Abstract
Background and purpose
The Barrel vascular reconstruction device (Barrel VRD) is a novel stent with design features that allow endovascular coiling of wide-necked bifurcation aneurysms while preserving adjacent branches, without necessitating dual stent implantation. This study aimed to assess the safety and effectiveness of the Barrel VRD at 12-month follow-up.
Materials and methods
The Barrel VRD trial is a prospective, multicenter, observational post-marketing registry evaluating the use of the Barrel VRD for treatment of wide-necked bifurcation aneurysms. The primary effectiveness endpoint was successful aneurysm treatment measured by digital subtraction angiography with a Raymond–Roy occlusion grade of 1 or 2 in the absence of retreatment, parent artery stenosis (>50%), or target aneurysm rupture at 12 months. The primary safety endpoint was the absence of neurological death or major stroke at 12 months.
Results
Twenty patients were enrolled from December 2013 to December 2014. The device was implanted in 19 patients with 19 aneurysms (8 middle cerebral artery, 4 anterior communicating artery, 1 internal carotid artery terminus, 4 basilar artery aneurysms; mean dome height 5.7±1.91 mm; mean neck length 4.8±1.35 mm, mean dome-to-neck ratio 1.6±2.0). Coiling was performed in all cases. The primary effectiveness endpoint was achieved in 78.9% of subjects (15/19; 12 complete occlusions, 3 neck remnants), and the primary safety endpoint was 5.3% (1/19).
Conclusions
This prospective study demonstrates that the Barrel VRD device resulted in ~80% occlusion rates and ~5% rates of neurological complications at 1 year after endovascular treatment of wide-necked bifurcation intracranial aneurysms.
Registered clinical trial
NCT02125097;Results.
Keywords: aneurysm, device, stent, technology
Introduction
Endovascular coiling is the reference therapy to treat both ruptured and unruptured intracranial aneurysms in the majority of neurovascular centers, based on the results of randomized clinical trials and large observational series.1–3 However, coiling of wide-necked bifurcation or branch intracranial aneurysms is often challenging or even not feasible. Balloon-assisted and stent-assisted coiling has increased the ability for coiling of such aneurysms.4 5 Complex stenting, especially in ‘Y’ or ‘X’ configurations, has been reported to increase the rate of procedure-related morbidity and mortality compared with coiling alone.4 To date, several devices have been created for the endovascular treatment of such wide-necked aneurysms arising at bifurcations, including WEB,6 LUNA, and pCONus.7 8
A new stent, the Barrel vascular reconstruction device (Barrel VRD; Medtronic/Covidien, Irvine, California, USA) has been developed to obviate the need for treatment of bifurcation aneurysms with double stent implantation. The Barrel VRD is a self-expanding, fully retrievable laser cut nitinol stent. The device has a bulged central component which allows for greater neck coverage and a double spiral strut construction which allows it to hinge in order to conform to tortuous anatomy at vessel branch points. It has 12 platinum marker bands. The proximal marker band attaches to a wire that pushes the device through a 0.021 inch inner diameter microcathether to the intended treatment site. Because the Barrel VRD can provide neck protection with a single implant, it is possible that the safety of coiling of wide-necked bifurcation aneurysms will be improved over current approaches.
The purpose of this study was to evaluate the safety and effectiveness of the Barrel VRD in a post-marketing setting when used to facilitate coiling of wide-necked bifurcating or branch intracranial aneurysms with any approved embolic coils.
Materials and methods
Study design
The Barrel VRD trial is a prospective, multicenter, observational registry of the Barrel VRD for treatment of wide-necked bifurcation aneurysms. A maximum of 20 subjects were to be enrolled at a maximum of six clinical evaluation centers located in France between December 2013 and December 2014 and followed up for 1 year post-treatment. Subjects were considered qualified and were enrolled in the clinical evaluation after informed consent was obtained, screening and baseline assessments were completed, and eligibility was confirmed. Inclusion criteria were as follows: male or female adult (≥18 years old) patients with an intracranial bifurcating or branch aneurysm with either a wide (≥4 mm) neck or a dome-to-neck ratio of <2, including non-de novo aneurysms where no stent was utilized; each subject’s aneurysm arose from a parent vessel with a diameter of 2–4 mm as measured by two-dimensional digital subtraction angiography. Subjects were excluded if they presented with a ruptured aneurysm that occurred within 30 days prior to screening. Follow-up was completed at 12 months after the procedure.
Written informed consent was obtained from all subjects prior to inclusion in this prospective study. This study was conducted in accordance with ethical principles based on the Declaration of Helsinki concerning medical research in humans and applicable regulations. The study complied with the pertinent requirements in ISO 14155 and national regulatory requirements, and adhered to the principles of the International Conference on Harmonization and Good Clinical Practice.
Endovascular procedure
All patients were treated under general anesthesia and received full anticoagulation. Double antiplatelet therapy was administered before the procedure according to the practice of each center (see online supplementary table 1). An antiplatelet inhibition test could be performed before treatment at the discretion of the operator.
neurintsurg-2017-013602supp001.docx (50KB, docx)
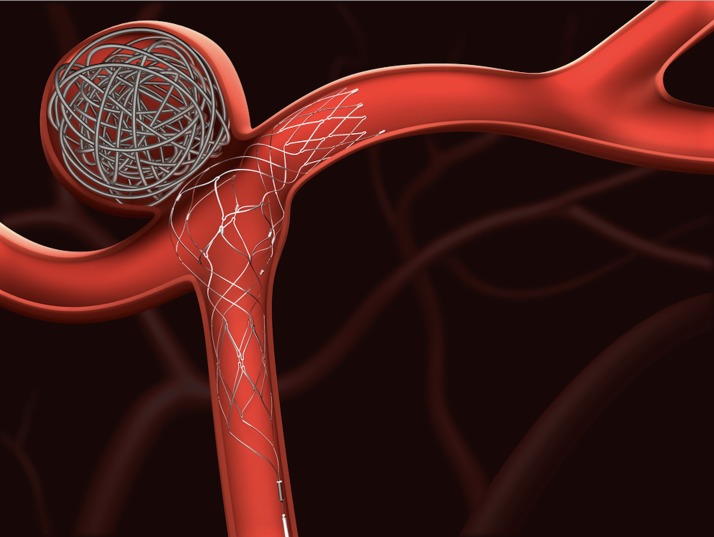
The Barrel VRD was used as an adjunctive treatment to embolic coils in all cases (figure 1). It was advanced through a 0.021 inch (1.6 Fr or 0.533 mm) inner diameter straight tip microcathether to the desired position. The appropriate device size was chosen based on the bifurcation span length, defining the neck height (figure 2). In a suitable working projection, the appropriately sized device was deployed across the neck of the aneurysm with the distal portion in the larger of the two bifurcation arteries. The marker bands were visualized to verify device placement. Thereafter, a second microcatheter (such as an Echelon microcatheter, Excelsior SL10 microcathether, or another microcathether adequate for coiling) was inserted through the stent strut into the aneurysm fundus and coiling was performed. The delivery wire was detached from the Barrel VRD by electrolytic means after deployment and then removed from the subject.
Figure 1.
The Barrel vascular reconstruction device (VRD) is intended for use with detachable coils for endovascular therapy of wide-necked intracranial aneurysms originating on vessel bifurcation.
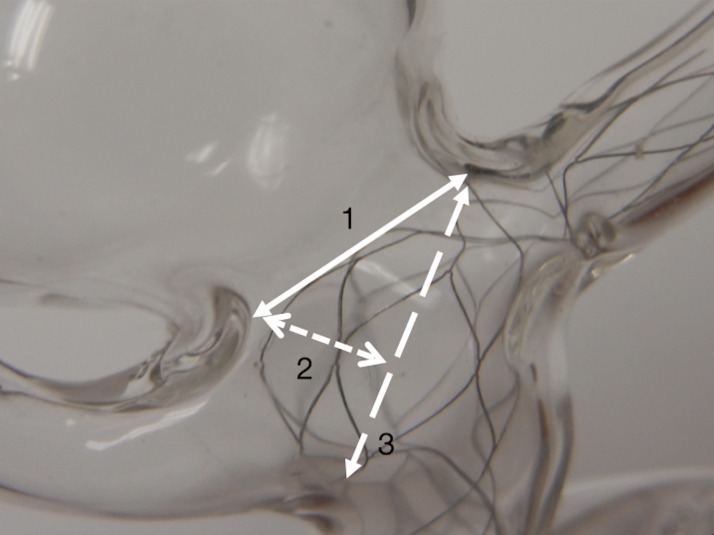
Figure 2.
Sizing the Barrel vascular reconstruction device (VRD): 1, aneurysm neck size; 2, neck height; 3, neck span. The neck height corresponds to the ideal radius of the Barrel VRD when fully deployed at the level of the six equatorial radio-opaque markers.
Endpoints
The primary endpoint for effectiveness was successful aneurysm treatment with the Barrel VRD, as measured by digital subtraction angiography, with an aneurysm Raymond scale grade of 1 or 2 at 12 months post-procedure in the absence of retreatment, parent artery stenosis (>50%), or target aneurysm rupture. A Raymond scale grade of 1 was defined as complete occlusion (complete obliteration of the aneurysm) and grade 2 was defined as residual neck (persistence of any portion of the original defect of the arterial wall as seen on any single projection, but without opacification of the aneurysm sac).9 The primary safety measures were the absence of neurological death or major stroke at 12 months post-treatment. A major stroke was defined as a new neurological event that persisted for more than 24 hours and resulted in at least a 4-point increase in the National Institutes of Health Stroke Scale (NIHSS) score compared with baseline or compared with any subsequent lower score.
Secondary endpoints included the following: all ischemic strokes up to 12 months after implantation (defined as episodes of focal or global neurological dysfunction due to brain or retinal infarction with signs that persisted 24 hours or longer); successful technical delivery of the device, which was defined as access to the lesion, successful deployment of the Barrel VRD, and correct positioning of the device over the aneurysm; significant stenosis (>50%) of the parent artery at 12 months after implantation; Barrel VRD migration at 12 months, defined as movement of the Barrel VRD by more than 5 mm as documented by 12-month follow-up angiogram; device-related and procedure-related serious adverse events (SAEs) occurring up to 12 months after the procedure; intracranial hemorrhages including subarachnoid, intraventricular, or intraparenchymal hemorrhages (symptomatic or asymptomatic); functional outcome as defined by modified Rankin Scale (mRS) at 12 months; all causes of mortality; aneurysm recanalization; and unplanned coil embolization or clipping within 12 months after the procedure.
Clinical Events Committee
A Clinical Events Committee (CEC) was put in place for the study composed of three expert physicians in the field of interventional neuroradiology and neurology who were not directly involved in the conduct of this clinical study. The CEC was responsible for the review of all site reported adverse events and adjudicated each event for its start date, stop date, relatedness, outcome, events of interest, and categorization per a predefined CEC charter. The CEC also adjudicated for safety endpoints of major stroke and neurological death. Antiplatelet medications, mRS score, and adverse events were evaluated at 30 (±7) days, 6 months (±4 weeks), and 12 months (±8 weeks). The NIHSS score was evaluated at 12 months (±8 weeks).
Angiographic follow-up
Angiographic images were acquired in anteroposterior, lateral, and working projections before and immediately after treatment. Angiographic images obtained immediately after treatment were compared with those obtained at 12-month follow-up. Aneurysm occlusion immediately after the procedure and at 12-month follow-up was classified using the simplified 3-point scale (Raymond–Roy scale grades 1, 2, or 3) by an independent core laboratory.9 Core laboratory evaluations included the baseline characteristics of the aneurysm as well as assessments of aneurysm occlusion, parent artery stenosis, and implant migration.
Results
Enrollment and background characteristics
Twenty subjects were enrolled and 19 subjects (12 women and 7 men; mean age 51±10 years) were treated with the Barrel VRD between December 2013 and December 2014. The Barrel VRD was not implanted in one subject because the protrusion of the Barrel VRD into the aneurysm neck was judged insufficient by the operator to protect the branch artery emerging from the neck.
Baseline demographics are shown in table 1. Baseline mRS was 0 in 94.7% (18/19) of subjects and 1 in 5.3% (1/19) of subjects.
Table 1.
Baseline demographics and medical history
| Characteristics | Results (n=19) Mean±SD or n/N (%) |
| No of subjects | 19 |
| Patient age, years | 51±10 |
| Sex | |
| Female | 12/19 (63.2%) |
| Male | 7/19 (36.8%) |
| Modified Rankin Scale (mRS) score | |
| 0 | 18/19 (94.7%) |
| 1 | 1/19 (5.3%) |
| ≥ 2 | 0 |
| Arrhythmia | 1/19 (5.3%) |
| Hearing impairment | 1/19 (5.3%) |
| Dizziness | 1/19 (5.3%) |
| Gastritis | 1/19 (5.3%) |
| Hiatus hernia | 1/19 (5.3%) |
| Hepatitis | 1/19 (5.3%) |
| Sinusitis | 1/19 (5.3%) |
| Ligament sprain | 1/19 (5.3%) |
| Hypercholesterolemia | 2/19 (10.5%) |
| Carotid artery aneurysm | 5/19 (26.3%) |
| Carotid artery dissection | 1/19 (5.3%) |
| Intracranial aneurysm | 7/19 (36.8%) |
| Transient ischemic attack | 1/19 (5.3%) |
| Alcoholism | 1/19 (5.3%) |
| Chronic obstructive pulmonary disease | 1/19 (5.3%) |
| Pleurisy | 1/19 (5.3%) |
| Ex-tobacco user | 1/19 (5.3%) |
| Familial risk factor | 3/19 (15.8%) |
| Tobacco user | 5/19 (26.3%) |
| Appendectomy | 1/19 (5.3%) |
| Knee operation | 1/19 (5.3%) |
| Malignant breast lump removal | 1/19 (5.3%) |
| Tonsillectomy | 1/19 (5.3%) |
| Arterial disorder | 1/19 (5.3%) |
| Arterial rupture | 1/19 (5.3%) |
| Hypertension | 5/19 (26.3%) |
Baseline aneurysm characteristics are shown in table 2. These are reported for 17 subjects; images for two subjects were not available for core laboratory analysis. The majority of aneurysms were located in the middle cerebral artery (8/17, 47.1%) and were small in size (<7 mm: 11/17, 64.7%; 7–12 mm: 6/17, 35.3%). The mean neck length was 4.8±1.35 mm and the mean dome-to-neck ratio was 1.6±2.0. Three aneurysms (15.8%) were previously coiled prior to study enrollment, with all three having a Raymond grade 3 (residual) aneurysm. Coiling was performed in all cases.
Table 2.
Baseline aneurysm characteristics
| Characteristics | Results (n=19) Mean±SD or n/N (%) |
| Aneurysm previous coiled | 3/19 (15.8%) |
| Aneurysm dome height, mm* | 5.7±1.91 |
| Aneurysm neck length, mm* | 4.8±1.35 |
| Dome-to-neck ratio* | 1.6±2.00 |
| Aneurysmal maximal diameter, mm* | 6.6±2.6 |
| Aneurysm location * | |
| Middle cerebral artery | 8/17 (47.1%) |
| Anterior communicating artery | 4/17 (23.5%) |
| Basilar artery | 4/17 (23.5%) |
| Internal carotid artery | 1/17 (5.9%) |
| Aneurysm size | |
| Small (<7 mm) | 11/17 (64.7%) |
| Medium (7–12 mm) | 6/17 (35.3%) |
| Large (13–24 mm) | 0 |
| Giant (≥25 mm) | 0 |
*Core laboratory reported (n=17). The angiograms of two patients were unavailable for core laboratory analysis.
Primary outcome measure
Aneurysm occlusion results are provided in table 3. ectiveness measure was met in 78.9% (15/19) of subjects at 12 months (Figure 3). Complete occlusion was observed in 63.2% (12/19) of subjects at 12 months (Figure 4)
Table 3.
Angiographic outcomes at 1-year follow-up
| Results (n=19) n/N (%) | |
| Post-procedure results | |
| Complete occlusion | 8/19 (42.1%) |
| Residual neck | 5/19 (26.3%) |
| Residual aneurysm | 6/19 (31.6%) |
| 12-month follow-up results | |
| Complete occlusion | 12/19 (63.2%) |
| Residual neck | 3/19 (15.8%) |
| Residual aneurysm | 4/19 (21.1%) |
Antiplatelet medications are shown in online supplementary table 1.
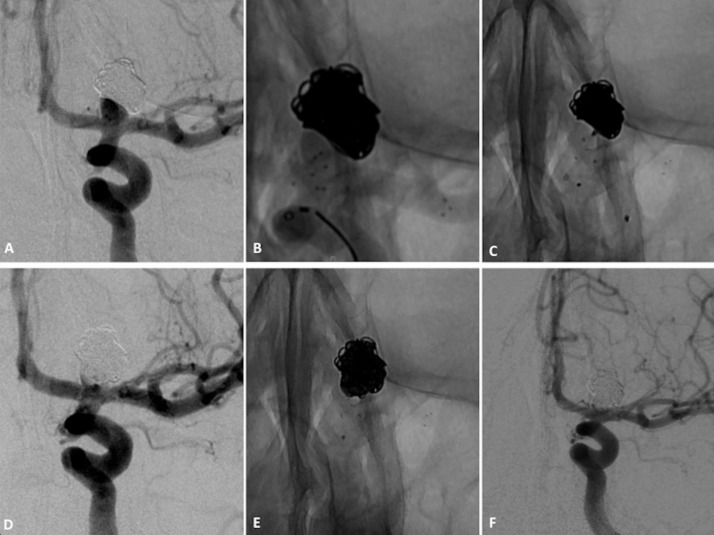
Figure 3.
Patient with left unruptured internal carotid artery bifurcation aneurysm. (A) Subtracted angiogram of internal carotid artery shows a wide-necked terminus carotid aneurysm. (B) Non-subtracted image shows Barrel vascular reconstruction device (VRD). The six markers of the cage covering aneurysmal neck are shown. (C) Non-subtracted image shows the placement of a second microcatheter through struts into the aneurysm fundus. (D) Non-subtracted image at the end of coiling. (E) Subtracted angiograms of the internal carotid artery at the end of the procedure show small neck remnant and one in-stent thromboembolic event. (F) Subtracted angiogram of internal carotid artery at 12-month follow-up shows near-complete occlusion with neck remnant that is stable in size.
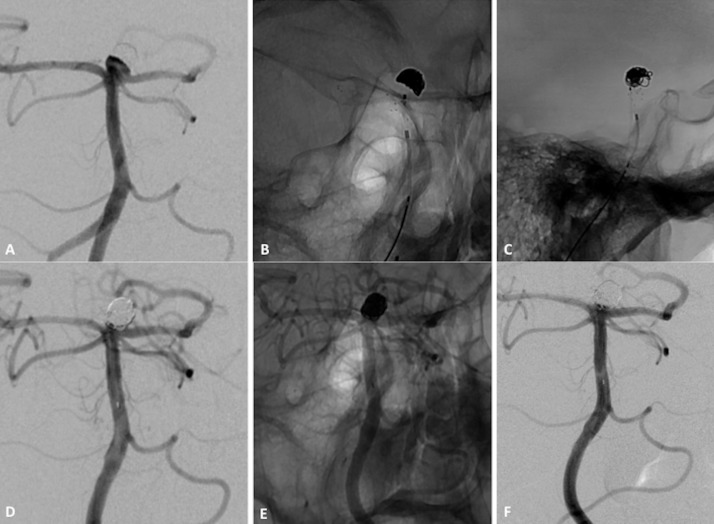
Figure 4.
Patient with wide-necked basilar tip aneurysm. (A) Subtracted angiogram of left vertebral artery shows small basilar tip aneurysm with 4 mm neck. (B, C) Non-subtracted image after placement of Barrel vascular reconstruction device (VRD). (D, E) Subtracted and non-subtracted angiograms of vertebral artery at procedure end show complete obliteration of neck remnant. (F) Angiogram at 12-month follow-up shows complete aneurysm occlusion.
The primary safety outcome measure was observed in one of the 19 subjects (5.3%) at the 12-month follow-up time point. One subject experienced a major stroke 9 months after Barrel VRD implantation during a scheduled orthopedic surgery to treat painful coxarthrosis with a hip replacement. Immediately following hip replacement surgery, rapid neurological deterioration was observed after an episode of low blood pressure. The subject experienced ipsilateral weakness of the upper and lower limbs which persisted for more than 24 hours. MRI showed ischemic changes in the treated vascular territory. The event was adjudicated as related to the study device due to the infarction noted in the treated vascular territory. The event was considered resolved with sequelae in outcome due to persisting neurological deficit in the right upper and lower limbs through the 12-month visit.
Secondary outcome measures
Two subjects (10.5%) had experienced an ischemic stroke at the 12-month visit. One of the two subjects who experienced ischemic stroke was the same previously mentioned subject who had experienced a major stroke at the 9-month visit. The other event was a minor stroke experienced by a subject immediately post-procedure, with left hemiparesis and ischemic (with hemorrhagic transformation) complications noted on CT on day 1 post-procedure. The subject made a complete recovery from the symptoms by the time of discharge from hospital. This minor stroke was adjudicated as related to the study procedure by the CEC. Device deployment was not successful in one of the 20 subjects. The remaining 19 subjects (the analytic sample) had successful deployment and positioning of the Barrel VRD over the aneurysm. There were no cases of either significant stenosis (>50%) of the parent artery at 12 months post-procedure or device migration (movement >5 mm) as reported by the core laboratory.
Device and procedure-related SAEs were observed in four subjects (21%), as adjudicated by the CEC. These included one device-related SAE of major stroke, as described above, and three procedure-related SAEs including a retroperitoneal hematoma, a vascular pseudoaneurysm, and a minor stroke (ischemic with hemorrhagic component, described above) as adjudicated by the CEC.
Intracranial hemorrhage was observed in two subjects (10.5%) during the 12-month follow-up period. The first was an intraparenchymal hemorrhage observed in a subject experiencing an acute ischemic stroke (minor), described above. The other was a subarachnoid hemorrhage that occurred due to intraoperative rupture of the dome of the target aneurysm during delivery of the last coil during the index procedure. There was minimal interpeduncular bleeding which resolved the next day. The subject remained asymptomatic with no reported deficit. Both intracranial hemorrhage events were adjudicated to be procedure-related. There were no deaths during the 12-month follow-up period. Overall, at 12 months, the mRS score was 0 for 16 patients (84.2%), 1 for two patients (10.5%), and >2 for one patient (5.3%).
One of the 19 subjects had aneurysm recanalization. The subject had a Raymond scale grade of 1 after the procedure and a Raymond scale grade of 2 at 12-month follow-up. There were no cases of the target aneurysm requiring further embolization (planned retreatment) with coiling or clipping within 12 months. A summary of the adverse events of interest is provided in the supplemental material in table 2.
Discussion
The Barrel VRD trial, a prospective multicenter study of this device, provides evidence to support its effectiveness and safety for coiling of wide-necked bifurcation aneurysms. Twelve-month angiographic follow-up showed that concomitant use of the Barrel VRD with coils resulted in complete occlusion or residual aneurysm neck with no retreatment, parent artery stenosis (>50%), or target aneurysm rupture observed in 79.0% of subjects. Additionally, we observed a high rate of technical success, which shows the utility of the Barrel VRD design in managing wide-necked bifurcation aneurysms. Without the use of the Barrel VRD, most of the treated aneurysms in this series would have been addressed with Y-stenting or a combination of stent and balloon remodeling in order to protect bifurcation arterial branches while providing adequate support for coiling and avoiding coil protrusion. The incidence of major stroke was low and no cases of neurological death during the 12 months following the procedure were reported. Moreover, there were no deaths in this study. Overall, our results demonstrate the safety of the Barrel VRD for the treatment of wide-necked bifurcation aneurysms.
The significance of the new Barrel VRD device to the treatment landscape is highlighted by the fact that, unlike sidewall aneurysms, complex wide-necked bifurcation aneurysms are difficult to treat endovascularly with simple coiling. Coiling of these aneurysms often requires complex stenting in association with dual antiplatelet therapy, whereas the morbidity and mortality rates of complex intracranial stenting are not negligible. In fact, in a retrospective study including 97 patients with complex and wide-necked bifurcation aneurysms who were treated with stent-assisted coiling in a ‘Y’ or ‘X’ configuration, the rate of procedure-related permanent neurological deficits was 10%.4 To address the complexity of treating wide-necked bifurcation aneurysms, three devices dedicated to endovascular treatment of wide-necked bifurcation aneurysms have been developed to date: the WEB (Sequent Medical, Palo Alto, California, USA), the pCONus (Phenox GmbH, Bochum, Germany), and the PulseRider (PulsarVascular, San Jose, California, USA) devices. Treatment of wide-necked bifurcation aneurysms using the WEB device, an intrasaccular flow disrupter, has been associated with variable outcomes. One recent study of the WEB device reported low rates of adverse events, including 0% mortality (0/113) and 2.7% morbidity (3/113) at 1 month.10 Similar safety results were observed with the pCONus device in a multicenter retrospective analysis of 40 wide-necked middle cerebral artery aneurysms.7 8 The permanent morbidity rate was 2.5% (1/40) and the mortality rate was 0.0% at 1-year follow-up.8 In addition, one study of treatment with the PulseRider for stent-assisted coiling reported a permanent morbidity rate of 5.3% and a 0% mortality rate at 6 months in 15 patients.11 A head-to-head comparison of these various devices could be useful to establish safer tools for endovascular treatment of intracranial aneurysms with a wide neck.
In the Barrel VRD trial with independent core laboratory evaluation, at 12-month angiographic follow-up concomitant use of the Barrel VRD with coils resulted in 79.0% successful aneurysm treatment (neck remnant or complete occlusion). The Barrel VRD achieved complete occlusion rates of 63% (12/19) at 12 months. Interestingly, a progressive aneurysmal thrombosis was observed after Barrel VRD placement, as previously reported after stenting.12 13 Nonetheless, a longer follow-up is needed to evaluate the effectiveness of this device. No studies have directly compared the anatomic outcome between devices dedicated to bifurcation aneurysms. The efficacy of the WEB device at 1-year follow-up was reported in the large WEBCAST and French Observatory series, which showed rates of complete aneurysm occlusion and neck remnant of 56% and 26%, respectively.10 However, less optimal results were recently described by Sivan-Hoffmann et al because of WEB compression at follow-up.14 Cognard and Januel reported a similar phenomenon in a small series of 15 consecutive patients.15 In a report of 40 bifurcation aneurysms treated with the pCONus, adequate aneurysm occlusion was obtained in 96.7% (30/31 aneurysms) at 12-month follow-up.8 However, retreatment was performed in nine aneurysms between 3 and 6 months after first treatment. PulseRider stenting has also yielded interesting results at 6 months. In a series of 19 patients with 19 wide-necked aneurysms (mean dome size 8.8 mm; mean neck size 5.8 mm), adequate occlusion was observed in 94.7% (18/19 aneurysms).16
The limitations of our study include the small number of patients and follow-up period of only 12 months to evaluate the effectiveness of the Barrel VRD. Although an independent core laboratory evaluated the angiographic outcomes and the safety outcomes were adjudicated by a CEC providing an unbiased evaluation of clinical outcomes, longer follow-up is needed to evaluate the effectiveness of this device.
Conclusions
This prospective study found that the use of the Barrel VRD device for the endovascular treatment of wide-necked bifurcation aneurysms resulted in ~80% occlusion rates and ~5% rates of neurological complications at 1 year.
Footnotes
Contributors: All authors made substantial contributions to (1) the conception or design of the work or the acquisition, analysis, or interpretation of data for the work; (2) drafting the work or revising it critically for important intellectual content; (3) final approval of the version to be published; and (4) agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Competing interests: BG: None. RB: Consultancy for Stryker, Medtronic, Microvention, Balt, and Penumbra. FT: Consultancy for Stryker, Medtronic, and Codman. JB: None. MP: Consultancy for Stryker, Medtronic, Microvention, Balt, and Penumbra.
Ethics approval: Comité de Protection des Personnes Ile de France XI.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Gory B, Turjman F. Endovascular treatment of 404 intracranial aneurysms treated with nexus detachable coils: short-term and mid-term results from a prospective, consecutive, European multicenter study. Acta Neurochir 2014;156:831–7. 10.1007/s00701-014-2047-3 [DOI] [PubMed] [Google Scholar]
- 2. Molyneux AJ, Birks J, Clarke A, et al. . The durability of endovascular coiling versus neurosurgical clipping of ruptured cerebral aneurysms: 18 year follow-up of the UK cohort of the International Subarachnoid Aneurysm Trial (ISAT). The Lancet 2015;385:691–7. 10.1016/S0140-6736(14)60975-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gory B, Huot L, Riva R, et al. . One-year efficacy and safety of the Trufill DCS Orbit and Orbit Galaxy detachable coils in the endovascular treatment of intracranial aneurysms: results from the TRULINE study. Interv Neuroradiol 2017;23:485–91. 10.1177/1591019917717576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bartolini B, Blanc R, Pistocchi S, et al. “Y” and “X” stent-assisted coiling of complex and wide-neck intracranial bifurcation aneurysms. AJNR Am J Neuroradiol 2014;35:2153–8. 10.3174/ajnr.A4060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gory B, Rouchaud A, Saleme S, et al. . Endovascular treatment of middle cerebral artery aneurysms for 120 nonselected patients: a prospective cohort study. AJNR Am J Neuroradiol 2014;35:715–20. 10.3174/ajnr.A3781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pierot L, Moret J, Turjman F, et al. . WEB treatment of intracranial aneurysms: clinical and anatomic results in the French observatory. AJNR Am J Neuroradiol 2016;37:655–9. 10.3174/ajnr.A4578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gory B, Aguilar-Pérez M, Pomero E, et al. . pCONus device for the endovascular treatment of wide-neck middle cerebral artery aneurysms. AJNR Am J Neuroradiol 2015;36:1735–40. 10.3174/ajnr.A4392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gory B, Aguilar-Pérez M, Pomero E, et al. . One-year angiographic results after pCONus stent-assisted coiling of 40 wide-neck middle cerebral artery aneurysms. Neurosurgery 2017;80:925–33. 10.1093/neuros/nyw131 [DOI] [PubMed] [Google Scholar]
- 9. Roy D, Milot G, Raymond J. Endovascular treatment of unruptured aneurysms. Stroke 2001;32:1998–2004. 10.1161/hs0901.095600 [DOI] [PubMed] [Google Scholar]
- 10. Pierot L, Spelle L, Molyneux A, et al. . Clinical and anatomical follow-up in patients with aneurysms treated with the WEB device: 1-year follow-up report in the cumulated population of two prospective, multicenter series (WEBCAST and French Observatory). Neurosurgery 2016;78:133–41. 10.1227/NEU.0000000000001106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gory B, Spiotta AM, Mangiafico S, et al. . PulseRider stent-assisted coiling of wide-neck bifurcation aneurysms: periprocedural results in an international series. AJNR Am J Neuroradiol 2016;37:130–5. 10.3174/ajnr.A4506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gory B, Klisch J, Bonafé A, et al. . Solitaire AB stent-assisted coiling of wide-necked intracranial aneurysms: mid-term results from the SOLARE study. Neurosurgery 2014;75:215–9. 10.1227/NEU.0000000000000415 [DOI] [PubMed] [Google Scholar]
- 13. Piotin M, Blanc R, Spelle L, et al. . Stent-assisted coiling of intracranial aneurysms: clinical and angiographic results in 216 consecutive aneurysms. Stroke 2010;41:110–5. 10.1161/STROKEAHA.109.558114 [DOI] [PubMed] [Google Scholar]
- 14. Sivan-Hoffmann R, Gory B, Riva R, et al. . One-year angiographic follow-up after WEB-SL endovascular treatment of wide-neck bifurcation intracranial aneurysms. AJNR Am J Neuroradiol 2015;36:2320–4. 10.3174/ajnr.A4457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cognard C, Januel AC. Remnants and recurrences after the use of the WEB intrasaccular device in large-neck bifurcation aneurysms. Neurosurgery 2015;76:522–30. 10.1227/NEU.0000000000000669 [DOI] [PubMed] [Google Scholar]
- 16. Gory B, Spiotta AM, Di Paola F, et al. . PulseRider for treatment of wide-neck bifurcation intracranial aneurysms: 6-month results. World Neurosurg 2017;99:605–9. 10.1016/j.wneu.2016.12.065 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
neurintsurg-2017-013602supp001.docx (50KB, docx)