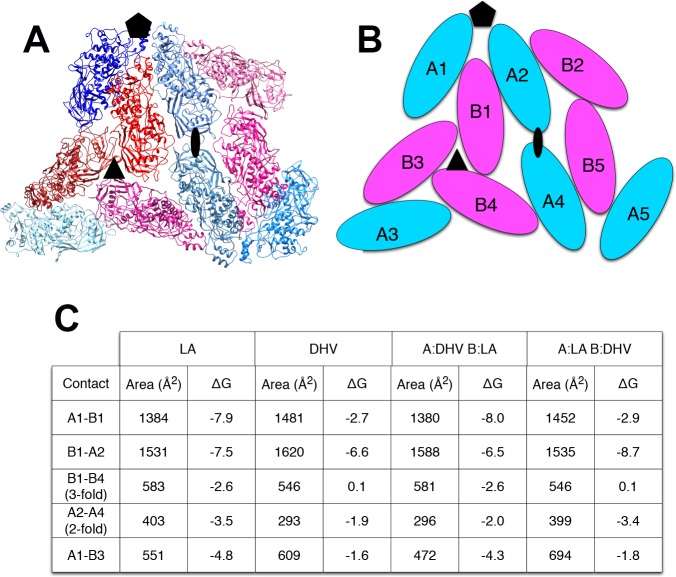
Figure 2. Predicted stability of DhVcp1-ScVcap interactions.
Energy of formation of subunit surfaces. (A) Ribbon diagram showing two-fold, three-fold, and five-fold axes of symmetry. (B) Subunit labels corresponding to the free energies calculated in (C). (C) Free-energies of formation of subunit interaction interfaces. Note that in the ScVL1(L-A) virus, there are two copies of the capsid protein in the icosahedral asymmetric unit that are not in identical environments, labeled ‘A’ and ‘B’ in (B). As shown in the figure, the A subunits are clustered around the five-fold and two-fold axes while the B subunits dominate the three-fold axes.