Abstract
The depth of proteomic analyses is often limited by the overwhelming proportion of confounding background ions that compromise the identification and quantification of low abundance peptides. To alleviate these limitations, we present a new high field asymmetric waveform ion mobility spectrometry (FAIMS) interface that can be coupled to the Orbitrap Tribrid mass spectrometers. The interface provides several advantages over previous generations of FAIMS devices, including ease of operation, robustness, and high ion transmission. Replicate LC-FAIMS-MS/MS analyses (n = 100) of HEK293 protein digests showed stable ion current over extended time periods with uniform peptide identification on more than 10,000 distinct peptides. For complex tryptic digest analyses, the coupling of FAIMS to LC-MS/MS enabled a 30% gain in unique peptide identification compared with non-FAIMS experiments. Improvement in sensitivity facilitated the identification of low abundance peptides, and extended the limit of detection by almost an order of magnitude. The reduction in chimeric MS/MS spectra using FAIMS also improved the precision and the number of quantifiable peptides when using isobaric labeling with tandem mass tag (TMT) 10-plex reagent. We compared quantitative proteomic measurements for LC-MS/MS analyses performed using synchronous precursor selection (SPS) and LC-FAIMS-MS/MS to profile the temporal changes in protein abundance of HEK293 cells following heat shock for periods up to 9 h. FAIMS provided 2.5-fold increase in the number of quantifiable peptides compared with non-FAIMS experiments (30,848 peptides from 2,646 proteins for FAIMS versus 12,400 peptides from 1,229 proteins with SPS). Altogether, the enhancement in ion transmission and duty cycle of the new FAIMS interface extended the depth and comprehensiveness of proteomic analyses and improved the precision of quantitative measurements.
Keywords: Quantification, Tandem Mass Spectrometry, Protein Turnover, Mass Spectrometry, Protein Identification, Heat shock, Ion mobility, isobaric peptide labeling, Proteostasis, tandem mass tag
Over the past decade, significant improvements in sensitivity, resolution, and scan speed of mass spectrometers have extended the depth of proteomic analyses. Peptide identification by mass spectrometry (MS) relies on the detection of precursor ions with predefined charge state and their sequential selection for fragmentation as they elute from the chromatographic column (1, 2). For low sample complexity, most capillary LC-MS systems are capable of acquiring at least one product ion spectrum for every precursor ion detected.
However, large-scale proteomic analyses represent a sizable analytical challenge in view of the overwhelming peptide density, the occurrence of chimeric tandem mass spectra from co-eluting isobaric ions and the difficulty of sequencing low abundance peptide ions that are often confounded with background noise. These limitations not only affect the dynamic range of peptide identification and the depth of proteome analyses but can also compromise quantitative measurements. For label-free quantitation, the desired precursor ion must be selected in every injection and assigned based on the MS/MS spectra to quantify the peptide between different samples. Congested MS spectra hinder the selection of the precursor ion of choice between the various samples due to the acquisition of MS/MS spectra on other ions in the chromatography window. This difficulty can be partly addressed using spectral alignment and “match between run” that correlate relevant features based on recalibrated peptide coordinates across LC-MS runs (3). Quantitation approaches such as label-free and metabolic labeling that rely on the correlation of peptide abundances from the survey scan can be affected by the occurrence of confounding ions that share similar ion coordinates (m/z, charge, and retention time). Furthermore, the presence of co-eluting interfering ions of similar m/z values can also affect the accuracy and precision of multiplex quantitative proteomics when using isobaric peptide labeling (e.g. TMT or iTRAQ). Indeed, the co-isolation and co-fragmentation of interfering ions results in distorted reporter ion ratios arising from isobaric tags of the target peptide and interfering ions that cannot be distinguish from each other (4, 5). The resolution of conflicting ions often requires multistage ion fragmentation and synchronous precursor selection (SPS-MS3) (6, 7).
To tackle sample complexity and alleviate issues associated with interfering ions, several approaches based on peptide fractionation, improved MS resolution, and peak assignments were proposed. For example, two-dimensional chromatography fractionation (8, 9) and increased reverse-phase column length (10, 11) were reported to enhance peak capacity for proteome analyses. Improvements in quadrupole performance through higher resolution and scanning rate have enabled more efficient isolation and transmission of target ions with narrow isolation windows in turn reducing chimeric tandem mass spectra (12). Newer generation of MS instruments that combine high resolution of survey scan with fast scanning rate of MS/MS acquisition are progressively bridging the gap that exists between detectable and identifiable peptide ions (13, 14). The higher resolution of MS instruments also highlights the presence of convoluted isobaric ions with unassigned charge states that prevent their selection for MS/MS acquisition. The recent development of algorithms that facilitate advanced peak determination can rapidly and iteratively annotate several isotope envelopes in the same m/z window, and can enhanced peptide identification by more than 40% in a single run (15). While advanced peak determination can distinguish precursors with overlapping isotope envelopes, its application for quantitative proteomics using isobaric peptide labeling is mitigated by the occurrence of chimeric MS/MS spectra and often requires SPS-MS3.
These remarkable technological advances have also unveiled the depth of sample complexity that exists in cell extracts as we improve sensitivity and sample peptide ions of lower abundance. While improvements in MS resolution and peak assignment algorithms have expanded the dynamic range of peptide detection, the relative proportion of identifiable and quantifiable peptides remains significantly small (13). This is particularly true for ion trap instruments where the ion population is constrained to minimize space-charging effects for optimal ion isolation and analysis (16). Further enhancement of proteome coverage thus requires alternate approaches that can enhance the chromatographic peak capacity of current LC-MS systems while simultaneously enabling the identification of peptides over several orders of magnitude in intensity.
In this context, gas-phase ion fractionation using ion mobility spectrometry (IMS) opens new perspectives to overcome sample complexity of proteomic samples. Previous contributions highlighted a broad range of application of IMS to the separation of peptides and proteins, including the study of protein conformation dynamics (17–21). IMS can be divide into two broad categories that regroup drift-tube ion mobility spectrometry (DTIMS) and differential mobility spectrometry, the latter being mostly represented by high-field asymmetric waveform ion mobility spectrometry (FAIMS)1. In DTIMS, ions are separated in a drift tube filled with an inert background gas based on their mobilities in a low but constant electric field (22). For appropriate separation, ions are introduced as a narrow pulse into the drift tube, and resolution up to 200 can be achieved using DTIMS (23). In FAIMS, ions are separated based on their differences in mobility when subjected to low and high electric fields (24, 25). A carrier gas entrains ions between two electrodes to which is applied an asymmetric dispersion voltage (DV) that progressively deflects ions toward one of the electrodes. To counter this drift and allow ions to move across the device, a compensation voltage (CV) is applied, and ions are transmitted in turn by scanning the CV (26). When compared with DTIMS, FAIMS with cylindrical electrodes has several advantages for large-scale proteomic studies. First, FAIMS operates at atmospheric pressure and facilitates the separation of co-eluting isobaric interferences, including undesired singly charged contaminant ions. Second, FAIMS does not need periodic pulsing of ions to achieve separation and resolution, which confers a significant advantage in terms of sensitivity compared with DTIMS. Third, the electric field gradient in the cylindrical FAIMS enables ion focusing and provides a further gain in sensitivity compared with planar FAIMS where the electric field is homogenous (27). Finally, FAIMS ion transit time is typically less than 50 ms and can be parallelized with the LC-MS duty cycle. Previous studies have reported the benefits of FAIMS to improve proteome coverage and to reduce the extent of co-fragmentation that impedes the identification of low-abundance peptide ions (28–33). The separation capability of FAIMS also facilitates the resolution of isomeric peptides, including histone variants and isomeric phosphopeptides (34–43). Furthermore, the reduction of peptide co-elution and co-fragmentation observed with FAIMS can significantly improve the accuracy and the comprehensiveness of multiplex proteomic analyses (44).
Despite these advantages, a wider acceptance of FAIMS particularly among the recent MS instruments has been relatively limited. This can be explained by the fact that previous generation FAIMS devices were attenuating ion signal by up to an order of magnitude and limiting its usefulness only to applications suffering from overwhelming high chemical background (32). Another deterrent from routine use of FAIMS has been the long transit time of ions through the ion separation gap leading to ∼100 ms delay between CV switching. Poor sensitivity and long CV switch time combined with limited ruggedness and complexity of operation were seen as key drawbacks of the older generation FAIMS for use with modern MS. Here, we introduce a novel FAIMS device that addresses these limitations and demonstrate its application for proteomics analyses on a Tribrid Orbitrap mass spectrometer. We also compare its analytical performances in multiplexed proteomic experiments with that of SPS-MS3 to profile the temporal changes in protein abundance of HEK293 cells during heat shock.
EXPERIMENTAL PROCEDURES
Direct Infusion
Angiotensin II human (Sigma-Aldrich, Oakville, ON, Canada) for direct infusion was prepared in 50% in methanol (Fisher Scientific, Whitby, ON, Canada) with 0.2% formic acid (FA; Fisher Scientific). Bovine serum albumin (BSA; Bioshop, Burlington, ON, Canada) was dissolved in 50 mm ammonium bicarbonate (Sigma-Aldrich), reduced with 5 mm tris(2-carboxyethyl)phosphine (Thermo Scientific, Logan, UT) and alkylated with 10 mm 2-chloroacetamide (Sigma-Aldrich) prior to digestion with trypsin (Promega, Madison, WI, USA) at a ratio 1:50 (w/w) overnight. The sample was desalted on an Oasis hydrophilic lipophilic balance (HLB) extraction cartridge (Waters, Milford, MA): The HLB cartridge was first conditioned with 1 ml of 50% MeOH/0.2% FA and 2 ml 0.2% FA. The acidified sample was applied and the cartridge washed with 1 ml 0.2% FA. Peptides were eluted with 1 ml 50% MeOH/0.2% FA and dried down. For direct infusions, the protein mix was diluted to 0.4 μg/μl in 50% MeOH/0.2% FA.
Cell Culture
HEK293 cells were cultured at 37 °C in a 5% CO2 constant atmosphere unless indicated otherwise. Dulbecco's Modified Eagles Medium (Fisher Scientific) was supplemented with 10% fetal bovine serum (Wisent Inc., St-Bruno, QC, Canada), 1% penicillin/streptomycin solution (Fisher Scientific), and 1% l-glutamine (Fisher Scientific). For heat stress experiments, HEK293 cells were grown in 15-cm Petri plates to near confluency (70–80%), the media were removed and replaced by media prewarmed at 43 °C. The Petri dishes were placed in a 43 °C heated incubator and collected at 1-h intervals for a total of 9 h. Control and heat-shock-treated cells were collected simultaneously and washed twice with 37 °C PBS. PBS-washed HEK293 cell pellets were mechanical lysed (3 × 10 s sonicate pulses) in a buffer composed of 8 m urea (Fisher Scientific), 50 mm HEPES (Bio Basic, Inc., Markham, ON, Canada), and 75 mm sodium chloride (Fisher Scientific), pH 8.2. The total cell extracts were centrifuged for 5 min at 17,000 g to remove cellular debris. The protein concentration of the supernatants were determined by Bradford assay (Bio Rad, Mississauga, ON, Canada).
Tryptic Digestion
The total cell extracts were reduced with tris(2-carboxyethyl)phosphine (5 mm final concentration) for 30 min and alkylated with 2-chloroacetamide (10 mm final concentration) for 30 min at 37 °C in the dark. HEK293 total cell extracts were diluted to 1 m urea with 50 mm ammonium bicarbonate. Trypsin was added to each sample at a 1:50 (trypsin:protein) ratio and incubated overnight at 37 °C. The reaction was quenched by acidifying the samples with FA to pH 3. Samples were desalted on HLB and dissolved in 0.2% FA for LC-MS analyses. Peptide concentrations were determined using the Thermo Scientific Pierce Quantitative Colorimetric Peptide assay (Thermo Scientific).
Isobaric Peptide Labeling
For TMT (Thermo Scientific) labeling, 100 μg of peptides from each time point were dissolved in 100 μl 200 mm HEPES, pH 8.2, and mixed with 41 μl anhydrous acetonitrile (Thermo Scientific) containing 0.2 mg of individual TMT channel for 1 h. The reaction was quenched for 15 min with 8 μl of 5% hydroxylamine (Thermo Scientific). The 10 TMT channels were mixed together in equal volumes and desalted on HLB. The combined TMT samples were dissolved in 0.2% aqueous FA. An aliquot of 500 ng of this mixture (50 ng of each channel) was injected for each LC-MS/MS analysis.
MS
For chromatographic reversed-phase separation, an in-house packed 150-μm inner diameter × 25-cm capillary LC column (C18, 3 μm, 300 Å; Phenomenex, Torrance, CA) with eluent A (0.2% FA in water) and eluent B (0.2% FA in acetonitrile) was operated through an Easy nLC 1000 Thermo system. Samples were loaded on column and separated at a flow rate of 600 nl/min using a linear gradient starting from 5% eluent B to 32% over 125 min, a 10 min hold at 90% eluent B prior to a 10 min analytical column equilibration with 5% eluent B. A 1-h gradient was employed for the instrumental reproducibility experiments using 100 replicate injections. For electrospray ionization, a nanospray Flex ion source (Thermo Scientific) at 320 °C was coupled to an Orbitrap Fusion Tribrid (Thermo Scientific, San Jose, CA) controlled by XCalibur. Spray voltage was set to 3,000 V and 2,800 V with and without FAIMS, respectively. Full MS (range from m/z 300 to m/z 1,200) in the Orbitrap were acquired at 60 k resolution followed by a top speed MS2 acquisition of 3 s at 15 k. Maximal injection time for full MS was 50 ms with an AGC of 5e5. Maximal injection time for MS2 was 100 ms with an AGC of 2e4. For MS2 triggering, only charge states 2–6 were selected with normalized higher-energy collisional dissociation collision energy activation of 30% and an isolation window 1.6 Th. For the TMT 10-plex analysis with FAIMS, the MS2 approach described above was used with minor modifications. The MS2 resolution was set at 50 k with a maximum injection time of 120 ms and a normalized collision energy of 35% (45). For the SPS-MS3 method, the parameters for the MS scan were the same as for the regular method (scan range m/z 300–1,200, Orbitrap resolution 60 k, AGC 5e5, and maximum injection time 50 ms) followed by a 3 s top speed approach for MS2 in the ion trap (Isolation window 0.7 Th, collision-induced dissociation at 35% collision energy, turbo scan rate mode, AGC 1e4 with maximal injection time of 50 ms) followed by the selection of 10 synchronous precursor ions for MS3 acquisition (scan range m/z 100–500, Orbitrap resolution of 50 k, AGC of 2e4, maximum injection time of 100 ms, 10 notches, isolation window of 2.0 Th, and a collision energy of up to 65% using 5% stepping).
FAIMS
The new FAIMS interface was re-engineered with the goal of reducing the footprint, improving sensitivity, speeding up CV switch time, and removing helium from the carrier gas. A schematic of the FAIMS interface is presented in supplemental Fig. 1 with additional details on the main control board, adapter flange, and electrode assembly. Briefly, FAIMS inner and outer electrodes were separated by a 1.5-mm gap and can be independently heated between room temperature to 100 °C. For this study, the inner and outer electrodes were heated to a common temperature of 100 °C to maximize ion transmission. The inlet on the outer electrode was modified to improve gas dynamics for ion entrainment as described previously by Prasad et al. (46). Nitrogen (N2) was used as the temperature control gas (12 l/min) and user FAIMS carrier gas (1.6 l/min). The DV was set to -5,000 V with a 3-MHz frequency for the high electric field. The FAIMS transit time was 40 ms. For CV scanning injections, an individual scan experiment was defined using CV Scan Tool in the Tune User Interface Software. MS parameters for every scan experiment were the same as explained in the previous paragraph.
Data Analysis and Visualization
RAW files were searched with PEAKS engine (Bioinformatics Solutions, Inc., Version 8.5) against the Uniprot Human database (http://www.uniprot.org/) release 2016_02 (February 17, 2016), including the reversed database with 74,508 entries. The precursor tolerance was set to 12 ppm and fragment tolerance to 0.015 Da (Orbitrap) and 0.5 Da (Iontrap). The maximum number of missed cleavages for trypsin was set to 3. Deamidation (NQ), oxidation (M), and carbamidometylation were set as variable modifications with a maximum of three modifications per peptide. For TMT quantification, the TMT 10-plex was added as a fixed modification while adding phosphorylation (STY) and methylation (C-term) as extra variable modifications. General false discovery rate for peptides was at 1.0% with decoy removal. Furthermore, only unique peptides within a mass error tolerance of 0.01 Da of the reporter ion and with a 1% false discovery rate were considered for TMT quantification. Proteins were quantified with ≥2 unique peptides presenting reporters in at least seven channels (intensity threshold > 500). TMT dynamic ratios were normalized to the control time point (TMT 126). Coefficient of determination (R2) for polynomial order up to n = 4 were calculated, and protein groups with R2 ≥0.75 were selected for further processing using fuzzy clustering, see Experimental Design and Statistical Rationale for further details (47). Protein groups were classified into four different dynamic profiles. R Studio Version 1.1.414 (https://www.rstudio.com/) was used for data processing and visualization. RawMeat (VAST Scientific, Version 2.1) was used to extract information about the duty cycle metrics for the various LC-MS injections.
Protein interaction networks were constructed with the STRING database using experimentally verified interaction sources only with a medium confidence (0.400) (48) using proteins whose abundances changed in response to the challenge and fit one of the four dynamic profiles described in the results section. The resulting interacting proteins were visualized with Cytoscape version 3.4.0. (49). Gene Ontology (GO) enrichment analyses were performed and visualized using the BiNGO plugin for Cytoscape (50).
Experimental Design and Statistical Rationale
All regular LC-MS/MS or LC-FAIMS-MS/MS optimization and benchmarking experiments were conducted on the same HEK293 digest to directly compared datasets. The tests for system reproducibility were conducted with n = 100 for the LC-FAIMS-MS experiments and n = 27 for the regular LC-MS experiments. 27 replicates without FAIMS were conducted to limit instrument use while providing enough replicates to directly compare the LC-MS/MS configuration to three instrumental replicates with LC-FAIMS-MS/MS for the nine individual CV injections.
For the experiments that compared three injections for LC-MS versus LC-FAIMS-MS with HEK293 digests (Figs. 3 and 4), three instrumental replicates were conducted without FAIMS, while the three-stepped CV combination was used for the LC-FAIMS-MS (CV -37 V/-44 V/-51 V, CV -58 V/-65 V, and CV -72 V/-79 V/-86 V/-93 V) providing one instrumental replicate, thereby normalizing the instrument usage time between both analysis methods.
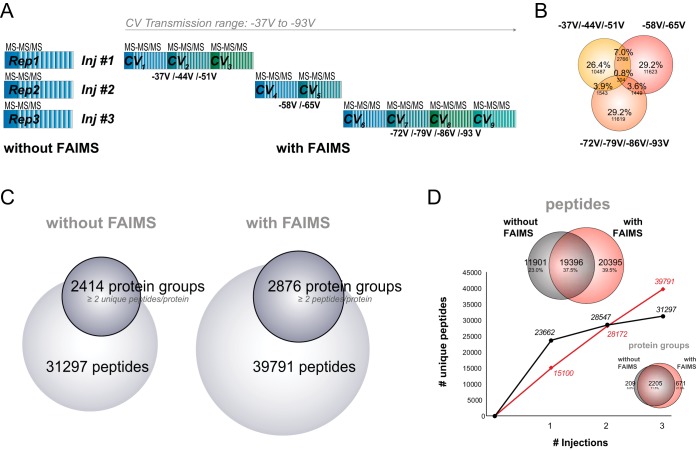
Fig. 3.
LC-FAIMS-MS CV stepping improves coverage of the proteome compared with regular LC-MS. (A) Overview of the three injection LC-FAIMS-MS CV stepping program with optimal CV stepping of CV -37 V/-44 V/-51 V, CV -58 V/-65 V, and CV -72 V/-79 V/-86 V/-93 V. The CV stepping cycle consists of a full MS and a top 3 s MS/MS acquisition at each CV. (B) Venn diagram representation of the overlap in peptide identifications between the three CV stepping injections. (C) Number of identified peptides and protein groups with at least two unique peptides per protein for the three CV stepping injections with FAIMS or three repeat injections without FAIMS. (D) Cumulative number of unique peptides identified as a function of repeat injections without FAIMS (black) or CV stepping program with FAIMS (red). Overlap in peptide and protein identifications between the two methods are depicted in Venn diagrams above and below the curves, respectively.
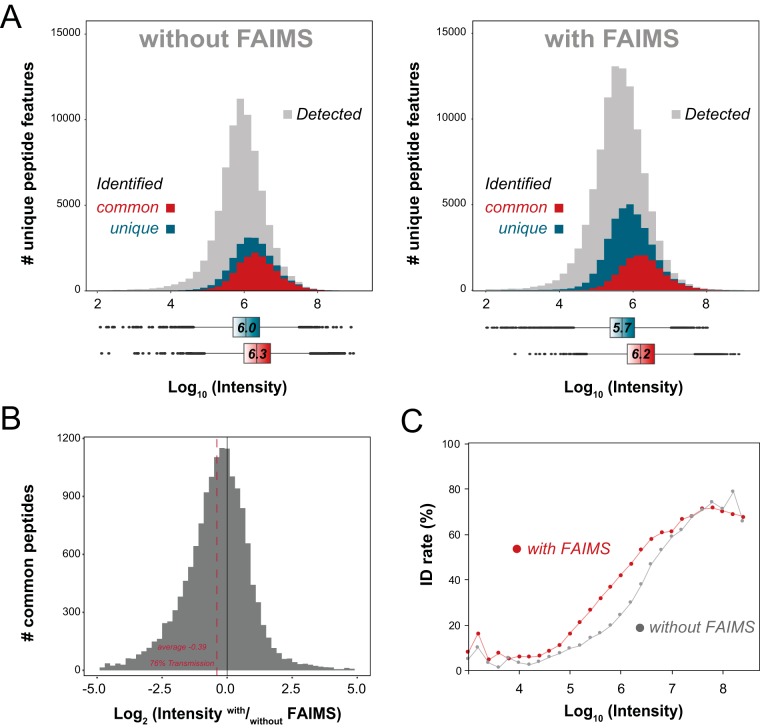
Fig. 4.
Depth and coverage of proteomic analyses. (A) Intensity distribution for detected peptide ions (gray), peptides identified by both with and without FAIMS (red), and peptides identified only (turquoise) without FAIMS (left) or with FAIMS (right). The corresponding boxplots for the common and unique peptides for both methods are represented below the plots with the same color scheme. (B) FAIMS peptide transmission rates for common peptides identified by both methods with median transmission of 76%. (C) Plots for peptide identification rates based on precursor intensity without FAIMS (gray) and with FAIMS (red).
The quantitative kinetic analysis of the changes in the proteome upon heat stress were conducted with a single biological replicate per time point (Figs. 5–7). One 15-cm Petri was used for each time point by removing the Petri from the 43 °C incubator at the indicated times and processing the sample as indicated above. The TMT10 126 labeled peptide sample was used as an internal nonstimulated control while the other TMT10 tags were used to label peptides from cells that were subjected to the heat shock. A different TMT10 reagent was used to label peptides from the various time points with a 1-h resolution. The 10 TMT-labeled samples were combined in an equimolar fashion prior to their analysis by MS. For the regular LC-MS experiment the samples was injected three times to obtain three instrumental replicates. For the LC-FAIMS-MS experiment, the same sample that was used for the regular LC-MS experiment was injected three times using the three-stepped CV combination (CV -37 V/-44 V/-51 V, CV -58 V/-65 V, and CV -72 V/-79 V/-86 V/-93 V) providing one instrumental replicate, thereby normalizing the instrument usage time between both analysis methods.
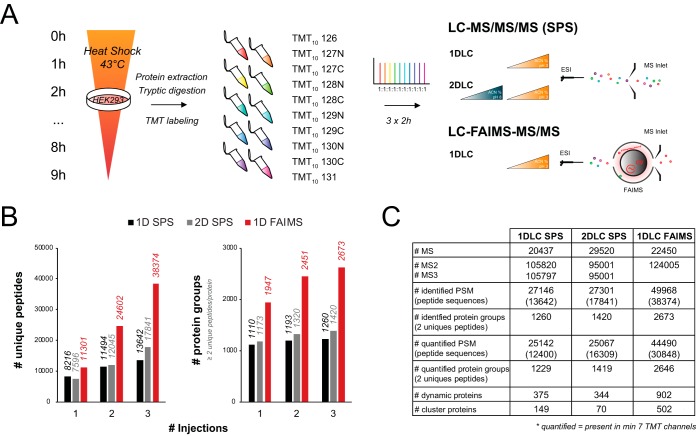
Fig. 5.
FAIMS improves TMT quantification of the human proteome. (A) TMT labeling and quantification workflow. HEK293 cells were exposed to a 43 °C heat stress for up to 9 h in 1-h increments. At each time point, cells were collected, lysed, digested with trypsin, and labeled with a different TMT10 channel. The 10 TMT-labeled samples were combined in an equimolar amount and analyzed by 2-h reversed-phase LC gradient. Triplicate injections were performed for the SPS method with and without high pH/basic reverse-phase fractionation while three different CV combinations (CV -37 V/-44 V/-51 V, CV -58 V/-65 V, and CV -72 V/-79 V/-86 V/-93 V) were used with FAIMS. (B) Cumulative number of unique peptides and protein groups identified as a function of repeat injections for 1D SPS (black), 2D SPS (gray), or CV stepping program with FAIMS (red). (C) Summary table comparing MS analysis parameters between SPS and FAIMS.
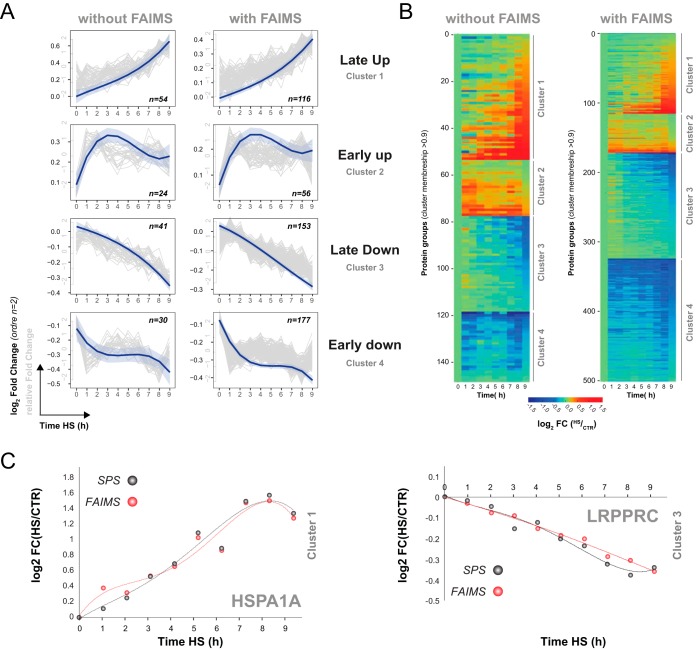
Fig. 6.
The heat shock response in HEK293 cells alters protein abundances with distinct dynamics. (A) Dynamic clusters for heat shock regulated proteins without FAIMS (left) and with FAIMS (right). The gray lines show the relative fold changes of the individual proteins with high membership (≥0.9), the blue lines the average fold changes of all the proteins in the corresponding cluster. (B) Heat map for all proteins in the clusters from (A). (C) Representative dynamic profile of HSPA1A (assigned to a late up-regulation) and LRPPRC (assigned to a late up down-regulation) for SPS (gray) and FAIMS analysis (red), highlighting virtually identical profiles/quantifications for both acquisition methods.
Fig. 7.
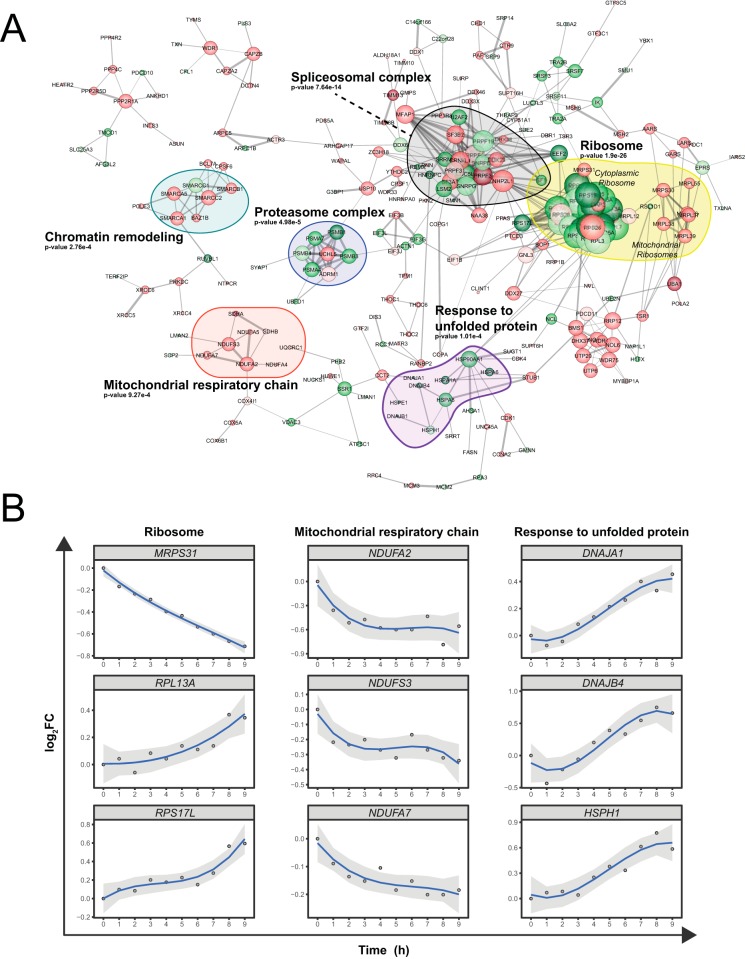
Heat stress affects several key cellular processes that impact protein homeostasis. (A) Interaction network for up-regulated proteins (green) and down regulated proteins (red) based on the clustering shown in Fig. 6A. The darkest shades of green and red correspond to proteins that were quantified by SPS and FAIMS, the intermediate shades to proteins that were quantified by LC-FAIMS-MS only and the lightest shades to proteins that were quantified by SPS only. Proteins that belong to enriched GO-terms are outlined by colored shapes. (B) Examples of kinetic profiles for proteins associated with the ribosome, the mitochondrial respiratory chain, and the response to unfolded proteins.
We selected protein groups that contained at least two unique peptides and that were quantified (FC = heat shock/control) in at least seven of the nine time points. Each profile was fitted to a polynomial with orders of up to n = 4. As a first criteria, proteins were selected as “dynamic proteins” if their kinetic profiles fit a polynomial curve with a R2 value ≥0.75. Due to the low proportion and amplitude of the changes in protein levels with respect to the proteome as a whole false discovery rate value based cutoff on the dynamic profiles by generating a randomized dataset was not possible. Instead an R2 cutoff value ≥0.75 had to be employed. This cutoff value was chosen since this threshold has been shown to correspond to a false discovery rate of ∼5% in large-scale proteomic analyses (51). These “dynamic proteins” were subsequently fit to four different dynamic trends based on their c-means fuzzy clustering (52). “Dynamic proteins” assigned to a cluster with membership ≥0.9 confidence were retained as “clustered proteins.”
RESULTS
Ruggedness and Stability of the FAIMS Interface
This is the first report of a novel FAIMS interface that is compatible with the newest generation of Orbitrap Fusion Tribrid™ mass spectrometers. An exploded view of the FAIMS device and its coupling to the Orbitrap Fusion instrument are depicted in Fig. 1A. One advantage of the new FAIMS system is its small footprint that makes interfacing FAIMS to the MS as trivial as interfacing an ion source to the MS. The FAIMS electrodes can be disassembled/assembled for maintenance within a few minutes without interrupting the MS vacuum.
Fig. 1.
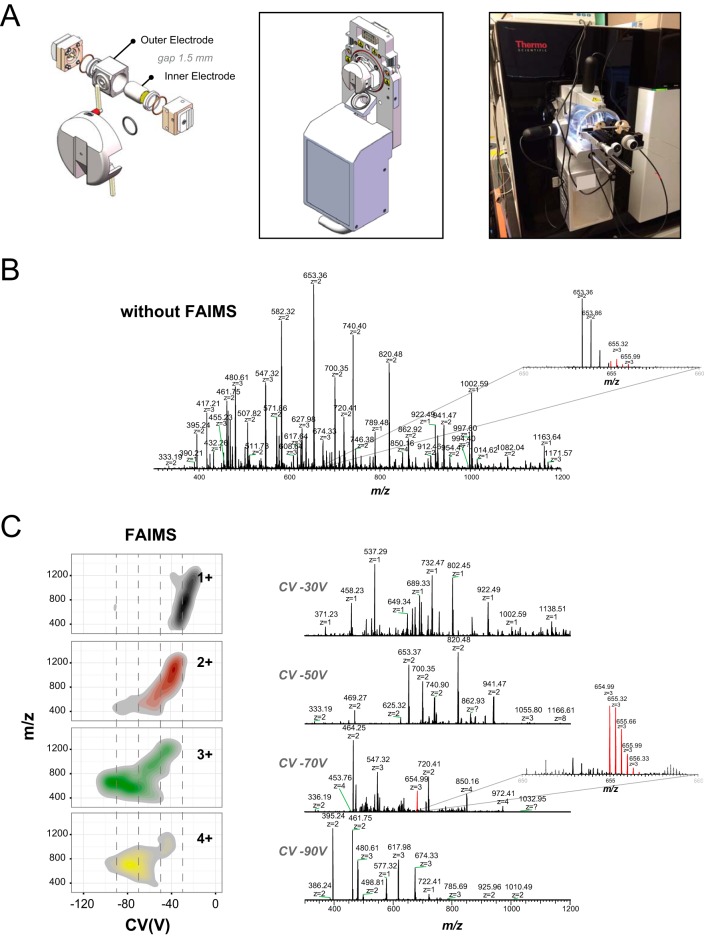
Architecture and separation capacity of a novel FAIMS interface. (A) Scheme of the new FAIMS components with the narrowed electrode gap of 1.5 mm (left). 3D-rendered representation of the FAIMS unit (middle) with the clip in installation mechanism that allows the FAIMS unit to be installed like an ion source on the FUSION Orbitrap front end (right). (B) Direct infusion of BSA tryptic digest by standard ESI-MS showing the ion at m/z 654.99 in background noise of the instrument. (C) Direct infusion of BSA tryptic digest by ESI-FAIMS-MS by switching the CV in 2-V steps from -8 V up to -98 V at DV -5,000 V. Left, charge state distribution of ions as a function of the CV transmission range. Right, examples of MS spectra for the BSA digest infusion at CV -30 V, CV -50 V, CV -70 V, and CV -90 V with the improved detection of the ion at m/z 654.99 highlighted.
Benchmark experiments were performed to determine ion distribution and evaluate the stability and ruggedness of the FAIMS interface using infusion and LC-MS experiments. A solution of 2 μm of angiotensin II (m/z 523.782+) was infused at a 1 μl/min flow rate via the nanospray Flex ion source while maintaining identical MS parameters between FAIMS and non-FAIMS experiments. Intensity of angiotensin II at m/z 523.782+ monitored at CV = -78 V was 6.0e7 with FAIMS and 8.1e7 without FAIMS, corresponding to an ion transmission of 74% (supplemental Fig. 2A). The comparison of MS spectra acquired with and without FAIMS also indicate clear visual differences between the two configurations. Experiments performed without FAIMS display different ion populations including singly charged ions and interfering ions (e.g. solvent clusters, convoluted isotopic profiles). In contrast, the FAIMS MS spectrum only showed the transmission of the doubly protonated peptide ion with no contribution of interfering ions. Under these conditions, the width at half height for angiotensin II is about 8 V when using an inner and outer electrode temperature of 100 °C. The prototype was evaluated for short-term stability using the same angiotensin II solution infused over a period of 24 h. The robustness of CV and DV voltages as well the temperatures of the inner and outer electrode were evaluated. Supplemental Fig. 2B shows that the relative standard deviation for the signal of angiotensin II was 5.3%, highlighting the stability of the spray over this period. The plots for CV, DV, and electrode stability over the course of 24 h further emphasize the robustness of the FAIMS interface for sustained operation.
Next, we determined the distribution of peptide ions with and without FAIMS for a tryptic digest of BSA infused using the nanospray Flex ion source (Figs. 1B and 1C). A heat map representation of ion features as a function of their charge state is shown in Fig. 1C along with representative MS spectra taken at CV -30 V, -50 V, -70 V, and -90 V. The data revealed that gas-phase fractionation of the peptide ion current with FAIMS enabled the detection of more low abundance peptide ions that are typically underrepresented with conventional electrospray. This is highlighted with the triply charged peptide ion at m/z 654.993+, which shows improved coverage of the isotopic cluster with FAIMS at CV -70 V. These results also show the selectivity of FAIMS for gas-phase ion fractionation and the enrichment of specific charge state ion populations. The analyses of the CV distribution of all observed BSA peptide ions indicated that the median width at half peak height was 8 V (supplemental Fig. 3A). The distribution of ions across the range of CV steps can also be used to determine the optimal CV values for maximal transmission of specific multiply charged ions and to reduce the contribution of singly charged interferences (supplemental Fig. 3B).
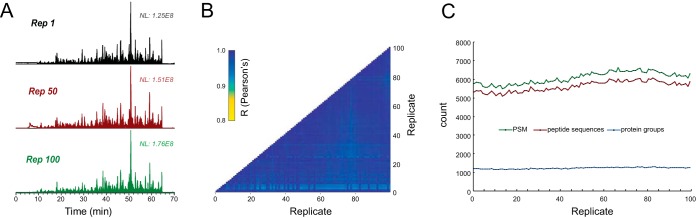
We evaluated the robustness of the new FAIMS interface over repeated LC-MS/MS analyses (CV = -45 V) of 500 ng injections of a HEK293 tryptic digest taken over a 120-h consecutive period (n = 100). Peptides were separated with a 70-min gradient and individually searched against the Human database using the PEAKS search engine. The peptide intensities from the various injections were obtained using the label-free quantification option in the PEAKS software. The replicates were normalized based on the total ion current, and the representation of all the identified peptides present in at least 98 out of 100 replicates were used to create the heat map shown in supplemental Fig. 4A. The chromatographic separation for the 100 replicates was reproducible and constant over the various replicates as exemplified by the total ion chromatograms of replicates 1, 50, and 100 (Fig. 2A). Similar experiments were performed for LC-MS/MS analysis without FAIMS for 27 consecutive injections; the corresponding results are displayed in the heat map of supplemental Fig. 5A. In the FAIMS experiments, 4,427 out of a total of 11,964 unique peptide sequences were present in 98% of all replicates. The reproducibility of peptide detection across replicates can be obtained from supplemental Table 1. The reproducibility of ion transmission of the FAIMS interface is also shown in supplemental Fig. 4A, where the intensity of common peptides is highly reproducible across the different runs. The relative standard deviation for the intensity of each peptide had a median deviation of 18.7% between replicates for FAIMS (supplemental Fig. 4B), compared with a median deviation of 22% for non FAIMS experiments (supplemental Fig. 5B). As expected, we observed an increasing variation in relative standard deviation ranging from 9–35% for decreasing ion intensity (supplemental Fig. 3C). LC-MS/MS experiments performed without FAIMS showed relative standard deviation values ranging from 16–30% (supplemental Fig. 5C). The Pearson correlation coefficients were extracted for each pair of replicates as a metric for replicate reproducibility. All replicates showed coefficient values greater than 0.91 for FAIMS (Fig. 2B), similar to that observed for LC-MS/MS experiments performed without FAIMS (supplemental Fig. 5D). The number of peptide spectrum matches, unique peptide sequences, and protein groups were consistent across the entire duration of the LC-FAIMS-MS/MS analyses (Fig. 2C). After 5 days of constant usage the electrodes showed some trace of accumulation but without a loss of sensitivity. This accumulation of debris indicates that the FAIMS electrodes can serve as a neutral blocker that prevents particulate matter from entering the orifice of the MS and could extend the sensitivity and lifetime of the instrument. The FAIMS device can be operated for more than a month without having to take the assembly apart for cleaning.
Fig. 2.
Reproducibility and robustness of FAIMS-Fusion LC-MS system with 100 replicate injections of HEK293 digest at a fix CV value of -45 V. (A) Ion chromatogram for replicate number 1, 50, and 100. (B) Heat map for Pearson correlation coefficients between all 100 replicate injections. (C) Number of peptide spectrum matches, unique peptide sequences, and protein groups for all 100 replicates.
Optimization of FAIMS for LC-MS/MS Analyses
To optimize the selection of CV steps for maximal use of MS duty cycle, we first performed LC-FAIMS-MS/MS analyses at individual CVs ranging from -37 V up to -93 V in 7-V steps. The 7-V step was selected based on the distribution of peak width observed for the infusion of BSA tryptic peptides as well as the range of CVs that needed to be covered (supplemental Figs. 3A and 3B). Each LC-FAIMS-MS/MS was performed on a 500-ng injection of a HEK293 tryptic digest using a 2-h gradient. The distribution of acquired MS/MS spectra per survey scan is shown in supplemental Fig. 6A and indicates that peripheral CVs were less populated as evidenced from their lower number of MS/MS acquisition events. To make greater use of the MS duty cycle, the number of injections was reduced by combining different CVs into three separate runs. CV combinations (run1: -37 V/-44 V/-51 V, run2: -58 V/-65 V, and run3: -72 V/-79 V/-86 V/-93 V) were selected to maximize the number of acquisition events with an average of 15–20 MS/MS spectra triggered per survey scan. The nine CVs were further combined into two runs (CV -37 V/-44 V/-51 V/-58 V and CV -65 V/-72 V/-79 V/-86 V/-93 V), which reached the maximum duty cycle. The distribution of the number of unique peptides per run for the nine different CV steps (supplemental Fig. 6B) shows that the outermost CV generated fewer identifications, supporting the notion that these ion chromatograms were not densely populated in peptide ions. In contrast, the number of unique peptides obtained for runs that combined multiple CVs indicated that the number of MS/MS events were uniformly distributed for each run, suggesting that the CV combinations were optimal. When analyzing each CV separately (nine runs), the overlap in common peptides ranged from 0.3% to 17.5%, whereas the maximum peptide overlap obtained for three and two runs were 10.7% and 10.9%, respectively (supplemental Fig. 6C).
We evaluated the reproducibility of the peptide identification for separate CV steps and combined within two or three individual LC-FAIMS-MS/MS analyses. Triplicate analyses performed for nine separate CV steps led to the identification of 59,044 unique peptides on average with an overlap of 37,662 common peptides (supplemental Fig. 6D). The combination of CV steps within two or three separate LC-FAIMS-MS/MS analyses enabled an average identification of 29,890 and 38,540 unique peptides with an overlap of 17,353 and 21,456 common peptides, respectively. While LC-FAIMS-MS/MS runs performed at individual CV step provided a greater depth of proteome analysis, the combination of CV steps in three separate runs identified ∼60% of the corresponding peptides and offered a reasonable compromise to maximize sample utilization.
Next, we compared the three-injection combined CV stepping method (2–4 CVs per run) with conventional LC-MS/MS analysis. Three replicates of 500-ng HEK293 tryptic digest were analyzed by LC-MS/MS with and without FAIMS according to the experimental workflow shown in Fig. 3A. LC-MS/MS analyses performed with and without FAIMS enabled the identification of 39,791 and 31,297 and unique peptides, respectively. The acquisition of different peptide ion populations across CVs was clearly evident in FAIMS as only 15.3% of the total number of identified peptides appeared in more than one injection (Fig. 3B). The gain of 27% in peptide identification with FAIMS also translated in a 19% increase in the number of protein groups containing at least two unique peptides (Fig. 3C). The cumulative number of unique identification obtained for each injection is shown in Fig. 3D and indicate that a comparable number of identification was obtained in both configurations after two injections. Beyond this point the FAIMS setup outperformed the standard LC-MS/MS approach. Despite the 38% overlap in identified peptides in the two configurations the overlap in protein groups was excellent with 72% of protein groups being shared. It should be noted that with FAIMS no replicate experiments were performed, which would add further identifications (supplemental Fig. 6D for 2–3 and nine injection methods).
Next, we investigated the proportion of detected versus identified peptide ions across the range of precursor intensities for LC-MS/MS performed with and without FAIMS. These analyses indicated that FAIMS enabled a 50% gain in the detection of new features (Fig. 4A). From the detected ions, 33% and 29% were identified with and without FAIMS, respectively. Interestingly, peptides that were common to both FAIMS and conventional LC-MS/MS followed similar distributions with median log10 intensities of 6.3 and 6.2, respectively. As shown in Fig. 4B an average transmission of 78% was observed for LC-FAIMS-MS, which correlates well with the transmission that was obtained earlier with angiotensin II. The peptides that were identified only with FAIMS (median log10 intensity of 5.7) were less intense ions than those identified only without FAIMS (median log10 intensity of 6.0). This gain in sensitivity favors the identification of lower intensity features (Fig. 4C).
FAIMS Extends the Comprehensiveness of Quantitative Proteomics Using Isobaric Labeling
The ability of FAIMS to reduce spectral complexity and precursor co-selection was advantageously exploited on previous generations of Orbitrap mass spectrometers to improve the accuracy and precision of quantitative measurements in proteomic analyses (28, 44). In the present study, we compared the analytical benefits of FAIMS for multiplex proteomics using isobaric labeling with that of the synchronous precursor selection SPS-MS3 method which is commonly used to reduce peptide co-fragmentation and distorted reporter ion ratios (53–54). Accordingly, we profiled the temporal change in protein abundance of HEK293 cells upon heat shock in increments of 1 h, up to a total period of 9 h (Fig. 5A). After protein extraction and tryptic digestion, peptides from each of the ten time points were labeled individually with the 10-plex TMT reagent and then combined prior to MS analysis. To directly compare LC-MS/MS with FAIMS or SPS-MS3, 500 ng of the TMT labeled sample was analyzed using three injections, similar to that described previously in Fig. 3A. In addition, we also compared FAIMS with SPS-MS3 for three concatenated fractions obtained from off-line high pH/basic reverse-phase fractionation of 1.5 μg of the TMT-labeled sample, as this is the most common method for achieving depth and avoiding/decreasing interference when using isobaric labeling (Fig. 5A). Results obtained for each experiment are presented in Fig. 5B where a progressive increase in the number of peptide identification is observed for each successive injection. Without FAIMS, replicate injections lead to modest gains in identification with a total of 13,642 peptide sequences (27,146 peptide spectrum matches). A ∼30% increase in the number of identification (17,841 peptide sequences) was achieved using basic reverse-phase fractionation. In comparison, LC-FAIMS-MS/MS analyses enabled up to threefold increase in the number of identified peptides compared with SPS-MS3 (Fig. 5C). A major advantage of using FAIMS for TMT quantification is the increased precursor ion purity that enables high-resolution peptide sequencing and reporter ion quantification from the same MS2 spectrum. Although more dependent scan events were registered with SPS-MS3, only half are MS2 events that provide meaningful peptide identification. Indeed, for SPS-MS3, two spectra must be acquired for identification and quantification, where identifications occur at the MS2 level, while quantification of the reporter ions is achieved using the MS3 spectra.
To determine the number of relevant kinetic profiles that showed dynamic changes in protein abundance, we selected protein groups that contained at least two unique peptides and that were observed in at least seven time points. For convenience, we compared kinetic profiles for LC-MS/MS analyses performed using FAIMS and SPS-MS3. These analyses enabled the identification of 1,260 and 2,673 protein groups, of which 1,229 and 2,646 protein groups were quantified using SPS-MS3 and FAIMS, respectively. Dynamic profiles were determined by computing the coefficient of determination (R2) for polynomial functions with orders of up to n = 4. As a first criterion, proteins were selected as “dynamic proteins” if their kinetic profiles fit a polynomial curve with a R2 value ≥0.75. Secondly, the dynamic proteins were subsequently fit to four different dynamic trends based on their fuzzy clustering. Dynamic proteins assigned to a cluster with membership ≥0.9 confidence were retained as “clustered proteins” and resulted in 149 and 502 profiles for SPS-MS3 and FAIMS, respectively. A list of quantified dynamic protein groups (cluster membership > 0.9, with at least two unique peptides and TMT reporters present in ≥ 7 TMT channels) is provided in supplemental Table 4. Next we grouped these profiles into four dynamic trends corresponding to 1) up-regulated early, 2) up-regulated late, 3) downregulated early, and 4) downregulated late (Fig. 6A). The gray lines in Fig. 6A represent the relative fold changes for all proteins that fit the trend, while the blue curve represents the smoothed average log2 fold changes for all clustered proteins from that group. As illustrated by the heat maps in Fig. 6B, the overall changes in protein abundances observed by SPS-MS3 and FAIMS followed similar trends. More importantly, proteins that were quantified by both SPS-MS3 and FAIMS showed comparable fold changes, as illustrated in the representative plots for HSPA1A and leucine-rich PPR motif-containing protein (LRPPRC) in Fig. 6C. A comparison of fold changes observed for common peptides and proteins is provided in supplemental Fig. 7 and highlight the correlation of protein quantification measurements between SPS-MS3 and FAIMS.
Dynamic Proteomics Enabled the Profiling of Cellular Response Upon Hyperthermia
The protein network shown in Fig. 7 provides an overview of the heat shock response in the HEK293 cells. Proteins whose abundances vary in response to the heat shock stimulus were grouped together. The majority of proteins depicted in the network are known to interact with multiple partners whose abundance also varies during the heat stress. Proteins that are grouped into specific GO terms (outlined shapes) have a tendency to alter their abundance in the same demeanor except for proteins from the spliceosomal complex.
Using TMT 10-plex to follow the kinetics of the heat shock process allowed for a greater understanding of the underlying dynamics. Interestingly, the acute heat shock response was primarily mediated by the down-regulation of proteins. Several proteins involved in chromatin remodeling were rapidly decreased in abundance upon heat shock, suggesting that chromatin remodeling is an important adaptation response to hyperthermia. Chromatin remodeling is taking place in the early phase of the heat stress prior to up-regulation of heat shock proteins (response to unfolded protein GO term), and has been reported previously (55). Indeed, all regulated proteins that are involved in the response to unfolded protein (DNAJA1, DNAJB1, DNAJB4, HSP90AA1, HSPA1A, HSPA6, HSPA8, HSPE1, and HSPH1) were up-regulated in the later stages of the heat shock response.
The heat shock response is believed to be initiated in part by HSF1 (56). HSF1 is translocated to the nucleus upon heat stress to promote the transcription of heat shock proteins to favor the refolding of proteins that may have denatured as a result of the stress (57). The efficient transcription of the heat shock proteins requires chromatin remodeling as exemplified by the delay of the transcription of the HSP70 gene by nucleosome formation (55). This is in line with the rapid change that was observed with chromatin associated proteins followed by the subsequent induction of proteins involved in the cellular response to unfolded proteins (supplemental Fig. 8). It is well documented that, upon heat stress, proteins can denature and promote their aggregation or misfolding. Induction of heat shock proteins favors their refolding or prevents denatured protein from aggregating, which can have detrimental effects on the cell. An increase in abundance of several heat shock proteins was observed, including the HSP90 protein HSP90AA1. This chaperon not only prevents the aggregation and favors refolding of proteins but also serves to inactivate HSF1 (58). The up-regulation of HSP90AA1 in the later stages of the heat stress could serve as a feedback loop during the process to inhibit the induction of more heat shock proteins beyond a set threshold.
Proteins that are localized in the mitochondria decreased rapidly in response to heat shock. A rapid down-regulation in proteins involved in the electron transport chain was observed upon hyperthermia consistent with earlier reports (59). Proteins that are part of complex I (NDUFA2, NDUFA4, NDUFA5, NDUFA7, and NDUFS3), complex II (SDHA and SDHB), complex III (UQCRC1), and complex IV (COX4I1, COX5A, and COX6B1) were all downregulated. Moreover, a depletion in the levels of the mitochondrial ribosomes was observed throughout the heat shock process (acute and delayed). The rapid depletion of the electron transport chain proteins from complexes I-IV during the heat shock is known to lead to a rapid and drastic drop in ATP levels in the cell. This process is coupled to the release of reactive oxygen species (60). These observations are congruent with the notion that hyperthermia promotes the disassembly of the mitochondrial wall, resulting in the release of its content.
Prolonged heat stress causes protein misfolding and requires the removal of impaired and nonfunctional proteins. As a result, 5 of the 14 core proteins that make up the 20S proteasome catalytic complex were up-regulated in the later stages of the heat shock. In contrast, the two proteasomal proteins that were not part of the 20S proteasome subunit (ADRM1 and UCHL5) were downregulated in response to the stress (supplemental Fig. 8). ADRM1, a component of the 19S proteasome complex, interacts and activates the deubiquitinase protein UCHL5 (61). ADRM1 favors the association of UCHL5 to the proteasome to promote the removal of poly Lys-48 linked ubiquitin from proteins that are targeted to the proteasome as a means to reduce their aberrant proteolysis. Overall, the levels of proteasome in the cell are increased and output of this machinery is augmented at the potential cost of error by eliminating the proof reading capability of UCHL5 to alleviate cytotoxic protein aggregates.
Interestingly, only components of the spliceosomal complex were increased in abundance in the acute adaptation response to the heat stress. Heat stress has been shown to adversely affect the splicing machinery in HeLa cells (62). Due to the temperature sensitivity of the splicing machinery, the low temperatures that can inhibit this process do not affect transcription. This leads to the buildup of mRNA precursors that can erroneously be translated into toxic proteins. The buildup of mRNA precursors can be partially mitigated by increasing the copy number of the splicing machinery in order to keep up with the downstream pre-mRNA production.
In contrast to the mitochondrial ribosomal content that was reduced, the heat shock led to a late increase in nonmitochondrial ribosomal content (supplemental Fig. 8). Of the 28 nonmitochondrial ribosomal proteins that were quantified, only RPS26 was downregulated in an acute manner. Interestingly, RPS26 is involved in the recognition of the Kozak sequence (63). Indeed, ribosomes that lack RPS26 have a greater propensity to translate proteins that do not start with canonical Kozak leader sequences. More importantly, proteins from the stress response pathway tend to begin with non-Kozak sequences. These findings indicate that both increasing the level of ribosomes in the cell while altering the ribosomal complex by eliminating RPS26 favors the heat shock response by promoting the translation of key proteins.
Overall, the early decrease in proteins involved in cell-cell adhesion, ATP-dependent chromatin remodeling and mitochondrial proteins, and the rapid increase in proteins involved in mRNA splicing during the stress are presumably the catalysts that prompt the up-regulation of nonmitochondrial ribosomal proteins, the cellular response to unfolded proteins, and the down-regulation of the mitochondrial ribosome in the later phase of the challenge. Clearly, the heat shock response includes a number of cellular pathways that result in the regulation of the toxic effects of protein aggregates, alteration in protein synthesis, increased catabolic activities, and targeted translation of heat shock protein (HSP) gene products.
DISCUSSION
This report described the analytical benefits of a re-engineered FAIMS device that can be interfaced on the latest generation of Orbitrap Tribrid instruments and can provide significant advantages for proteomic analyses. FAIMS devices were previously available on earlier generations of mass spectrometers but had several drawbacks in terms of ease of operation, ruggedness, and sensitivity that prevented their wider acceptance. Indeed, the installation and operation of previous FAIMS devices were tedious due to their large sizes and the use of nonstandard ionization sources that were not equipped with camera to assist spray optimization. The use of capillary LC-MS/MS system operating at nanoflow was not straightforward with previous interface due to the spray instability arising from the high flow rate of gas flow exiting the FAIMS device. Also, the entry of droplets into the FAIMS device led to DV instability that prevented the use of LC-FAIMS-MS/MS over extended time periods. However, the more compact footprint of the new FAIMS unit facilitates its installation on the newest generation of Orbitrap Tribrid mass analyzers and can be assembled, mounted, and operated within a few minutes. While LC-FAIMS-MS/MS experiments described here were performed with 10 cm × 150 μm inner diameter capillary columns and a flow rate of 600 nl/min, smaller columns (e.g. 75 μm inner diameter) operating at lower flow rates can also be used equally well with the new FAIMS interface. The FAIMS interface can be used in combination with standard ionization sources equipped with camera to rapidly optimize the position of the electrospray emitter. The stability of the new FAIMS device is vastly improved and can be used for time periods extending over 120 h without significant losses in sensitivity. The use of FAIMS in LC-MS/MS analyses also prevented the direct entry of droplets and contaminants in the transfer tube and S-lens assembly and can extend the continuous operation of the mass spectrometer.
The ion transmission through the FAIMS device was significantly improved and led to ∼80% ion transmission for both direct infusion and LC-FAIMS-MS/MS analyses. This is in stark contrast with previous generation of FAIMS units that provided transmission efficiency of 13–77% (64). Several improvements in the electrode assembly contributed to this enhanced transmission. First, nitrogen flow was supplied to the FAIMS inlet to maximize Coandã effects and prevent ions from preferentially striking the inner electrode upon entry (65). Second, the gap space between the outer and inner electrode of the unit was reduced from 2.5 mm to 1.5 mm (64, 66). This in addition to the smaller FAIMS electrodes translated into an increased electric field and a decreased transit time of ions into the FAIMS device from 130 ms to 40 ms to improve the duty cycle and the number of MS/MS spectra acquired per cycle.
For proteomics applications, the ability of FAIMS to fractionate ions based on their gas-phase differential mobility between high and low electric fields lead to significant benefits in reducing the inherent complexity of cell extracts and improving the depth of proteome coverage. Indeed, FAIMS not only reduces the proportion of singly charged contaminating ions but also separates overlapping multiply charged peptide ions of similar m/z values that lead to chimeric MS/MS spectra. This results in the selection of precursor ions of higher purity and the detection of lower abundance peptide ions. To maximize the acquisition of MS/MS spectra, multiple CV steps can be combined in a single LC-FAIMS-MS/MS run. The CV stepping method can be easily modified depending on the desired depth of the proteome needed. We optimized the CV stepping method where the entire CV range can be covered with 2–3 injections, which provides a 30% gain in the number of unique peptide identifications compared with traditional LC-MS/MS analyses for the same number of injections (Figs. 3 and 4). It is noteworthy that parallel acquisition of ion trap MS/MS spectra available on the Orbitrap Tribrid instrument can improve the duty cycle and proteome coverage in LC-FAIMS-MS/MS experiments and can facilitate the incorporation of multiple CVs for single-shot proteomics.
The reduced proportion of mixed precursor ions in FAIMS experiments also has important advantages for any type of quantitative proteomics workflows and more, specifically, for isobaric labeling where both peptide sequencing and abundance measurements are obtained from the MS/MS spectra. Different strategies, including SPS-MS3, were devised to reduce reporter ions suppression and chimeric MS/MS spectra that undermine the precision and comprehensiveness of quantitative measurements using isobaric labeling. Here, we evaluated the benefits of LC-MS/MS analysis performed using SPS-MS3 and FAIMS to profile the temporal changes in protein abundances using TMT 10-plex reagents. These analyses indicated that FAIMS led to a 2.5-fold increase in the number of quantified peptides compared with SPS-MS3. Since the differential ion mobility reduces the occurrence of co-selection and co-fragmentation, there is a substantial reduction in ratio compression with more accurate TMT quantification (44). Since the TMT quantification can be performed at the MS2 level with FAIMS, the number of scan events leading to peptide sequencing and quantification is higher than that achievable with SPS-MS3 for the same sample amount and analysis time. In addition, the higher-energy collisional dissociation spectra acquired in the Orbitrap increases the identification success rate for FAIMS compared with the collision-induced dissociation ion trap spectra in SPS-MS3.
The improved duty cycle imparted by FAIMS for TMT-based quantification over SPS-MS3 helped to increase the coverage of the dynamic protein profiles upon heat shock (502 versus 149 for FAIMS and SPS, respectively). The added proteins that were quantified as a result of using FAIMS revealed how vast the effects of heat shock is on the cell by monitoring the dynamics of the heat shock response in greater detail, as evidenced in Fig. 7A. Indeed several processes were altered in response to the stimulus and with varying kinetics. Moreover, the processes that were affected by the heat shock were varied and independent from each other, highlighting the global effect of the heat shock response. Cells subjected to heat stress are known to adapt to this challenge by altering several key cellular processes, which include but are not limited to: aggregation of ribosomal proteins, alterations in the RNA-splicing machinery (67), disruption of the cytoskeleton structure, mitochondrial dysfunction (68), and increased production of heat shock proteins (69, 70). In HEK293 cells, the order in which these events occur with regards to each other is not known. The dynamics of these events holds great value to better understand how cells can adapt to stress because the heat shock response is not unique to mild heat stress but is also shared with adaptation to oxidative stress and exposure to heavy metals (70). Indeed the thermotolerance that is imparted to cells by heat stress has been shown to increase cellular resistance to anticancer drugs and trophic factor withdrawal, indicating that the heat shock response is versatile and robust (71).
By following the changes in the proteome mediated by hyperthermia in a time resolved manner, we expanded the comprehensiveness of the cellular response. We found that proteins that shared similar functions had a tendency to alter in abundance with similar dynamics. Seminal work by the Savitski group, which followed protein turnover using a stable isotope labeling with amino acids in cell culture (SILAC) quantification methodology in several primary cells highlighted that proteins that make up complexes have similar half-lives, suggesting that complexes as a whole degrade together (72). Our results are consistent with this earlier study, where the proteins that share the same GO term in the network not only possess similar functions but interact readily with one another, explaining their shared dynamics.
This work highlights several advantages of the new FAIMS interface with the most valuable being the ability of the electrodes to perform gas-phase fractionation of the complex ion population from an ESI within a 40-ms period. An important outcome from such a fractionation mechanism was the enrichment of multiply charged species that facilitated a deeper sampling of precursors in the MS survey scan that could have otherwise remained unsampled. Due to its novelty and equal or superior performance over condensed phase fractionation, FAIMS devices will gain wider acceptance as an important technology in the field of MS-based proteomics. There are possibilities to improve duty cycle usage by invoking the parallelization that is accessible on the Orbitrap Tribrid mass spectrometers. Ideally, the instrument duty cycle can be improved to cover the whole CV transmission range with a single injection. This would allow reduction in the instrument usage time by 2–3-fold. During the review of this manuscript, this application was demonstrated on the Orbitrap Tribrid LUMOS mass spectrometer, and enabled increased depth for single-shot proteome analyses (73). The application of FAIMS on the new Tribrid MS instruments in the field of proteomics is in infancy and has the potential to flourish in the coming years.
DATA AVAILABILITY
The MS proteomics data have been deposited to the Proteome-Xchange Consortium via the massIVE partner repository which can be accessed with the dataset identifier: PXD009547.
Supplementary Material
Acknowledgments
We thank Philip Remes and Jesse D. Canterbury at Thermo Fisher Scientific in San Jose (CA) for technical assistance and Susan Abbatiello for constructive criticism and valuable comments during the review of this manuscript. S.Pf is the recipient of a Swiss national science foundation scholarship (P1SKP3–168335). This work was carried out with financial support from the Natural Sciences and Engineering Research Council (NSERC 311598) and the Genomic Applications Partnership Program (GAPP) of Genome Canada. The Institute for Research in Immunology and Cancer (IRIC) receives infrastructure support from IRICoR, the Canadian Foundation for Innovation, and the Fonds de Recherche du Québec–Santé (FRQS). The IRIC proteomics facility is a Genomics Technology platform funded in part by the Canadian government through Genome Canada.
Footnotes
* The authors declare the following competing financial interest(s): M.B., S.P., D.B., and J.-J.D., are employees at Thermo Fisher Scientific, which develops and distributes MS instruments, including the FAIMS device.
 This article contains supplemental material Tables 1–4 and Figs. 1–8.
This article contains supplemental material Tables 1–4 and Figs. 1–8.
1 The abbreviations used are:
- FAIMS
- high-field asymmetric waveform ion mobility spectrometry
- LC-MS/MS
- liquid chromatography-tandem mass spectrometry
- Hz
- hertz
- IMS
- ion mobility spectrometry
- CV
- compensation voltage
- DV
- dispersion voltage
- TMT
- tandem mass tag
- SPS
- synchronous precursor selection
- HEK293
- human embryonic kidney cells 293
- FA
- formic acid
- HEPES
- 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid)
- AGC
- automatic gain control
- m/z
- mass-to-charge
- Th
- Thomson
- FDR
- false discovery rate
- ESI
- electrospray ionization
- GO
- gene ontology.
REFERENCES
- 1. Beck S., Michalski A., Raether O., Lubeck M., Kaspar S., Goedecke N., Baessmann C., Hornburg D., Meier F., Paron I., Kulak N. A., Cox J., and Mann M. (2015) The Impact 2 a very high resolution qTOF for deep shotgun proteomics. Mol. Cell. Proteomics 14, 2014–2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Eliuk S., and Makarov A. (2015) Evolution of the Orbitrap mass spectrometry instrumentation. Annu. Rev. Anal. Chem. 8, 61–80 [DOI] [PubMed] [Google Scholar]
- 3. Cox J., Hein M. Y., Luber C. A., Paron I., Nagaraj N., and Mann M. (2014) Accurate proteome-wide label-free quantification by delayed normalization and maximal peptide ratio extraction, termed MaxLFQ. Mol. Cell. Proteomics 13, 2513–2526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Karp N. A., Huber W., Sadowski P. G., Charles P. D., Hester S. V., and Lilley K. S. (2010) Addressing accuracy and precision issues in iTRAQ quantitation. Mol. Cell. Proteomics 9, 1885–1897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ow S. Y., Salim M., Noirel J., Evans C., Rehman I., and Wright P. C. (2009) iTRAQ underestimation in simple and complex mixtures: “The good, the bad and the ugly.” J. Proteome Res. 8, 5347–5355 [DOI] [PubMed] [Google Scholar]
- 6. McAlister G. C., Huttlin E. L., Haas W., Ting L., Jedrychowski M. P., Rogers J. C., Kuhn K., Pike I., Grothe R. A., Blethrow J. D., and Gygi S. P. (2012) Increasing the multiplexing capacity of TMTs using reporter ion isotopologues with isobaric masses. Anal. Chem. 84, 7469–7478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McAlister G. C., Nusinow D. P., Jedrychowski M. P., Wühr M., Huttlin E. L., Erickson B. K., Rad R., Haas W., and Gygi S. P. (2014) MultiNotch MS3 enables accurate, sensitive, and multiplexed detection of differential expression across cancer cell line proteomes. Anal. Chem. 86, 7150–7158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bekker-Jensen D. B., Kelstrup C. D., Batth T. S., Larsen S. C., Haldrup C., Bramsen J. B., Sørensen K. D., Høyer S., Ørntoft T. F., Andersen C. L., Nielsen M. L., and Olsen J. V. (2017) An optimized shotgun strategy for the rapid generation of comprehensive human proteomes. Cell Syst. 4, 587–599.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kelstrup C. D., Jersie-Christensen R. R., Batth T. S., Arrey T. N., Kuehn A., Kellmann M., and Olsen J. V. (2014) Rapid and deep proteomes by faster sequencing on a benchtop quadrupole ultra-high-field Orbitrap mass spectrometer. J. Proteome Res. 13, 6187–6195 [DOI] [PubMed] [Google Scholar]
- 10. Iwasaki M., Sugiyama N., Tanaka N., and Ishihama Y. (2012) Human proteome analysis by using reversed-phase monolithic silica capillary column with enhanced sensitivity. J. Chromatogr. A 1228, 292–297 [DOI] [PubMed] [Google Scholar]
- 11. Nagaraj N., Kulak N. A., Cox J., Neuhauser N., Mayr K., Hoerning O., Vorm O., and Mann M. (2012) System-wide perturbation analysis with nearly complete coverage of the yeast proteome by single-shot ultra HPLC runs on a bench top Orbitrap. Mol. Cell. Proteomics 11, M111.013722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Scheltema R. A., Hauschild J. P., Lange O., Hornburg D., Denisov E., Damoc E., Kuehn A., Makarov A., and Mann M. (2014) The Q Exactive HF, a benchtop mass spectrometer with a pre-filter, high-performance quadrupole and an ultra-high-field Orbitrap analyzer. Mol. Cell. Proteomics 13, 3698–3708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Michalski A., Cox J., and Mann M. (2011) More than 100,000 detectable peptide species elute in single shotgun proteomics runs but the majority is inaccessible to data-dependent LC-MS/MS. J. Proteome Res 10, 1785–1793 [DOI] [PubMed] [Google Scholar]
- 14. Shishkova E., Hebert A. S., and Coon J. J. (2016) Now, more than ever, proteomics needs better chromatography. Cell Syst. 3, 321–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hebert A. S., Thöing C., Riley N. M., Kwiecien N. W., Shiskova E., Huguet R., Cardasis H. L., Khuen A., Eliuk S., Zabrouskov V., Westphall M. S., McAlister G. C., and Coon J. J. (2018) Improved precursor characterization for data-dependent mass spectrometry. Anal. Chem. 90, 2333–2340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. O'Brien J. J., O'Connell J. D., Paulo J. A., Thakurta S., Rose C. M., Weekes M. P., Huttlin E. L., and Gygi S. P. (2018) Compositional proteomics: Effects of spatial constraints on protein quantification utilizing isobaric tags. J. Proteome Res. 17, 590–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cumeras R., Figueras E., Davis C. E., Baumbach J. I., and Gràcia I. (2015) Review on ion mobility spectrometry. Part 2: Hyphenated methods and effects of experimental parameters. Analyst 140, 1391–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lapthorn C., Pullen F., and Chowdhry B. Z. (2013) Ion mobility spectrometry-mass spectrometry (IMS-MS) of small molecules: Separating and assigning structures to ions. Mass Spectrom. Rev. 32, 43–71 [DOI] [PubMed] [Google Scholar]
- 19. Schneider B. B., Nazarov E. G., Londry F., Vouros P., and Covey T. R. (2016) Differential mobility spectrometry/mass spectrometry history, theory, design optimization, simulations, and applications. Mass Spectrom. Rev. 35, 687–737 [DOI] [PubMed] [Google Scholar]
- 20. Swearingen K. E., and Moritz R. L. (2012) High-field asymmetric waveform ion mobility spectrometry for mass spectrometry-based proteomics. Expert Rev. Proteomics 9, 505–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wyttenbach T., Pierson N. A., Clemmer D. E., and Bowers M. T. (2014) Ion mobility analysis of molecular dynamics. Annu. Rev. Phys. Chem. 65, 175–196 [DOI] [PubMed] [Google Scholar]
- 22. Ruotolo B. T., McLean J. A., Gillig K. J., and Russell D. H. (2005) The influence and utility of varying field strength for the separation of tryptic peptides by ion mobility-mass spectrometry. J. Am. Soc. Mass Spectrom. 16, 158–165 [DOI] [PubMed] [Google Scholar]
- 23. Harvey S. R., MacPhee C. E., and Barran P. E. (2011) Ion mobility mass spectrometry for peptide analysis. Methods 54, 454–461 [DOI] [PubMed] [Google Scholar]
- 24. Guevremont R. (2004) High-field asymmetric waveform ion mobility spectrometry: A new tool for mass spectrometry. J. Chromatogr. A 1058, 3–19 [PubMed] [Google Scholar]
- 25. Purves R. W., and Guevremont R. (1999) Electrospray ionization high-field asymmetric waveform ion mobility spectrometry-mass spectrometry. Anal. Chem. 71, 2346–2357 [DOI] [PubMed] [Google Scholar]
- 26. Barnett D. A., Ells B., Guevremont R., and Purves R. W. (2002) Application of ESI-FAIMS-MS to the analysis of tryptic peptides. J. Am. Soc. Mass Spectrom. 13, 1282–1291 [DOI] [PubMed] [Google Scholar]
- 27. Shvartsburg A. A., Tang K., and Smith R. D. (2004) Modeling the resolution and sensitivity of FAIMS analyses. J. Am. Soc. Mass Spectrom. 15, 1487–1498 [DOI] [PubMed] [Google Scholar]
- 28. Bonneil E., Pfammatter S., and Thibault P. (2015) Enhancement of mass spectrometry performances for proteomics analyses using high-field asymmetric waveform spectrometry (FAIMS). J. Mass Spectrom. 50, 1181–1195 [DOI] [PubMed] [Google Scholar]
- 29. Canterbury J. D., Yi X., Hoopmann M. R., and MacCoss M. J. (2008) Assessing the dynamic range and peak capacity of nanoflow LC-FAIMS-MS on an ion trap mass spectrometer for proteomics. Anal. Chem. 80, 6888–6897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Creese A. J., Shimwell N. J., Larkins K. P., Heath J. K., and Cooper H. J. (2013) Probing the complementarity of FAIMS and strong cation exchange chromatography in shotgun proteomics. J. Am. Soc. Mass Spectrom. 24, 431–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Saba J., Bonneil E., Pomiès C., Eng K., and Thibault P. (2009) Enhanced sensitivity in proteomics experiments using FAIMS coupled with a hybrid linear ion trap/Orbitrap mass analyzer. J. Proteome Res. 8, 3355–3366 [DOI] [PubMed] [Google Scholar]
- 32. Swearingen K. E., Hoopmann M. R., Johnson R. S., Saleem R. A., Aitchinson J. D., and Moritz R. L. (2012) Nanospray FAIMS fractionation provides significant increases in proteome coverage of unfractionated complex protein digests. Mol. Cellular Proteomics 11, M111.014985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Venne K., Bonneil E., Eng K., and Thibault P. (2005) Improvement in peptide detection for proteomics analyses using nanoLC-MS and high-field asymmetry waveform ion mobility mass spectrometry. Anal. Chem. 77, 2176–2186 [DOI] [PubMed] [Google Scholar]
- 34. Baird M. A., and Shvartsburg A. A. (2016) Localization of post-translational modifications in peptide mixtures via high-resolution differential ion mobility separations followed by electron transfer dissociation. J. Am. Soc. Mass Spectrom. 27, 2064–2070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Garabedian A., Baird M. A., Dit Fouque J., Shliaha P. V., Jensen O. N., Williams T. D., Fernandez-Lima F., and Shvartsburg A. A. (2018) Linear and differential ion mobility separations of middle-down proteoforms. Anal. Chem. 90, 2918–2925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kaszycki J. L., Bowman A. P., and Shvartsburg A. A. (2016) Ion mobility separation of peptide isotopomers. J. Am. Soc. Mass Spectrom. 27, 795–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shliaha P. V., Baird M. A., Nielsen M. M., Gorshkov V., Bowman A. P., Kaszycki J. L., Jensen O. N., and Shvartsburg A. A. (2017) Characterization of complete histone tail proteoforms using differential ion mobility spectrometry. Anal. Chem. 89, 5461–5466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shvartsburg A. A., Creese A. J., Smith R. D., and Cooper H. J. (2010) Separation of peptide isomers with variant modified sites by high-resolution differential ion mobility spectrometry. Anal. Chem. 82, 8327–8334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shvartsburg A. A., Singer D., Smith R. D., and Hoffmann R. (2011) Ion mobility separation of isomeric phosphopeptides from a protein with variant modification of adjacent residues. Anal. Chem. 83, 5078–5085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shvartsburg A. A., Haris A., Andrzejewski R., Entwistle A., and Giles R. (2018) Differential ion mobility separations in the low-pressure regime. Anal. Chem. 90, 936–943 [DOI] [PubMed] [Google Scholar]
- 41. Shvartsburg A. A., Seim T. A., Danielson W. F., Norheim R., Moore R. J., Anderson G. A., and Smith R. D. (2013) High-definition differential ion mobility spectrometry with resolving power up to 500. J. Am. Soc. Mass Spectrom. 24, 109–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shvartsburg A. A., Zheng Y., Smith R. D., and Kelleher N. L. (2012) Separation of variant methylated histone tails by differential ion mobility. Anal. Chem. 84, 6317–6320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shvartsburg A. A., Zheng Y., Smith R. D., and Kelleher N. L. (2012) Ion mobility separation of variant histone tails extending the middle-down range. Anal. Chem. 84, 4271–4276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pfammatter S., Bonneil E., and Thibault P. (2016) Improvement of quantitative measurements in multiplex proteomics using high-field asymmetric waveform spectrometry. J. Proteome Res. 15, 4653–4665 [DOI] [PubMed] [Google Scholar]
- 45. Keshishian H., Burgess M. W., Specht H., Wallace L., Clauser K. R., Gillette M. A., and Carr S. A. (2017) Quantitative, multiplexed workflow for deep analysis of human blood plasma and biomarker discovery by mass spectrometry. Nat. Protoc. 12, 1683–1701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Prasad S., Belford M. W., Dunyach J. J., and Purves R. W. (2014) On an aerodynamic mechanism to enhance ion transmission and sensitivity of FAIMS for nano-electrospray ionization-mass spectrometry. J. Am. Soc. Mass Spectrom. 25, 2143–2153 [DOI] [PubMed] [Google Scholar]
- 47. Schwämmie V., and Jensen O. N. (2010) A simple and fast method to determine the parameters for fuzzy c-means cluster analysis. Bioinformatics 26, 2841–2848 [DOI] [PubMed] [Google Scholar]
- 48. Franceschini A., Szklarczyk D., Frankild S., Kuhn M., Simonovic M., Roth A., Lin J., Minguez P., Bork P., von Mering C., and Jensen L. J. (2013) STRING v9.1: Protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res. 41, D808–D815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Smoot M. E., Ono K., Ruscheinski J., Wang P. L., and Ideker T. (2011) Cytoscape 2.8: New features for data integration and network visualization. Bioinformatics 27, 431–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Maere S., Heymans K., and Kuiper M. (2005) BiNGO: a Cytoscape plugin to assess overrepresentation of gene ontology categories in biological networks. Bioinformatics 21, 3448–3449 [DOI] [PubMed] [Google Scholar]
- 51. Kubiniok P., Lavoie H., Therrien M., and Thibault P. (2017) Time-resolved phosphoproteome analysis of paradoxical RAF activation reveals novel targets of ERK. Mol. Cell. Proteomics 16, 663–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nock R., and Nielsen F. (2006) On weighting clustering. IEEE Trans. Pattern Anal. Mach. Intell. 28, 1223–1235 [DOI] [PubMed] [Google Scholar]
- 53. McAlister G. C., Huttlin E. L., Haas W., Ting L., Jedrychowski M. P., Rogers J. C., Kuhn K., Pike I., Grothe R. A., Blethrow J. D., and Gygi S. P. (2012) Increasing the multiplexing capacity of TMTs using reporter ion isotopologues with isobaric masses. Anal. Chem. 84, 7469–7478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. McAlister G. C., Nusinow D. P., Jedrychowski M. P., Wühr M., Huttlin E. L., Erickson B. K., Rad R., Haas W., and Gygi S. P. (2014) MultiNotch MS3 enables accurate, sensitive, and multiplexed detection of differential expression across cancer cell line proteomes. Anal. Chem. 86, 7150–7158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Brown S. A., Imbalzano A. N., and Kingston R. E. (1996) Activator-dependent regulation of transcriptional pausing on nucleosomal templates. Genes Dev. 10, 1479–1490 [DOI] [PubMed] [Google Scholar]
- 56. de Nadal E., Ammerer G., and Posas F. (2011) Controlling gene expression in response to stress. Nat. Rev. Genet. 12, 833–845 [DOI] [PubMed] [Google Scholar]
- 57. Anckar J., and Sistonen L. (2011) Regulation of HSF1 function in the heat stress response: Implications in aging and disease. Annu. Rev. Biochem. 80, 1089–1115 [DOI] [PubMed] [Google Scholar]
- 58. Zou J., Guo Y., Guettouche T., Smith D. F., and Voellmy R. (1998) Repression of heat shock transcription factor HSF1 activation by HSP90 (HSP90 complex) that forms a stress-sensitive complex with HSF1. Cell 94, 471–480 [DOI] [PubMed] [Google Scholar]
- 59. Patriarca E. J., and Maresca B. (1990) Acquired thermotolerance following heat shock protein synthesis prevents impairment of mitochondrial ATPase activity at elevated temperatures in Saccharomyces cerevisiae. Exp. Cell Res. 190, 57–64 [DOI] [PubMed] [Google Scholar]
- 60. Kikusato M., Yoshida H., Furukawa K., and Toyomizu M. (2015) Effect of heat stress-induced production of mitochondrial reactive oxygen species on NADPH oxidase and heme oxygenase-1 mRNA levels in avian muscle cells. J .Therm. Biol. 52, 8–13 [DOI] [PubMed] [Google Scholar]
- 61. Yao T., Song L., Xu W., DeMartino G. N., Florens L., Swanson S. K., Washburn M. P., Conaway R. C., Conaway J. W., and Cohen R. E. (2006) Proteasome recruitment and activation of the Uch37 deubiquitinating enzyme by Adrm1. Nat. Cell Biol. 8, 994–1002 [DOI] [PubMed] [Google Scholar]
- 62. Bond U. (1988) Heat shock but not other stress inducers leads to the disruption of a sub-set of snRNPs and inhibition of in vitro splicing in HeLa cells. EMBO J. 7, 3509–3518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ferretti M. B., Ghalei H., Ward E. A., Potts E. L., and Karbstein K. (2017) Rps26 directs mRNA-specific translation by recognition of Kozak sequence elements. Nat. Struct. Mol. Biol. 24, 700–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Barnett D. A., and Ouellette R. J. (2011) Elimination of the helium requirement in high-field asymmetric waveform ion mobility spectrometry (FAIMS): Beneficial effects of decreasing the analyzer gap width on peptide analysis. Rapid Commun. Mass Spectrom. 25, 1959–1971 [DOI] [PubMed] [Google Scholar]
- 65. Prasad S., Belford M. W., Dunyach J. J., and Purves R. W. (2014) On an aerodynamic mechanism to enhance ion transmission and sensitivity of FAIMS for nano-electrospray ionization-mass spectrometry. J. Am. Soc. Mass Spectrom. 25, 2143–2153 [DOI] [PubMed] [Google Scholar]
- 66. Swearingen K. E., Winget J. M., Hoopmann M. R., Kusebauch U., and Moritz R. L. (2015) Decreased gap width in a cylindrical high-field asymmetric waveform ion mobility spectrometry device improves protein discovery. Anal. Chem. 87, 12230–12237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Shukla R. R., Dominski Z., Zwierzynski T., and Kole R. (1990) Inactivation of splicing factors in HeLa cells subjected to heat shock. J. Biol. Chem. 265, 20377–20383 [PubMed] [Google Scholar]
- 68. Welch W. J., and Suhan J. P. (1985) Morphological study of the mammalian stress response: Characterization of changes in cytoplasmic organelles, cytoskeleton, and nucleoli, and appearance of intranuclear actin filaments in rat fibroblasts after heat-shock treatment. J. Cell Biol. 101, 1198–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Richter K., Haslbeck M., and Buchner J. (2010) The heat shock response: Life on the verge of death. Mol. Cell 40, 253–266 [DOI] [PubMed] [Google Scholar]
- 70. Tabuchi Y., Takasaki I., Wada S., Zhao Q. L., Hori T., Nomura T., Ohtsuka K., and Kondo T. (2008) Genes and genetic networks responsive to mild hyperthermia in human lymphoma U937 cells. Int. J. Hyperthermia 24, 613–622 [DOI] [PubMed] [Google Scholar]
- 71. Fulda S., Gorman A. M., Hori O., and Samali A. (2010) Cellular stress responses: Cell survival and cell death. Int. J. Cell Biol. 2010, 214074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mathieson T., Franken H., Kosinski J., Kurzawa N., Zinn N., Sweetman G., Poeckel D., Ratnu V. S., Schramm M., Becher I., Steidel M., Noh K. M., Bergamini G., Beck M., Bantscheff M., and Savitski M. M. (2018) Systematic analysis of protein turnover in primary cells. Nat. Commun. 9, 689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hebert A. S., Prasad S., Belford M. W., Bailey D. J., McAlister G. C., Abbatiello S. E., Huguet R., Wouters E. R., Dunyach J. J., Brademan D. R., Westphall M. S., and Coon J. J. (2018) Rapid and comprehensive single shot proteomics with LC-FAIMS-MS/MS. Anal. Chem., in press [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The MS proteomics data have been deposited to the Proteome-Xchange Consortium via the massIVE partner repository which can be accessed with the dataset identifier: PXD009547.