Abstract
Epithelial-mesenchymal transition (EMT) is an important mechanism in cancer progression and malignancy including colorectal cancer (CRC). Importantly, inflammatory mediators are critical constituents of the local tumor environment and an intimate link between CRC progression and inflammation is now validated. We and others have reported key role of the deregulated claudin-1 expression in colon carcinogenesis including colitis-associated colon cancer (CAC). However, the causal association between claudin-1 expression and inflammation-induced colon cancer progression remains unclear. Here we demonstrate, TNF-α, a pro-inflammatory cytokine, regulates claudin-1 to modulate epithelial to mesenchymal transition (EMT) and migration in colon adenocarcinoma cells. Importantly, colon cancer cells cultured in the presence of TNF-α (10 ng/ml), demonstrated a sharp decrease in E-cadherin expression and an increase in vimentin expression (versus control cells). Interestingly, TNF-α treatment also upregulated (and delocalized) claudin-1 expression in a time-dependent manner accompanied by increase in proliferation and wound healing. Furthermore, similar to our previous observation that claudin-1 overexpression in CRC cells induces ERK1/2 and Src- activation, signaling associated with colon cancer cell survival and transformation, TNF-α-treatment induced upregulation of phospho-ERK1/2 and -Src expression. The shRNA-mediated inhibition of claudin-1 expression largely abrogated the TNF-α-induced changes in EMT, proliferation, migration, p-Erk and p-Src expression. Taken together, our data demonstrate TNF-α mediated regulation of claudin-1 and tumorigenic abilities of colon cancer cells and highlights a key role of deregulated claudin-1 expression in inflammation-induced colorectal cancer growth and progression, through the regulation of the ERK and Src-signaling.
Keywords: TNF-alpha, Claudin-1, EMT, Migration
1. Introduction
The tight junction complex (TJ) is located in the paracellular space between epithelial and endothelial cells and selectively regulates the passage of molecules and ions via the paracellular pathway. Dysregulated TJ structure/function associates with the loss of differentiated and polarized phenotype and immune activation. Notably, recent studies have confirmed key role of inflammation in colon cancer progression. Furthermore, myriad of studies including ours focused upon the cancer promoting role of claudin family of proteins, integral to the TJ-structure/function, have confirmed an important role of the dysregulated claudin-1 protein in CRC progression and metastasis [1–5]. EMT, an important trait of colon cancer cells, modified by changes in claudin-1 expression, is central to the colon cancer malignancy and metastasis [1]. As described, inflammatory signaling dysregulate mucosal barrier composition and function and promote colon carcino-genesis [6,7]. Furthermore, a sharp increase in claudin-1 expression in colon cancer has been found in Inflammatory Bowel Disease (IBD)-patients which associates primarily with epithelial cell transformation [8]. However, a causal contribution of claudin-1 protein in inflammation driven colon cancer progression remains unexamined.
Notably, one of the key mediators implicated in inflammation and inflammation driven cancer progression is TNF-α [9–12]. Animal model studies further demonstrate the pro-cancer property of TNF-α as TNF-α −/− and TNFR1 −/− mice are resistant to chemically induced carcinogenesis in the skin [13,14]. TNFR1 −/− mice are similarly resistant to the development of liver metastasis in experimental colon cancer [15,16]. Moreover, TNF-α is frequently detected in human cancers with poor prognosis, such as ovarian, renal and breast cancers [17]. In cancer cells, TNF-α is involved in epithelial-mesenchymal transition (EMT) and regulates malignant progression of epithelial tumors by controlling cell migration, invasion and metastasis [18].
TNF-α also induces internalization of TJ proteins [19], decreases trans-epithelial electrical resistance, and increases the paracellular permeability of ions and normally impermeable molecules [20]. In current study, we demonstrate using human colon cancer HT29 cells as model that TNF-α treatment induces EMT in these cells in claudin-1 dependent manner. We demonstrate that TNF-α induced deregulated expression of claudin-1 helps promote TNF-α induced cell proliferation, cell migration and altered cellular permeability in colon cancer cells. To our knowledge, this is the first report demonstrating the causal association of TNF- α and claudin-1 in regulating CRC progression.
2. Materials and methods
2.1. Plasmids and reagents
The antibodies against claudin-1,−2,−3,−4,−7 and ZO-1 were obtained from Invitrogen Inc. (Carlsbad, CA, USA). β-actin and vimentin antibodies were purchased from Sigma-Aldrich. Anti–β-catenin and E-cadherin antibodies were obtained from BD Biosciences, NJ, USA. Horseradish peroxidase (HRP)-conjugated goat anti-mouse and anti-rabbit secondary antibody were from Santa Cruz Biotechnology (Santa Cruz, CA). FITC labeled goat anti-mouse and anti-rabbit secondary antibodies, were from Jackson Laboratories, Inc (West Grove, PA). All other antibodies were from Cell Signaling Technology, Inc. (Danvers, MA). The human TNF-α was from R & D Systems Inc. (Minneapolis, MN). Human claudin-1-specific and control short-hairpin RNA constructs were obtained from Sigma Aldrich (St Louis, MO, USA).
2.2. Cell culture and transfection
The human colon cancer cell line, HT29 was obtained from ATCC and cultured in RPMI-1640 containing 10% FBS. Cells were cultured in a standard humidified incubator at 37 °C with 5% CO2. Cells were transfected using Effectene Transfection Reagent (QIAGEN, Valencia, CA) as described previously [21]. HT29 cells with stable knockdown of claudin-1 were generated using puromycin (1 mg/ml) as selection reagent.
2.3. Immunofluorescent staining and microscopy
Cells were stained as previously described [21]. In brief, HT29 cells were cultured in 8-well glass chamber slides (Lab-Tek, NY, USA). After different treatments, cells were fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton X-100, blocked with 1% BSA and stained with designated antibodies. After gentle washing, slides were mounted with vectashield fluorescence mounting medium (Vector Laboratories, Inc, CA). Slides were examined under the confocal microscope. Isotype-matched mouse or rabbit IgG were used as negative control with the same dilutions as the primary antibodies.
2.4. Protein studies
Immunoblotting was done using antigen-specific antibody using standard protocol, as described previously [22]. The signal was visualized with horseradish peroxidase-conjugated secondary antibodies using enhanced chemiluminescence (Amersham Biosciences, Piscataway, NJ). Triton X-100 soluble and insoluble protein fractions were prepared as described previously [23]. Briefly, cells were lysed with modified radioimmune precipitation assay buffer containing 1% Triton X-100. Cell lysate was centrifuged (12,000 rpm for 10 min at 4 °C), and the supernatant was collected as the Triton X soluble fraction. The remaining pellet was resuspended in 60 ml of lysis buffer containing 1% SDS. The resulting suspension was centrifuged (12,000 rpm for 10 min at 4 °C) and the supernatant was collected as the Triton-X insoluble fraction.
2.5. RNA isolation, semiquantitative reverse transcription–PCR and real-time PCR
Total RNA isolation and reverse transcription–PCR was performed using standard protocols as described earlier [24]. For real-time PCR, complementary DNA (cDNA) was synthesized and PCR was carried out using SYBR PCR master kit (Applied Biosystems Inc., Foster City, CA, USA). The sequences of the claudin-1 primers are 5’-TCACTCCCAGGAGGATGC-3′(Forward Primer) and 5′-GGCAGATCCAGTGCAAAGTC-3′(Reverse Primer). PCR was carried out in triplicates for each gene being validated. The gene expression levels were normalized to the level of β-actin as a house-keeping gene.
2.6. Wound-healing assay
Wounds were created in confluent cells using a pipette tip [1]. The cells were then rinsed with medium to remove floating cells and debris and treated with 10 μg/ml mitomycin O/N. After that these cells were treated with TNF-α (10 ng/ml). The culture plates were incubated at 37 °C. Wounds were measured at 0, 4, 24 and 48 h. Assays were repeated three times for each condition.
2.7. Cell proliferation assay
The proliferation assays were performed to determine the effect of TNF-α on the cell growth over time as described previously [1]. In brief, HT29 cells were seeded into 96-well cell culture plates at a density of 5×103 cells/well and incubated for 48 h prior to the treatments. The medium was replaced with 100 μl fresh one containing 10 ng/ml TNF-α. The assay was performed at 0, 4, 24 and 48 h after the treatments in quadruplicates with CellTiter 96R Aqueous One Solution Cell Proliferation Assay (Promega, Madison, WI). At each time point, 20 μl of the assay solution was added to each well and incubated for 1 h at 37 °C. The plate was then read on a 96 well plate reader at 490 nm. The absorbance values were plotted as a function of time for each treatment to show the cell proliferation profile.
2.8. Measurement of paracellular permeability
Paracellular permeability was quantified by measuring the flux of FITC-labeled dextran (FD-4) across cell monolayers as described previously [25]. HT29 cell monolayers were incubated for 0, 4, 24 and 48 h with TNF-α. Then (FD-4) was added to the apical side of the monolayer and the samples were taken from the basolateral chambers, FITC-dextran fluorescence was measured using a fluorescent plate reader (Synergy 2, BioTek, VT USA), and FD-4 concentrations transported were extrapolated from a standard curve and expressed as % fold change. Values are means ± SEM.
2.9. Statistical analysis
Statistical analyses were performed using Graphpad Prism software (San Diego, CA) for t-test analysis where comparisons between two groups were involved and analysis of variance when applicable using Bonferroni’s correction for multiple testing. A difference of P < 0.05 was considered significant.
3. Results
3.1. TNF-α treatment induces epithelial to mesenchymal transition (EMT) in colon cancer cells
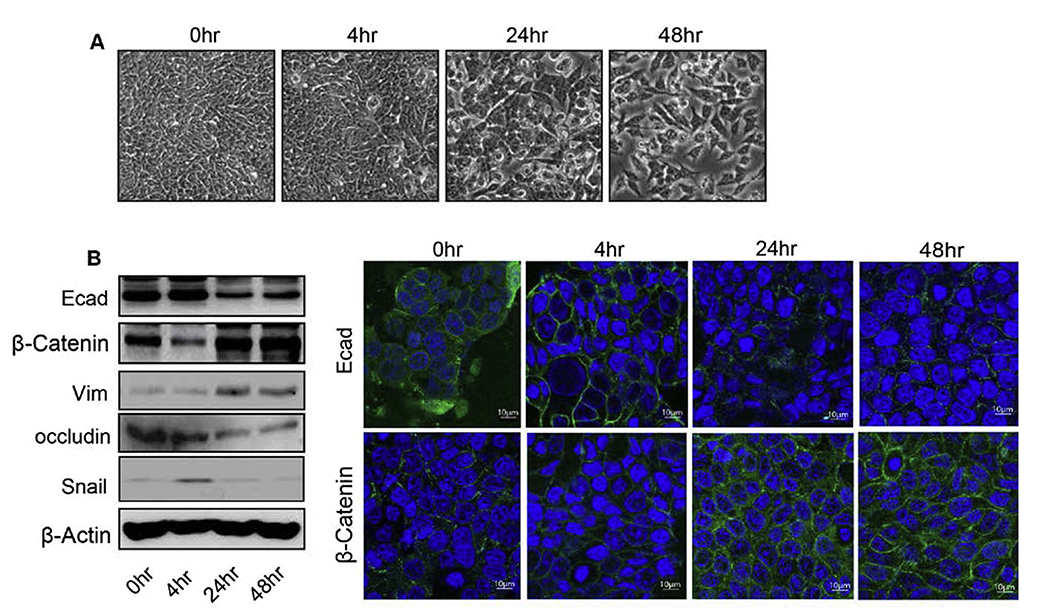
A key mediator in inflammation, TNF-α is also involved in EMT in multiple cell types. To test its role in EMT in colon cancer cells, we treated HT29 cells with TNF-α (10 ng/ml) for 0–48 h. Phase-contrast microscopy demonstrated spindle shape of the HT29 cells with long processes in comparison with the control cells at 24 and 48 h after treatment of TNF-α (Fig. 1A). To investigate whether change in the morphology of treated cells associate with changes in EMT markers, expression of established EMT marker proteins was determined. Immunofluorescent analysis demonstrated that the treatment with TNF-α reduced the expression of E-cadherin while increased and mislocalized β-catenin expression (Fig. 1C). Immunoblotting demonstrated decreased levels of the cellular E-Cadherin and Occludin, typical epithelial adherens and TJ marker proteins, and contrasting increase in expressions of snail, vimentin and β-catenin, mesenchymal markers (Fig. 1B). These changes were significant at 24 and 48 h post-treatment (Fig. 1B and C).
Fig. 1.
TNFα induces epithelial to mesenchymal transition (EMT) in human colon adenocarcinoma HT29 cells. A. Representative phase-contrast images of HT29 cells growing in monolayer cultures. Treatment of TNFα (10 ng/ml) induced morphological alterations characterized as fibroblast-like cells with significant changes at 24 and 48 h. B. Immunoblot analysis of HT29 cells treated with TNFα for 0–48 h. Cells were lysed in RIPA Buffer, and a total of 20 μg proteins for each sample were loaded onto the SDS-polyacrylamide gel. Membranes were blotted against E-cadherin, β –catenin, vimentin, and occludin whereas actin was used as a loading control. C. Immunofluorescence illustration for localization of E-cadherin and β-catenin with DAPI in HT29 cells treated with TNFα for time dependent manner (0–48 h).
3.2. TNF-α treatment induces claudin-1 expression in human colon adenocarcinoma HT29 cells
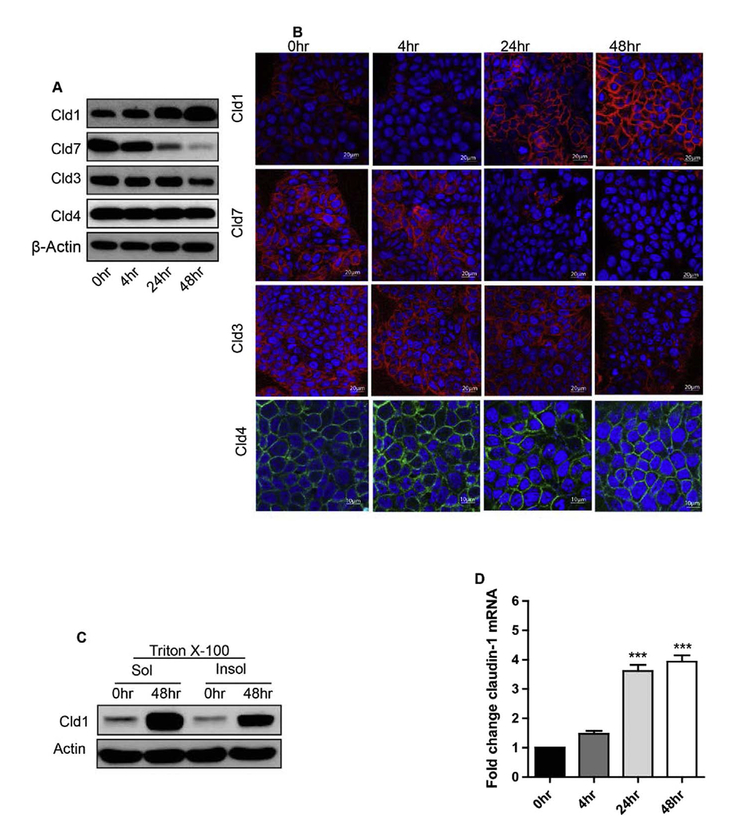
Next, we investigated the effect of TNF-α (10 ng/ml) on claudin-1 expression in HT29 cells. In the untreated monolayer, there was a small but measurable amount of claudin-1 protein. In contrast, TNF-α treatment upregulated claudin-1 expression at 4 h which further increased at 24 and 48 h post-treatment, suggesting a time dependent increase. In contrast, claudin-7 expression sharply decreased in TNF-α treated cells at 24 and 48 h. These findings correlated well with our previous studies demonstrating that an increase in claudin-1 expression induces EMT while upregulated claudin-7 expression promotes MET in colon cancer cells [1,21]. We did not observe any significant changes in Claudin-3 or Claudin-4 expression with TNF-α treatment at different time points of TNF-α treatment (Fig. 2A).
Fig. 2.
TNFα induces increase in claudin-1 expression and also its delocalization in human colon adenocarcinoma HT29 cells. A. Immunoblot analysis of HT29 cells treated with TNFα were immune-blotted against claudin-1, claudin-7, claudin-3 and claudin-4. Actin was used as a loading control. B. Immunofluorescence microscopy was performed to analyze the localization of claudin-1, claudin-7, claudin-3 and claudin-4 with DAPI in HT29 cells treated with TNFα for 0–48 h. C. Claudin-1 expression increased in both Tritox-100 soluble and insoluble fractions during the time course of TNFα in HT-29 cells. D. Quantitative Real-Time PCR analysis. HT29 cells were treated with TNFα for 0–48 h, and quantitative RT-PCR was performed. After treatment, total RNA was isolated and subjected to real-time PCR using claudin-1 gene specific primers. Results were plotted as mean ± SD from three independent experiments and presented as fold change. ***p < 0.001 when compared with 24 h and 48 h time points.
Since TNF-α treatment altered the expression of claudin-1, immunofluorescent staining was applied to determine potential alterations in its cellular localization. TNF-α induced claudin-1 protein expression was found to be predominantly localized in cell cytoplasm and membrane while claudin-7 expression simply decreased (Fig. 2B). Similar to the immunoblotting, we did not observe much change in claudin-3 or claudin-4 expression. Notably, assembly of tight junction recruits tight junction proteins into complexes and thus make them resistant to detergent-salt extractions. Conversely, disassembly of tight junction may result in internalization or diffuse cytoplasmic distribution of tight junction proteins, making them more extractable with detergent salt solutions [26]. Thus, to further determine whether TNF-α treatment induced redistribution of claudin-1 protein, we used Triton-X100 extraction followed by western blotting. In concurrence with the immunofluorescence staining, claudin-1 expression was present predominantly in Triton X-100 soluble fractions, and increased after 48 h of TNF-α stimulation (Fig. 2C). Overall, our results suggest that TNF-α induced increased and delocalized claudin-1 expression.
Next, to determine if transcriptional regulation contributes to the increased claudin-1 protein expression, we performed qRT-PCR (Fig. 2D). There was minimal claudin-1 mRNA expression detected in control and untreated cells, which however increased at 4 h after TNF-α treatment. However, a statistically significant increase in claudin-1 transcription was documented only at 24 and 48 h post-treatment (***p < 0.001) (Fig. 2D).
3.3. TNF-α treatment alters barrier function, cell viability and migration in human colon adenocarcinoma HT29 cells
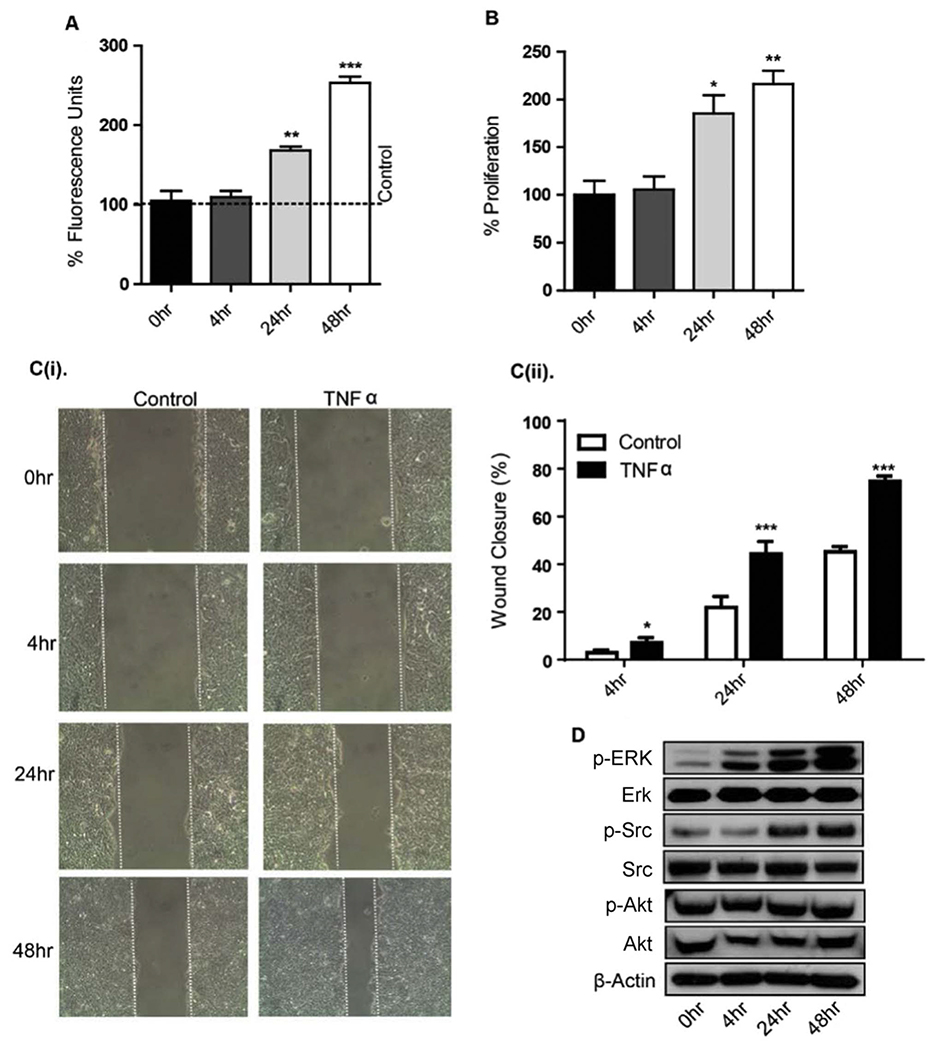
Since the proinflammatory cytokines IFN-γ and TNF-α are reported to influence TJ composition and function [27], we analyzed the effect of TNF-α on HT29 epithelial monolayer barrier properties. Confluent HT29 monolayers grown on permeable filter supports were incubated with TNF-α and paracellular permeability was determined by measuring the apico-basal flux of a paracellular solute, FD-4 (4-kDa). Apical incubation with TNF-α induced a significant increase in paracellular flux of FD-4 after 24 and 48 h TNF-α treatment (Fig. 3A) suggesting increased permeability (**p < 0.01, ***p < 0–001 respectively).
Fig. 3.
TNF-α promotes permeability, proliferation, and migration of HT29 cells. A. TNFα caused a time dependent increase in paracellular permeability for FITC-dextran (4 kDa). Medium containing FITC-dextran (4 kDa) was added to the top (inner) chamber of the transwell and samples were collected from the bottom chamber after 0, 4, 24 and 48 h after TNFα treatment. B. Cellular proliferation was measured in HT29 cells treated with TNFα (10 ng/ml) for 0–48 h using MTS reagent after plating equal number of cells. C(i). TNF-α enhanced cell migration in a wound healing assay. After mechanical wounding, confluent HT29 cells were pretreated with mitomycin O/N and then treated with TNFα (10 ng/ml) for 0–48 h. Representative photomicrographs of the wounded cell monolayer are shown. C(ii). Percentage of wound closure at 4, 24 and 48 h was calculated. D. Effects of TNF-α on phosphorylation of Erk, Src, and Akt. HT29 cells, which had been serum starved for 1 d, were treated with 10 ng/ml TNFα for the indicated times. Western immunoblots of cellular extracts separated on SDS-polyacrylamide gels were probed with antibodies specific for P-Erk, P-Src, and, P-Akt and these blots were re-probed with respective total Erk, Src, and Akt antibodies. Results were plotted as mean ± SD from three independent experiments and presented as percent change. *p < 0.05**p < 0.01, ***p < 0.001 were considered significant change when compared with control.
We next determined whether TNF-α treatment affects cell proliferation and migration in HT29 cells. Indeed, TNF-α induced a significant increase in proliferation at 24 h which further increased at 48 h post-treatment (*p < 0.05, **p < 0.01) (Fig. 3B). To determine whether TNF-α treatment promotes cell migration, we examined cell migration using the wound-healing assay in HT29 cells pretreated with mitomycin O/N and then treated with TNF-α for 0–48 h. TNF-α treatment also significantly increased motility in HT29 cells at 24 and 48 h, as evidenced by increased wound closure (**p < 0.01, ***p < 0–001 respectively) (Fig. 3C).
TNF-α can induce its effects on EMT and TJs function through the activation of multiple signaling cascades via tyrosine kinase Src, PI3K and MAPK signaling [18]. Therefore, we further determined the effect of TNF-α treatment on Erk1/2, Src and Akt signaling. Addition of TNF-α induced a robust activation of ERK1/2 and Src signaling without affecting the AKT phosphorylation. Here, the phosphorylation of ERK 1/2 was evident within at 4 h while maximal Src activation was seen at 24 and 48 h treatment (Fig. 3D).
3.4. Claudin-1 is important for TNF-α induced alteration in barrier function and changes in the EMT markers in human colon adenocarcinoma HT29 cells
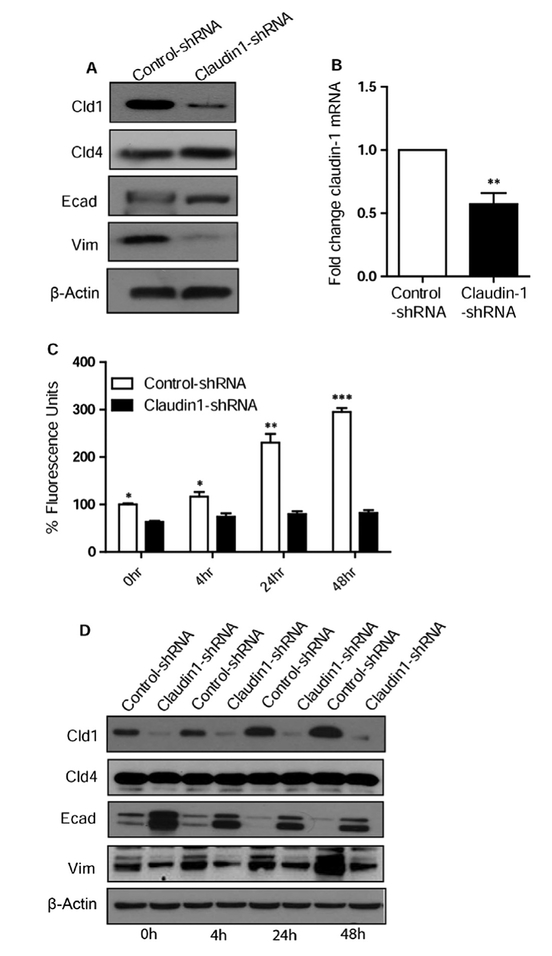
To determine whether claudin-1 is involved in TNF-α induced changes, we performed claudin-1 knockdown in HT29 cells using anti-human claudin-1 specific shRNA. Knockdown was confirmed by observing reduced protein and mRNA expression (Fig. 4A and B). Effect of the knockdown of claudin-1 on cell permeability was determined (Fig. 4C). The increase in cell permeability by TNF-α was abrogated by the knockdown of claudin-1 (Fig. 4C) expression. To determine whether claudin-1 is similarly involved in TNF-α induced EMT, control shRNA and claudin-1 shRNA transfected HT29 cells were first tested for the expression of E-cadherin and vimentin. Claudin-4 was used to show that shRNA was specific for claudin-1. We observed increased E-cadherin (epithelial marker) and decreased vimentin (mesenchymal marker) expression in claudin-1 knockdown cells. Control shRNA and claudin-1 shRNA HT29 cells were then treated with TNF-α for 0–48 h. As expected, TNF-α treatment did not increase the expression of claudin-1 in shRNA claudin-1 cells though there was a significant increase in control shRNA transfected cells at 24 h and 48 h. We did not observe any change in claudin-4 in control and shRNA claudin-1 cells with or without TNF-α treatment (Fig. 4D). Most importantly, we did not observe a decrease in E-cadherin expression or increased vimentin expression in TNF-α treated claudin-1 knock down cells at 24 and 48 h, as was the case with control cells (Fig. 4D).
Fig. 4.
Loss of claudin-1 induces the mesenchymal to epithelial transition (MET) in HT-29 cells. A. Immunoblot analysis of HT29 control and claudin-1 shRNA transfected cells. Cells were lysed in RIPA Buffer, and a total of 20 μg proteins for each sample were loaded onto the SDS-polyacrylamide gel. Membranes were blotted against claudin-1, claudin-4, E-cadherin, and vimentin. Actin was used as a loading control. B. Quantitative Real-Time PCR analysis of claudin-1 in HT-29 cells, transfected with control and claudin-1 shRNA. C. FITC dextran permeability remained significantly less during the time course in claudin-1 knockdown cells compared to control. D. Immuno-blot analysis showed that Claudin-1 knockdown induces MET with time and abrogates the TNF-α mediated changes. Results were plotted as mean ± SD from three independent experiments and presented as percent change. *p < 0.05**p < 0.01, ***p < 0.001 were considered signifi-cant change when compared with control.
3.5. Claudin-1 plays an important role in TNF-α induced cell proliferation and migration through the regulation p-ERK1/2 and p-Src in human colon adenocarcinoma HT29 cells
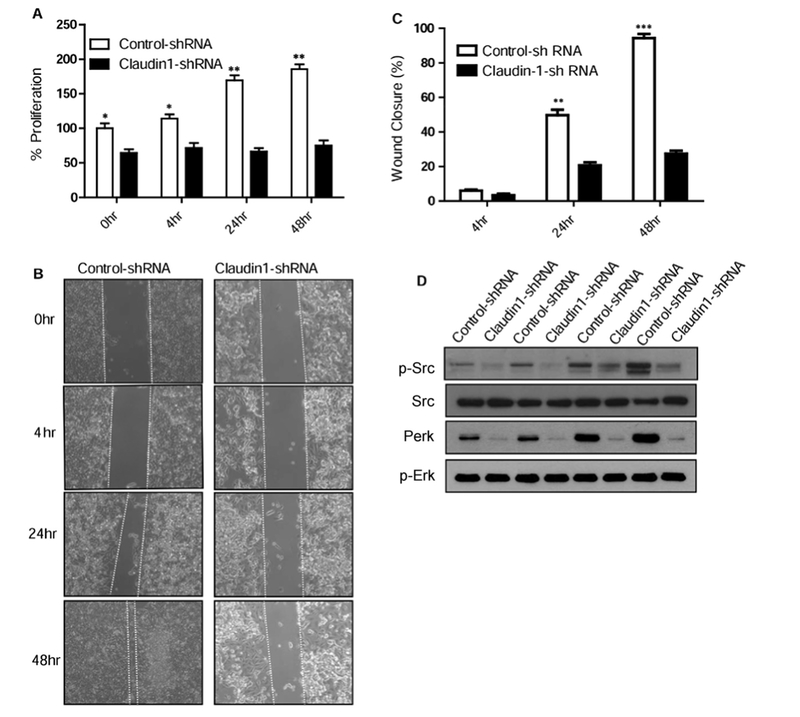
We and others have shown a role of claudin-1 in regulating migration of colon cancer cells [1,2,28]. To determine if claudin-1 plays a similar role in TNF-α mediated cell proliferation and migration, we determined effect of claudin-1 inhibition on cell proliferation in TNF-α treated cells at 4, 24 and 48 h post-treatment. Genetic inhibition of claudin-1 expression decreased proliferation in HT29 cells at basal levels (Fig. 5A). Furthermore, the time-dependent increase in TNF-α induced cell proliferation in control cells was also inhibited in claudin-1 knockdown cells (Fig. 5A). Next to determine whether TNF-α mediated cell migration is mediated by claudin-1, we performed wound healing assay in control shRNA and claudin-1 shRNA cells. We observed a significant wound closure at 48 h in control cells when treated with TNF-α but not in claudin-1 knockdown cells (Fig. 5B and C). Collectively, these results indicate that claudin-1 has important role in mediating TNF-α induced changes in cell proliferation and migration.
Fig. 5.
Claudin-1 knockdown reduced TNFα -induced changes in proliferation, Migration, EMT markers and Erk/Src signaling in HT29 cells. A. Cellular proliferation was measured in control or Claudin-1 shRNA transfected HT29 cells treated with or without TNFα (10 ng/ml) using MTS reagent after plating equal number of cells. B. Claudin-1 expression levels affect cell migration. Down regulation of Claudin-1 with shRNA reduced TNFα enhanced migration of HT29 cells. The control or Claudin-1 shRNA transfected HT29 cells were cultured until confluent, mechanically wounded, and then treated with or without 10 ng/ml TNFα. Representative photomicrographs of wounded cell monolayer are shown. C. Percentage of wound closure in each condition was calculated. D. Immunoblot analysis of HT29 control and claudin-1 shRNA transfected cells treated with TNFα for 0–48 h. Cells were lysed in RIPA Buffer, and a total of 20 μg proteins for each sample were loaded onto the SDS-polyacrylamide gel. Membranes were blotted against P-Erk, P-Src, and antibodies against total Erk and Src. Results were plotted as mean ± SD from three independent experiments and presented as percent change. *p < 0.05**p < 0.01, ***p < 0.001 were considered significant change when compared control.
We have previously reported [22] that claudin-1 may mediate its effect on carcinogenesis through the regulation of ERK1/2 and Src activity and TNF-α treatment induces ERK and Src activity, so we were interested to test if claudin-1induces TNF-α mediated effects on these signaling molecules. To examine, we determined expression of these kinases in TNF-α treated control and claudin-1 knockdown cells. Inhibition of claudin-1 inhibited Src and Erk activity at basal level as well in TNF-α induced induction of Src and Erk signaling (Fig. 5D). Combined together, our studies suggest a major role of claudin-1 in mediating the TNF-α induced EMT and cellular signaling in human colon adenocarcinoma HT29 cells.
4. Discussion
A link between the chronic inflammation and cancer progression has long been suspected, yet the causal relationship was deciphered only in the past few years [29–31]. Up to 20% of all human cancers result from chronic inflammation and/or persistent infections. Unresolved chronic inflammation is implicated in all stages of cancer development and an inflammatory tumor microenvironment is considered an important hallmark of cancer and contributor to the resistance to anti-cancer treatment. The pro-inflammatory cytokines such as TNF-α, IFN-γ, IL-1β etc play critical role in almost every developmental stages of inflammation-induced cancers, from initiation, promotion, and progression to malignant metastasis [18,32]. Among all, TNF-α has been identified as a potent pro-inflammatory cytokine exerting pleiotropic effects on various cell types and plays a critical role in the pathogenesis of many inflammatory diseases. Of note, patients suffering from inflammatory bowel disease (IBD), a chronic inflammatory condition of the mucosa thought to be primed by TNF-α, are at increased risk of developing colon cancer [8]. Also, it was recently demonstrated that TNF-α mRNA transcripts are more abundant in colorectal tumor cells than in their normal epithelial counterparts [18]. The anti-TNF-α agents have changed the way of treating IBD refractory to conventional medications (corticosteroids, immuno-modulators) [33]. However, how TNF-α regulates inflammation and EMT are not clearly established. Understanding novel insights into the mechanism of action of TNF-α in IBD may contribute to the development of novel anti-TNF-α therapy with improved efficacy.
The role of tight junction constituent proteins in regulating inflammation driven neoplastic growth in the colon is essential, and based upon the pivotal role of the TJ in regulating barrier function, immune homeostasis and regulating the polarized colonic epithelial phenotype [22]. Claudins are the major transmembrane components of TJs and play major role in regulating the barrier function, as well as proliferation and migration. Importantly, claudin-1 is one of the most deregulated claudin family member in human cancers, and studies in our and other laboratories have confirmed its tumor promotive function role in IBD and colorectal cancer [1,34]. In this regard, in colon cancer cells forced claudin-1 overexpression induced EMT, cell migration and invasion while inhibiting claudin-1 expression induced MET and inhibited tumor growth and metastasisin vivo [1]. These findings were further verified by the studies that APCmin/Cldn-1 mice generated by crossing claudin-1 transgenic mice (mice overexpressing claudin-1 in colonic epithelium) and APCmin mice possess highly aggressive and increased colon cancer burden [4]. Here, it is worthy of mentioning that dysregulation of colonic epithelial differentiation, increased cell mobility and invasiveness have emerged as key outcomes of claudin-1 manipulation in vitro or in vivo [1]. The in vivo constitutive claudin-1 overexpression in mouse colon epithelium led to dysregulated colonocyte differentiation program and goblet cell differentiation [22]. The cancer-promoting role of claudin-1 via its effect on invasion or motility of cancer cells has been described in various cancers [35,36]. Claudin-1 is a barrier-integral protein and its homozygous loss in mice results in death due to dysregulated barrier function [37]. Thus, another aspect how deregulated claudin-1 protein may help facilitate colon cancer progression is by promoting mucosal inflammation. In this regard, studies including ours have demonstrated marked increase in claudin-1 expression in IBD-associated colon cancer where increased claudin-1 expression associated with neoplastic transformation of the epithelium [1]. Moreover, claudin-1 transgenic mice are not only susceptible to experimentally induced colitis but also demonstrate sustained (chronic) inflammation when subjected to the DSS-induced acute colitis [22]. Most notably, APCmin/Cldn-1 mice demonstrate increased and aggressive colon tumor burden when subjected to DSS-administration as compared to the APCmin alone [4]. Taken together, a precocious causal association between increased claudin-1 expression, mucosal inflammation and the risk of developing colon cancer seems to exist. Our current study supports such a proposition and also uncovers a functional role for claudin-1 in TNF-α mediated EMT in colon cancer. Also, the TNF-α induced proliferation in HT-29 cells was dependent on claudin-1 expression. In our recently published study, we showed that claudin-1Tg mice demonstrate significantly increased and sustained cytokine production including TNF-α and IFN γ at the mRNA and protein levels when subjected to DSS-colitis [4]. Of note, these conditions are also associated with modified barrier permeability as seen in TNF-α treated HT29 cells. Taken together, our findings suggest a possible feedforward loop between EMT and inflammation in regulating colon cancer progression where claudin-1 may play the role of a rheostat.
To further elucidate the signaling pathways that are triggered by cytokine stimulation and that contribute to the EMT in claudin-1 dependent manner, we observed increased p-ERK1/2 and p-Src expression with TNF-α treatment where the peak activation was observed at 48 h of treatment. Studies including ours have revealed that the induction of EMT by claudin-1 requires the activation of the c-Abl-Ras-Raf-1/ERK1/2 signaling pathway, supporting the importance of p-ERK1/2 signaling in claudin-1-induced acquisition of a malignant phenotype [35]. Furthermore, down or upregulation of claudin-1 was accompanied with changes of membrane β-catenin expression as well as Akt and Src activities [38]. Notably, Src kinase regulates EMT initiation, cytoskeleton rearrangement, cell motility, and expression of mesenchymal markers, in response to many stimulators [38]. Src suppression also allows restoration of E-cadherin, and inhibits expression of vimentin and other mesenchymal markers [38]. We have further found that claudin-1 regulates Notch-signaling through the regulation of matrix metalloproteinase-9 (MMP-9) and p-ERK signaling to regulate colonocyte proliferation and differentiation [22]. Activation of the ERK1/2 signaling positively regulates expression of Snail and Slug, repressor of E-cadherin [39]. Taken together, our data suggests that inflammatory signaling modulate ERK1/2 and Src signaling by modulating claudin-1 expression to promote cancer supporting cell phenotype. Our studies are supported by other previous studies demonstrating TNF-α-induced changes in claudin-2 levels which contribute to TER changes and proliferation regulation [40]. TNF-α also induced CLDN1 expression in human lung, gastric and pancreatic carcinoma cells [41–43].
TJ and adherens junction proteins are usually down-regulated during the progression of EMT [44–46]. Thus, claudin 1 expression is generally thought to decrease by TNF-α stimulation, and the decreased protein expression leading to the increase in the paracellular permeability and EMT of epithelial cells [47,48]. However, recent studies have demonstrated that TNF-α treatment rather increases claudin-1 protein expression in pancreatic and airway smooth muscle cancer cells [49]. Similarly, we here show that TNF-α induced delocalized claudin-1 expression in colon cancer HT29 cells, and knocking down claudin-1 blocked the TNF-α-induced alteration in cellular permeability, EMT and cell migration. In summary, we here describe that TNF-α stimulation induces the gene expression of claudin-1 in human HT29 cancer cells, and the latter acts as the signal mediator to regulate cell multiplication, migration and signaling cascade. In the near future, we plan to clarify the mechanisms and significance of claudin-1 in cell proliferation and a functional role in the Erk/Src-signaling pathways in regulating human colon cancer cells in conjunction with cancer promoting inflammatory signaling. A deeper understanding of this pathway may serve as a mean to establish a new therapeutic target for colon carcinoma.
Acknowledgement
This work was supported by VA-Merit BX002086 (P.D.), DK088902, BX002761 (A.B.S).
References
- [1].Dhawan P, Singh AB, Deane NG, No Y, Shiou SR, Schmidt C, et al. , Claudin-1 regulates cellular transformation and metastatic behavior in colon cancer, J. Clin. Investig 115 (7) (2005) 1765–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kinugasa T, Akagi Y, Yoshida T, Ryu Y, Shiratuchi I, Ishibashi N, et al. , Increased claudin-1 protein expression contributes to tumorigenesis in ulcerative colitis-associated colorectal cancer, Anticancer Res 30 (8) (2010) 3181–3186. [PubMed] [Google Scholar]
- [3].Suren D, Yildirim M, Kaya V, Alikanoglu AS, Bulbuller N, Yildiz M, et al. , Loss of tight junction proteins (Claudin 1, 4, and 7) correlates with aggressive behavior in colorectal carcinoma, Med. Sci. Monit 20 (2014) 1255–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Pope JL, Ahmad R, Bhat AA, Washington MK, Singh AB, Dhawan P, Claudin-1 overexpression in intestinal epithelial cells enhances susceptibility to adenamatous polyposis coli-mediated colon tumorigenesis, Mol. Cancer 13 (2014) 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Nakagawa S, Miyoshi N, Ishii H, Mimori K, Tanaka F, Sekimoto M, et al. , Expression of CLDN1 in colorectal cancer: a novel marker for prognosis, Int. J. Oncol 39 (4) (2011) 791–796. [DOI] [PubMed] [Google Scholar]
- [6].Engel MA, Neurath MF, New pathophysiological insights and modern treatment of IBD, J. Gastroenterol 45 (6) (2010) 571–583. [DOI] [PubMed] [Google Scholar]
- [7].Grivennikov SI, Inflammation and colorectal cancer: colitis-associated neoplasia, Semin. Immunopathol 35 (2) (2013) 229–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Weber CR, Nalle SC, Tretiakova M, Rubin DT, Turner JR, Claudin-1 and claudin-2 expression is elevated in inflammatory bowel disease and may contribute to early neoplastic transformation, Lab Investig 88 (10) (2008) 1110–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Balkwill F, TNF-alpha in promotion and progression of cancer, Cancer Metastasis – Rev 25 (3) (2006) 409–416. [DOI] [PubMed] [Google Scholar]
- [10].Popivanova BK, Kitamura K, Wu Y, Kondo T, Kagaya T, Kaneko S, et al. , Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis, J. Clin. Investig 118 (2) (2008) 560–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Grivennikov SI, Karin M, Inflammatory cytokines in cancer: tumour necrosis factor and interleukin 6 take the stage, Ann. Rheum. Dis 70 (Suppl 1) (2011) S104–S108. [DOI] [PubMed] [Google Scholar]
- [12].Balkwill F, Tumour necrosis factor and cancer, Nat. Rev. Cancer 9 (5) (2009) 361–371. [DOI] [PubMed] [Google Scholar]
- [13].Moore RJ, Owens DM, Stamp G, Arnott C, Burke F, East N, et al. , Mice deficient in tumor necrosis factor-alpha are resistant to skin carcinogenesis, Nat. Med 5 (7) (1999) 828–831. [DOI] [PubMed] [Google Scholar]
- [14].Arnott CH, Scott KA, Moore RJ, Robinson SC, Thompson RG, Balkwill FR, Expression of both TNF-alpha receptor subtypes is essential for optimal skin tumour development, Oncogene 23 (10) (2004) 1902–1910. [DOI] [PubMed] [Google Scholar]
- [15].Kitakata H, Nemoto-Sasaki Y, Takahashi Y, Kondo T, Mai M, Mukaida N, Essential roles of tumor necrosis factor receptor p55 in liver metastasis of intrasplenic administration of colon 26 cell, Cancer Res 62 (22) (2002) 6682–6687. [PubMed] [Google Scholar]
- [16].Orosz P, Echtenacher B, Falk W, Ruschoff J, Weber D, Mannel DN, Enhancement of experimental metastasis by tumor necrosis factor, J. Exp. Med 177 (5) (1993) 1391–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Balkwill F, Tumor necrosis factor or tumor promoting factor, Cytokine Growth Factor Rev 13 (2) (2002) 135–141. [DOI] [PubMed] [Google Scholar]
- [18].Bates RC, Mercurio AM, Tumor necrosis factor-alpha stimulates the epithelialto-mesenchymal transition of human colonic organoids, Mol. Biol. Cell 14 (5) (2003) 1790–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bruewer M, Luegering A, Kucharzik T, Parkos CA, Madara JL, Hopkins AM, et al. , Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanism, J. Immunol 171 (11) (2003) 6164–6172. [DOI] [PubMed] [Google Scholar]
- [20].Coyne CB, Vanhook MK, Gambling TM, Carson JL, Boucher RC, Johnson LG, Regulation of airway tight junctions by proinflammatory cytokine, Mol. Biol. Cell 13 (9) (2002) 3218–3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bhat AA, Pope JL, Smith JJ, Ahmad R, Chen X, Washington MK, et al. , Claudin-7 expression induces mesenchymal to epithelial transformation (MET) to inhibit colon tumorigenesis, Oncogene 34 (35) (2015) 4570–4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Pope JL, Bhat AA, Sharma A, Ahmad R, Krishnan M, Washington MK, et al. , Claudin-1 regulates intestinal epithelial homeostasis through the modulation of Notch-signalling, Gut 63 (4) (2014) 622–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Singh AB, Harris RC, Epidermal growth factor receptor activation differentially regulates claudin expression and enhances transepithelial resistance in Madin-Darby canine kidney cell, J. Biol. Chem 279 (5) (2004) 3543–3552. [DOI] [PubMed] [Google Scholar]
- [24].Bhat AA, Sharma A, Pope J, Krishnan M, Washington MK, Singh AB, et al. , Caudal homeobox protein Cdx-2 cooperates with Wnt pathway to regulate claudin-1 expression in colon cancer cell, PLoS One 7 (6) (2012) e37174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Dhawan P, Ahmad R, Chaturvedi R, Smith JJ, Midha R, Mittal MK, et al. , Claudin-2 expression increases tumorigenicity of colon cancer cells: role of epidermal growth factor receptor activation, Oncogene 30 (29) (2011) 3234–3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Nishiyama R, Sakaguchi T, Kinugasa T, Gu X, MacDermott RP, Podolsky DK, et al. , Interleukin-2 receptor beta subunit-dependent and -independent regulation of intestinal epithelial tight junction, J. Biol. Chem 276 (38) (2001) 35571–35580. [DOI] [PubMed] [Google Scholar]
- [27].Baker OJ, Camden JM, Redman RS, Jones JE, Seye CI, Erb L, et al. , Proinflammatory cytokines tumor necrosis factor-alpha and interferon-gamma alter tight junction structure and function in the rat parotid gland Par-C10 cell line, Am. J. Physiol. Cell Physiol 295 (5) (2008) C1191–C1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Huo Q, Kinugasa T, Wang L, Huang J, Zhao J, Shibaguchi H, et al. , Claudin-1 protein is a major factor involved in the tumorigenesis of colorectal cancer, Anticancer Res 29 (3) (2009) 851–857. [PubMed] [Google Scholar]
- [29].Wang K, Karin M, Chapter five – tumor-elicited inflammation and colorectal cancer, in: Xiang-Yang W, Paul BF (Eds.), , Advances in Cancer Research 128, Academic Press, 2015, pp. 173–196. [DOI] [PubMed] [Google Scholar]
- [30].Ben-Neriah Y, Karin M, Inflammation meets cancer, with NF-[kappa]B as the matchmaker, Nat. Immunol 12 (8) (2011) 715–723. [DOI] [PubMed] [Google Scholar]
- [31].Terzić J, Grivennikov S, Karin E, Karin M, Inflammation and colon cancer, Gastroenterology 138 (6) (2010) (2101–14.e5). [DOI] [PubMed] [Google Scholar]
- [32].Turner JR, Intestinal mucosal barrier function in health and disease, Nat. Rev. Immunol 9 (11) (2009) 799–809. [DOI] [PubMed] [Google Scholar]
- [33].Peyrin-Biroulet L, Anti-TNF therapy in inflammatory bowel diseases: a huge review, Minerva Gastroenterol. Dietol 56 (2) (2010) 233–243. [PubMed] [Google Scholar]
- [34].Kinugasa T, Akagi Y, Ochi T, Tanaka N, Kawahara A, Ishibashi Y, et al. , Increased claudin-1 protein expression in hepatic metastatic lesions of colorectal cancer, Anticancer Res 32 (6) (2012) 2309–2314. [PubMed] [Google Scholar]
- [35].Suh Y, Yoon CH, Kim RK, Lim EJ, Oh YS, Hwang SG, et al. , Claudin-1 induces epithelial-mesenchymal transition through activation of the c-Abl-ERK signaling pathway in human liver cell, Oncogene 32 (41) (2013) 4873–4882. [DOI] [PubMed] [Google Scholar]
- [36].Stebbing J, Filipovic A, Giamas G, Claudin-1 as a promoter of EMT in hepatocellular carcinom, Oncogene 32 (41) (2013) 4871–4872. [DOI] [PubMed] [Google Scholar]
- [37].Furuse M, Hata M, Furuse K, Yoshida Y, Haratake A, Sugitani Y, et al. , Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice, J. Cell Biol 156 (6) (2002) 1099–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Singh AB, Sharma A, Dhawan P, Claudin-1 expression confers resistance to anoikis in colon cancer cells in a Src-dependent manner, Carcinogenesis 33 (12) (2012) 2538–2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Smith BN, Burton LJ, Henderson V, Randle DD, Morton DJ, Smith BA, et al. , Snail promotes epithelial mesenchymal transition in breast cancer cells in part via activation of nuclear ERK, PLoS One 9 (8) (2014) e104987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Amoozadeh Y, Dan Q, Xiao J, Waheed F, Szászi K, Tumor necrosis factor-α induces a biphasic change in claudin-2 expression in tubular epithelial cells: role in barrier function, Am. J. Physiol. – Cell Physiol 309 (1) (2015) C38–C50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Shiozaki A, Shimizu H, Ichikawa D, Konishi H, Komatsu S, Kubota T, et al. , Claudin 1 mediates tumor necrosis factor alpha-induced cell migration in human gastric cancer cell, World J. Gastroenterol.: WJG 20 (47) (2014) 17863–17876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Iitaka D, Moodley S, Shimizu H, Bai X-H, Liu M, PKCδ–iPLA2–PGE2–PPARγ signaling cascade mediates TNF-α induced Claudin 1 expression in human lung carcinoma cell, Cell. Signal 27 (3) (2015) 568–577. [DOI] [PubMed] [Google Scholar]
- [43].Shiozaki A, Bai X.-h., Shen-Tu G, Moodley S, Takeshita H, Fung S-Y, et al. , Claudin 1 mediates TNFα-induced gene expression and cell migration in human lung carcinoma cell, PLoS ONE 7 (5) (2012) e38049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Chua HL, Bhat-Nakshatri P, Clare SE, Morimiya A, Badve S, Nakshatri H, NF-[kappa]B represses E-cadherin expression and enhances epithelial to mesenchymal transition of mammary epithelial cells: potential involvement of ZEB-1 and ZEB-, Oncogene 26 (5) (2006) 711–724. [DOI] [PubMed] [Google Scholar]
- [45].Ikenouchi J, Matsuda M, Furuse M, Tsukita S, Regulation of tight junctions during the epithelium-mesenchyme transition: direct repression of the gene expression of claudins/occludin by snail, J. Cell Sci 116 (10) (2003) 1959–1967. [DOI] [PubMed] [Google Scholar]
- [46].Ohkubo T, Ozawa M, The transcription factor snail downregulates the tight junction components independently of E-cadherin downregulation, J. Cell Sci 117 (9) (2004) 1675–1685. [DOI] [PubMed] [Google Scholar]
- [47].Baker OJ, Camden JM, Redman RS, Jones JE, Seye CI, Erb L, et al. , Proinflammatory cytokines tumor necrosis factor-α and interferon-γ alter tight junction structure and function in the rat parotid gland Par-C10 cell line, Am. J. Physiol. – Cell Physiol 295 (5) (2008) C1191–C1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Poritz LS, Garver KI, Tilberg AF, Koltun WA, Tumor necrosis factor alpha disrupts tight junction assembly1,2, J. Surg. Res 116 (1) (2004) 14–18. [DOI] [PubMed] [Google Scholar]
- [49].Fujita H, Chalubinski M, Rhyner C, Indermitte P, Meyer N, Ferstl R, et al. , Claudin-1 expression in airway smooth muscle exacerbates airway remodeling in asthmatic subjects, J. Allergy Clin. Immunol 127 (6) (2011) (1612–21.e8). [DOI] [PubMed] [Google Scholar]