Abstract
Background.
The government of Tanzania launched an opioid treatment program (OTP), using methadone, in Dar es Salaam in February of 2011. Hepatitis C virus (HCV) is a leading cause of morbidity and mortality globally, especially among people who inject drugs (PWID). We conducted a cross-sectional study among PWID engaged in OTP in Dar es Salaam to describe the prevalence and predictors of HCV antibody serostatus.
Methods.
Routine programmatic data on patients enrolled in Muhimbili National Hospital’s OTP clinic from February 2011 to January 2013 were utilized. Multivariable Poisson regression was used to examine factors associated with HCV antibody serostatus.
Results.
A total of 630 PWID enrolled into the OTP clinic during the study period, seven percent of which were women. The overall seroprevalence of HCV antibody was 57% (95% Confidence interval: 53%-61%). In adjusted analysis, methadone patients who used heroin for 5-10 years (adjusted prevalence ratio; aPR=1.41; 95% CI: 1.10-1.81) and >10 years (aPR=1.48; 95% CI: 1.17-1.88) were more likely to be HCV antibody positive, compared to patients who used heroin for < 5 years. Patients who reported sharing needles or other equipment at their last injection (aPR=1.20; 95% CI: 1.01-1.41; p=0.022), being arrested (aPR=1.20; 95% CI: 1.04-1.40; p=0.012) and who were HIV-positive (aPR=1.84; 95% CI: 1.56-2.16; p<0.001) were also more likely to be HCV antibody positive than their counterparts.
Conclusion.
Our observed HCV antibody prevalence among PWID engaged in OTP is higher than previously reported estimates in Dar es Salaam. Predictors of HCV antibody serostatus in this sample were similar to those found among PWID in many other settings. Integrating HCV care and treatment into OTP clinics should be considered, leveraging lessons learned from the integration of HIV services into OTP. Global efforts to develop HCV care and treatment programs in low and middle-income countries are critical, especially among PWID who have a high burden of HCV.
Background
Hepatitis C virus (HCV) is a leading cause of morbidity and mortality globally.1,2 HCV is a bloodborne pathogen most commonly transmitted through sharing of drug injection equipment, transfusion of infected blood or blood products or improperly sterilized medical equipment.3 Current estimates suggest that the general population of Tanzania has an HCV prevalence of 3.2%, which is higher than estimates from the surrounding countries of Kenya (0.9%), Zambia (0.2%), Mozambique (2.8%) and Malawi (0.3%).4–6 Recent research among a community-based sample of PWID estimated an HCV prevalence of 22% among PWID in Zanzibar7 and 27.7% among PWID in Dar es Salaam.8 A recent World Health Organization report noted the lack of reliable estimates with regards to coverage of HCV testing and treatment in the region.9
With no HCV vaccine currently available, treatment is paramount to controlling the HCV epidemic. In the past, treatments with pegylated interferon and ribavirin were 6-12 months in duration, required weekly clinic visits for injections, caused severe side effects, and had clearance rates ranging from 45% to 80% depending on genotype.10 By contrast, new treatments with directing acting antivirals are shorter in duration (3-6 months) and have fewer side effects, and 95% of patients achieve sustained viral response after completing treatment.11,12 With these treatment improvements, health system requirements, including clinical monitoring, laboratory procedures and pharmacy dispensing are less burdensome, paving the way for treatment possibilities in resource-limited settings - provided medications are available and affordable.
An estimated 500,000 people in East Africa use opioids for non-medical reasons, and on the mainland of Tanzania, there are an estimated 30,000 people who inject drugs (PWID), primarily with heroin.13,14 The first publicly-funded opioid treatment program (OTP), using methadone, on the mainland of sub-Saharan Africa was launched in February 2011, offering daily directly observed methadone services at Muhimbili National Hospital (MNH) in Dar es Salaam.15 The estimated prevalence of HIV at 39%16 and tuberculosis (TB) at 4%17 among PWID enrolling into the MNH OTP clinic is over 7 times and 20 times,18,19 respectively, the prevalence in the general population, and is an important challenge for the national HIV/TB response.
Understanding the epidemiology of HCV among the highest risk populations, such as PWID, is critical to design an effective and efficient HCV prevention and treatment program, and these data are scarce for many countries in sub-Saharan Africa, including Tanzania. We conducted a cross-sectional study among a cohort of PWID engaged in OTP in Dar es Salaam, Tanzania to describe the seroprevalence and predictors of HCV antibody.
Methods
Study Setting
In February 2011, the Ministry of Health and Social Welfare, Muhimbili University of Health and Allied Sciences, and the Drug Control Commission, with funding from the US President’s Emergency Plan for AIDS Relief (PEPFAR) launched an OTP clinic at Muhimbili National Hospital in Dares Salaam, Tanzania. Detailed procedures have been explained elsewhere.15 Briefly, enrollment into OTP required referral from a community-based organization. In preparation for OTP initiation, individuals were also required to attend a series of educational sessions on HIV, sexually transmitted infections, medication adherence, and supportive services provided by community based organizations. To be eligible for OTP, individuals had to (1) present with opioid dependence, (2) have evidence of recent drug injection, and (3) test positive for opioids through urine drug screening.
Once enrolled in OTP, methadone was administered to patients 7 days a week at the clinic. Patients visited the clinic on a daily basis to receive in-person, directly observed methadone dosing. Screening for HCV and HIV were offered to OTP patients. Upon enrollment in OTP, patients were encouraged to obtain rapid HCV and HIV screening via provider-initiated testing and counseling on-site at the methadone clinic. However, patients could opt out of these disease screening procedures and still receive methadone treatment.
Study Population
Study subjects included patients who enrolled into Muhimbili National Hospital’s OTP clinic from February 2011 to January 2013.
Data Sources
This study utilized de-identified routine clinical and program monitoring data from the OTP clinic at Muhimbili National Hospital. As part of routine care, health care providers and social workers conducted an in-person baseline assessment to collect demographic, health history, legal history, mental health and HIV risk behavior data. Clinical data, including HCV and HIV test results, were obtained through medical chart review and abstraction.
Laboratory Procedures
As part of routine care, patients were offered voluntary testing and counseling for HCV and HIV once enrolled into the methadone clinic. Patients were screened for HCV using the Standard Diagnostics (SD) Bioline HCV assay – a rapid, immunochromatographic test for detecting the presence of HCV-specific antibodies in human plasma, serum or whole blood.20 The SD Bioline HCV test has a sensitivity of 100% and specificity of 99.4%.20 HIV status was determined using the Ministry of Health and Social Welfare’s HIV rapid test algorithm including a Standard Diagnostic’s Bioline HIV rapid test,21 followed by confirmation with Alere Determine21 and Uni-Gold21 as a tiebreaker in the case of discrepancy between Bioline and Alere Determine. Patients could also opt-out of receiving their results.
Measures
Outcome
Our primary outcome of interest was HCV antibody serostatus. Patients were considered to be HCV antibody positive if they had HCV antibodies as determined through the SD Bioline HCV assay. A recent study estimated that 26% of people who are initially infected and are HCV-antibody positive will subsequently clear the virus but remain antibody positive.22
Exposures
Demographic risk factors included age (≤30 years old; >30 years old), sex (male; female), education level (primary-level education or below; secondary level education and above) and marital status (currently married; not married). Sexual risk factors included multiple sex partners in the last 6 months, defined as >1 sex partner (yes; no), and risky sexual behavior in the last 6 months, defined as vaginal or anal intercourse with no or inconsistent condom use (yes; no). Drug use and injection-related risk factors included years of heroin use (<5 years; 5-10 years; >10 years), flashblood, defined as injecting blood from another drug user who has recently injected heroin,23 (yes; no), shared needles at last injection (yes; no), polysubstance use, defined as heroin and alcohol, cocaine, benzodiazepine or amphetamine use, (yes; no) and HIV infection (yes; no). Criminal justice involvement included a lifetime history of arrest (yes; no).
Statistical Methods
Descriptive statistics, including frequencies, medians, and interquartile ranges (IQR) were calculated to describe the distribution of variables in the study population. We calculated the prevalence and accompanying 95% confidence intervals (Cl) for HCV antibody positivity. Poisson regression models with robust variances were built to estimate prevalence ratios (PR) and adjusted prevalence ratios (aPR)24. We examined the associations of demographic characteristics, sexual risk factors, injection and drug use-related risk factors, HIV serostatus and criminal justice involvement with HCV serostatus. We present unadjusted estimates and adjusted estimates from the full model. Statistical significance was set at p<0.05. All statistical analyses were conducted in Stata v14.1. (College Station, Texas).
Patient data were missing for some variables. To address this, we utilized multiple imputation procedures to fill in missing values in the dataset. Ten imputations were performed, using fully conditional specifications. Results from the imputations were combined into a single set of parameter estimates for the “final” Poisson regression models that incorporated the uncertainty from the imputations.25
Ethics Statement
The use and analysis of de-identified, programmatic data was approved by E&I Review Services in the United States as well as the ethical review committees at Muhimbili University of Health and Allied Sciences and the National Institute of Medical Research in Tanzania as program evaluation and non-human subjects research.
Results
A total of 630 PWID enrolled into the methadone clinic during the study period (Table 1). The median age of patients was 32 years (IQR: 29-37), and only 7% of the enrolled patients were women. A third of the patients had received at least secondary levels of education and 87% were not currently married. The median time since initiating heroin use was 10 years (IQR: 5-15), and 40% (95% CI: 35%−44%) of patients were living with HIV. More than half of methadone patients had previously been arrested.
Table 1.
Characteristics of MAT Clients at Muhimbili National Hospital in Dar es Salaam, Tanzania, (n=630)
| n (%) | |
|---|---|
| Male | 581 (93) |
| Age | |
| ≤30 years old | 209 (33) |
| >30 years old | 417 (67) |
| Primary education or below | 370 (64) |
| Currently married | 81 (13) |
| Multiple Sex Partners in last 6 months | 117 (19) |
| Risky Sex in last 6 months | 291 (47) |
| Years of Heroin Use | |
| <5 years | 97 (17) |
| 5-10 years | 223 (40) |
| >10 years | 240 (43) |
| Flashblood | 43 (7) |
| Shared needles at last injection | 81 (13) |
| Polysubstance use | 191 (34) |
| Infection with HIV | 187 (40) |
| Ever been arrested | 315 (55) |
The overall seroprevalence of HCV antibody was 57% (95% CI: 53-61%), and among people who were HIV-positive, the seroprevalence of HCV antibody was 81% (75-86%). Figure 1 portrays HCV antibody prevalence by exposure groups as well as unadjusted and adjusted prevalence ratios with corresponding confidence intervals. In the unadjusted analysis, methadone patients who were over 30 years of age were more likely to be HCV antibody positive than patients who were 30 years of age or younger (PR=1.31; 95% CI: 1.09-1.57). Compared to patients who used heroin for <5 years, methadone patients who used heroin for 5-10 years (PR=1.52; 95% CI: 1.16-1.98) and >10 years (PR=1.68; 95% CI: 1.31-2.15) were more likely to be HCV antibody positive. Also, patients who reported the practice of flashblood (PR=1.40; 95% CI: 1.14-1.72; p=0.002), sharing needles at their last injection (PR=1.36; 95% CI: 1.15-1.61; p<0.001), a history of arrest (PR=1.20; 95% CI: 1.02-1.42; p=0.029) and were HIV-positive (PR=1.97; 95% CI: 1.68-2.33; p<0.001) were also more likely to be HCV antibody positive than their counterparts.
Figure 1.
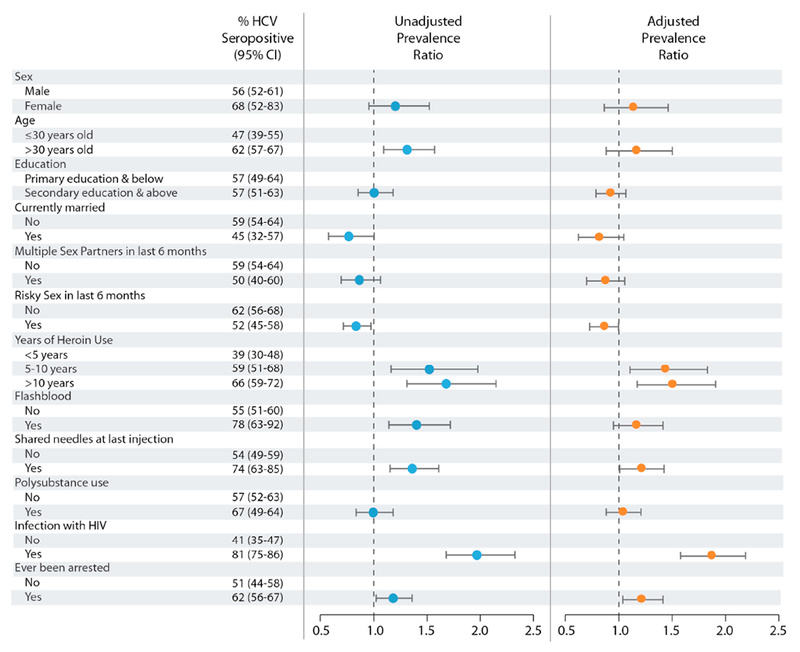
Associations of Client Characteristics with HCV Seropositivity
In adjusted analysis, methadone patients who used heroin for 5-10 years (aPR=1.41; 95% CI: 1.10-1.81) and >10 years (aPR=1.48; 95% CI: 1.17-1.88) were more likely to be HCV antibody positive, compared to patients who used heroin for < 5 years. Patients who reported a history of sharing needles or other equipment at their last injection (aPR=1.20; 95% CI: 1.01-1.41; p=0.022), a history of arrest (aPR= 1.20; 95% CI: 1.04-1.40; p=0.012) and who were HIV-positive (aPR=1.84; 95% CI: 1.56-2.16; p<0.001) were also more likely to be HCV antibody positive than their counterparts.
Discussion
We assessed the prevalence and predictors of HCV among 630 patients enrolled in the opioid treatment program at Muhimbili National Hospital in Dar es Salaam, Tanzania. This is the first study to our knowledge that assesses the prevalence and predictors of HCV in East Africa among PWID enrolled into OTP. Our data suggest a high prevalence of HCV among OTP patients. We also found that patients who used heroin for longer periods of time, had a history of sharing needles, were living with HIV and reported a history of arrest had a higher prevalence of HCV than others. These findings have important implications not only to understanding the burden of HCV among PWID in Tanzania, but also for future prevention and treatment programs.
The high levels of observed HCV prevalence among people who inject drugs is consistent with estimates from other regions around the world.26 Our observed HCV prevalence among PWID engaged in OTP is higher than previously reported estimates of 22% among PWID in Zanzibar7 and 27.7% among community-based sample of PWID in Dar es Salaam.8 This finding could reflect differences between the community-based sample of PWID and OTP patients, who could have a longer history of heroin injection with potentially more risks. Alternatively, these results could reflect a worsening HCV epidemic among PWID, with similarities to HCV epidemic trends observed in other countries.27–29 In addition, the estimated HCV prevalence was 18-fold greater than the estimated HCV prevalence in the general population of 3.2% in Tanzania.6 HCV prevalence among people who were HIV-positive was 81% – an important finding as HIV has a significant impact on the natural history of HCV. Co-infection with HIV is associated with higher HCV viral load, acceleration of liver disease progression and higher mortality, compared to HCV mono-infected individuals.30–33
Predictors of HCV antibody in this sample were similar to those found among PWID in many other settings. The robust association between sharing of injection equipment and infection is a common finding,34–36 as is the increase in antibody prevalence with years of drug use.37–40 Among PWID who have HIV infection, the prevalence of HCV is typically high - for example, approximately 80% of PWID living with HIV in the United States are HCV antibody positive.41 People with a history of arrest are likely to have more exposure to carceral settings, environments that typically have an elevated prevalence of HCV.42–45 Thus, people with a history of criminal justice encounters may have had more exposure to HCV.
As a health service model, opioid treatment programs can facilitate the improvement of health-related outcomes for their patients46,47 and have proven to be a successful venue for treatment of infectious diseases such as HIV,48,49 and tuberculosis.50,51 Opioid treatment programs have successfully delivered treatment for HCV infection interferon-based therapies, despite their long duration and substantial side effects.52 OTP clinics in Tanzania provide a unique opportunity for comprehensive HCV prevention and treatment to this high-risk population for several reasons. First, the HCV burden among PWID is 18-fold greater than what is observed in the general population. Second, methadone helps to stabilize PWID and can facilitate adherence and completion of treatment regimens.53,54 Third, methadone treatment decreases injecting behaviors and therefore decreases transmission and the potential for reinfection.55,56 Fourth, OTP providers are perfectly positioned to provide culturally competent services for PWID, compared to most other health care providers, and can assist in the cultivation of a welcoming environment for the provision of services. Fifth, as the majority of patients present daily for their methadone dose, OTP clinics are ideal venues to dispense medication, monitor treatment, and provide proper follow-up care, including reinfection prevention counseling.
In considering HCV care and treatment delivery, lessons learned from the successes of HIV scale-up should be considered. For example, the OTP clinic at Muhimbili National Hospital has seen significant results from efforts to integrate HIV treatment into the OTP clinic that include training OTP providers in HIV care and treatment procedures, utilizing point-of-care diagnostics and offering multiple treatment dispensing modalities based on the patients’ needs.47,57–59 These same approaches could be useful for building an effective HCV treatment response.
The principal limitation of our study is the generalizability of these results, which require careful consideration. Participants were included in the study based on whether they enrolled into OTP and were not selected through a random process. In addition, we were only able to test for HCV antibody not viremia. Past studies based have shown that approximately 74% of people who are HCV antibody positive will be chronically infected with Hepatitis C virus.22 HCV RNA levels will need to be obtained in those who are seropositive to provide a more accurate prevalence of chronic HCV infection. Although we included a number of variables from routine data sources to understand the independent relationships with HCV, the potential for unmeasured or mismeasured factors to influence our results existed. Another limitation was the self-report of behaviors, and as a result, recall and social desirability were potential biases impacting the metrics collected as part of the baseline survey. In particular, specific metrics for sexual risk factors (e.g., multiple sex partners) and injection risk factors (e.g., sharing needles) were prone to social desirability bias. Due to the stigmatization of behaviors, these types of biases have been common when studying PWID.60 The resulting misclassification would be non-differential with regard to HCV antibody serostatus as they are asked prior to HCV screening, and as a result, our results would be biased toward the null, on average.61 Strengths of the study included the standardization of protocols for care delivery, patient tracing, and data recording for the patients included in the study. Furthermore, the database had high levels of completeness with regards to clinical and laboratory data.
In conclusion, the time to mobilize HCV treatment efforts is upon us. If the results of this study reflect an increasing HCV prevalence, these findings underpin the urgency of an effective and evidence-based response. For nearly 15 years, the Global Fund to Fight AIDS, Tuberculosis and Malaria and the President’s Emergency Plan for AIDS Relief have mobilized resources and partnerships to reduce HIV and tuberculosis-related mortality globally. In doing so, health systems have been strengthened to better treat and care for chronic infectious diseases. However, no global financing efforts have been established, to date, to enhance the provision of HCV treatment in low and middle-income countries. In developing HCV treatment programs, it will be critical to leverage the HIV treatment infrastructure and build programs similar to PEPFAR’s data-driven approach that focuses on geographic areas and populations that maximize impact from investments. Research has highlighted that focusing HCV treatment efforts on PWID will be a key component to reduce the prevalence of and eliminate HCV.62,63 Future studies that address attitudes and beliefs about HCV as well as implementation science research that seeks to identify approaches that facilitate integration of HCV care and treatment within the health system will be critical to identify the most effective and efficient treatment approaches.
Acknowledgements.
We would like to thank the research staff and study participants for sharing their time and experience. This research was conducted with funding from the National Institute of Drug Abuse (R34DA037787) and the National Institute of Mental Health (R01MH094090).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
We declare that we have no conflicts of interest.
References
- 1.Cooke GS, Lemoine M, Thursz M, et al. Viral hepatitis and the Global Burden of Disease: a need to regroup. Journal of viral hepatitis. 2013;20(9):600–601. [DOI] [PubMed] [Google Scholar]
- 2.Cowie BC, Carville KS, MacLachlan JH. Mortality due to viral hepatitis in the Global Burden of Disease Study 2010: new evidence of an urgent global public health priority demanding action. Antiviral therapy. 2013;18(8):953–954. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Hepatitis C FAQs for the Public. 2015; http://www.cdc.gov/hepatitis/hcv/cfaq.htm#cFAQ22. Accessed 12/15/2015.
- 4.Fox JM, Newton R, Bedaj M, et al. Prevalence of hepatitis C virus in mothers and their children in Malawi. Trop Med Int Health. 2015;20(5):638–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karoney MJ, Siika AM. Hepatitis C virus (HCV) infection in Africa: a review. Pan Afr Med J. 2013;14:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petruzziello A, Marigliano S, Loquercio G, Cozzolino A, Cacciapuoti C. Global epidemiology of hepatits C virus infection: An update of the distribution and circulation of hepatitis C virus genotypes. World J Gostroenterol. 2016. September 2016;14(22(34)):7824–7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnston LG, Holman A, Dahoma M, et al. HIV risk and the overlap of injecting drug use and high-risk sexual behaviours among men who have sex with men in Zanzibar (Unguja), Tanzania. International Journal of Drug Policy. 2010;21(6):485–492. [DOI] [PubMed] [Google Scholar]
- 8.Bowring AL, Luhmann N, Pont S, et al. An urgent need to scale-up injecting drug harm reduction services in Tanzania: prevalence of blood-borne viruses among drug users in Temeke District, Dar-es-Salaam, 2011. Int J Drug Policy. 2013;24(1):78–81. [DOI] [PubMed] [Google Scholar]
- 9.Organization WH. Global Report on Access to Hepatitis C Treatment. http://appswhoint/iris/bitstream/10665/250625/1/WHO-HIV-201620-engpdf (Accessed 4/6/17). 2016.
- 10.Liang TJ, Ghany MG. Current and future therapies for hepatitis C virus infection. The New England journal of medicine. 2013;368(20):1907–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yau AH, Yoshida EM. Hepatitis C drugs: the end of the pegylated interferon era and the emergence of all-oral interferon-free antiviral regimens: a concise review. Canadian journal of gastroenterology & hepatology. 2014;28(8):445–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.AASLD. Initial Treatment of HCV Infection. http://wwwhcvguidelinesorg/printpdf/71 (Accessed 10/18/16). 2016.
- 13.Csete J, Kamarulzaman A, Kazatchkine M, et al. Public health and international drug policy. Lancet. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.NACP. Consensus Estimates on Key Population Size and HIV Prevalence in Tanzania. In: National AIDS Control Programme MoHaSW, Government of Tanzania, ed. http://www.healthpolicyproject.com/pubs/391_FORMATTEDTanzaniaKPconsensusmtgreport.pdf2014. [Google Scholar]
- 15.Lambdin BH, Masao F, Chang O, et al. Methadone treatment for HIV prevention-feasibility, retention, and predictors of attrition in Dar es Salaam, Tanzania: a retrospective cohort study. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2014;59(5):735–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tran OC, Bruce RD, Masao F, et al. Implementation and operational research: linkage to care among methadone clients living with HIV in Dar es Salaam, Tanzania. Journal of acquired immune deficiency syndromes. 2015;69(2):e43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta A, Mbwambo J, Mteza I, et al. Active case finding for tuberculosis among people who inject drugs on methadone treatment in Dar es Salaam, Tanzania. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2014;18(7):793–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanzania TURo. Global AIDS Response Country Progress Report. http://wwwunaidsorg/sites/default/files/country/documents/TZA_narrative_report_2014pdf (Accessed 10/11/2016). 2014. March 31.
- 19.World Health Organization. Global Tuberculosis Report 2012. http://appswhoint/iris/bitstream/10665/75938/l/9789241564502_engpdf (Accessed 10/11/2016). 2012.
- 20.Standard Diagnostics. Standard Diagnostics Bioline HCV: One Step Hepatitis C Virus test. http://wwwstandardiacom/en/home/product/Rapid_Diagnostic_Test/Anti-HCVhtml (Accessed 10/18/16). 2016.
- 21.Diagnostics S. Standard Diagnostics Bioline HIV-1/2 3.0. http://wwwstandardiacom/en/home/product/Rapid_Diagnostic_Test/Anti-HIV12html (Accessed 10/18/16). 2016.
- 22.Micallef JM Kaldor JM, Dore GJ. Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. Journal of viral hepatitis. 2006;13(1):34–41. [DOI] [PubMed] [Google Scholar]
- 23.McCurdy SA, Ross MW, Williams ML, Kilonzo GP, Leshabari MT. Flashblood: blood sharing among female injecting drug users in Tanzania. Addiction (Abingdon, England). 2010; 105(6): 1062–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zou G A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. [DOI] [PubMed] [Google Scholar]
- 25.Rubin DB. Multiple Imputation for Non-Response in Surveys. New York, NY: John Wiley & Sons; 1987. [Google Scholar]
- 26.Nelson PK, Mathers BM, Cowie B, et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet. 2011;378(9791):571–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pybus OG. The hepatitis C virus epidemic among injecting drug users. Infection, Genetics and Evolution. 2005;5:131–139. [DOI] [PubMed] [Google Scholar]
- 28.Mizokami M, Tanaka Y. Tracing the evolution of hepatitis C virus in the United States, Japan, and Egypt by using the molecular clock. Clin Gastroenterol Hepatol. 2005;3(10 Suppl 2):S82–85. [DOI] [PubMed] [Google Scholar]
- 29.Mizokami M, Tanaka Y, Miyakawa Y. Spread times of hepatitis C virus estimated by the molecular clock differ among Japan, the United States and Egypt in reflection of their distinct socioeconomic backgrounds. Intervirology. 2006;49(1-2):28–36. [DOI] [PubMed] [Google Scholar]
- 30.Rotman Y, Liang TJ. Coinfection with Hepatitis C Virus and Human Immunodeficiency Virus: Virological, Immunological, and Clinical Outcomes▿. Journal of Virology. 2009;83(15):7366–7374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thein HH, Yi Q, Dore GJ, Krahn MD Natural history of hepatitis C virus infection in HIV-infected individuals and the impact of HIV in the era of highly active antiretroviral therapy: a meta-analysis. Aids. 2008;22( 15): 1979–1991. [DOI] [PubMed] [Google Scholar]
- 32.Operskalski EA, Kovacs A. HIV/HCV Co-infection: Pathogenesis, Clinical Complications, Treatment,. Current HIV/AIDS Reports. 2015;8(1): 12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benhamou Y, Bochet M, Di Martino V, et al. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. The Multivirc Group. Hepatology. 1999;30(4):1054–1058. [DOI] [PubMed] [Google Scholar]
- 34.Pouget ER, Hagan H, Des Jarlais DC. Meta-analysis of hepatitis C seroconversion in relation to shared syringes and drug preparation equipment. Addiction (Abingdon, England). 2012; 107(6): 1057–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thorpe LE, Ouellet LJ, Hershow R, et al. Risk of hepatitis C virus infection among young adult injection drug users who share injection equipment. Am J Epidemiol. 2002;155(7):645–653. [DOI] [PubMed] [Google Scholar]
- 36.Craine N, Hickman M, Parry JV, et al. Incidence of hepatitis C in drug injectors: the role of homelessness, opiate substitution treatment, equipment sharing, and community size. Epidemiol Infect. 2009;137(9):1255–1265. [DOI] [PubMed] [Google Scholar]
- 37.Amon JJ, Garfein RS, Ahdieh-Grant L, et al. Prevalence of hepatitis C virus infection among injection drug users in the United States, 1994-2004. Clin Infect Dis. 2008;46(12):1852–1858. [DOI] [PubMed] [Google Scholar]
- 38.Hagan H, Pouget ER, Des Jarlais DC, Lelutiu-Weinberger C. Meta-regression of hepatitis C virus infection in relation to time since onset of illicit drug injection: the influence of time and place. Am J Epidemiol. 2008;168(10):1099–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Backmund M, Meyer K, Wachtler M, Eichenlaub D. Hepatitis C virus infection in injection drug users in Bavaria: risk factors for seropositivity. Eur J Epidemiol. 2003;18(6):563–568. [DOI] [PubMed] [Google Scholar]
- 40.Diaz T, Des Jarlais DC, Vlahov D, et al. Factors associated with prevalent hepatitis C: differences among young adult injection drug users in lower and upper Manhattan, New York City. Am J Public Health. 2001;91(1):23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.CDC. HIV and Viral Hepatitis. http://wwwcdcgov/hiv/pdf/library_factsheets_hiv_and_viral_hepatitispdf (Accessed September 29, 2016). March 2014.
- 42.Adjei AA, Armah HB, Gbagbo F, et al. Correlates of hepatitis C virus infection among incarcerated Ghanaians: a national multicentre study. J Med Microbiol. 2007;56(3):391–397. [DOI] [PubMed] [Google Scholar]
- 43.Heijnen M, Mumtaz GR, Abu-Raddad LJ. Status of HIV and hepatitis C virus infections among prisoners in the Middle East and North Africa: review and synthesis. Journal of the International Aids Society. 2016; 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.He T, Li K, Roberts MS, et al. Prevention of Hepatitis C by Screening and Treatment in U.S. Prisons. Annals of internal medicine. 2016;164(2):84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Varan AK, Mercer DW, Stein MS, Spaulding AC. Hepatitis C Seroprevalence Among Prison Inmates Since 2001: Still High but Declining. Public Health Rep. 2014;129(2):187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kresina TF, Lubran R. Improving Public Health Through Access to and Utilization of Medication Assisted Treatment. Int J Env Res Pub He. 2011;8(10):4102–4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lambdin BH, Mbwambo JK, Josiah RM, Bruce RD. Service integration: opportunities to expand access to antiretroviral therapy for people who inject drugs in Tanzania. J Int AIDS Soc. 2015;18(1):19936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lucas GM, Mullen BA, Weidle PJ, Hader S, McCaul ME, Moore RD. Directly administered antiretroviral therapy in methadone clinics is associated with improved HIV treatment outcomes, compared with outcomes among concurrent comparison groups. Clin Infect Dis. 2006;42(11):1628–1635. [DOI] [PubMed] [Google Scholar]
- 49.Berg KM, Litwin A, Li X, Heo M, Arnsten JH. Directly observed antiretroviral therapy improves adherence and viral load in drug users attending methadone maintenance clinics: a randomized controlled trial. Drug and alcohol dependence. 2011;113(2–3):192–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Batki SL, Gruber VA, Bradley JM, Bradley M, Delucchi K. A controlled trial of methadone treatment combined with directly observed isoniazid for tuberculosis prevention in injection drug users. Drug and alcohol dependence. 2002;66(3):283–293. [DOI] [PubMed] [Google Scholar]
- 51.Altarac D, Dansky SF. Tuberculosis treatment through directly observed therapy in a large multisite methadone maintenance treatment program: addressing the public health needs of a high-risk population. Journal of public health management and practice : JPHMP. 1995;1(4):40–47. [PubMed] [Google Scholar]
- 52.Litwin AH, Harris KA Jr., Nahvi S, et al. Successful treatment of chronic hepatitis C with pegylated interferon in combination with ribavirin in a methadone maintenance treatment program. Journal of substance abuse treatment. 2009;37(1):32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spire B, Lucas GM, Carrieri MP. Adherence to HIV treatment among IDUs and the role of opioid substitution treatment (OST). International Journal of Drug Policy. 2007;18(4):262–270. [DOI] [PubMed] [Google Scholar]
- 54.Kapadia F, Vlahov D, Wu Y, et al. Impact of drug abuse treatment modalities on adherence to ART/HAART among a cohort of HIV seropositive women. Am J Drug Alcohol Ab. 2008;34(2):161–170. [DOI] [PubMed] [Google Scholar]
- 55.Tsui JI, Evans J, Lum PJ, Hahn JA, Page K. Opioid agonist therapy is associated with lower incidence of hepatitis C virus infection in young adult persons who inject drugs. Hepatology. 2014;60:284a–284a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nolan S, Lima VD, Fairbairn N, et al. The impact of methadone maintenance therapy on hepatitis C incidence among illicit drug users. Addiction (Abingdon, England). 2014;109(12):2053–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hassan S, Mushi D, Cooke A, Mbwambo J, Lambdin BH. Increasing Antiretroviral Therapy Initiation Rates among People Who Inject Opioids in Tanzania: The Integration of Methadone and Anti-Retroviral Therapy (IMAT) Strategy. Paper presented at: International AIDS Society2017; Paris, France. [Google Scholar]
- 58.Lambdin BH, Hassan S, Mushi D, Cooke A, Mbwambo J. Viral Suppression at the First Integrated Methadone and Antiretroviral Therapy Program for People who Inject Drugs in sub-Saharan Africa. Paper presented at: International AIDS Society 2017; Paris, France. [Google Scholar]
- 59.Lambdin BH, Hassan S, Cooke A, Mbwambo J, Mushi D. Engaging People Who Use Drugs to Integrate HIV Care into Methadone Maintenance Treatment in Dar es Salaam, Tanzania Paper presented at: Harm Reduction Conference2016; San Diego, CA. [Google Scholar]
- 60.Perlis T, Des Jarlais D, Friedman S, Arasteh K, Turner C. Audiocomputerized self-interviewing versus face-to-face interviewing for research data collection at drug abuse treatment programs. Addiction (Abingdon, England). 2004;99:1–7. [DOI] [PubMed] [Google Scholar]
- 61.Koepsell T, Weiss N. Epidemiologic methods: studying the occurrence of illness. New Yorik: Oxford University Press; 2003. [Google Scholar]
- 62.Hellard M, Sacks-Davis R, Doyle J. Hepatitis C elimination by 2030 through treatment and prevention: think global, act in local networks. J Epidemiol Community Health. 2016. June 24;Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 63.Hellard M, Doyle JS, Sacks-Davis R, Thompson AJ, McBryde E. Eradication of hepatitis C infection: the importance of targeting people who inject drugs. Hepatology. 2014;59(2):366–369. [DOI] [PMC free article] [PubMed] [Google Scholar]


