Abstract
Aims:
The present study evaluates association between physical activity and Gestational Diabetes Mellitus (GDM), for it can be an effective intervention for its management. Though physical activity helps maintain glucose homeostasis, evidences of GDM risk are less extensive. Therefore, this study also identifies its correlation with maternal blood glucose levels.
Materials and Methods:
A prospective case-control study was carried out among pregnant women attending regular antenatal clinic at two private hospitals. The study comprised of 100 cases and 273 matched controls. Data was collected by personal interviews using a standard questionnaire. Physical activity was assessed using long form of International Physical Activity Questionnaire (IPAQ) reported as Metabolic Equivalent-Minutes per week (MET-Minutes/Week). Statistical Package for Social Sciences (SPSS) was used for analysis.
Results:
Results shows high exposure rates for low-to-moderate physical activity among cases, across all domains and sub-activities. The odds of GDM engaged in domestic and gardening activities for <2999 MET-minutes per week are 10 times higher than involved for ≥3000 MET-minutes per week (P < 0.001). The study also shows poor or no correlation between physical activity during pregnancy and maternal blood glucose levels.
Conclusion:
Despite existence of poor or no relationship with maternal blood glucose levels, prolonged sedentary behavior and decreased physical activities, especially domestic, are potential risk factors for GDM, a major finding of the study.
Keywords: Exercise in pregnancy, gestational diabetes mellitus, International Physical Activity Questionnaire, maternal blood glucose level, oral glucose tolerance test, physical activity
INTRODUCTION
One of the most popular remedies to gestational diabetes mellitus (GDM) widely suggested is enhanced physical activity during pregnancy. It is of common experience that GDM is usually managed through glycemic control.[1,2] To provide adequate protection against adverse perinatal outcomes, it is prudent to achieve euglycemia in women with GDM until there is absolute evidence of normal fetal growth in ultrasonography.[3] Medical nutrition therapy propped up with physical activity, insulin therapy, self-care, and intensive blood glucose monitoring is the cornerstone of GDM management, which ultimately aims to attain and maintain euglycemia.[3]
So far, there has no guideline for GDM-specific exercise prescription until recently Padayachee and Coombes drafted the first guideline on exercise for GDM management, which states that a GDM-affected woman should perform exercise, both aerobic and resistance, at moderate intensity for a minimum period of 30–60 min at a frequency of three times/week.[2] Moderate activities are referred to those requiring normal physical effort that make pregnant women breathe slightly harder and their heart beat a little faster than normal.[4] Given the lack of large cohort studies implementing exercise as the management of GDM, these recommendations are drawn from “exercise in pregnancy” and “exercise in Type II diabetes mellitus” guidelines.[2] Meta-analysis has shown aerobic/resistance exercise to be an adjunct to standard care, significantly improved postprandial glycemic control (mean difference [MD]: −0.33 mmol/L, 95% confidence interval [CI]: −0.49 to −0.17) and lowered fasting blood glucose (MD: −0.31 mmol/L, 95% CI: −0.56 to − 0.05) compared to standard care alone.[5]
It is well known that hyperglycemia in GDM mainly occurs due to maternal insulin resistance. Physical activity, on the other hand, helps in improving insulin sensitivity and secretion ensuring glucose homeostasis.[6,7,8] Despite this fact, evidences regarding benefits of physical activity on GDM prevention are less extensive and less convincing,[9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35] largely due to non-uniformity in frequency, intensity, time/duration or intensity of physical activity.[36] Several studies,[14,17,19,21,22,23,24,25,27,32,33,34,35,37,38,39] but not all,[9,10,11,12,13,15,16,18,20,26,28] reported the existence of inverse association between higher physical activity and subsequent GDM risk with varying strength of association. There exists a wide disparity in the reduction of relative GDM risk due to physical activity ranging from 10–30%[22,23,24,35] to 50%–90%.[14,17,19,21,25,32,33,34,37] A meta-analysis of cohort and case–control study revealed an inverse relationship between physical activity and GDM.[40] This study however did not study the effect of dose on GDM. Other meta-analyses[41,42] of randomized trials showed two different kinds of results – one showing an inverse[41] and the other no association.[42] None of the published literature, however, reported the impact of the amount of physical activity on GDM.[36] Only few studies reported dose-dependent inverse relationship between physical activity and GDM risk.[21,22,29,33,35] However, studies found benefits of physical activity level from low to moderate.[23,25,32] A thorough investigation of the dose-dependent physical activity–GDM (linear or nonlinear) relationship is necessary to identify the threshold physical activity level for adequate recommendations on GDM risk reduction.[36] It will help health planners conduct large-scale randomized trials to prevent GDM in the future.[36]
Although health-care providers advise pregnant women to maintain or increase physical activity during pregnancy,[43] it hardly convinces them against their traditional belief/perception that pregnancy is a state that requires extra care/rest and recuperation.[44] Consequently, exercise during pregnancy remains at very low level of acceptability throughout the world.[45] One such study evidenced a nonlinear association between physical activity in early pregnancy and GDM with pnonlinearity = 0.008 and no further decline in risk with physical activity >8 h/week.[36]
It is thus seen that the literature available in this area of research is too limited. Therefore, the present prospective case–control study was designed to identify the association between physical activity during pregnancy and GDM risk. In addition, the study also explores the existence of a correlation between physical activity during pregnancy and maternal blood glucose levels following oral glucose tolerance test (OGTT), a by-product of the study.
MATERIALS AND METHODS
Study design and participants
This study was carried out at two private hospitals of Udupi district situated along the coastline in the southern part of Karnataka (India). It covers a population of 1.18 million spread over a geographical area of 3575 km2. As per the report of District Level Household and Facility Survey-4 (2012–2013), 53.4% of the antenatal women residing in district availed complete antenatal care.[46] The district has been identified with better maternal and child health indicators than the national average. Most deliveries (98.8%) are institutional in the district, out of which 68.1% were in private health-care institutions.[46]
The study population included all those pregnant women who were coming to secondary care hospitals for routine antenatal checkup. Potential cases included incident cases of GDM reporting to antenatal outpatient department. All those pregnant women, who were beyond 20 weeks of gestation, were universally screened for GDM by subjecting them to 1-h 50 g glucose challenge test (GCT). Their GCT reports reviewed after a week time. All those women whose 1-h 50 g GCT exceeded the cutoff of 140 mg/dL were called for diagnostic 3-h 100 g OGTT the next day. A pregnant woman who was newly diagnosed with GDM in her present pregnancy by 3-h 100 g OGTT following 20 weeks of gestation using Carpenter and Coustan criteria[47] at the study setting was enrolled as an incident case. The next pregnant woman, frequency-matched with period of gestation (POG) (±2 weeks), whose 1-h 50 g GCT value fell below 140 mg/dL was identified as non-GDM (or control). Women who were diagnosed with DM before their pregnancy were excluded from the study. Physical inactivity as a risk factor for GDM was considered for sample size estimation. Expecting 57.7% of the cases to be not physically active[48] and anticipating a difference of at least 20% between the cases and controls to be clinically significant for a power of 80%, at 5% level of significance, and 10% nonresponse rate, a minimum of 73 cases and 219 controls, frequency matched with POG, were required to be recruited with a case-to-control ratio of 1:3. Data were collected over a period of 24 months during 2014–2016.
GDM was diagnosed using Carpenter and Coustan criteria[47] at the onset of the study. Subsequently, Diabetes in Pregnancy Study Group India (DIPSI) criteria[49] was adopted as the new diagnostic criterion since November 2015. It was uniformly adopted by the treating obstetricians at both the study settings by consensus following a departmental review. The data collection was continued to interview the predetermined number of cases and controls as per the sample size calculation. However, to ascertain the similarity between risk factors among cases and controls, additional number of cases and POG frequency-matched controls at a case-to-control ratio of 1:2 was required to be recruited as per the new criterion. This additional number was decided based on the time available to the investigator for data collection as per the stipulated study period until the last recruitment of a study subject.
Accordingly, operational definition of both the cases and controls was changed according to the new DIPSI guidelines.[49] An antenatal woman who was newly diagnosed with GDM in her present pregnancy by 2-h 75 g single venous plasma glucose following 20 weeks of gestation exceeding the cutoff of 140 mg/dL (irrespective of the last meal timings) at a health-care setting was included as a new case. The next pregnant woman, frequency matched with POG (±2 weeks), whose 2-h 75 g single venous plasma glucose value was <140 mg/dL was included as a new control in 1:2 ratio.
Data collection methodology
Institutional ethical committee (IEC: 623/2014) approval was obtained before the beginning of the study. Subsequent modification due to the change in the diagnostic criterion and sample size was duly notified to the ethics committee. Subject information sheet was distributed to, and written informed consent obtained from, all participants before data collection.
At the study setting, newly diagnosed GDM cases visiting hospitals for routine antenatal care were identified from the outpatient records. On the same day, POG frequency-matched controls were also identified and included in the study. Fulfilling the inclusion criteria, cases and controls were interviewed using a pretested questionnaire that included details on sociodemographic variables. Socioeconomic status (SES) was assessed using modified Udai Pareek Scale.[50] Accordingly, a score of <40 was identified as belonging to low, 40–70 middle, and ≥70 high SES.[50] Stress was assessed using Cohen Perceived Stress Scale: score of <20 was graded as low stress whereas ≥20 was considered as high stress.[51,52]
As a part of anthropometric measurements, weight at first antenatal registration visit in the first trimester was considered as prepregnancy weight. Height was measured using a measuring tape or stadiometer (cm) to the nearest 1 cm. Women were required to stand upright barefoot with their back against the wall, heels together, and looking forward.[53] Prepregnancy body mass index (BMI) was calculated as the ratio of prepregnancy weight (kg) to the square of height (m).[53] A woman was considered to be overweight if BMI ≥25 kg/m2.
Assessment of physical activity
Physical activity was assessed through the administration of a standardized questionnaire – the long form of International Physical Activity Questionnaire (IPAQ).[54] The long version of IPAQ, assessed physical activity in the last 7 days of the interview, has been validated for estimating physical activity among pregnant women,[55] showing poor correlation between the questionnaire and an accelerometer, 0.03 for moderate physical activity, 0.15 for total physical activity.
The questionnaire was undertaken across a comprehensive set of four domains: work-related, transport-related, domestic and garden (yard)-related, and leisure time-related physical activities. Work-related activities included all kinds of paid and unpaid jobs that the woman did outside her house. Details pertaining to both long- and short-distance travel were included under transport-related activities, for example, travel from one place to other including places of work, stores, and movies. Domestic and garden (yard)-related activities included those carried out in and around home such as housework, gardening, yard work, general maintenance work, and caring for own family. Activities solely carried out for recreation, sport, exercise, or leisure were covered under leisure time-related physical activities.[54]
Under each domain, details pertaining to specific type of physical activities were interviewed, viz., walking, moderate-intensity, and vigorous-intensity physical activities. The last referred to hard physical effort requiring the woman breathe much harder than normal. On the other hand, moderate activities required moderate physical effort making woman breathe somewhat harder than normal.[54]
Domain-specific scores were assigned pertaining to each type of physical activity. The total score was taken as the summation of the duration (in minutes) and frequency (days) for all types of activities in all domains. Data so collected were reported as metabolic equivalent-minutes per week (MET-minutes/week), which can be computed by weighing each type of activity by its energy requirements defined in METs. These METs are multiples of the resting metabolic rates. MET-minute/week is then computed for each activity as follows:[54]
MET-minutes/week = MET level × minutes of activity/day × days/week
Total score was calculated for each domain, and then, the overall grand total was estimated. Domain-specific scores or activity-specific subscores may also be computed as summation of the scores for walking, moderate-intensity, and vigorous-intensity activities within the specific domain, whereas activity-specific scores are summation of the scores for the specific type of activity across domains:[54]
Total physical activity (MET-minutes/week) = Total MET-minutes/week (at work + for transport + in domestic chores + in leisure).
MET-minute scores are equivalent to kilocalories for a 60-kg person = MET-min × (weight in kilograms/60 kg).
MET-minutes/day can also be presented as more popularly used MET-minutes/week. As there exist no established thresholds for presenting MET-minutes, the IPAQ Research Committee proposed to report as median values and interquartile range. However, the overall grand total scores so computed can be categorized into three levels of physical activity: high: total physical activity ≥3000 MET-minutes/week; moderate: 600 < total physical activity <3000 MET-minutes/week; low: total physical activity <600 MET-minutes/week.[54]
The above criteria were also used to subcategorize domain- and activity-specific scores. Details pertaining to time spent in sedentary activity were considered as an additional indicator but not included in the summary score of physical activity. It gives an estimate of sitting on typical weekdays and weekend days excluding the time spent in sitting during travel covered under transport domain. For the assessment of time spent in sitting, “minutes” was used as an indicator instead of MET-minutes which refers to energy expenditure. Data on sitting were reported as categorical variable although there exist no well-accepted thresholds till date.[54]
Statistical analysis
The above data were analyzed using Statistical Package for the Social Sciences (SPSS) version 15 for windows (SPSS South Asia, Bangalore, Karnataka, India) in four steps: (1) Results were expressed as percentages and proportions for categorical variables. Cases and controls were compared for exposure using univariate logistic regression. Odds ratio (OR) with 95% confidence interval (CI) was reported to study the association between different variables; P < 0.05 was considered statistically significant. (2) Stratification technique was employed to control confounding to assess the true association between exposure (physical activity during pregnancy) and outcome of interest (GDM risk) within the homogenous strata of each potential confounding variable. Pooled summary OR estimate adjusted for probable confounder was derived using Mantel and Haenszel test statistic assuming uniformity in stratum-specific estimates over the range of confounding variables. (3) The strength of relationship between IPAQ scores as independent variable (in MET-minutes/week) and maternal venous plasma glucose levels as dependent variable (in mg/dL) was determined using coefficient of determination (R2). (4) The relationship was quantified and described using regression analysis.
RESULTS AND DISCUSSION
As above, a total of 100 GDM cases and 273 POG frequency-matched controls (1:2.7 ratio) were recruited, as shown in Figure 1. As seen, among the total, 52 cases and 156 controls were recruited based on Carpenter and Coustan criteria (1:3 ratio). After change in diagnostic criteria, remaining 21 cases and 63 controls were enrolled as per the DIPSI criteria to meet the sample size (1:3 ratio). An additional of 27 cases and 54 controls were recruited based on the time available to the investigator as per the stipulated study period. This enrollment was in accordance with new criteria (1:2 ratio), making a total of 48 cases and 117 controls (1:2.4 ratio). An interim analysis was carried out at the end of data collection to assess comparability of risk factors profile with respect to diagnostic criteria. The two criteria were found to be similar. Mean (±standard deviation [SD]) POG of diagnosis for cases and controls was 27 weeks 2 days (±5 weeks 2 days) and 26 weeks 2 days (±4 weeks 2 days), respectively.
Figure 1.
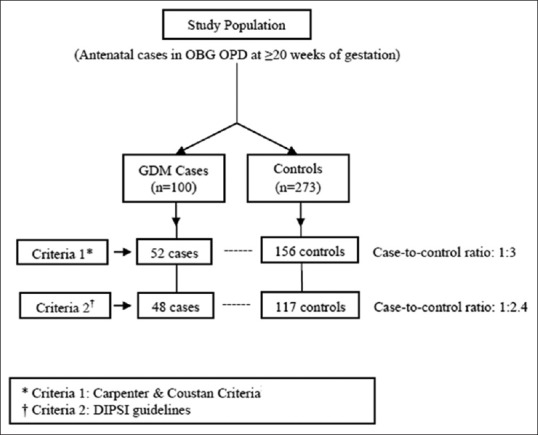
Recruitment of Study Subjects
Table 1 shows the baseline sociodemographic characteristics of the studied women. Visibly, the odds of GDM aged ≥30 years are 17 times higher than those aged ≤25 years (P < 0.001). All study participants were literate, except one who was an illiterate (P = 0.392). Over 90% of the women were homemakers (P = 0.05) whereas most belonged to middle- and high-socioeconomic class. Higher odds of GDM were identified among high-socioeconomic class (P = 0.021), those being overweight and obese (BMI ≥25 kg/m2), weighing ≥60 kg during their pre-pregnancy period (P = 0.001), and those with high stress were also higher (P < 0.001) than controls.
Table 1.
Demographic characteristics of cases (n=100) and controls (n=273)
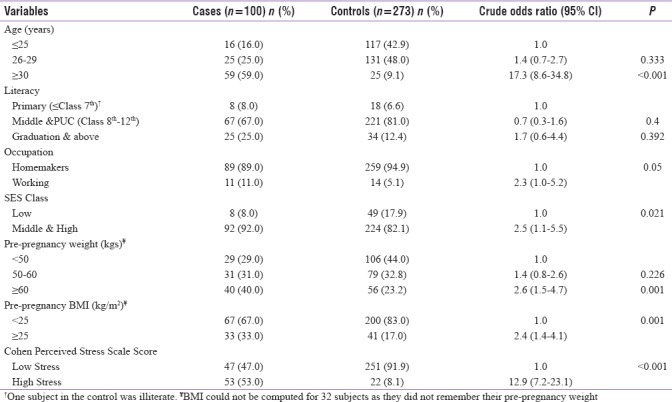
It can be further inferred from the results of Table 1 that GDM risk among ≥30 years of age was similar to that observed in prospective cohort studies carried out in Uttar Pradesh (India)[56] and Tianjin (China),[57] when employed the World Health Organization (WHO) criteria and International Association of the Diabetes and Pregnancy Study Group-WHO criteria, respectively. On the other hand, when used the DIPSI criteria, other prospective cohort studies carried out in Rohtak (Haryana),[58] Hyderabad (Andhra Pradesh),[59] and Jodhpur (Western Rajasthan)[60] in India revealed high GDM prevalence among those ≥25 years of age. Two hospital-based studies carried out in Wardha (Maharashtra)[61] and Rohtak (Haryana)[62] showed GDM prevalence significantly associated with higher education when identified using the WHO and American Diabetes Association (ADA) criteria, respectively. However, other community-based cross-sectional studies carried out in Punjab,[63] Gujarat,[64] and Kashmir[65] invariably showed positive association between GDM and illiteracy, irrespective of the criteria used. In another community-based study,[64] the authors found the prevalence of GDM positively associated with nonworking group when diagnosed using ADA criteria, though the association was statistically nonsignificant (OR = 1.14; 95% CI: 0.20–6.35; P = 0.28). However, in another retrospective matched case–control study carried out in Malaysia, the authors found a significant association between GDM and occupation (χ2 = 4.01; P = 0.045).[66]
Similar to above, high odds of GDM in high-socioeconomic class were consistent with the works of Kalyani et al.[61] and Rajput et al.[62] when scored using Kuppuswamy classification. These studies, however, diagnosed GDM using the WHO and ADA criteria, respectively. In contrast, Raja et al.[65] found high GDM prevalence in lower socioeconomic class following modified BG Prasad classification and DIPSI guidelines (P < 0.05).
A retrospective ADA-based study of Varghese et al.[67] revealed increased GDM prevalence in subjects weighing >60 kg consistent with the results of the present study. Another study carried out in Canada also revealed a positive association between National Diabetes Data Group-defined GDM and pregravid obesity (weight >91 kg).[68] Similarly, various prospective cohort Indian studies also found high GDM prevalence in subjects with prepregnancy BMI ≥25 kg/m2, irrespective of the criteria used,[56,60,69,70] whereas Kalyani et al.[61] and Nanda et al.[71] found high GDM prevalence among BMI ≥30 kg/m2 based on the WHO criteria. Increased stress from early to mid-pregnancy, as identified by Cohen Perceived Stress Scale-14, found positively associated with increased GDM risk.[72]
Physical activity during pregnancy and gestational diabetes mellitus risk
Table 2 shows that more than half of the cases (57.0%) were low-to-moderately active physically while 81.7% of the controls were highly active during pregnancy. High odds of GDM were among those involved in low-to-moderate physical activities (P < 0.001). These results are consistent with the findings of several other studies carried out elsewhere but with varying strengths of association,[14,17,19,21,22,23,24,25,27,32,33,34,35,37,38,39] except for a few[9,10,11,12,13,15,16,18,20,26,28] describing no association.
Table 2.
Association between physical activity during pregnancy and risk of gestational diabetes mellitus
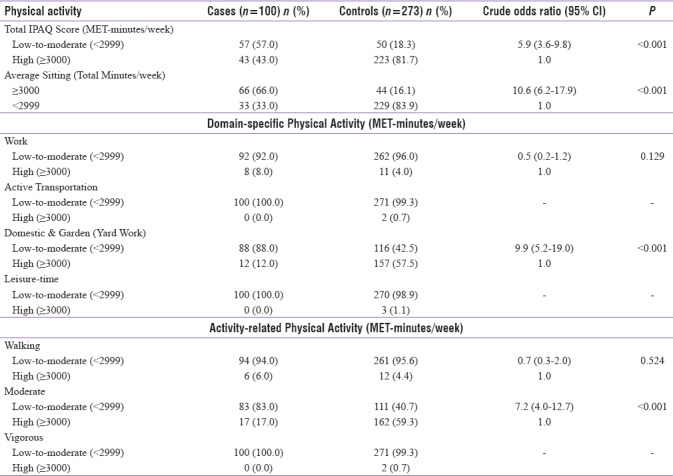
Similar to the above, it can be seen from Table 2 that the odds of GDM in those spending ≥3000 min/week of their time in sitting are 11 times higher than those spending <2900 min/week (P < 0.001), consistent with the works of Anjana et al.[73] and Oken et al.,[23] who found increased sedentary behavior during pregnancy associated with abnormal glucose tolerance.
Based on subgroup analysis, Table 2 exhibits high-exposure rates in cases exhibiting low-to-moderate physical activity across all domains and subactivities. For example, 88% of the reported cases were engaged in domestic and gardening activities for <2999 MET-minutes/week while 57.5% of the controls were involved for ≥3000 MET-minutes/week (OR = 9.9; 95% CI: 5.2–19.0; P < 0.001). Odds of GDM cases being less-moderately active (<2999 MET-minutes/week) during their pregnancy period are seven times higher than controls (P < 0.001), consistent with the study of Tobias et al.[40] dealing with physical activity associated with GDM. However, the case–control study of Nasiri-Amiri et al.[74] revealed significantly high risk but in transportation domain.
Stratified analysis
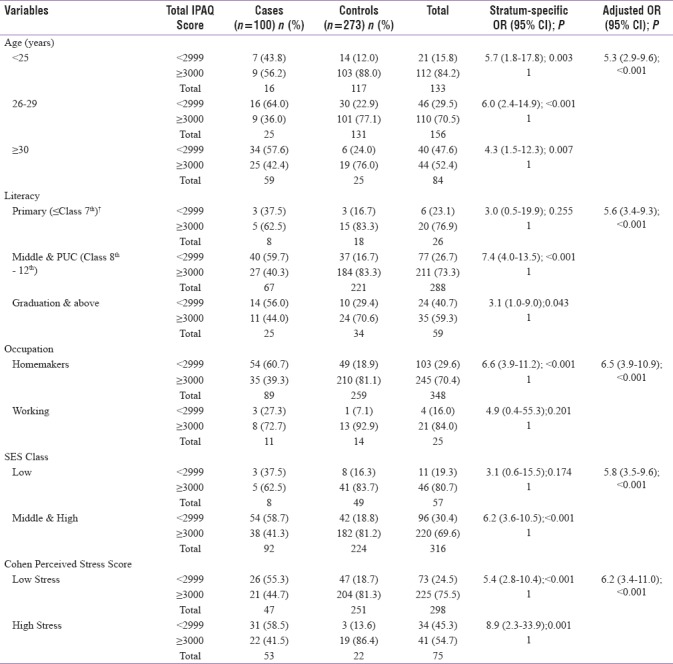
As seen from Table 3, the stratum-specific estimates adjusted for age and stress levels identified decreased physical activity levels as a risk factor for GDM uniformly across all defined age and stress subcategories. However, when controlled for maternal education, occupation, and socioeconomic class, these behaved as effect modifier. Notably, no confounding was indicated for a reason that overall crude and adjusted risk estimates were similar.
Table 3.
Stratified analysis of confounding variables between physical activity during pregnancy and risk of gestational diabetes mellitus
Physical activity versus maternal blood glucose levels
Figures 2-6 generally show poor or no relationship between physical activity during pregnancy and maternal blood glucose levels, when OGTT is followed. Figure 2 exhibits weak-positive association between IPAQ scores and fasting venous plasma glucose levels, with increasing physical activity during pregnancy. There exists a concurrent rise in fasting glucose levels (R2 = 0.036). Expressed mathematically,
Figure 2.
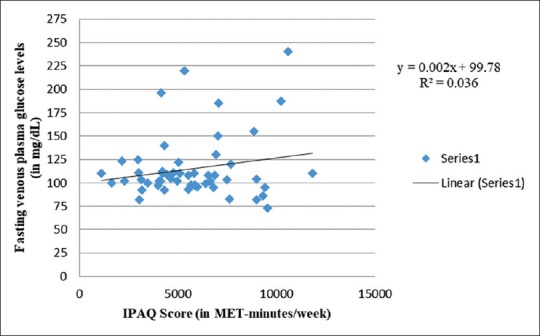
Correlation between fasting glucose levels and IPAQ score
Figure 6.
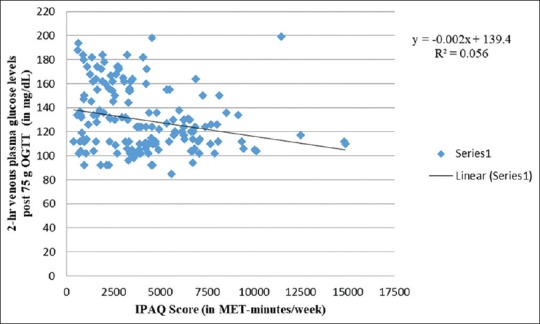
Correlation between 2-hr venous plasma glucose levels post 75g OGTT and IPAQ score
y = 0.002x + 99.78+ ε
where ε represents an error between observed and expected values of the dependent variable. Assuming this error to follow a normal distribution (mean = 3.96 and SD = 33.52 mg/dl), the dependent variable can be predicted with 95% confidence level as below:
y = 0.002x + 103.74 ± 33.52
The corresponding mathematical relations between IPAQ scores and maternal glucose levels at varying timings post-OGTT [Figures 2-6] can be described as shown in Table 4.
Table 4.
Regression analysis for maternal venous plasma glucose levels versus physical activity (IPAQ) scores

In contrast to the above [Figure 2], Anjana et al.[73] reported low levels of fasting blood glucose among physically active pregnant women. However, Idowu et al.[75] and Oostdam et al.[11] exhibited no correlation between physical activity levels and maternal fasting plasma glucose levels. The latter study findings might be due to low compliance as explained earlier.
Figure 3 shows a negative association between physical activity and 1-h venous plasma glucose levels after 100 g OGTT (R2 = 0.011), thus implying protective impact of physical activity on 1-h venous plasma glucose levels. This is consistent with the work of Anjana et al.[73] revealing lower 1-h postprandial glucose levels among physically active women. Nonetheless, similar to Figure 2, Figure 4 shows a comparable weak-positive trend for 2-h venous plasma glucose levels after 100 g OGTT (R2 = 0.078) with increasing physical activity levels. Therefore, similar to fasting venous plasma glucose levels, exposure to physical activity also increases 2-h venous plasma glucose levels after 100 g OGTT. In contrast, Anjana et al.[73] observed low 2-h postprandial glucose levels among physically active women. In an observational cohort study, Idowu et al.[75] documented no correlation between physical activity levels and 2-h plasma glucose levels during late pregnancy. On the other hand, 2-h postprandial glucose in late pregnancy was found associated and predictive of 2-h glucose in early pregnancy (Spearman's correlation coefficient [rho] ρ = 0.468; P ≤ 0.001).
Figure 3.
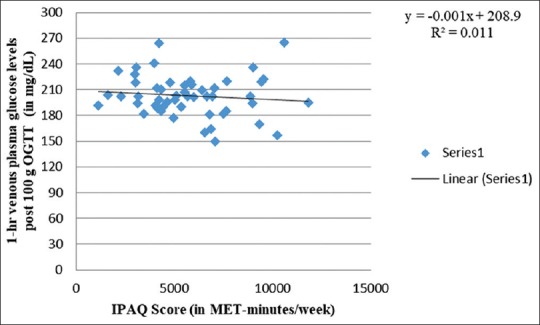
Correlation between 1-hr venous plasma glucose levels post 100g OGTT and IPAQ score
Figure 4.
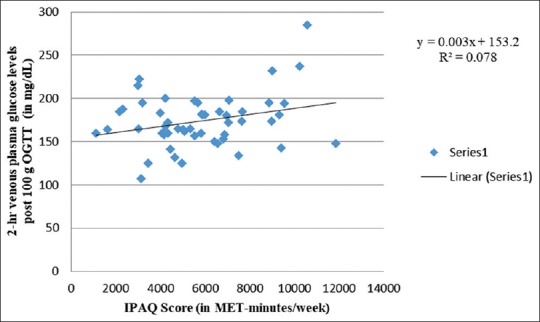
Correlation between 2-hr venous plasma glucose levels post 100g OGTT and IPAQ score
Similar to Figures 3, 5 and 6 also exhibit weak-negative trends of physical activity scores with 3-h and 2-h venous plasma glucose levels after 100 g and 75 g OGTT, respectively. Halse et al.[76] showed low mean daily postprandial glucose concentrations in bicycling intervention group compared to controls. They, however, found no difference in postprandial glucose among both the groups when assessed after 6 weeks of intervention. Similarly, Ong et al.[77] observed at 2-h postintervention OGGT and found blood glucose to remain elevated from baseline among controls compared to intervention group following 10-week supervised exercise program among 12 obese pregnant women. It may largely be attributed to small sample size. A total of seven trials that included five randomized trials, revealed that physical activity for a mean duration of six weeks during last trimester of pregnancy led to significant fall in glycemic parameters.[78]
Figure 5.
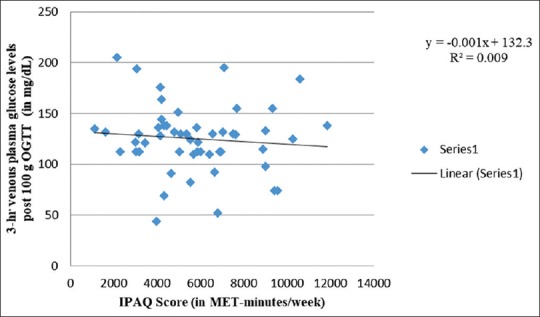
Correlation between 3-hr venous plasma glucose levels post 100g OGTT and IPAQ score
The prospective enrolment of GDM cases in 1:3 case-to-control ratio, in a hospital setting, is the main highlight of the study. Change of diagnostic criteria during the study, a possible limitation in any prospective design, indirectly helped compare the two criteria with respect to physical activity. Although the study was not powered enough to detect subgroup differences, overall power of the study might not be compromised as the primary objective was to ascertain association between physical activity and GDM. Identifying similarity between exposure variables among cases and controls was a by-product of the study. In addition, the sample size was calculated based on a risk factor of physical inactivity. Furthermore, nonuniformity in the diagnostic GDM criteria across centers and geographical areas makes comparisons difficult. Hospital-based data collection limits the generalization of the study findings, but community-based identification of GDM cases was a difficult task, due to varying time periods of diagnosis, multitude of tests, and varying modifications of diagnostic criteria in practice. Thus, this approach was most feasible. Moreover, in a region where institutional antenatal care is universal, the findings would reflect the true population scenario.
CONCLUSION
The present study showed higher exposure rates for low-to-moderate levels of physical activities among cases across all domains, especially among those involved in domestic and gardening activities during pregnancy. Risk of GDM was higher among those less moderately active in their pregnancy. Prolonged sitting was also a significant risk factor. There was no or poor relationship existing between physical activity and blood glucose levels. It is thus advisable that pregnant women should perform moderate-intensity domestic chores that can help reduce GDM risk.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.American Diabetes Association. (12) Management of diabetes in pregnancy. Diabetes Care. 2015;38(Suppl 1):S77–9. doi: 10.2337/dc15-S015. [DOI] [PubMed] [Google Scholar]
- 2.Padayachee C, Coombes JS. Exercise guidelines for gestational diabetes mellitus. World J Diabetes. 2015;6:1033–44. doi: 10.4239/wjd.v6.i8.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morampudi S, Balasubramanian G, Gowda A, Zomorodi B, Patil AS. The challenges and recommendations for gestational diabetes mellitus care in India: A Review. Front Endocrinol (Lausanne) 2017;8:56. doi: 10.3389/fendo.2017.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang C, Guelfi KJ, Yang HX. Exercise and its role in gestational diabetes mellitus. Chronic Dis Transl Med. 2016;2:208–14. doi: 10.1016/j.cdtm.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harrison AL, Shields N, Taylor NF, Frawley HC. Exercise improves glycaemic control in women diagnosed with gestational diabetes mellitus: A systematic review. J Physiother. 2016;62:188–96. doi: 10.1016/j.jphys.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Sato Y, Iguchi A, Sakamoto N. Biochemical determination of training effects using insulin clamp technique. Horm Metab Res. 1984;16:483–6. doi: 10.1055/s-2007-1014825. [DOI] [PubMed] [Google Scholar]
- 7.Regensteiner JG, Mayer EJ, Shetterly SM, Eckel RH, Haskell WL, Marshall JA, et al. Relationship between habitual physical activity and insulin levels among nondiabetic men and women. San Luis Valley diabetes study. Diabetes Care. 1991;14:1066–74. doi: 10.2337/diacare.14.11.1066. [DOI] [PubMed] [Google Scholar]
- 8.Helmrich SP, Ragland DR, Leung RW, Paffenbarger RS., Jr Physical activity and reduced occurrence of non-insulin-dependent diabetes mellitus. N Engl J Med. 1991;325:147–52. doi: 10.1056/NEJM199107183250302. [DOI] [PubMed] [Google Scholar]
- 9.Callaway LK, Colditz PB, Byrne NM, Lingwood BE, Rowlands IJ, Foxcroft K, et al. Prevention of gestational diabetes: Feasibility issues for an exercise intervention in obese pregnant women. Diabetes Care. 2010;33:1457–9. doi: 10.2337/dc09-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Price BB, Amini SB, Kappeler K. Exercise in pregnancy: Effect on fitness and obstetric outcomes – A randomized trial. Med Sci Sports Exerc. 2012;44:2263–9. doi: 10.1249/MSS.0b013e318267ad67. [DOI] [PubMed] [Google Scholar]
- 11.Oostdam N, van Poppel MN, Wouters MG, Eekhoff EM, Bekedam DJ, Kuchenbecker WK, et al. No effect of the FitFor2 exercise programme on blood glucose, insulin sensitivity, and birthweight in pregnant women who were overweight and at risk for gestational diabetes: Results of a randomised controlled trial. BJOG. 2012;119:1098–107. doi: 10.1111/j.1471-0528.2012.03366.x. [DOI] [PubMed] [Google Scholar]
- 12.Stafne SN, Salvesen KÅ, Romundstad PR, Eggebø TM, Carlsen SM, Mørkved S, et al. Regular exercise during pregnancy to prevent gestational diabetes: A randomized controlled trial. Obstet Gynecol. 2012;119:29–36. doi: 10.1097/AOG.0b013e3182393f86. [DOI] [PubMed] [Google Scholar]
- 13.Barakat R, Cordero Y, Coteron J, Luaces M, Montejo R. Exercise during pregnancy improves maternal glucose screen at 24-28 weeks: A randomised controlled trial. Br J Sports Med. 2012;46:656–61. doi: 10.1136/bjsports-2011-090009. [DOI] [PubMed] [Google Scholar]
- 14.Tomić V, Sporiš G, Tomić J, Milanović Z, Zigmundovac-Klaić D, Pantelić S, et al. The effect of maternal exercise during pregnancy on abnormal fetal growth. Croat Med J. 2013;54:362–8. doi: 10.3325/cmj.2013.54.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barakat R, Pelaez M, Lopez C, Lucia A, Ruiz JR. Exercise during pregnancy and gestational diabetes-related adverse effects: A randomised controlled trial. Br J Sports Med. 2013;47:630–6. doi: 10.1136/bjsports-2012-091788. [DOI] [PubMed] [Google Scholar]
- 16.Barakat R, Perales M, Bacchi M, Coteron J, Refoyo I. A program of exercise throughout pregnancy? Is it safe to mother and newborn. Am J Health Promot. 2014;29:2–8. doi: 10.4278/ajhp.130131-QUAN-56. [DOI] [PubMed] [Google Scholar]
- 17.Renault KM, Nørgaard K, Nilas L, Carlsen EM, Cortes D, Pryds O, et al. The treatment of obese pregnant women (TOP) study: A randomized controlled trial of the effect of physical activity intervention assessed by pedometer with or without dietary intervention in obese pregnant women. Am J Obstet Gynecol. 2014;210:134.e1–9. doi: 10.1016/j.ajog.2013.09.029. [DOI] [PubMed] [Google Scholar]
- 18.Ko CW, Napolitano PG, Lee SP, Schulte SD, Ciol MA, Beresford SA, et al. Physical activity, maternal metabolic measures, and the incidence of gallbladder sludge or stones during pregnancy: A randomized trial. Am J Perinatol. 2014;31:39–48. doi: 10.1055/s-0033-1334455. [DOI] [PubMed] [Google Scholar]
- 19.Cordero Y, Mottola MF, Vargas J, Blanco M, Barakat R. Exercise is associated with a reduction in gestational diabetes mellitus. Med Sci Sports Exerc. 2015;47:1328–33. doi: 10.1249/MSS.0000000000000547. [DOI] [PubMed] [Google Scholar]
- 20.Nobles C, Marcus BH, Stanek EJ, 3rd, Braun B, Whitcomb BW, Solomon CG, et al. Effect of an exercise intervention on gestational diabetes mellitus: A randomized controlled trial. Obstet Gynecol. 2015;125:1195–204. doi: 10.1097/AOG.0000000000000738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dempsey JC, Sorensen TK, Williams MA, Lee IM, Miller RS, Dashow EE, et al. Prospective study of gestational diabetes mellitus risk in relation to maternal recreational physical activity before and during pregnancy. Am J Epidemiol. 2004;159:663–70. doi: 10.1093/aje/kwh091. [DOI] [PubMed] [Google Scholar]
- 22.Zhang C, Solomon CG, Manson JE, Hu FB. A prospective study of pregravid physical activity and sedentary behaviors in relation to the risk for gestational diabetes mellitus. Arch Intern Med. 2006;166:543–8. doi: 10.1001/archinte.166.5.543. [DOI] [PubMed] [Google Scholar]
- 23.Oken E, Ning Y, Rifas-Shiman SL, Radesky JS, Rich-Edwards JW, Gillman MW, et al. Associations of physical activity and inactivity before and during pregnancy with glucose tolerance. Obstet Gynecol. 2006;108:1200–7. doi: 10.1097/01.AOG.0000241088.60745.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iqbal R, Rafique G, Badruddin S, Qureshi R, Cue R, Gray-Donald K, et al. Increased body fat percentage and physical inactivity are independent predictors of gestational diabetes mellitus in South Asian women. Eur J Clin Nutr. 2007;61:736–42. doi: 10.1038/sj.ejcn.1602574. [DOI] [PubMed] [Google Scholar]
- 25.Chasan-Taber L, Schmidt MD, Pekow P, Sternfeld B, Manson JE, Solomon CG, et al. Physical activity and gestational diabetes mellitus among Hispanic women. J Womens Health (Larchmt) 2008;17:999–1008. doi: 10.1089/jwh.2007.0560. [DOI] [PubMed] [Google Scholar]
- 26.van der Ploeg HP, van Poppel MN, Chey T, Bauman AE, Brown WJ. The role of pre-pregnancy physical activity and sedentary behaviour in the development of gestational diabetes mellitus. J Sci Med Sport. 2011;14:149–52. doi: 10.1016/j.jsams.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 27.Ramos-Leví AM, Pérez-Ferre N, Fernández MD, Del Valle L, Bordiu E, Bedia AR, et al. Risk factors for gestational diabetes mellitus in a large population of women living in Spain: Implications for preventative strategies. Int J Endocrinol 2012. 2012:312529. doi: 10.1155/2012/312529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mørkrid K, Jenum AK, Berntsen S, Sletner L, Richardsen KR, Vangen S, et al. Objectively recorded physical activity and the association with gestational diabetes. Scand J Med Sci Sports. 2014;24:e389–97. doi: 10.1111/sms.12183. [DOI] [PubMed] [Google Scholar]
- 29.Zhang C, Tobias DK, Chavarro JE, Bao W, Wang D, Ley SH, et al. Adherence to healthy lifestyle and risk of gestational diabetes mellitus: Prospective cohort study. BMJ. 2014;349:g5450. doi: 10.1136/bmj.g5450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Currie LM, Woolcott CG, Fell DB, Armson BA, Dodds L. The association between physical activity and maternal and neonatal outcomes: A prospective cohort. Matern Child Health J. 2014;18:1823–30. doi: 10.1007/s10995-013-1426-3. [DOI] [PubMed] [Google Scholar]
- 31.Chasan-Taber L, Silveira M, Lynch KE, Pekow P, Braun B, Manson JE, et al. Physical activity before and during pregnancy and risk of abnormal glucose tolerance among Hispanic women. Diabetes Metab. 2014;40:67–75. doi: 10.1016/j.diabet.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dempsey JC, Butler CL, Sorensen TK, Lee IM, Thompson ML, Miller RS, et al. A case-control study of maternal recreational physical activity and risk of gestational diabetes mellitus. Diabetes Res Clin Pract. 2004;66:203–15. doi: 10.1016/j.diabres.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 33.Liu J, Laditka JN, Mayer-Davis EJ, Pate RR. Does physical activity during pregnancy reduce the risk of gestational diabetes among previously inactive women? Birth. 2008;35:188–95. doi: 10.1111/j.1523-536X.2008.00239.x. [DOI] [PubMed] [Google Scholar]
- 34.Harizopoulou VC, Kritikos A, Papanikolaou Z, Saranti E, Vavilis D, Klonos E, et al. Maternal physical activity before and during early pregnancy as a risk factor for gestational diabetes mellitus. Acta Diabetol. 2010;47(Suppl 1):83–9. doi: 10.1007/s00592-009-0136-1. [DOI] [PubMed] [Google Scholar]
- 35.Redden SL, LaMonte MJ, Freudenheim JL, Rudra CB. The association between gestational diabetes mellitus and recreational physical activity. Matern Child Health J. 2011;15:514–9. doi: 10.1007/s10995-010-0586-7. [DOI] [PubMed] [Google Scholar]
- 36.Aune D, Sen A, Henriksen T, Saugstad OD, Tonstad S. Physical activity and the risk of gestational diabetes mellitus: A systematic review and dose-response meta-analysis of epidemiological studies. Eur J Epidemiol. 2016;31:967–97. doi: 10.1007/s10654-016-0176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rudra CB, Williams MA, Lee IM, Miller RS, Sorensen TK. Perceived exertion in physical activity and risk of gestational diabetes mellitus. Epidemiology. 2006;17:31–7. doi: 10.1097/01.ede.0000184474.33629.cd. [DOI] [PubMed] [Google Scholar]
- 38.Baptiste-Roberts K, Ghosh P, Nicholson WK. Pregravid physical activity, dietary intake, and glucose intolerance during pregnancy. J Womens Health (Larchmt) 2011;20:1847–51. doi: 10.1089/jwh.2010.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Campbell SK, Lynch J, Esterman A, McDermott R. Pre-pregnancy predictors of diabetes in pregnancy among aboriginal and torres strait islander women in North Queensland, Australia. Matern Child Health J. 2012;16:1284–92. doi: 10.1007/s10995-011-0889-3. [DOI] [PubMed] [Google Scholar]
- 40.Tobias DK, Zhang C, van Dam RM, Bowers K, Hu FB. Physical activity before and during pregnancy and risk of gestational diabetes mellitus: A meta-analysis. Diabetes Care. 2011;34:223–9. doi: 10.2337/dc10-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Russo LM, Nobles C, Ertel KA, Chasan-Taber L, Whitcomb BW. Physical activity interventions in pregnancy and risk of gestational diabetes mellitus: A systematic review and meta-analysis. Obstet Gynecol. 2015;125:576–82. doi: 10.1097/AOG.0000000000000691. [DOI] [PubMed] [Google Scholar]
- 42.Yin YN, Li XL, Tao TJ, Luo BR, Liao SJ. Physical activity during pregnancy and the risk of gestational diabetes mellitus: A systematic review and meta-analysis of randomised controlled trials. Br J Sports Med. 2014;48:290–5. doi: 10.1136/bjsports-2013-092596. [DOI] [PubMed] [Google Scholar]
- 43.Gaston A, Cramp A. Exercise during pregnancy: A review of patterns and determinants. J Sci Med Sport. 2011;14:299–305. doi: 10.1016/j.jsams.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 44.Lee DT, Ngai IS, Ng MM, Lok IH, Yip AS, Chung TK, et al. Antenatal taboos among Chinese women in Hong Kong. Midwifery. 2009;25:104–13. doi: 10.1016/j.midw.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 45.Nascimento SL, Surita FG, Cecatti JG. Physical exercise during pregnancy: A systematic review. Curr Opin Obstet Gynecol. 2012;24:387–94. doi: 10.1097/GCO.0b013e328359f131. [DOI] [PubMed] [Google Scholar]
- 46.Mumbai: International Institute for Population Sciences, Ministry of Health & Family Welfare; 2012. [Last accessed on 2016 Aug 15]. Government of India. District Level Household and Facility Survey – 4. District Fact Sheet: Udupi (2012-13) Available from URL: https://nrhm-mis.nic.in/DLHS4/State%20and%20District%20Factsheets/Karnataka/District%20Factsheets/Udupi.pdf . [Google Scholar]
- 47.Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144:768–73. doi: 10.1016/0002-9378(82)90349-0. [DOI] [PubMed] [Google Scholar]
- 48.Jali MV, Desai BR, Gowda S, Kambar S, Jali SM. A hospital based study of prevalence of gestational diabetes mellitus in an urban population of India. Eur Rev Med Pharmacol Sci. 2011;15:1306–10. [PubMed] [Google Scholar]
- 49.Seshiah V, Das AK, Balaji V, Joshi SR, Parikh MN, Gupta S, et al. Gestational diabetes mellitus – Guidelines. J Assoc Physicians India. 2006;54:622–8. [PubMed] [Google Scholar]
- 50.Pareek U, Trivedi G. Reliability and validity of socio-economic scales. Indian J Appl Psychol. 1964;1:34–40. [Google Scholar]
- 51.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–96. [PubMed] [Google Scholar]
- 52.Scoring for Cohen Perceived Stress Scale. [Last accessed on 2016 Sep 10]. Available from: http://www.podcast.uctv.tv/webdocuments/COHEN-PERCEIVED-STRESS-Scale.pdf .
- 53.World Health Organization. Waist Circumference and Waist-Hip Ratio. Report of a WHO Expert Consultation. Geneva, 8-11 December 2008. Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- 54.International Physical Activity Questionnaire. Guidelines for Data Processing and Analysis of the International Physical Activity Questionnaire (IPAQ) – Short and Long Forms; November. 2005. [Last accessed on 2016 Sep 10]. Available from: https://www.sites.google.com/site/theipaq/scoring.protocol .
- 55.Harrison CL, Thompson RG, Teede HJ, Lombard CB. Measuring physical activity during pregnancy. Int J Behav Nutr Phys Act. 2011;8:19. doi: 10.1186/1479-5868-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sharma A, Gupta M, Agarwal A. Comparison of diagnostic accuracy of two one step procedures for screening of gestational diabetes mellitus. Int J Reprod Contracept Obstet Gynecol. 2015;4:81–5. [Google Scholar]
- 57.Leng J, Shao P, Zhang C, Tian H, Zhang F, Zhang S, et al. Prevalence of gestational diabetes mellitus and its risk factors in Chinese pregnant women: A prospective population-based study in Tianjin, China. PLoS One. 2015;10:e0121029. doi: 10.1371/journal.pone.0121029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dahiya K, Sahu J, Dahiya A. Maternal and fetal outcome in gestational diabetes mellitus – A study at tertiary health centre in Northern India. Open Access Libr J. 2014;1:1–5. [Google Scholar]
- 59.Singh A, Uma B. Incidence of gestational diabetes mellitus and its outcomes in a rural population. J Evol Med Dent Sci. 2013;2:1982–6. [Google Scholar]
- 60.Kalra P, Kachhwaha CP, Singh HV. Prevalence of gestational diabetes mellitus and its outcome in Western Rajasthan. Indian J Endocrinol Metab. 2013;17:677–80. doi: 10.4103/2230-8210.113760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kalyani KR, Jajoo S, Hariharan C, Samal S. Prevalence of gestational diabetes mellitus, its associated risk factors and pregnancy outcomes at a rural setup in central India. Int J Reprod Contracept Obstet Gynecol. 2014;3:219–24. [Google Scholar]
- 62.Rajput R, Yadav Y, Nanda S, Rajput M. Prevalence of gestational diabetes mellitus & associated risk factors at a tertiary care hospital in Haryana. Indian J Med Res. 2013;137:728–33. [PMC free article] [PubMed] [Google Scholar]
- 63.Arora GP, Thaman RG, Prasad RB, Almgren P, Brøns C, Groop LC, et al. Prevalence and risk factors of gestational diabetes in Punjab, North India: Results from a population screening program. Eur J Endocrinol. 2015;173:257–67. doi: 10.1530/EJE-14-0428. [DOI] [PubMed] [Google Scholar]
- 64.Dave VR, Rana BM, Sonaliya KN, Chandwani SJ, Sharma SV, Khatri SO, et al. Screening of gestational diabetes and hypertension among antenatal women in rural West India. Central Asian J Global Health. 2014. [Last accessed on 2016 Aug 15]. p. 3. ISSN 2166-7403. doi:https://doi.org/10.5195/cajgh.2014.140. Available from: https://cajgh.pitt.edu/ojs/index.php/cajgh/article/view/140/196 . [DOI] [PMC free article] [PubMed]
- 65.Raja MW, Baba TA, Hanga AJ, Bilquees S, Rasheed S, Haq IU, et al. A study to estimate the prevalence of gestational diabetes mellitus in an urban block of Kashmir Valley (North India) Int J Med Sci Public Health. 2014;3:191–5. [Google Scholar]
- 66.Areefa SM, Nik MZ, Yousef IA, Soon LK. Prevalence and associated demographic characteristics of gestational diabetes mellitus in Gaza. Health Environ J. 2014;5:10–25. [Google Scholar]
- 67.Varghese R, Thomas B, Hail MA, Rauf A, Sadi MA, Sualiti AA, et al. The prevalence, risk factors, maternal and fetal outcomes in gestational diabetes mellitus. Int J Drug Dev Res. 2012;4:356–68. [Google Scholar]
- 68.Xiong X, Saunders LD, Wang FL, Demianczuk NN. Gestational diabetes mellitus: Prevalence, risk factors, maternal and infant outcomes. Int J Gynaecol Obstet. 2001;75:221–8. doi: 10.1016/s0020-7292(01)00496-9. [DOI] [PubMed] [Google Scholar]
- 69.Seshiah V, Balaji V, Balaji MS, Paneerselvam A, Arthi T, Thamizharasi M, et al. Prevalence of gestational diabetes mellitus in South India (Tamil Nadu) – A community based study. J Assoc Physicians India. 2008;56:329–33. [PubMed] [Google Scholar]
- 70.Zargar AH, Sheikh MI, Bashir MI, Masoodi SR, Laway BA, Wani AI, et al. Prevalence of gestational diabetes mellitus in Kashmiri women from the Indian subcontinent. Diabetes Res Clin Pract. 2004;66:139–45. doi: 10.1016/j.diabres.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 71.Nanda SS, Dash K, Dash S, Misra S, Das S. Screening of gestational diabetes mellitus with 75 gms OGTT and its effects on feto-maternal outcome. Sch J Appl Med Sci. 2014;2:340–4. [Google Scholar]
- 72.Silveira ML, Whitcomb BW, Pekow P, Braun B, Markenson G, Dole N, et al. Perceived psychosocial stress and glucose intolerance among pregnant Hispanic women. Diabetes Metab. 2014;40:466–75. doi: 10.1016/j.diabet.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Anjana RM, Sudha V, Lakshmipriya N, Anitha C, Unnikrishnan R, Bhavadharini B, et al. Physical activity patterns and gestational diabetes outcomes – The wings project. Diabetes Res Clin Pract. 2016;116:253–62. doi: 10.1016/j.diabres.2016.04.041. [DOI] [PubMed] [Google Scholar]
- 74.Nasiri-Amiri F, Bakhtiari A, Faramarzi M, Adib Rad H, Pasha H. The association between physical activity during pregnancy and gestational diabetes mellitus: A case-control study. Int J Endocrinol Metab. 2016;14:e37123. doi: 10.5812/ijem.37123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Idowu AT. Physical Activity and Glucose Tolerance in Overweight and Obese Pregnant Women (Master's Thesis). Norway, Trondheim. 2015. [Last accessed on 2017 Sep 15]. Available from: https://www.brage.bibsys.no/xmlui/bitstream/handle/11250/296890/Aminat%20Titilola%20Idowu_Master%27s%20%20Thesis%20May%202015_New.pdf?sequence=1 .
- 76.Halse RE, Wallman KE, Newnham JP, Guelfi KJ. Home-based exercise training improves capillary glucose profile in women with gestational diabetes. Med Sci Sports Exerc. 2014;46:1702–9. doi: 10.1249/MSS.0000000000000302. [DOI] [PubMed] [Google Scholar]
- 77.Ong MJ, Guelfi KJ, Hunter T, Wallman KE, Fournier PA, Newnham JP, et al. Supervised home-based exercise may attenuate the decline of glucose tolerance in obese pregnant women. Diabetes Metab. 2009;35:418–21. doi: 10.1016/j.diabet.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 78.Kokic IS. Exercise and gestational diabetes mellitus. Period Biol. 2014;116:83–7. [Google Scholar]


