Batayha WQ. Periodontal status of diabetics compared with nondiabetics: A meta-analysis. J Diabetes Complications. 2006;20:59–68. doi: 10.1016/j.jdiacomp.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 20.Chávarry NG, Vettore MV, Sansone C, Sheiham A. The relationship between diabetes mellitus and destructive periodontal disease: A meta-analysis. Oral Health Prev Dent. 2009;7:107–27. [PubMed] [Google Scholar]
- 21.Bascones-Martinez A, Matesanz-Perez P, Escribano-Bermejo M, González-Moles MÁ, Bascones-Ilundain J, Meurman JH, et al. Periodontal disease and diabetes-review of the literature. Med Oral Patol Oral Cir Bucal. 2011;16:e722–9. doi: 10.4317/medoral.17032. [DOI] [PubMed] [Google Scholar]
- 22.22Gürsoy UK, Yildiz Çiftlikli S, Könönen E, Gürsoy M, Doğan B. Salivary interleukin-17 and tumor necrosis factor-α in relation to periodontitis and glycemic status in type 2 diabetes mellitus. J Diabetes. 2015;7:681–8. doi: 10.1111/1753-0407.12228. [DOI] [PubMed] [Google Scholar]
- 23.American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care. 2017;40:s11–24. [Google Scholar]
- 24.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 25.American Diabetes Association. Standards of medical care in diabetes--2006. Diabetes Care. 2006;29(Suppl 1):S4–42. [PubMed] [Google Scholar]
- 26.Misra A, Chowbey P, Makkar BM, Vikram NK, Wasir JS, Chadha D, et al. Consensus statement for diagnosis of obesity, abdominal obesity and the metabolic syndrome for Asian Indians and recommendations for physical activity, medical and surgical management. J Assoc Physicians India. 2009;57:163–70. [PubMed] [Google Scholar]
- 27.Borrell LN, Papapanou PN. Analytical epidemiology of periodontitis. J Clin Periodontol. 2005;32(Suppl 6):132–58. doi: 10.1111/j.1600-051X.2005.00799.x. [DOI] [PubMed] [Google Scholar]
- 28.Bandyopadhyay D, Marlow NM, Fernandes JK, Leite RS. Periodontal disease progression and glycaemic control among Gullah African Americans with type-2 diabetes. J Clin Periodontol. 2010;37:501–9. doi: 10.1111/j.1600-051X.2010.01564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsai C, Hayes C, Taylor GW. Glycemic control of type 2 diabetes and severe periodontal disease in the US adult population. Community Dent Oral Epidemiol. 2002;30:182–92. doi: 10.1034/j.1600-0528.2002.300304.x. [DOI] [PubMed] [Google Scholar]
- 30.Taylor GW, Burt BA, Becker MP, Genco RJ, Shlossman M. Glycemic control and alveolar bone loss progression in type 2 diabetes. Ann Periodontol. 1998;3:30–9. doi: 10.1902/annals.1998.3.1.30. [DOI] [PubMed] [Google Scholar]
- 31.American Diabetes Association. Standards of medical care for patients with diabetes mellitus. Diabetes Care. 2003;26(Suppl 1):S33–50. doi: 10.2337/diacare.26.2007.s33. [DOI] [PubMed] [Google Scholar]
- 32.Morita I, Inagaki K, Nakamura F, Noguchi T, Matsubara T, Yoshii S, et al. Relationship between periodontal status and levels of glycated hemoglobin. J Dent Res. 2012;91:161–6. doi: 10.1177/0022034511431583. [DOI] [PubMed] [Google Scholar]
- 33.Nassar H, Kantarci A, van Dyke TE. Diabetic periodontitis: A model for activated innate immunity and impaired resolution of inflammation. Periodontol 2000. 2007;43:233–44. doi: 10.1111/j.1600-0757.2006.00168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mealey BL, Rose LF. Diabetes mellitus and inflammatory periodontal diseases. Curr Opin Endocrinol Diabetes Obes. 2008;15:135–41. doi: 10.1097/MED.0b013e3282f824b7. [DOI] [PubMed] [Google Scholar]
- 35.Lim LP, Tay FB, Sum CF, Thai AC. Relationship between markers of metabolic control and inflammation on severity of periodontal disease in patients with diabetes mellitus. J Clin Periodontol. 2007;34:118–23. doi: 10.1111/j.1600-051X.2006.01032.x. [DOI] [PubMed] [Google Scholar]
- 36.Shimazaki Y, Saito T, Yonemoto K, Kiyohara Y, Iida M, Yamashita Y, et al. Relationship of metabolic syndrome to periodontal disease in Japanese women: The hisayama study. J Dent Res. 2007;86:271–5. doi: 10.1177/154405910708600314. [DOI] [PubMed] [Google Scholar]
- 37.Nepomuceno R, Pigossi SC, Finoti LS, Orrico SRP, Cirelli JA, Barros SP, et al. Serum lipid levels in patients with periodontal disease: A meta-analysis and meta-regression. J Clin Periodontol. 2017;44:1192–207. doi: 10.1111/jcpe.12792. [DOI] [PubMed] [Google Scholar]
- 38.D’Aiuto F, Sabbah W, Netuveli G, Donos N, Hingorani AD, Deanfield J, et al. Association of the metabolic syndrome with severe periodontitis in a large U.S. Population-based survey. J Clin Endocrinol Metab. 2008;93:3989–94. doi: 10.1210/jc.2007-2522. [DOI] [PubMed] [Google Scholar]
- 39.Saxlin T, Suominen-Taipale L, Kattainen A, Marniemi J, Knuuttila M, Ylöstalo P, et al. Association between serum lipid levels and periodontal infection. J Clin Periodontol. 2008;35:1040–7. doi: 10.1111/j.1600-051X.2008.01331.x. [DOI] [PubMed] [Google Scholar]
- 40.Nesbitt MJ, Reynolds MA, Shiau H, Choe K, Simonsick EM, Ferrucci L, et al. Association of periodontitis and metabolic syndrome in the baltimore longitudinal study of aging. Aging Clin Exp Res. 2010;22:238–42. doi: 10.1007/bf03324802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gomes-Filho IS, das Mercês MC, de Santana Passos-Soares J, Seixas da Cruz S, Teixeira Ladeia AM, Trindade SC, et al. Severity of periodontitis and metabolic syndrome: Is there an association? J Periodontol. 2016;87:357–66. doi: 10.1902/jop.2015.150367. [DOI] [PubMed] [Google Scholar]
- 42.Albandar JM. Periodontal diseases in North America. Periodontol 2000. 2002;29:31–69. doi: 10.1034/j.1600-0757.2002.290103.x. [DOI] [PubMed] [Google Scholar]
- 43.Bouchard P, Boutouyrie P, Mattout C, Bourgeois D. Risk assessment for severe clinical attachment loss in an adult population. J Periodontol. 2006;77:479–89. doi: 10.1902/jop.2006.050128. [DOI] [PubMed] [Google Scholar]
- 44.Poitou C, Coussieu C, Rouault C, Coupaye M, Cancello R, Bedel JF, et al. Serum amyloid A: A marker of adiposity-induced low-grade inflammation but not of metabolic status. Obesity (Silver Spring) 2006;14:309–18. doi: 10.1038/oby.2006.40. [DOI] [PubMed] [Google Scholar]
- 45.Rangé H, Poitou C, Boillot A, Ciangura C, Katsahian S, Lacorte JM, et al. Orosomucoid, a new biomarker in the association between obesity and periodontitis. PLoS One. 2013;8:e57645. doi: 10.1371/journal.pone.0057645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khader YS, Bawadi HA, Haroun TF, Alomari M, Tayyem RF. The association between periodontal disease and obesity among adults in Jordan. J Clin Periodontol. 2009;36:18–24. doi: 10.1111/j.1600-051X.2008.01345.x. [DOI] [PubMed] [Google Scholar]


