Abstract
Background–
HSPGs are glycoproteins containing covalently attached heparan sulfate (HS) chains which bind to growth factors, chemokines, etc., and regulate various aspects of inflammation including cell recruitment. We previously showed that deletion of endothelial N-acetylglucosamine N-deacetylase-N-sulfotransferase-1 (Ndst1), an enzyme responsible for N-sulfation during HS biosynthesis, reduces airway allergic inflammation (AAI). Here, we investigated the importance of O-sulfation mediated by uronyl 2-O-sulfotransferase (Hs2st) in development of AAI relative to N-sulfation.
Methods–
Mice deficient in endothelial and leukocyte Hs2st (Hs2stf/fTie2Cre+) or Ndst1 (Ndst1f/fTie2Cre+) and WT mice were challenged with Alternaria alternata and evaluated for airway inflammation. Trafficking of murine eosinophils on lung endothelial cells was examined in vitro under conditions of flow.
Results–
Exposure to Alternaria decreased expression level of Hs2st in WT mice while level of Ndst1 remained unchanged. Compared to WT mice, Alternaria-challenged Hs2stf/fTie2Cre+ mice exhibited significantly increased eosinophils in the bone marrow, bronchoalveolar lavage fluid [BALF] and lung tissue associated with persistent airway hyperresponsiveness, airway mucus hypersecretion and elevated Th2 cytokines. In contrast, Alternaria-challenged Ndst1f/fTie2Cre+ mice exhibited a marked reduction in airway eosinophilia, mucus secretion and smooth muscle mass compared to WT counterparts. While BALF eotaxins were lower in Alternaria-challenged Hs2stf/fTie2Cre+ relative to WT mice, they were not reduced to background levels as in allergen-challenged Ndst1f/fTie2Cre+ mice. Trafficking of murine eosinophils under conditions of flow in vitro was similar on Hs2st-deficient and WT endothelial cells. Expression of ZO-1 in Hs2st-deficient lung blood vessels in control and allergen-challenged mice was significantly lower than in WT counterparts.
Conclusions–
Our study demonstrates that allergen exposure reduces expression of Hs2st; loss of uronyl 2-O-sulfation in endothelial and leukocyte HSPG amplifies recruitment of eosinophils likely due to a compromised vascular endothelium resulting in persistent inflammation whereas loss of N-sulfation limits eosinophilia and attenuates inflammation underscoring the importance of site-specific sulfation in HSPG to their role in AAI.
Keywords: Hs2st, Ndst1, eosinophilia, allergic asthma, trafficking, endothelial barrier
Introduction
Allergic airway inflammation (AAI) is associated with increased pulmonary recruitment of inflammatory cells, especially eosinophils, along with elevated levels of Th2 cytokines, pro-inflammatory chemokines and growth factors that together contribute to the overall pathogenesis of disease including the development of bronchoconstriction and airway hyperresponsiveness (AHR) [1]. Heparan sulfate proteoglycans (HSPGs) are ubiquitously expressed glycoproteins containing covalently attached heparan sulfate (HS) chains [2] and constitute the most abundant sulfated glycosaminoglycans in the lung parenchyma [3]. Patients with asthma exhibit elevated levels of proteoglycans, including small HSPG, with levels correlating to severity of disease and AHR [4, 5]. Further, we [6] and others [5] have demonstrated increased HSPG expression in the airways in animal models of allergic asthma. Because of their ability to bind to growth factors and their receptor tyrosine kinases as well as chemokines, interleukins, enzymes, extracellular matrix components and plasma proteins via their HS chains, HSPGs are critically involved in various physiological processes such as normal development and growth control, cell signaling and morphogenesis, cellular crosstalk, organization of basement membrane barriers, nutritional metabolism, injury and wound repair [7], and antibacterial innate immunity [8]. Further, these sulfated glycans play an important role in regulating molecular and cellular events during inflammatory responses [9] by participating in leukocyte trafficking on inflamed endothelium, transendothelial migration and extravasation to sites of inflammation [10, 11].
We have previously shown that targeted inactivation of endothelial N-acetylglucosamine N-deacetylase-N-sulfotransferase-1 (Ndst1), an enzyme that is responsible for N-deacetylation/N-sulfation of N-acetyl glucosamine residues of the HS backbone [12], resulting in reduced overall sulfation leads to decreased eosinophil recruitment, inflammation and airway remodeling in experimental models of acute and chronic allergic asthma [6, 13]. These studies clearly indicate a pro-inflammatory role for endothelial-expressed HSPG, and specifically the importance of N-sulfation, in the development of AAI. Since the pattern of sulfation of the glycan residues of the HS backbone is a crucial determinant of HSPG function in biological processes, we examined the role of heparan sulfate 2-O-sulfotransferase (Hs2st), another enzyme in the HS biosynthetic pathway which catalyzes the transfer of sulfate to the C2-position of selected hexuronic acid residues within the HS chain [12], during experimental AAI in the current study. We demonstrate that allergen exposure reduces expression of Hs2st in the lungs and that altered 2-O-sulfation in the HS backbone of endothelial HSPG in mice deficient in leukocyte and endothelial Hs2st leads to enhanced recruitment of eosinophils associated with persistent airway inflammation during allergic asthma.
Materials and Methods
Mouse model of AAI, sample collection and analysis
Hs2stf/fTie2Cre+ and Ndst1f/fTie2Cre+ mice fully backcrossed on C57Bl/6 background were generated as described previously [14, 15]. Hs2stf/fTie2Cre+, Ndst1f/fTie2Cre+ and wild-type (WT) C57BL/6 mice (male and female, 8–12 weeks) were administered with 50 μg of an extract of the fungal allergen Alternaria alternata (Greer® Laboratories, Inc., Lenoir, NC) in 50 μl PBS or with PBS alone (control) intranasally on days 0, 3 and 6 under anesthesia as described previously [16]. Mice were sacrificed 24 h after the last challenge. Bronchoalveolar lavage fluid (BALF), lungs, blood and bone marrow (BM) were collected. Differential cell counts were determined as described previously [17]. BALF supernatants were stored at −80ºC for later analysis. Right lungs were snap-frozen and left lungs were perfused with 4% paraformaldehyde to preserve pulmonary structure, fixed in 4% paraformaldehyde and paraffin-embedded. All animal studies were performed following standards and procedures approved by the Institutional Animal Care and Use Committee at the University of Minnesota.
Measurement of airway responsiveness
Pulmonary function was assessed by invasive technique in control and Alternaria-challenged WT and Hs2stf/fTie2Cre+ mice using FinePointe™ RC system (Buxco, Wilmington, NC) as described in our previous studies [18]. Changes in pulmonary resistance (RL) and dynamic lung compliance (Cdyn) were monitored continuously in response to saline followed by increasing concentrations of inhaled methacholine (3–50 mg/ml) nebulized for 18–20 seconds and expressed as percent baseline following each dose of methacholine.
Lung histology
Paraffin-embedded tissue sections (4 μm thick) were stained with Harris Modified Hematoxylin and Shandon Instant Eosin (H & E, Thermo Fisher Scientific Co., Pittsburgh, PA) to determine cellular infiltration. Analysis of lung tissue for infiltrated eosinophils was performed by immunohistochemical staining of sections for eosinophil-specific major basic protein (MBP) with rat mAb against murine MBP as described in our previous studies [13]. Positively stained cells (reddish brown) in the lung sections were counted (at 400× magnification) in randomly selected non-overlapping fields and expressed as the average number of MBP-positive cells/microscopic field. In addition, the number of blood vessels with adherent eosinophils was enumerated in each lung section and expressed as a percentage of the total number of blood vessels (excluding very small vessels). Next, the number of MBP-positive cells adherent on the endothelium in each of these blood vessels was counted and expressed as number of adherent eosinophils per vessel as described previously [17]. Expression of the tight junction protein ZO-1 in the vascular endothelium of peribronchial blood vessels was evaluated by immunofluorescent staining with rabbit polyclonal antibodies against ZO-1 (10 μg/ml, Santa Cruz Biotechnology, Inc.) with rabbit IgG as a control antibody. The number of peribronchial blood vessels in non-overlapping microscopic fields of the entire section was counted under a confocal microscope (FLUOVIEW FV1000/BX61, Olympus, Melville, NY, at 600× magnification) and the number of these vessels positive for expression of ZO-1 was identified. Results were expressed as percent ZO-1-positive blood vessels. To detect airway mucus production, deparaffinized lung sections were stained with Periodic acid-Schiff’s (PAS) reagent (Sigma Chemical Co.). PAS-positive areas (dark pink) in horizontally sectioned airways were quantitated using ImageJ image analysis program [19] and expressed as μm2 PAS-positive area/100 μm basement membrane length (BML) [18]. Expression of α-smooth muscle actin (α-SMA) was evaluated by immunohistochemistry using mAb against α-SMA (0.25 μg/ml, Sigma-Aldrich) and area of the positively-stained (brown) peribronchial smooth muscle layer was quantitated from captured images using ImageJ image analysis software as described [20]. To determined perivascular inflammation, inflammatory infiltrates around all peribronchial blood vessels in H & E stained lung sections were assessed (at 400× magnification). The maximum number of rows of inflammatory cells in the perivascular space was counted for each vessel. In all cases, stained slides were examined using a Nikon Microphot EPI-FL microscope and images were captured with an Olympus DP71 camera.
Measurement of lung cytokines and chemokines
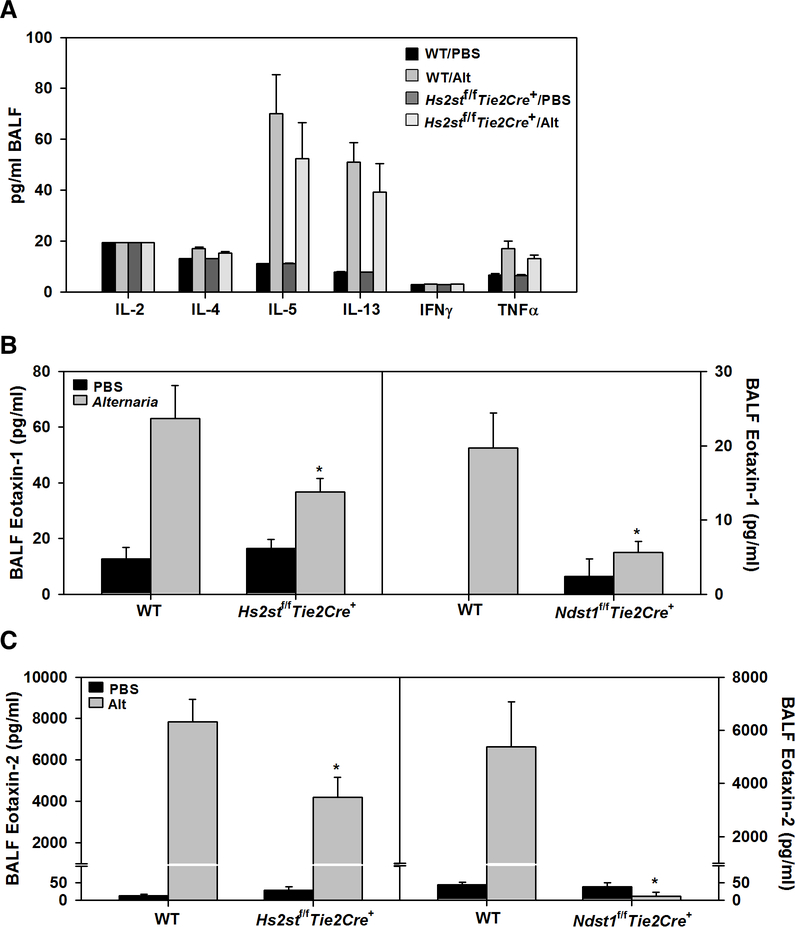
Th1 (IL-2, IFN-γ)/Th2 (IL-4, IL-5, IL-13) cytokine and TNFα levels in BALF were determined by flow cytometry using CBA Flex Set kits (BD Biosciences) according to the manufacturer and as described in our previous studies [18]. Results were expressed as pg/ml BALF. Eotaxin-1(CCL11) and eotaxin-2 (CCL24) in the BALF were measured using ELISA kits (R & D Systems, Minneapolis, MN) according to the manufacturers’ recommendations.
Quantitative real-time PCR (qPCR)
Total RNA was extracted from cells with TRIzol® and reverse-transcribed into cDNA using the iScript™ cDNA synthesis kit (Bio-Rad, Hercules, CA) according to the manufacturer’s protocol. Expression levels of Ndst1 and Hs2st in the lung tissue were measured by qPCR using previously published primers specific for each [21]. qPCR was performed using iTaq™ Universial SYBR® Green Supermix 200 (Bio-Rad) and carried out in a iQ™5 multicolor real-time PCR detection System (Bio-Rad). After initial denaturation, conditions for gene amplification were as follows: 50 cycles with 45 sec at 92ºC followed by 45 sec at 55°C each cycle. The amount of Ndst1 or Hs2st mRNA in each sample was calculated based on its threshold cycle, Ct, suggested by the software (IQ™5 Optical System software) after subtraction of the Ct of the housekeeping gene GAPDH. Results are expressed as fold change in expression relative to expression in control lungs.
Western blot
Lung tissue was homogenized in radioimmunoprecipitation assay (RIPA) buffer and total protein in the supernatants was measured (BCA Protein Assay Kit, Thermo Fisher Scientific Co.). Expression of Hs2st in lung lysates was evaluated using a 10% SDS polyacrylamide gel with 80 μg protein loaded per lane. After transfer to PVDF membrane, mAb against Hs2st (2 μg/ml, R and D Systems, Minneapolis, MN) followed by HRP-conjugated goat anti-mouse IgG (0.2 μg/ml, Jackson ImmunoResearch Laboratories, Inc, West Grove, PA) were used for detection of Hs2st. HRP-conjugated anti-mouse β-actin (0.04 μg/ml, Santa Cruz Biotechnology, Inc.) was used to monitor levels of β-actin expression in lung tissue as an internal control. Bound antibodies were detected using WesternBright ECL HRP substrate (Advansta Corporation, Menlo Park, CA) and bands were visualized on X-ray films. Intensity of the detected band for Hs2st (~42 kDa band [22]) was quantified using ImageJ image analysis software and the expression level was normalized against that of β-actin.
Flow chamber studies
Eosinophils were cultured from BM of WT mice as described previously [23, 24]. Lung endothelial cells isolated from WT and Hs2stf/fTie2Cre+ mice as described previously [15] were cultured to confluence on gelatin-coated glass coverslips. Cells were treated with murine TNFα (50 ng/ml, BD Pharmingen) for 6 h at 37°C prior to placement in the flow chamber for dynamic flow studies (1 ml/min; wall shear stress ~1.0–2.0 dynes/cm2) as described previously [13, 14]. BM-derived murine eosinophils were perfused into the flow chamber for a period of 5 min. Interaction of the infused eosinophils with endothelial cells from WT versus Hs2stf/fTie2Cre+ mice on the cover-slips was observed using a Leitz Wetzlar inverted microscope and recorded for subsequent offline analysis to manually determine the number of interacting cells [25].
Statistical analysis
Data are presented as mean ± SEM. For in vitro studies, statistical significance between groups was determined using a two-tailed unpaired Student’s t-test. For in vivo studies, a two-tailed test was used to establish a statistically significant difference in recruitment of total inflammatory cells between allergen-challenged WT and Hs2stf/fTie2Cre+ or Ndst1f/fTie2Cre+ mice. A one-tailed test was used for all other analyses. A p value ≤0.05 was considered as significant.
Results
Allergen challenge alters expression of Hs2st.
We have previously demonstrated that Ndst1 plays a pro-inflammatory role during allergic asthma, wherein inactivation of leukocyte and endothelial Ndst1 decreased airway eosinophil recruitment, inflammation and remodeling in experimental models of acute and chronic ovalbumin (OVA)-induced allergic asthma [6, 13]. Expression levels of HSPG biosynthetic enzyme can vary under inflammatory and pathological conditions [26–28]. We first examined lung expression level of Ndst1 and Hs2st in a murine model of AAI induced by a physiologically relevant fungal allergen Alternaria alternata. Analysis of lung tissue from control and Alternaria-challenged WT mice by qPCR revealed a significant reduction in Hs2st mRNA in lungs of Alternaria-challenged mice compared to the control group (Fig. 1, A). Expression of Ndst1 mRNA, on the other hand, was not altered. Evaluation of Hs2st at the protein level by Western blot analysis of lung tissue lysates followed by densitometry confirmed decreased expression of Hs2st in Alternaria-challenged mice compared to control mice (Fig. 1, B).
Fig. 1. Allergen challenge alters expression of Hs2st.
(A) Hs2st and Ndst1 gene expression in the lungs of Alternaria-challenged WT mice by qPCR. Alt: Alternaria. (B) Hs2st protein expression in lungs of control and Alternaria-challenged WT mice by Western blot analysis using mAb against Hs2st. A representative Western blot with Hs2st expression in two mice from each group (PBS1, PBS2, Alt1, Alt2) is shown below graph. Data represent mean ± SEM. n = 5–6 mice/group in A and 4–5 mice/group in B. *p<0.01 in A versus control (PBS-challenged) mice.
Divergent airway cellular inflammation in Hs2stf/fTie2Cre+ versus Ndst1f/fTie2Cre+ mice in response to allergen challenge.
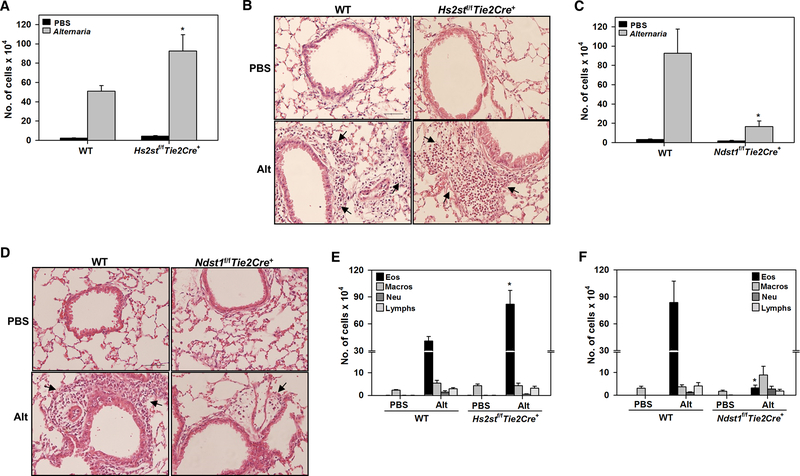
To further understand the significance of decreased Hs2st expression noted in Alternaria-challenged mice and examine the relative importance of Hs2st-mediated 2-O-sulfation versus Ndst1-mediated N-sulfation to the role played by endothelial and leukocyte HSPG in eosinophil recruitment during AAI, mice deficient in expression of endothelial and leukocyte Hs2st (Hs2stf/fTie2Cre+) or Ndst1 (Ndst1f/fTie2Cre+) were used. Hs2stf/fTie2Cre+, Ndst1f/fTie2Cre+ and WT mice were challenged with Alternaria and evaluated for AAI. As expected, WT mice-challenged with Alternaria exhibited an increase in the total number of inflammatory cells in the BALF compared to control mice. Interestingly, the number of inflammatory cells in the BALF of Alternaria-challenged Hs2stf/fTie2Cre+ mice was significantly higher compared to Alternaria-challenged WT mice (Fig. 2, A). Consistent with this finding, histological evaluation showed that peribronchial inflammation in response to allergen challenge was amplified in Hs2stf/fTie2Cre+ mice relative to corresponding WT (Fig. 2, B). On the other hand, the number of cells in the BALF as well as peribronchial inflammation in Alternaria-challenged Ndst1f/fTie2Cre+ mice was markedly reduced compared to WT counterparts (Fig, 2, C and D). Differential cell counts indicated that the number of allergen-induced eosinophils in the BALF of Hs2stf/fTie2Cre+ mice was significantly higher compared to WT mice (Fig. 2, E), while the number of eosinophils in Ndst1f/fTie2Cre+ mice was drastically lower (Fig. 2, F). No significant differences were noted with respect to the number of macrophages, neutrophils and lymphocytes between allergen-challenged Hs2stf/fTie2Cre+ or Ndst1f/fTie2Cre+ mice and corresponding WT mice.
Fig. 2. Divergent airway cellular inflammation in allergen-challenged Hs2stf/fTie2Cre+ and Ndst1f/fTie2Cre+ mice.
(A) Total cell counts in the BALF of control and Alternaria-challenged Hs2stf/fTie2Cre+ mice relative to corresponding WT mice. (B) H & E staining of lung tissue from mice identified in A. Representative image for each group is shown. Arrows indicate infiltrating inflammatory cells. Scale bar, 50 μm. (C) Total cell counts in the BALF of control and Alternaria-challenged Ndst1f/fTie2Cre+ mice relative to corresponding WT mice. (D) H & E staining of lung tissue from mice identified in C. Representative image for each group is shown. Arrows indicate infiltrating inflammatory cells. Scale bar, 50 μm. (E and F) Differential cell counts in the BALF of control and Alternaria-challenged Hs2stf/fTie2Cre+ and Ndst1f/fTie2Cre+ mice relative to corresponding WT mice, respectively. Eos; eosinophils, Macros; macrophages, Neu; neutrophils, Lymphs; lymphocytes. Data represent mean ± SEM. n=4–7 mice for PBS groups and n=8–10 mice for Alternaria groups *p<0.05 in A, <0.01 in B, < 0.04 in E and <0.01 in F versus Alternaria-challenged WT mice.
Hs2stf/fTie2Cre+ mice have increased eosinophilia while Ndst1f/fTie2Cre+ mice exhibit decreased eosinophilia after allergen challenge.
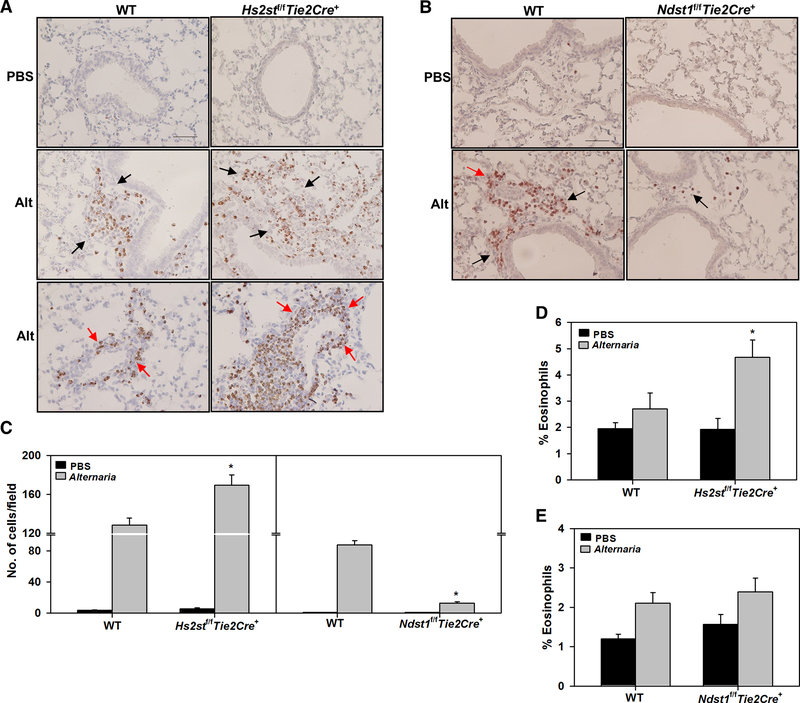
We next examined eosinophil infiltration in the lung tissue by immunohistochemical staining for eosinophil-specific MBP. Very few MBP-positive cells were detected in the lung tissue of PBS-exposed control Hs2stf/fTie2Cre+, Ndst1f/fTie2Cre+ and WT mice (Fig. 3, A and B, upper panels). WT mice exhibited notable infiltration of eosinophils around airways and peribronchial blood vessels after exposure to Alternaria (Fig. 3, A, left middle and lower panels). Compared to allergen-challenged WT mice, allergen-challenged Hs2stf/fTie2Cre+ mice showed enhanced eosinophil infiltration in the lungs (Fig. 3, A, right middle and lower panels). Consistent with our previous finding of decreased OVA-induced eosinophilia in Ndst1f/fTie2Cre+ mice [13], Ndst1f/fTie2Cre+ mice challenged with Alternaria exhibited significantly reduced pulmonary eosinophilia compared to WT counterparts (Fig. 3, B, lower panels). Quantitaion of MBP-positive cells in the lung tissue confirmed the enhanced eosinophilia in allergen-challenged Hs2stf/fTie2Cre+ and the marked reduction in eosinophilia in Ndst1f/fTie2Cre+ mice versus corresponding WT mice (Fig. 3, C, left and right panels, respectively). Along with the amplified airway eosinophilia in allergen-challenged Hs2stf/fTie2Cre+ mice, a significantly higher number of eosinophils were detected in the BM of these mice relative to allergen-challenged WT mice (Fig. 3, D). In contrast, the number of BM eosinophils after allergen challenge was similar in Ndst1f/fTie2Cre+ and WT mice (Fig. 3, E).
Fig. 3. Allergen-challenged Hs2stf/fTie2Cre+ mice exhibit increased airway eosinophilia.
(A and B) Infiltrated eosinophils in lung tissue of control and Alternaria-challenged Hs2stf/fTie2Cre+ and Ndst1f/fTie2Cre+ mice, respectively, relative to corresponding WT mice detected by immunohistochemical staining for MBP (stained dark brown). Representative image for each group is shown. Black arrows; eosinophils around airways, red arrows; eosinophils around peribronchial blood vessels. Scale bar, 50 μm. (C) Quantitation of MBP-positive cells in randomly selected non-overlapping microscopic fields of lung tissue sections from control and Alternaria-challenged Hs2stf/fTie2Cre+ (left panel) and Ndst1f/fTie2Cre+ mice (right panel) relative to corresponding WT mice at 400× magnification. (D and E) Percentage of eosinophils in the BM of control and Alternaria-challenged Hs2stf/fTie2Cre+ and Ndst1f/fTie2Cre+ mice, respectively, relative to corresponding WT mice determined based on cell morphology after Hema3 staining. Data represent mean ± SEM. n=5–7 mice/group in C, 5–8 mice for PBS groups and 8–11 mice for Alternaria groups in D and E. *p<0.01 (left panel) and <0.001 (right panel) in C, < 0.05 in D versus Alternaria-challenged WT mice.
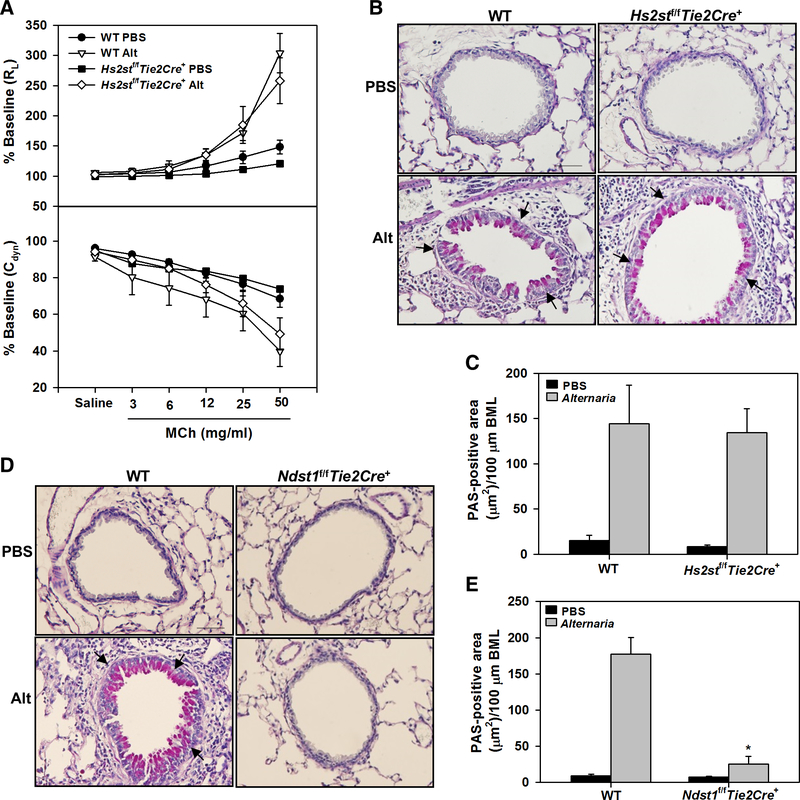
Persistent AHR, airway mucus hypersecretion and airway smooth muscle hypertrophy/hyperplasia in allergen-challenged Hs2stf/fTie2Cre+ mice.
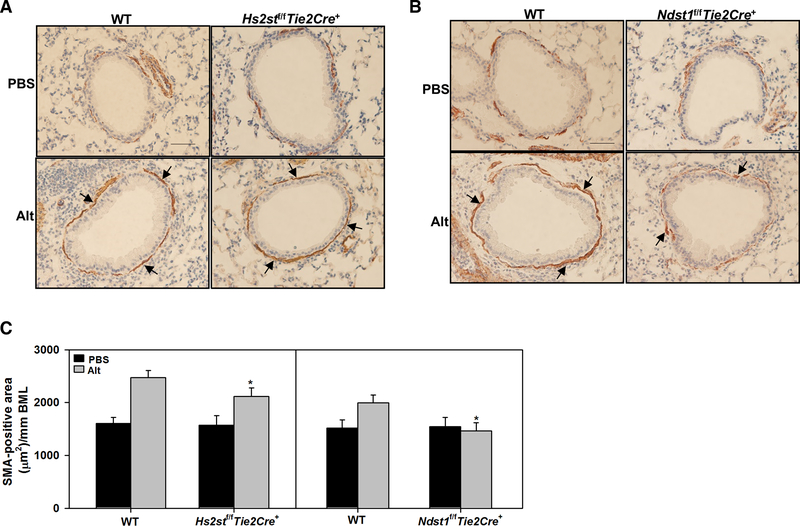
AHR is one of the hallmark features of allergic asthma. To assess airway responsiveness, pulmonary resistance and dynamic lung compliance in response to increasing concentrations of aerosolized methacholine was evaluated in Alternaria-challenged Hs2stf/fTie2Cre+ and WT mice. RL and Cdyn in control mice of both genotypes were similar. Allergen-challenged Hs2stf/fTie2Cre+ mice displayed elevated AHR with increased RL and decreased Cdyn which was similar to that noted in WT counterparts (Fig. 4, A). This is in contrast to Ndst1f/fTie2Cre+ mice [13]; while AHR was not assessed in Alternaria-challenged Ndst1f/fTie2Cre+ mice in the current study, our previous studies using OVA-challenged Ndst1f/fTie2Cre+ mice have shown that AHR in these mice is significantly lower than in WT mice. AHR can be brought on by structural changes in the airways such as increased mucus production that contributes to airflow obstruction [29]. Along with persistent AHR, allergen-challenged Hs2stf/fTie2Cre+ exhibited increased airway mucus secretion similar to that observed in allergen-challenged WT mice (Fig. 4, B and C). On the contrary, airway mucus production in Alternaria-challenged Ndst1f/fTie2Cre+ mice was almost completely inhibited and markedly lower than in corresponding WT mice (Fig. 4, D and E). Another contributory factor to AHR is airway smooth muscle hypertrophy/hyperplasia [30]. Although airway smooth muscle mass in Alternaria-challenged Hs2stf/fTie2Cre+ and Ndst1f/fTie2Cre+ mice was lower than in corresponding WT mice (Fig 5, A and B), the reduction in airway smooth muscle hypertrophy in Alternaria-challenged Hs2stf/fTie2Cre+ mice was small unlike Alternaria-challenged Ndst1f/fTie2Cre+ mice wherein airway smooth muscle mass was reduced to levels noted in control mice (Fig. 5, C, left and right panel, respectively). Thus, increased airway mucus production and airway smooth muscle mass in Alternaria-challenged Hs2stf/fTie2Cre+ mice along with the increased cellular inflammation may contribute to persistent AHR as noted in allergen-challenged WT mice.
Fig. 4. Persistent AHR and airway mucus in allergen challenged Hs2stf/fTie2Cre+ mice.
(A) Measurement of pulmonary resistance (RL) and dynamic lung compliance (Cdyn) in mechanically ventilated control and Alternaria-challenged Hs2stf/fTie2Cre+ and WT mice following exposure to increasing concentrations of aerosolized methacholine (MCh). (B and C) Airway mucus secretion in control and Alternaria-challenged Hs2stf/fTie2Cre+ and WT mice assessed by PAS staining (stained dark-pink, black arrows) and quantitation of the PAS-positive area, respectively. A representative image for each group is shown in B. Scale bar, 50 μm. (D and E) Airway mucus secretion in control and Alternaria-challenged Ndst1f/fTie2Cre+ and WT mice assessed as described in B and quantitation of the PAS-positive area, respectively. A representative image is shown for each group in D. Scale bar, 50 μm. Data represent mean ± SEM. n=6–7 mice for PBS groups and 6–9 for Alternaria groups *p<0.01 in E versus Alternaria-challenged WT mice.
Fig. 5. Persistent airway smooth muscle hypertrophy in allergen-challenged Hs2stf/fTie2Cre+ mice.
(A and B) Airway smooth muscle mass in control and Alternaria-challenged Hs2stf/fTie2Cre+ and Ndst1f/fTie2Cre+ mice, respectively, relative to corresponding WT mice assessed by immunohistochemical staining for α-SMA (stained brown, black arrows). A representative image for each group is shown. Scale bar, 50 μm. (C) Quantitation of the α-SMA-stained area in control and Alternaria-challenged Hs2stf/fTie2Cre+ (left panel) and Ndst1f/fTie2Cre+ mice (right panel) relative to corresponding WT mice. Data represent mean ± SEM. n=5–6 mice for PBS groups and 6–8 mice for Alternaria groups. *p<0.05 (left panel) and <0.01 (right panel) in C versus Alternaria-challenged WT mice.
Cytokine and chemokine levels in allergen-challenged Hs2stf/fTie2Cre+ mice
AAI is driven by elevated levels of Th2 cytokines in the lungs [31]. Our previous studies have shown that OVA-challenge induces IL-5 in Ndst1f/fTie2Cre+ mice but at significantly lower levels compared to corresponding WT mice [13]. IL-4 and IL-13 levels on the other hand were found to be similar in both groups of allergen-challenged mice. In the current study, Alternaria-challenge induced IL-5 to a similar level in allergen-challenged Hs2stf/fTie2Cre+ mice and WT mice (Fig. 6, A). Level of IL-4, IL-13 and the pro-inflammatory cytokine TNFα were also similar in these two groups. Eotaxin-1 and −2 are critical players in eosinophil recruitment and pulmonary eosinophilia [32]. We anticipated that eotaxin levels in Hs2stf/fTie2Cre+ mice may be higher than in WT mice in response to allergen exposure and thus lead to increased eosinophilia as noted in Figs. 2 and 3. Exposure to Alternaria induced expression of eotaxin-1 and −2 in the BALF of WT and Hs2stf/fTie2Cre+ mice but not in Ndst1f/fTie2Cre+ mice. However, level of both chemokines was significantly lower in allergen-challenged Hs2stf/fTie2Cre+ mice compared to corresponding WT mice (Fig. 6, B and C). This suggests that the amplified airway eosinophilia in allergen-challenged Hs2stf/fTie2Cre+ mice relative to WT mice may not be linked to eotaxin levels alone.
Fig. 6. Th1-Th2 cytokines and eotaxins in BALF.
(A) Th1-Th2 cytokine and TNFα levels in BALF of control and Alternaria-challenged Hs2stf/fTie2Cre+ and WT mice. (B and C) Eotaxin-1 and eotaxin-2 levels, respectively, in the BALF of control and Alternaria-challenged Hs2stf/fTie2Cre+ (left panels) and Ndst1f/fTie2Cre+ mice (right panels) relative to corresponding WT mice. Data represent mean ± SEM. n=6–7 mice for PBS groups and 6–9 mice for Alternaria groups. *p<0.04 (left panel) and <0.03 (right panel) in B and <0.02 in C versus Alternaria-challenged WT mice.
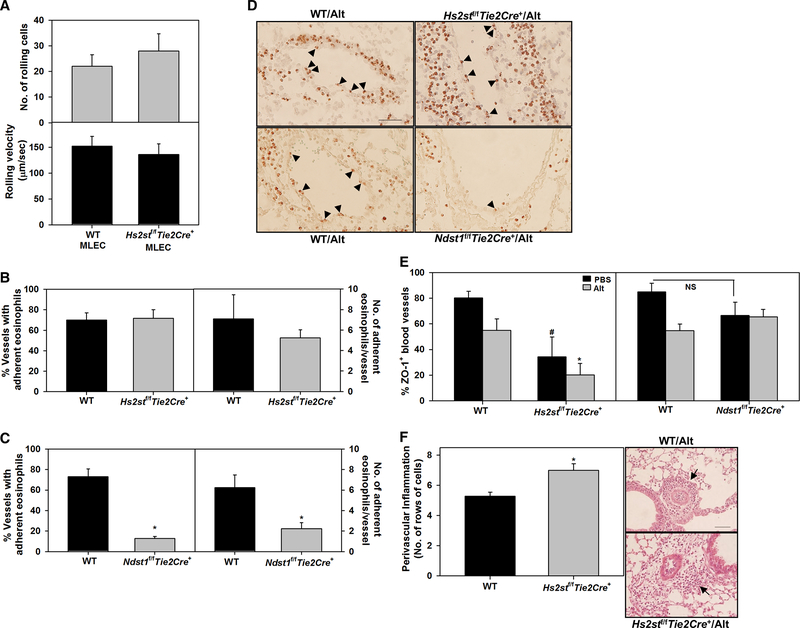
Eosinophil rolling on Hs2st-deficient endothelial cells
Under conditions of shear flow, eosinophil trafficking involves a multi-step paradigm mediated by cell adhesion molecules on leukocytes and endothelial cells of which rolling is an initial step [33]. In previous studies, WT neutrophils were found to roll with lower velocity and in greater numbers on Hs2stf/fTie2Cre+ endothelial cells than on WT cells and thus contribute to increased accumulation of neutrophil during acute inflammation [14]. We examined whether a similar phenomenon might be responsible for the amplified eosinophilia in allergen-challenged Hs2stf/fTie2Cre+ mice. Unlike neutrophils, the number of rolling cells and rolling velocity of WT eosinophils was similar on WT and Hs2stf/fTie2Cre+ mouse lung endothelial cells under shear stress in flow chamber assays (Fig. 7, A). This is in contrast to eosinophil rolling on Ndst1f/fTie2Cre+ mouse lung endothelial cells, wherein WT eosinophils have been shown to exhibit significantly reduced rolling on mutant cells relative to WT endothelial cells [13]. Further, in the current study, blood vessels in the lungs of allergen-challenged Hs2stf/fTie2Cre+ mice supported cell adhesion to the vessel wall in a manner similar to WT mice while blood vessels in the lungs of allergen-challenged Ndst1f/fTie2Cre+ mice did not. The number of blood vessels with adherent eosinophils (MBP-positive cells) and number of adherent eosinophils per blood vessel in the lungs of allergen-challenged Hs2stf/fTie2Cre+ and WT mice were similar, while they were significantly lower in Ndst1f/fTie2Cre+ mice than in corresponding WT mice (Fig. 7, B-D).
Fig. 7. Eosinophil rolling and adhesion to Hs2stf/fTie2Cre+ endothelium.
(A) Eosinophil rolling on cultured primary mouse lung endothelial cells (MLEC) from WT and Hs2stf/fTie2Cre+ mice assessed in vitro in a flow chamber under shear stress of 1–2 dynes/cm2. (B and C) Quantitation of the number of lung blood vessels with adherent eosinophils (MBP-positive cells, left panels) and the number of adherent eosinophils in the blood vessels (right panels) in Alternaria-challenged Hs2stf/fTie2Cre+ and Ndst1f/fTie2Cre+ mice relative to corresponding WT mice, respectively. (D) Representative images of adherent eosinophils (black arrowheads) in lung blood vessels of Alternaria-challenged Hs2stf/fTie2Cre+ and Ndst1f/fTie2Cre+ mice relative to corresponding WT mice. (E) Quantitation of ZO-1-positive peribronchial blood vessels in control and Alternaria-challenged Hs2stf/fTie2Cre+, Ndst1f/fTie2Cre+ and WT mice. 8–21 vessels were analyzed/mouse. (F) Semi-quantitative analysis of perivascular inflammation in lung sections from Alternaria-challenged Hs2stf/fTie2Cre+ versus corresponding WT mice. The maximum number of rows of inflammatory cells around each peribronchial blood vessel (total number of blood vessels, 132 for Hs2stf/fTie2Cre+ and 136 for WT mice) was counted at 400× magnification for each group. A representative image for each group is shown. Data represent mean ± SEM. Combined data of n = 5 independent experiments is shown in A. n = 4–6 mice/group in B and C and 6–8 mice/group in E and F. *p<0.01 (left panel) and <0.02 (right panel) in C and <0.01 in E and F versus Alternaria-challenged WT mice. #p<0.05 versus control (PBS) WT in E. NS; Not statistically significant.
As indicated earlier, infiltration of eosinophils was noted not only around airways but also peribronchial blood vessels after exposure to Alternaria. To determine whether a breakdown in endothelial barriers might contribute to increased eosinophil recruitment, we examined expression of the endothelial tight junction protein ZO-1 in peribronchial blood vessels of allergen-challenged Hs2stf/fTie2Cre+, Ndst1f/fTie2Cre+ and WT mice. The number of ZO-1-positive peribronchial blood vessels was lower in Alternaria-challenged WT mice compared to control mice (Fig. 7, E, left and right panels). Interestingly, the number of ZO-1-positive peribronchial blood vessels in control (non-allergen exposed) and Alternaria-challenged Hs2stf/fTie2Cre+ mice was significantly lower than in WT counterparts (Fig. 7, E, left panel). These data suggest that the vascular endothelium in Hs2stf/fTie2Cre+ mice may be defective/compromised. In contrast, the number of ZO-1-positive peribronchial blood vessels in control and allergen-challenged Ndst1f/fTie2Cre+ mice was essentially similar to that observed in their WT counterparts (Fig. 7, E, right panel). Additionally, semi-quantitative analysis of perivascular inflammation around blood vessels in H&E stained lung tissue sections from allergen-challenged Hs2stf/fTie2Cre+ and WT mice indicated increased inflammation around peribronchial blood vessels in allergen-challenged Hs2stf/fTie2Cre+ relative to WT mice (Fig. 7, F), further supporting the notion of a compromised vascular endothelium caused by decreased ZO-1 expression which could potentially lead to increased cellular inflammation.
Discussion
HSPGs play a multifunctional role in inflammation largely due to their highly diverse HS chains that interact with a variety of ligands, including pro-inflammatory mediators such as cytokines, chemokines and growth factors [10, 11]. Increased HSPG deposition has been noted in patients with asthma [4, 5] and in experimental models of allergic asthma [5, 6] correlating with disease severity. HS chains of HSPGs can be highly heterogeneous with respect to length as well as the degree and pattern of sulfation, which are critical determinants of ligand binding and functional role during inflammation [12, 34]. In the current study, we found that expression of Hs2st (mRNA and protein) is decreased in WT mice after exposure to Alternaria alternata, while expression of Ndst1 was unaltered. Expression levels of HSPG biosynthetic enzymes are known to be altered during inflammatory conditions. For example, inflammatory cytokines such as IFNγ and TNFα can induce Ndst1 expression in endothelial cells increasing sulfation of HS along with a corresponding increase in sequestration of RANTES at the apical surface of endothelial cells and leukocyte chemotaxis [26]. In the context of AAI, previous studies have shown that expression of xylosyltransferase-1, β1,3-glucuronosyltransferase-1, chondroitin-4, and chondroitin-6 sulfotransferase genes is increased in OVA-challenged rats [5].
To further understand the significance of decreased Hs2st expression observed in our study, we used mice lacking endothelial and leukocyte Hs2st or Ndst1. The most striking finding was the divergent outcome on cellular inflammation in the lungs of these mice. Allergen-challenged Hs2stf/fTie2Cre+ mice demonstrated significantly increased cellular inflammation, specifically of eosinophils, while allergen-challenged Ndst1f/fTie2Cre+ mice exhibited minimal cellular inflammation with markedly reduced airway eosinophils compared to respective allergen-challenged WT mice. Along these lines, our collaborative studies have previously shown that targeted disruption of endothelial Hs2st enhances neutrophil recruitment during acute inflammation [14]. On the other hand, inactivation of endothelial Ndst1 resulted in decreased neutrophil infiltration in a similar model [15]. These divergent findings in Hs2stf/fTie2Cre+ versus Ndst1f/fTie2Cre+ mice in inflammation models were found to be due to altered HS-ligand interactions [14, 15]. Although eosinophil recruitment in the lungs of Alternaria-challenged Hs2stf/fTie2Cre+ mice was significantly higher than in corresponding WT mice, other pathophysiological features of allergen-induced inflammation such as AHR, airway mucus secretion, smooth muscle mass and Th2 cytokine levels were comparable between the two groups. In contrast, Alternaria-challenged Ndst1f/fTie2Cre+ mice had marked attenuation of airway mucus secretion and smooth muscle proliferation relative to WT mice. Further, our previous studies have shown that AHR and IL-5 are both significantly lower in allergen-challenged Ndst1f/fTie2Cre+ mice, albeit in a model of OVA-induced allergic asthma [13]. Based on observations in WT mice, OVA [13] and A. alternata induce a similar immune response at least with respect to the prominent antigen-induced responses, i.e., AHR, eosinophilia, elevated Th2 cytokines and eotaxin-1, airway mucus hypersecretion and smooth muscle hypertrophy.
Since eotaxins play a dominant role in eosinophil recruitment in experimental asthma [32] and Alternaria-challenged Hs2stf/fTie2Cre+ mice exhibited exaggerated airway eosinophil recruitment relative to corresponding WT mice, we anticipated that levels of eotaxin-1 and/or eotaxin-2 in these mice might be elevated compared to WT counterparts. Surprisingly, levels of both chemokines in allergen-challenged Hs2stf/fTie2Cre+ mice were lower than in WT mice but not reduced to background levels as noted in allergen-challenged Ndst1f/fTie2Cre+ mice. While they may not be entirely accountable for the increased eosinophilia, eotaxin-1 and −2 levels in allergen-challenged Hs2stf/fTie2Cre+ mice were substantially higher than in control mice and may contribute in part to overall eosinophil recruitment. While endothelial HSPGs bind to and present various chemokines on the luminal surface to facilitate leukocyte transmigration [10], studies have shown that eotaxin binds selectively to heparin but not to HS [35]. Since Hs2st expression in endothelial cells and myeloid cells is targeted in Hs2stf/fTie2Cre+ mice, changes in eosinophil HS or other leukocyte HS could contribute to some of the effects noted. For example, reduced eotaxin levels observed in airways of Alternaria-challenged Hs2stf/fTie2Cre+ and Ndst1f/fTie2Cre+ mice may be due to reduced expression of this chemokine by Hs2st- or Ndst1-deficient inflammatory cells (e.g., Th2 cells, alveolar macrophages) or by other eotaxin-producing cells such as epithelial cells and fibroblasts affected indirectly by the mutation. Along these lines, we have previously shown that Ndst1-deficient eosinophils express significantly lower amounts of TGFβ1 mRNA relative to WT eosinophils upon activation [6].
In addition to chemokine presentation, HSPG also function as ligands for L-selectin [10]. Neutrophils have been shown to roll with reduced velocity in greater numbers on Hs2st-deficient endothelial cells than on WT cells under flow (in vitro) due to stronger binding of neutrophil L-selectin to mutant endothelial cells, thus contributing to increased neutrophil accumulation during acute inflammation [14]. Unlike neutrophils, we found that BM eosinophils did not differ in rolling velocity or the number of rolling cells on Hs2st-deficient endothelial cells relative to WT cells under flow in the current study. On the other hand, eosinophils exhibit reduced rolling on endothelial cells from Ndst1f/fTie2Cre+ mice compared with WT cells under flow in vitro and in vivo correlating with decreased airway eosinophil recruitment as shown in our previous studies [13]. We examined whether eosinophils adhered in greater numbers in lung blood vessels of allergen-challenged Hs2stf/fTie2Cre+ mice which could facilitate enhanced recruitment. Eosinophil adhesion to the vessel wall in the lungs of allergen-challenged Hs2stf/fTie2Cre+ mice was similar to that noted in WT mice while eosinophil adhesion to lung blood vessels of allergen-challenged Ndst1f/fTie2Cre+ mice was significantly lower both in terms of the number of blood vessels with adherent cells and number of adherent eosinophils per blood vessel.
Overall, deficiency of endothelial Hs2st does not appear to affect eosinophil rolling under flow or adhesion to allergen-challenged lung blood vessels and yet results in exaggerated recruitment in the lung tissue despite lower eotaxin-1 and −2 levels. A contributory factor may be decreased endothelial barrier function in Hs2stf/fTie2Cre+ mice that innately and after allergen exposure express reduced levels of endothelial ZO-1 relative to WT mice. Compromised endothelial barrier function, together with persistent levels of eotaxin-1 and −2 in allergen-challenged airways, may facilitate increased inflammatory cell (including eosinophils) recruitment and transmigration to extravascular spaces as observed in these mice. While little is known regarding the effect Hs2st or Ndst1 deficiency may have on endothelial junction proteins that preserve barrier function of the vascular endothelium, our study suggests that Hs2st, but not Ndst1, may play a role in regulating ZO-1 expression. Treatment of endothelial cells with a heparinase to reduce HSPG has been shown to abolish shear stress-induced expression of ZO-1 and VE-cadherin suggesting that HSPG can play a role in regulating expression of junction proteins [36]. Tight junction proteins are highly regulated by cytokines and growth factors [37, 38] several of which bind to and mediate their effects via HSPG. Thus modified HSPG structure due to Hs2st deficiency may alter cytokine/growth factor binding and affect tight junction protein expression.
Another factor contributing to exaggerated eosinophil recruitment in allergen-challenged Hs2stf/fTie2Cre+ mice is the increased proliferation of eosinophils in the BM of these compared to corresponding WT mice. Previous studies have shown that stromal HSPG containing higher 6-O-sulfation on the glucosamine residues are more supportive of hematopoiesis because of their ability to bind both matrix components and cytokines important for hematopoiesis, including IL-3 [39], a cytokine that promotes differentiation of eosinophils in the BM [40]. Inactivation of Hs2st (which functions downstream of Ndst1 during HS biosynthesis) in endothelial cells has been shown to result in increased 6-O-sulfation and N-sulfation of glucosamine residues with decreased 2-O-sulfation of uronic acid residues in endothelial HSPG [14] Thus, Hs2st-deficient endothelial cells in the BM of allergen-challenged Hs2stf/fTie2Cre+ mice may be more supportive of eosinophil differentiation resulting in increased generation of mature eosinophils. This notion is further supported by the observation that the number of eosinophils in the BM of allergen-challenged Ndst1f/fTie2Cre+ mice was similar to WT mice. Finally, activation of ERK (1/2) is an important signaling event required for cell migration [41, 42] and airway recruitment during allergic inflammation [43]. Hs2st knock-down results in hyperactivation of ERK signaling associated with increased migration of glial cells in the corpus callosum of the embryo in mice [44]. On the contrary, deletion of Ndst1 is associated with loss of ERK activation (phosphor-ERK expression) in lacrimal gland cells [45]. Albeit in different tissues/cell types, these studies suggest that Hs2st and Ndst1 exert opposing regulator effects on ERK signaling. While speculative, increased eosinophil recruitment to the lungs in allergen-challenged Hs2stf/fTie2Cre+ mice may in part be due to hyperactivation of ERK (1/2) while inhibition of ERK (1/2) activation may contribute to the decreased airway eosinophilia in allergen-challenged Ndst1f/fTie2Cre+ mice. Clearly, additional studies examining how Hs2st or Ndst1 deficiency affects signaling mechanisms in eosinophils and endothelial cells in the context of allergic inflammation are required.
Our study demonstrates that allergen exposure reduces expression of Hs2st in the lungs and changes in the overall sulfation pattern of endothelial HSPG caused by loss of Hs2st facilitates amplified airway recruitment of eosinophils likely due to decreased endothelial barrier function resulting in persistent inflammation. On the other hand, loss of endothelial N-sulfation limits eosinophil-endothelial interactions leading to attenuated airway eosinophilia and inflammation. These findings clearly underscore the importance of site-specific sulfation in endothelial HSPG to their role in eosinophil recruitment and outcome of Alternaria-induced AAI.
Acknowledgments
Funding
This work was supported by The National Heart Lung and Blood Institute grant number HL107150 to JDE.
Footnotes
Declaration of interest
The authors report no conflicts of interest.
References
- 1.Hamid Q, Tulic M. Immunobiology of Asthma. Ann Rev Physiol. 2009;71:489–507. [DOI] [PubMed] [Google Scholar]
- 2.Sarrazin S, Lamanna WC, Esko JD. Heparan Sulfate Proteoglycans. Cold Spring Harb Perspect Biol. 2011;3:a004952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papakonstantinou E, Karakiulakis G The ‘sweet’ and ‘bitter’ involvement of glycosaminoglycans in lung diseases: pharmacotherapeutic relevance. Br J Pharmacol. 2009;157:1111–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Westergren-Thorsson G, Chakir J, Lafrenière-Allard MJ, Boulet LP, Tremblay GM. Correlation between airway responsiveness and proteoglycan production by bronchial fibroblasts from normal and asthmatic subjects. Int J Biochem Cell Biol. 2002. 34:1256–1267. [DOI] [PubMed] [Google Scholar]
- 5.Venkatesan N, Siddiqui S, Jo T, Martin JG, Ludwig MS. Allergen-induced airway remodeling in brown norway rats. Am J Respir Cell Mol Biol. 2012;46:96–105. [DOI] [PubMed] [Google Scholar]
- 6.Ge XN, Ha SG, Rao A, Greenberg YG, Rushdi MN, Esko JD et al. Endothelial and leukocyte heparan sulfates regulate the development of allergen-induced airway remodeling in a mouse model. Glycobiology. 2014;24:715–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bishop JR, Schuksz M, Esko JD. Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature 2007;446:1030–1037. [DOI] [PubMed] [Google Scholar]
- 8.Xu D, Olson J, Cole JN, van Wijk XM, Brinkmann V, Zychlinsky A et al. Heparan sulfate modulates neutrophil and endothelial function in antibacterial innate immunity. Infect Immun. 2015;83:3648–3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parish CR. The role of heparan sulphate in inflammation. Nat Rev Immunol. 2006;6:633–643. [DOI] [PubMed] [Google Scholar]
- 10.Kumar AV, Katakam SK, Urbanowitz A-K, Gotte M. Heparan sulphate as a regulator of leukocyte recruitment in inflammation. Curr Protein Pep Sci. 2015;16:77–86. [DOI] [PubMed] [Google Scholar]
- 11.Pomin VH. Sulfated glycans in inflammation. Eur J Med Chem. 2015;92:353–369. [DOI] [PubMed] [Google Scholar]
- 12.Kreuger J, Kjellen L. Heparan Sulfate Biosynthesis: Regulation and Variability. J Histochem Cytochem. 2012;60:898–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zuberi RI, Ge X, Jiang S, Bahaie NS, Kang BN, Hosseinkhani RM et al. Deficiency of endothelial heparan sulfates attenuates allergic airway inflammation. J Immunol. 2009;183:3971–3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Axelsson J, Xu D, Na Kang B, Nussbacher JK, Handel TM, Ley K et al. Inactivation of heparan sulfate 2-O-sulfotransferase accentuates neutrophil infiltration during acute inflammation in mice. Blood. 2012;120:1742–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang L, Fuster MM, Sriramarao P, Esko JD. Endothelial heparan sulfate deficiency impairs L-selectin- and chemokine-mediated neutrophil trafficking during inflammatory responses. Nat Immunol. 2005;6:902–910. [DOI] [PubMed] [Google Scholar]
- 16.Ha S, Ge XN, Bahaie NS, Kang BN, Rao A, Rao SP et al. ORMDL3 promotes eosinophil trafficking and activation via regulation of integrins and CD48. Nat Commun. 2013; 4:2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ge XN, Ha SG, Greenberg YG, Rao A, Bastan I, Blidner AG et al. Regulation of eosinophilia and allergic airway inflammation by the glycan-binding protein galectin-1. Proc Natl Acad Sci. USA 2016;113:E4837–E4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bahaie NS, Hosseinkhani RM, Ge XN, Kang BN, Ha SG, Blumenthal MN et al. Regulation of eosinophil trafficking by SWAP-70 and its role in allergic airway inflammation. J Immunol. 2012;188:1479–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abramoff MD, Magalhaes PJ, Ram SJ. Image processing with ImageJ. Biophotonics Int. 2004;11:36–42. [Google Scholar]
- 20.Ge XN, Bahaie NS, Kang BN, Hosseinkhani RM, Ha SG, Frenzel EM et al. Allergen-induced airway remodeling is impaired in galectin-3 deficient mice J Immunol. 2010;185:1205–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yin X, Johns SC, Lawrence R, Xu D, Reddi K, Bishop JR et al. Lymphatic endothelial heparan sulfate deficiency results in altered growth responses to vascular endothelial growth factor-C (VEGF-C). J Biol Chem. 2011;286:14952–14962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pinhal MAS, Smith B, Olson S, Aikawa J-i, Kimata K, Esko JD Enzyme interactions in heparan sulfate biosynthesis: Uronosyl 5-epimerase and 2-O-sulfotransferase interact in vivo. Proc Natl Acad Sci USA. 2001;98:12984–12989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dyer KD, Moser JM, Czapiga M, Siegel SJ, Percopo CM, Rosenberg HF. Functionally competent eosinophils differentiated ex vivo in high purity from normal mouse bone marrow. J Immunol. 2008;181:4004–4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bahaie NS, Kang B, Frenzel EM, Hosseinkhani MR, Ge X, Greenberg Y et al. N-glycans differentially regulate eosinophil and neutrophil recruitment during allergic airway inflammation. J Biol Chem. 2011;286:38231–38241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rao SP, Wang Z, Zuberi RI, Sikora L, Bahaie NS, Zuraw BL et al. Galectin-3 functions as an adhesion molecule to support eosinophil rolling and adhesion under conditions of flow. J Immunol. 2007;179:7800–7807. [DOI] [PubMed] [Google Scholar]
- 26.Carter NM, Ali S, Kirby JA. Endothelial inflammation: the role of differential expression of N-deacetylase/N-sulphotransferase enzymes in alteration of the immunological properties of heparan sulphate. J Cell Sci. 2003;116:3591–3600. [DOI] [PubMed] [Google Scholar]
- 27.Nakayama K, Natori Y, Sato T, Kimura T, Sugiura A, Sato H, et al. Altered expression of NDST-1 messenger RNA in puromycin aminonucleoside nephrosis. J Lab Clin Med. 2004;143:106–114. [DOI] [PubMed] [Google Scholar]
- 28.Hull EE, Montgomery MR, Leyva KJ. Epigenetic regulation of the biosynthesis and enzymatic modification of heparan sulfate proteoglycans: Implications for tumorigenesis and cancer biomarkers. Int J Mol Sci. 2017;18:1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bai Tony R, Knight Darryl A Structural changes in the airways in asthma: observations and consequences. Clin Sci. 2005;108:463–477. [DOI] [PubMed] [Google Scholar]
- 30.Munakata M Airway remodeling and airway smooth muscle in asthma. Allergol Int. 2006;55:235–243. [DOI] [PubMed] [Google Scholar]
- 31.Lloyd CM, Hessel EM. Functions of T cells in asthma: more than just T(H)2 cells. Nat Rev Immunol. 2010;10:838–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pope SM, Zimmermann N, Stringer KF, Karow ML, Rothenberg ME. The Eotaxin chemokines and CCR3 are fundamental regulators of allergen-induced pulmonary eosinophilia. J Immunol. 2005;175:5341–5350. [DOI] [PubMed] [Google Scholar]
- 33.Rosenberg HF, Phipps S, Foster PS. Eosinophil trafficking in allergy and asthma. J Allergy Clin Immunol. 2007;119:1303–1310. [DOI] [PubMed] [Google Scholar]
- 34.Zhang X, Wang F, Sheng J. “Coding” and “Decoding”: hypothesis for the regulatory mechanism involved in heparan sulfate biosynthesis. Carbohydr Resh. 2016;428:1–7. [DOI] [PubMed] [Google Scholar]
- 35.Ellyard JI, Simson L, Bezos A, Johnston K, Freeman C, Parish CR. Eotaxin selectively binds heparin: An interaction that protects eotaxin from proteolysis and potentiates chemotactic activity in vivo. J Biol Chem. 2007;282:15238–15247. [DOI] [PubMed] [Google Scholar]
- 36.Nikmanesh M, Shi Z-D, Tarbell JM. Heparan sulfate proteoglycan mediates shear stress-induced endothelial gene expression in mouse embryonic stem cell-derived endothelial cells. Biotechnol Bioeng. 2012;109:583–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harhaj NS, Antonetti DA. Regulation of tight junctions and loss of barrier function in pathophysiology. Int J Biochem Cell Biol. 2004;36:1206–1237. [DOI] [PubMed] [Google Scholar]
- 38.Capaldo CT, Nusrat A. Cytokine regulation of tight junctions. Biochim Biophys Acta 2009;1788:864–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gupta P, Oegema TR Jr., Brazil JJ, Dudek AZ, Slungaard A, Verfaillie CM Structurally specific heparan sulfates support primitive human hematopoiesis by formation of a multimolecular stem cell niche. Blood. 1998;92:4641–4651. [PubMed] [Google Scholar]
- 40.Clutterbuck EJ, Sanderson CJ. Regulation of human eosinophil precursor production by cytokines: a comparison of recombinant human interleukin-1 (rhIL-1), rhIL-3, rhIL-5, rhIL-6, and rh granulocyte-macrophage colony-stimulating factor. Blood. 1990;75:1774–1779. [PubMed] [Google Scholar]
- 41.Boehme SA, Sullivan SK, Crowe PD, Santos M, Conlon PJ, Sriramarao P et al. Activation of mitogen-activated protein kinase regulates eotaxin- induced eosinophil migration. J Immunol. 1999;163:1611–1618. [PubMed] [Google Scholar]
- 42.Choi EN, Choi MK, Park C-S, Chung IY. A parallel signal-transduction pathway for eotaxin- and interleukin-5-induced eosinophil shape change. Immunology. 2003;108:245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duan W, Chan JHP, Wong CH, Leung BP, Wong WSF. Anti-inflammatory effects of mitogen-activated protein kinase kinase inhibitor U0126 in an asthma mouse model. J Immunol. 2004;172:7053–7059. [DOI] [PubMed] [Google Scholar]
- 44.Clegg JM, Conway CD, Howe KM, Price DJ, Mason JO, Turnbull JE et al. Heparan sulfotransferases Hs6st1 and Hs2st keep Erk in check for mouse corpus callosum development. J Neurosci. 2014;34:2389–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pan Y, Carbe C, Powers A, Zhang EE, Esko JD, Grobe K et al. Bud specific N-sulfation of heparan sulfate regulates Shp2-dependent FGF signaling during lacrimal gland induction. Development 2008;135:301–310. [DOI] [PubMed] [Google Scholar]