Abstract
Objectives
To assess bone marrow (BM) sampling in academic medical centers.
Methods
Data from 6,374 BM samples obtained in 32 centers in 2001 and 2011, including core length (CL), were analyzed.
Results
BM included a biopsy (BMB; 93%) specimen, aspirate (BMA; 92%) specimen, or both (83%). The median (SD) CL was 12 (8.5) mm, and evaluable marrow was 9 (7.6) mm. Tissue contraction due to processing was 15%. BMB specimens were longer in adults younger than 60 years, men, and bilateral, staging, and baseline samples. Only 4% of BMB and 2% of BMB/BMA samples were deemed inadequate for diagnosis. BM for plasma cell dyscrasias, nonphysician operators, and ancillary studies usage increased, while bilateral sampling decreased over the decade. BM-related quality assurance programs are infrequent.
Conclusions
CL is shorter than recommended and varies with patient age and sex, clinical circumstances, and center experience. While pathologists render diagnoses on most cases irrespective of CL, BMB yield improvement is desirable.
Keywords: Bone marrow biopsy, Bone marrow biopsy indications, Core biopsy length, Bone marrow adequacy, Bone marrow quality
Examination of bone marrow (BM) is useful for diagnosis, staging, and monitoring of various benign and malignant disorders. Two main types of specimens are usually obtained through a BM procedure: core BM biopsy (BMB) and BM aspirate (BMA). The biopsy specimen is ideal for evaluation of the architectural and structural relationship of normal or pathologic BM elements, whereas the aspirate specimen is better for assessing hematopoietic cytomorphology and for differential cell counts and best suited for flow cytometric immunophenotyping, cytogenetic tests, and molecular tests. Some ancillary studies performed on the core biopsy specimen (ie, immunohistochemistry) are less feasible on aspirate specimens. The core biopsy and aspirate specimens provide rapidly available complementary information, each with its own strengths and limitations; therefore, a quality BM sample comprising both components is ideal for most clinical indications. BM biopsy specimen yield is also dependent on operator training, skill and specialty, and individual patient factors, and it varies within and among different institutions.1,2
An adequate BM specimen is essential for reaching the correct diagnosis and avoiding sampling error. A minimum of evaluable marrow space should be represented in the core biopsy specimen, and an optimal trephine biopsy sample length has been defined to be in the 15- to 25-mm range3-8 and/or a minimum of 10 preserved intertrabecular spaces.4,6 Measurements of the core length (CL) include preprocessing (ie, length measured at the bedside or grossing station), postprocessing (ie, measurements on the slide), and evaluable hematopoietic marrow (the actual preserved marrow space, after excluding nonhematopoietic or extrinsic elements). The first two measure the quantity of the sample while the latter reflects the quality of the material.1
An appropriate biopsy specimen length is particularly important in lymphoma or solid tumor staging. The presence and extent of myelofibrosis can only be reliably estimated in a core of adequate length,4,9,10 important both at the initial diagnosis and to gauge therapy response11 using a predictive and reproducible fibrosis grading system for BMB specimens endorsed by the recent World Health Organization classification.4 What constitutes an inadequate specimen is not uniformly defined.
In this study, we analyze BM sample characteristics, including biopsy specimen length and quality, operator-related and technical aspects of sampling, processing, and ancillary studies utilization. We report on the practice changes from 32 teaching institutions from the United States and Canada between 2001 and 2011. Core biopsy specimen aggregate length was uniformly measured, and its variability with clinical, institution, and patient-related characteristics was assessed and compared with current guidelines.
Materials and Methods
Study Design
This was a retrospective, cross-sectional study of BM specimens obtained at academic centers in the United States and Canada.
Participating Centers
Thirty-two participating hospitals from several regions of the United States and Canada submitted data for analysis after local institutional review board approval of a protocol (RPCCC BDR 034213). No individual patient consent was required.
Data Collection
Slides and corresponding pathology reports of 100 consecutive in-house BM specimens sampled from the posterior iliac crest and accessioned beginning February 1, 2001, and February 1, 2011, respectively, were retrieved from institutional archives and reviewed, with data tabulated at each site by the study pathologists. BMB specimens from other anatomic sites, specimens with missing slides, and consultation or referral cases were excluded.
Three categories of data were obtained at each center: extracted from pathology reports, from core biopsy specimen slide review, and from a survey on clinical and laboratory practice (Supplemental Methods; all supplemental materials can be found at American Journal of Clinical Pathology online). Data were collected at each site, deidentified, and centralized for analysis. Uniform guidelines for data collection and entry and specific, detailed instructions for all measurements were provided. Core biopsy specimen measurements were performed by the study participants at each site on the glass slides using the same model of transparent ruler (C-thru Ruler, Bloomfield, CT) and recorded in millimeters. Preprocessing length was imported from the pathology report when available.
Statistical Analysis
All 32 data sets were deidentified and aggregated. After eliminating ineligible samples (consultations or referral specimens, n = 24; specimens missing all data entries, n = 2), validated data were assembled into a master database with 6,374 samples accepted for analysis.
Descriptive statistics such as frequencies and relative frequencies were computed for all categorical variables. Numeric variables were summarized using simple descriptive statistics, including mean, standard deviation, range, and so on. Ninety-five percent confidence intervals were computed when appropriate. Boxplots (minimum, lower quartile, median, upper quartile, maximum) were used to graphically display data, along with pie charts for frequency data. Differences among biopsy specimen lengths and correlation with multiple variables were assessed using a random-effects mixed model incorporating a log transformation of the continuous response measures and random effects for center and the center-time interaction and a fixed effect for time. A nominal significance level of .05 was used in all testing, and the Bonferroni adjustment was used to correct for multiple comparisons when evaluating pairwise differences. All statistical analysis and plots were performed using SAS (version 9.4 or higher) statistical software (SAS Institute, Cary, NC).
Results
Centers and Operators
Bone marrow biopsy procedures were performed primarily by hematologists and oncologists in more than half (59%) of centers, whereas pathologists obtained most samples in only 8% of centers. Nonphysician health care providers obtained most samples in 31% of centers overall and increased as prevalent operators from four to eight hospitals between 2001 and 2011. Laboratory personnel provided regular bedside assistance in 64% of centers. The volume of procedures for each center was variable, with most hospitals performing between 500 and 2,000 BM procedures per year (Supplemental Table 1).
Patient Demographics and Clinical Indications for Biopsy Procedure
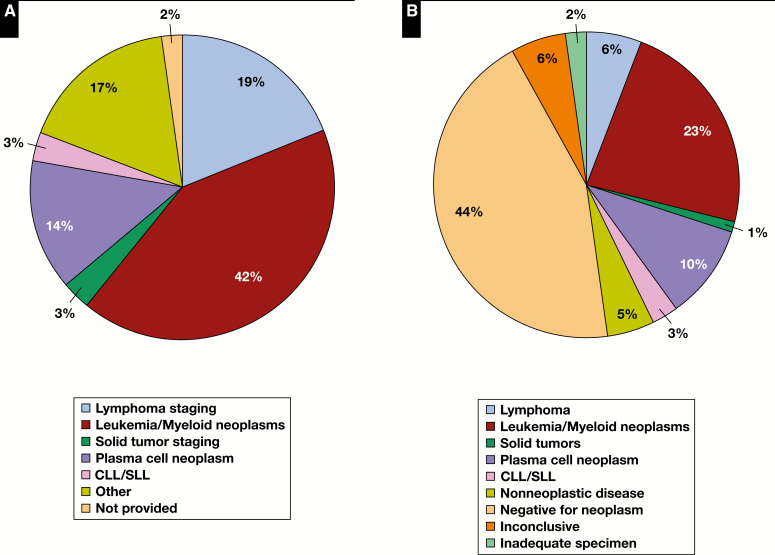
A total of 6,374 BM samples were obtained from 3,580 (56%) men and 2,792 (44%) women, who were predominantly adults (89%) with an average age of 52 (median, 57; range, 1-102) years. Clinical indications for BM examination are shown in Figure 1A , with the three most common being acute leukemia and myeloid neoplasms, lymphoma staging, and plasma cell neoplasms for both study years. BM evaluation for plasma cell neoplasms increased from 11% in 2001 to 18% in 2011. Overall, there were 23% staging and 77% nonstaging BM samples, 44% performed for initial diagnosis and 52% for follow-up (Supplemental Table 2).
Figure 1.
Clinical indications for bone marrow biopsy (A) and rendered pathologic diagnoses (B). CLL/SLL, chronic lymphocytic leukemia/small lymphocytic lymphoma.
Samples Type and Laterality
Most BM samples included a core biopsy specimen (93%), an aspirate specimen (92%), or both (83%). BMA-only samples proportionally decreased from 2001 to 2011 (9.2% vs 4.4%). Bilateral sampling was performed more often for staging than nonstaging indications (78% vs 2%, P < .001) and decreased from 2001 to 2011 from 10% to 4% of total BMB specimens. Aspirate specimens were also unilateral in most cases (86%) and only rarely obtained but not reviewed in the pathology department (6%) or not performed at all (4%). A clot section (including cytospin or filtered aspirate) was processed in 56% of all samples and in 70% (293 of 420) of specimens lacking the core biopsy. Touch preparations were obtained in about two-thirds of samples. Peripheral blood counts were provided in 78% of samples, and review of the peripheral blood smear was included in the pathology report in 71% of cases.
Histologic Processing and Routine and Ancillary Studies Performed
BM core biopsy and clot specimens were fixed in several different solutions, most commonly neutral buffered formalin or heavy metal fixatives (34% each), zinc-based formalin (16%), and Bouin’s (11%). Usage of zinc-based formalin fixatives increased fourfold (6%-25%), whereas use of heavy metal fixatives (eg, B5, Zenker) decreased from 53% to 16% of laboratories from 2001 to 2011.
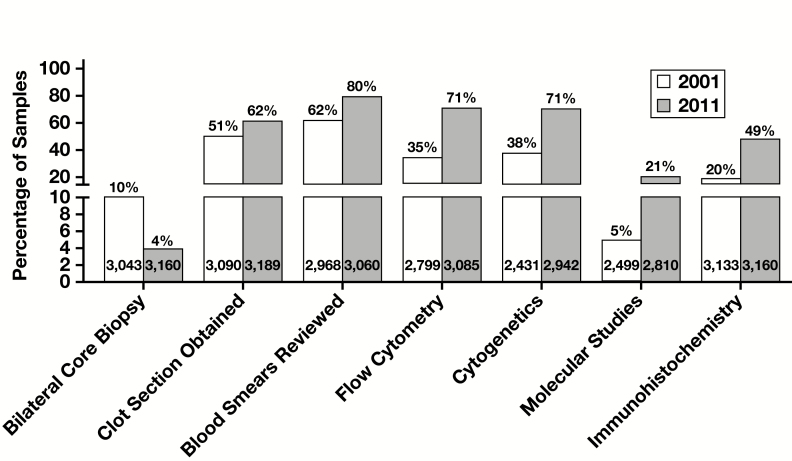
In addition to H&E staining, routine studies (ie, performed prior to microscopic evaluation) included iron and Wright-Giemsa stains for smears and iron, periodic acid–Schiff, Giemsa, and reticulin stains for core biopsy/clot specimens (Supplemental Table 1). The number of levels for each core biopsy sample was variable—in most cases (59%), three or more levels were examined. Ancillary study utilization significantly increased from 2001 to 2011: flow cytometry (35%-71%), cytogenetics (38%-71%), immunohistochemistry (20%-49%), and molecular studies (5%-21%) (all P < .001) (Supplemental Table 2 and Figure 2 ).
Figure 2.
Changes in bone marrow clinical and laboratory practice between 2001 and 2011 in 32 participating centers.
Core Biopsy Specimen Measurements
Overall BM core biopsy specimen measurements are listed in Table 1 . Preprocessing marrow CL (pre-CL) was available and extracted from the pathology reports of 3,137 (49%) samples. Ten centers lacked these data for both study years.
Table 1.
Core Biopsy Preprocessing, Postprocessing, and Evaluable Marrow Length
| Measurement | Core Biopsy Length Value, mm | |||
|---|---|---|---|---|
| Overall Median (Range) | Overall Mean (SD) | 2001 Mean (SD) | 2011 Mean (SD) | |
| Preprocessing core biopsy specimen length (n = 3,137) | 15 (2-103) | 16.8 (9.9) | 17.7 (11.8) | 16.0 (8.0) |
| Postprocessing core biopsy specimen length (n = 5,734) | 12 (1-115) | 14.1 (8.5) | 14.2 (9.4) | 14.0 (7.6) |
| Evaluable marrow length (n = 5,707) | 9 (0-79.5) | 10.6 (7.6) | 10.7 (8.5) | 10.4 (6.6) |
Mean postprocessing CL (post-CL) and mean evaluable marrow length (EML) measured 14.1 mm and 10.6 mm, respectively. On average, there was a 15% tissue contraction (ie, shrinkage) due to histologic processing. EML was widely variable (range, 0-79.5 mm) but on average measured 26% less than post-CL and 33% less than pre-CL. Median values of pre-CL, post-CL, and EML were 15 mm or more in 12 (55%) of 22, nine (28%) of 32, and two (6%) of 32 centers. Overall, only 53%, 38%, and 21% of samples measured 15 mm or more, whereas just 30%, 19%, and 10% of the cores were 20 mm or longer for pre-CL, post-CL, and EML, respectively.
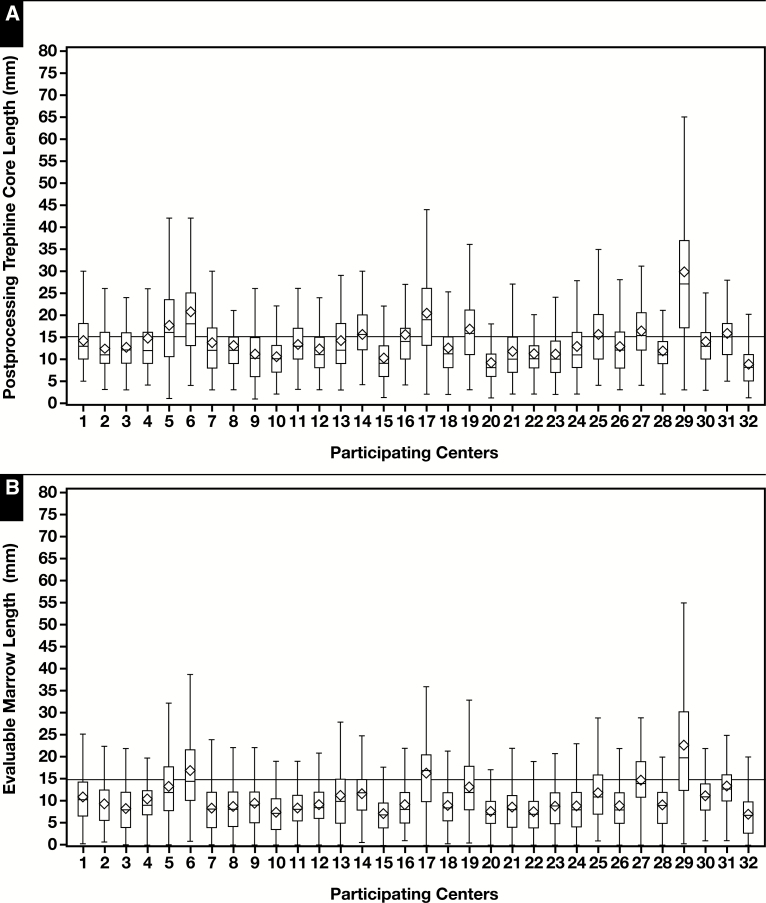
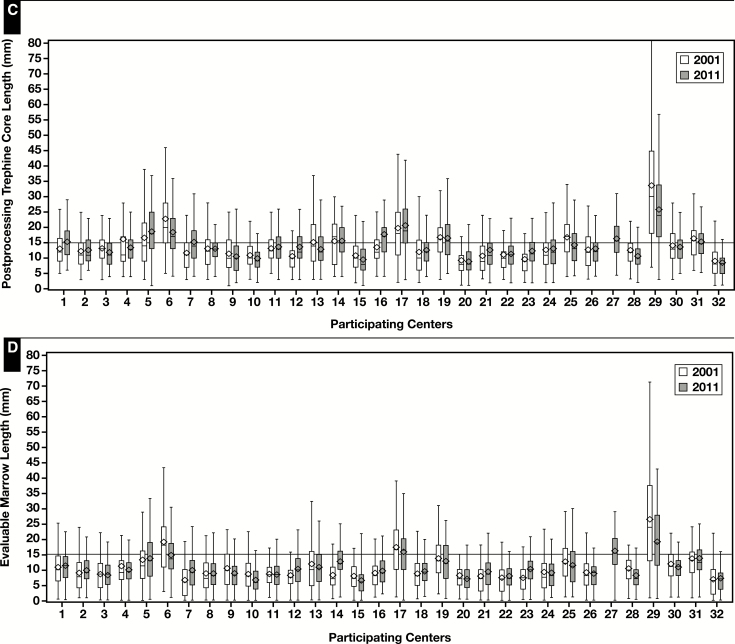
As shown in Table 2 , core biopsy specimen length was significantly longer in bilateral vs unilateral samples, men vs women, staging vs nonstaging, baseline rather than follow-up samples, and younger adults compared with older adults. Pediatric samples had comparable BMB length with that of adults but less evaluable marrow. Immunohistochemical studies were more often performed on BMB with more evaluable marrow. Centers where the pathologists were the primary sample procurers and where pathology bedside assistance was provided had longer cores but without reaching statistical significance. From the mixed model, as illustrated in Figure 3A and Figure 3B , we found that core length was widely variable among centers, with mean post-CL ranging from 8.8 to 29.6 mm and mean EML ranging from 7 to 22.8 mm (P < .001 for both values). In contrast, there was minimal within-center difference of CL over time Figure 3C and Figure 3D .
Table 2.
Core Biopsy Length and Center, Laboratory, and Patient-Related Parameters
| Characteristic | Postprocessing Length | Evaluable Core Length | ||||
|---|---|---|---|---|---|---|
| na | Mean (SD), mm | P Valueb | n | Mean (SD), mm | P Value | |
| Year | ||||||
| 2001 | 3,216 | 14.2 (9.4) | 3,197 | 10.8 (8.5) | ||
| 2011 | 2,517 | 14.0 (7.6) | .471 | 2,509 | 10.4 (6.6) | .509 |
| Sex | ||||||
| Men | 3,580 | 14.4 (8.6) | 3,197 | 10.8 (7.6) | ||
| Women | 2,792 | 13.8 (8.4) | <.001 | 2,509 | 10.3 (7.5) | .002 |
| Age, y | ||||||
| Children | 392 | 14.5 (7.4) | 391 | 8.9 (5.7) | ||
| Adult (≥18) | 5,341 | 14.1 (8.6) | .366 | 5,315 | 10.7 (7.6) | .02 |
| 18-60 | 2,888 | 14.3 (8.6) | 2,881 | 11.0 (7.8) | ||
| >60 | 2,453 | 13.8 (8.5) | .004 | 2,434 | 10.3 (7.6) | .106 |
| Clinical indication | ||||||
| Staging | 1,350 | 16.5 (11.0) | 1,347 | 12.4 (9.5) | ||
| Nonstaging | 4,248 | 13.5 (7.5) | <.001 | 4,224 | 10.0 (6.8) | <.001 |
| Initial diagnosis | 2,583 | 14.5 (8.9) | 2,572 | 11.0 (8.1) | ||
| Follow-up | 2,943 | 13.7 (8.0) | .008 | 2,931 | 10.1 (7.0) | <.001 |
| Core laterality | ||||||
| Unilateral | 5,295 | 13.3 (7.1) | 5,269 | 9.9 (6.5) | ||
| Bilateral | 423 | 25.0 (14.8) | <.001 | 422 | 18.9 (13.2) | <.001 |
| Core diagnostic contribution | ||||||
| Contributory | 5,119 | 14.5 (8.5) | 5,093 | 11.0 (7.5) | ||
| Noncontributory | 381 | 12.6 (7.4) | 380 | 8.5 (7.0) | ||
| Inadequate | 216 | 9.3 (6.1) | <.001c | 216 | 1.7 (2.7) | <.001 |
| Primary operators | ||||||
| Pathologists | 449 | 21.4 (14.6) | 444 | 12.6 (7.4) | ||
| Hematologists-oncologists | 3,352 | 13.8 (7.4) | 3,336 | 10.0 (6.5) | ||
| Nonphysicians | 1,835 | 12.6 (6.7) | .091d | 1,829 | 9.7 (6.4) | .824 |
| Bedside pathology assistance | ||||||
| Always or often | 3,679 | 14.8 (9.0) | 3,664 | 11.1 (8.2) | ||
| Rare or never | 2,055 | 13.0 (7.3) | .813 | 2,043 | 9.7 (6.2) | .868 |
| Number of BMBs per center | ||||||
| <1,000/y, 28 (44%) | 3,169 | 13.3 (7.3) | 3,153 | 11.0 (8.4) | ||
| ≥1,000/y, 36 (56%) | 2,565 | 14.8 (9.3) | .779 | 2,554 | 10.0 (6.4) | .938 |
| NCCN status | ||||||
| NCCN member | 1,670 | 13.2 (6.7) | 1,662 | 9.8 (6.2) | ||
| Not NCCN member | 4,064 | 14.5 (9.1) | .543 | 4,045 | 10.9 (8.0) | .668 |
| IHC performed on the core | ||||||
| Yes | 2,128 | 14.5 (8.5) | 2,117 | 10.9 (7.4) | ||
| No | 3,588 | 13.9 (8.5) | .139 | 3,573 | 10.4 (7.6) | .002 |
BMB, bone marrow biopsy; IHC, immunohistochemistry; NCCN, National Cancer Center Network.
aOnly valid datapoints were including in the analysis, and missing, out-of-range, or “unknown/not available” entries were excluded.
bStatistical analysis using the mixed model described in the Materials and Methods section.
cInadequate vs other (contributory and noncontributory).
dPathologists vs nonpathologists.
Figure 3.
Overall (A, B) and separate for 2001 and 2011 (C, D) postprocessing and evaluable marrow core length measurements in 32 participating centers (diamond, mean; line, median; bar, range; box, quartiles 1 to 3, values in millimeters).
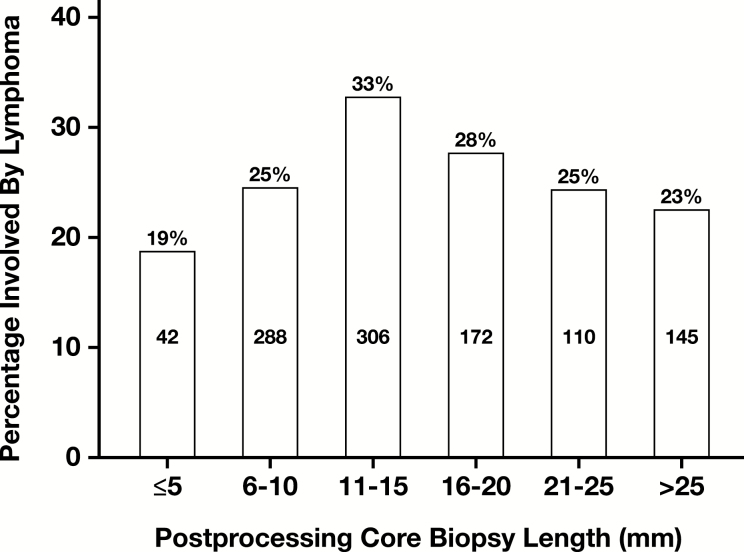
Lymphoma staging samples (n = 1,222) were obtained from adults (96%) and children (4%). Only 26% and 15% BMB specimens reached the post-CL of 20 mm and 25 mm, respectively, and only 15% and 8% samples reached the same EML values. The highest positive rate for lymphoma was seen in samples with post-CL of 11 to 20 mm, but neither the length nor the number of levels examined showed a statistically significant correlation Figure 4 .
Figure 4.
Distribution of samples involved by lymphoma relative to core biopsy specimen length.
Pathologic Diagnoses and BM Biopsy Specimen Quality
Final diagnoses rendered by the reporting pathologist on the BM specimen (including all of its components) are depicted in Figure 1B and were positive for a neoplastic (43%) or nonneoplastic (5%) disease, negative for neoplastic disease (44%), inconclusive (6%), and 2% of samples were deemed inadequate for diagnosis. The high rate of negative diagnoses may be due to the relatively high proportion of follow-up samples (52%) in this cohort. Most common neoplastic involvement was by acute leukemia or myeloid neoplasms (23%), plasma cell neoplasms (10%), and lymphoma (6%). Samples positive for plasma cell neoplasms roughly doubled in the interval studied, from 6.6% in 2001 to 12.3% in 2011. When study participants reviewed the core biopsy specimens and assessed their diagnostic value in isolation, 89% of cores were deemed contributory, 7% noncontributory, and 4% inadequate for diagnosis.
Quality Assurance Program
A BM quality assessment section existed in the pathology reports from 37 of 64 center-years (58%), increasing from 16 centers in 2001 to 21 in 2011. However, a formal, regular quality assurance (QA) program for BM samples was reported only by 36% of centers, and these results were regularly communicated to the clinicians in only 30% of centers as part of an institutional QA program.
Discussion
This retrospective study analyzing clinical and pathologic aspects of BM sampling and evaluation in 32 academic centers revealed several differences between 2001 and 2011: nonphysician operators increased, suggesting a practice pattern trend as recently reported in the United States and Europe12-14; plasma cell neoplasms comprised a greater proportion of clinical indications for BMB specimens, possibly due to improved survival15; and ancillary studies significantly increased while bilateral sampling and usage of heavy metal fixatives decreased.
Most BM procedures were unilateral and included both an aspiration and a core biopsy. There was a significant decrease in bilateral sampling between 2001 and 2011. Bilateral BM sampling had been standard clinical practice for lymphoma and solid tumor staging in the past due to higher positivity rates compared with unilateral samples.16,17 Exclusively unilateral BM space involvement, historically reported in 23% to 30% of cases,18,19 varies with underlying disease.20 Other studies, however, have indicated a comparable staging yield of unilateral cores provided that sufficient medullary tissue was obtained or showed that the added value of the contralateral core is limited.21-23 Unilateral marrow sampling for staging therefore has been recently widely accepted and included in various guidelines.5,7,8 This practice change is reflected in our findings. Currently, bilateral BM sampling is recommended mainly in the staging evaluation for neuroblastoma.24,25
The core biopsy and aspirate samples have complementary diagnostic value for various disorders,18,20,26 and each provides material for ancillary studies. Cytomorphology is best evaluated in stained aspirate films, and flow cytometry and molecular-genetic studies are optimally performed on the BMA specimen. The core biopsy specimen has higher sensitivity for detecting secondary involvement, has greater accuracy at estimating plasma cell tumor burden,27,28 and is more reliable at estimating hematopoietic cellularity.6,29-31 In addition, bone or stromal alterations, pattern of tumor infiltration, and fibrosis grading can be evaluated only in the core biopsy sample. Clot sections may also be used for immunohistochemistry, but they were not prepared in 30% of BMA-only samples. Results of ancillary studies are influenced by sample fixation and processing. Heavy metal fixatives have been largely replaced by either neutral buffered formalin or zinc formalin fixatives. Cytogenetics, flow cytometry, and immunohistochemistry usage roughly doubled and molecular testing quadrupled between 2001 and 2011. While greatly augmenting the diagnostic capabilities of a BM sample, ancillary tests do not supplant a good-quality BMB specimen since discrepancies with histopathologic examination exist.20,32-34
An area in which we have focused particular effort is determining how to judge adequacy of a BM core biopsy sample. This is influenced by the size of the sample, which can be measured in a variety of ways, and by the degree to which it is representative of overall hematopoiesis. There are three possible linear measurements of a core biopsy sample: before histologic processing (pre-CL), after decalcification and processing (post-CL), and EML. The first is perhaps the least reliable given the variability of technique settings and personnel involved in sample measurement at the bedside or at the pathology bench. In addition, this value was missing from half of the pathology reports, and in 10 centers, this information was lacking for both study years. A significant change in measured length may occur after decalcification and histologic processing. In our study, tissue contraction (ie, shrinkage) was on average 15%. This is slightly less than in the only other BM morphometric study that reported 25% shrinkage in a single institutional cohort.1 Neither study standardized measurements of pre-CL. Similar overall shrinkage was reported in soft tissue, 2.7% due to fixation and 12.6% due to histologic processing,35 whereas bone tissue contraction can be as low as 6%.36 Unlike pre-CL, post-CL and EML were uniformly measured in this study. Post-CL is a good indicator of sampled tissue quantity and EML of its quality. Post-CL and EML definition and measurements performed were uniform, the latter excluding crushed or aspirated areas, soft tissue, clots, or cortical bone as previously described1 with excellent reproducibility (see Supplemental Methods). We quantified the differences between the three measurements: on average, the evaluable marrow comprised 74% of post-CL and 67% of the pre-CL. All three CL measurements had smaller mean values in 2011 compared with 2001, without reaching statistical significance (Table 1). Aggregate CL was unsurprisingly longer in bilateral samples, roughly double the length of unilateral BMB specimens, but it is unclear why women’s cores were consistently shorter in both years studied. Anatomical differences are unlikely to be the sole explanation given that pediatric samples had comparable length with the adult ones. Older adults had shorter CL as reported in other cohorts,37,38 possibly due to increased bone fragility in this age group. Significant differences of CL between centers were detected and are illustrated in Figure 3. Despite the expected heterogeneity and diverse practice setting in 32 centers, a remarkable consistency of CL was noted between the 2 years for each hospital. These findings indicate that local experience may play an important role in a center’s performance, as previously reported in smaller cohorts.31,38-40 Centers with higher volume of procedures had on average longer cores (without reaching statistical significance), as reported by others.38 Training, technique, and personal experience of the operator are key in obtaining a good sample.2,38,39 Hematologists/oncologists obtained longer cores than pediatricians2 and trainees and students.41 In our cohort, centers where pathologists were the primary operators reported longer cores on average. The significantly longer BMB specimens obtained for staging than nonstaging indications and for baseline than follow-up procedures suggest a consistent effort of the operators to obtain larger samples in these clinical situations.
A core biopsy specimen of adequate length is important not only for accurate staging but also for diagnosis of myeloid neoplasms such as hypocellular myelodysplastic syndrome or acute myeloid leukemia29,42 and for quantifying residual posttherapy disease in various conditions such as myeloproliferative neoplasms, lymphomas, and neuroblastoma.7,8,11,25 In lymphoma staging, not only disease presence but also its extent, pattern, and type (concordant or discordant with the primary disease) carry diagnostic, staging, and prognostic significance.17,19,22,33,43-47 Some authors attributed the lower prevalence of discordant involvement in recent compared with older studies to the interval decrease in bilateral sampling.48 The rate of lymphoma positivity increases with core length before reaching a plateau.1,16,22 We found a similar trend in lymphoma staging BMs in this study, with highest positive rates detected in the samples with post-CL measuring between 10 and 20 mm. The lack of statistical significance is likely explained by disease aggregation without distinguishing between type (eg, Hodgkin vs non-Hodgkin lymphoma), cell lineage, grade, and extent of involvement in this cohort, all factors directly altering the positive detection rate. Studies of CL impact on positive diagnostic rate in a given neoplasm are the exception in the literature, with only rare series on neuroblastoma,2,39 follicular lymphoma23,34 and diffuse large B-cell lymphoma22 being available to date. In fact, reports of the BMB CL obtained in clinical practice are overall sparse. Otherwise excellent studies reporting on staging BM yield17,19,33,44-46; on relative diagnostic value of BMB, BMA, and ancillary studies18,20,49-52; and on diagnostic performance of various imaging modalities (recently complementing or even supplanting the BM in clinical practice, even when BM is used as a “gold standard”53-55) do not specify the core biopsy specimen length or evaluable marrow space (ie, the sample quantity or quality). Even in studies focusing on core biopsy specimen length, the type of measurement and its method are often unclear,19,21,22,37,56 and almost all guidelines include unspecified measurements, presumably referring to pre-CL Table 3 . This is relevant given the significant differences between the three values, as discussed above.
Table 3.
Core Biopsy Length Adequacy in the Literature
| Guideline or Consensus Statement | First Author | Year | Sample No. | Referenced or Reported Length | Reported Length Values,a mm | Optimal Core Length, mm | Inadequate Core Length, mm | Adequate Evaluable Space | No. of Levels | Disease or Clinical Indication |
|---|---|---|---|---|---|---|---|---|---|---|
| WHO | Arber4 | 2017 | — | NS | — | 15 | NS | 10 spaces | NS | Any |
| NCI-IWG | Cheson7 | 2007 | — | NS | — | 20 | NS | NS | NS | NHL |
| Lugano | Cheson8 | 2014 | — | NS | — | 25 | NS | NS | NS | Lymphomab |
| ICSH | Lee3 | 2008 | — | NS | — | 20 | NS | NS | 2-4 | Any |
| European Consensus | Thiele6 | 2005 | — | NS | — | 15 | NS | 10 spaces | NS | MF |
| NCCN | NCCN5 | 2018 | — | NS | — | 20 | NS | NS | NS | NMZL |
| NCCN | NCCN5 | 2018 | — | NS | — | 16 | NS | NS | NS | FL |
| NCCN | NCCN5 | 2018 | — | NS | — | 16 | NS | NS | NS | DLBCL |
| INRC BMWG | Burchill25 | 2017 | — | EML | — | 10 | NS | 10 mm | >3 | NB |
| Study Type | ||||||||||
| Single center | Campbell22 | 2003 | 172 | Post-CLc | Median 19 (6-73) | 20 | ≤5 | >5 mm | Mean 4 | DLBCL |
| Single center | Bishop1 | 1992 | 767 | Post-CL | Mean 11.5 | 16 | <16 | NS | NS | Any |
| Multicenter | Reid2 | 1996 | 822 | EML | 17% of all samples <5 | 10 | <5 | NS | NS | NB |
| Multicenter | Reid39 | 1999 | 605 | EML | 25% of all samples <5 | 10 | <5 | ≥5 mm | NS | NB |
| Multicenter | Rudzki38 | 2005 | 1,938 | EML | Mean 13.1 (8.3) | 10 | <10 | 10 mm | NS | Any |
| Single center | Rimmer41 | 2008 | 1,012 | Post-CL EML |
Mean 14.5 (5.3) Mean 10 (5.4) |
11 8 |
<11 <8 |
8 mm | NS | Any |
| Single center | Talaulikar56 | 2008 | 156 | Pre-CL or post-CLc,d | Mean 17.6 (8-36) | NS | NS | NS | Mean 3.7 (range, 1-8) | DLBCL |
| Single center | Iancu23 | 2007 | 114 | Pre-CL | Median 28 (8-48) | NS | NS | NS | NS | FL |
| Single center | Al-Ibraheemi31 | 2013 | 40 | Post-CLc,e | Mean 9.9 (3.1) Mean 11.7 (4.5) |
NS | NS | NS | NS | Any |
| Single center | Odejide37 | 2013 | 830 | Pre-CLc | 47% of all samples ≤10 | 10 | NS | NS | NS | Any |
| Single network/ multihospital | Dayton40 | 2014 | 40 | Pre-CLf | Mean 16.6 (4.5) Mean 18.5 (4.8) |
15 | NS | NS | NS | Any |
| Multicenter | Current study | 2018 | 6,374 | Post-CL EML |
Mean 14.1 (8.5) (1-115) Mean 10.6 (7.0) (0-79.5) |
NS | NS | NS | ≥3 (59% of all samples) | Any |
DLBCL, diffuse large B-cell lymphoma; EML, evaluable core length; FL, follicular lymphoma; INRC BMWG, International Neuroblastoma Response Criteria Bone Marrow Working Group; MF, myelofibrosis; NA, not applicable; NB, neuroblastoma; NCCN, National Cancer Center Network; NCI-IWG, National Cancer Institute–sponsored International Working Group, ICSH, International Council for Standardization in Hematology; NHL, non-Hodgkin lymphoma; NMZL, nodal marginal zone lymphoma; NS, not specified, post-CL, postprocessing core length; pre-CL, preprocessing core length; WHO, World Health Organization.
aValues are reported as mean or median (SD) (range) unless otherwise indicated.
bFor all lymphomas except classical Hodgkin lymphoma (CHL) and DLBCL, and in selected CHL and DLBCL.
cConfirmed by the corresponding author.
dIf pre-CL was not available, post-CL was recorded.
eTwo different techniques.
fTwo different hospitals.
An optimal BMB specimen should measure between 1.5 and 2 cm for any clinical indication and 1.6 to 2.5 cm for staging. Our findings indicate that these benchmarks are met in only a minority of samples and very few centers, particularly when the EML is considered, as previously suggested in several European2,38,39 and North American31,37,40,41 studies.
There is no uniform definition of an inadequate core biopsy specimen, as shown in Table 3, with both subjective and quantitative evaluations used in clinical practice. The latter are more frequent in published experience: BMB specimens with 5 mm or less evaluable marrow,2,22 less than five high-power fields of preserved marrow space,57 and all samples measuring less than 16 mm were considered inadequate by various authors,1 with inadequate samples ranging from 8.5% to 58% depending on the definition used.1,37 Inadequate samples comprised 17% to 25% of total BMB specimens if 5 mm EML was required2,39 and 33% if an 8-mm cutoff was used.41 In our cohort, while only 2% of the BMB/BMA specimens were considered inadequate by the reporting pathologist and 4% of the core biopsy specimens were deemed inadequate for evaluation at re-review by the study pathologists, a significantly higher proportion (15% of staging and 19% of all core biopsy specimens) would have been classified as insufficient had the suggested 5-mm minimum EML cutoff been used. This observation indicates that practicing pathologists regard (and report) as adequate core biopsy specimens that are much shorter than suggested in current recommendations and reject very few specimens. Reasons may include lack of a uniform definition of minimal marrow required to exclude a false-negative result, pathologists’ variable expectations related to training or local experience, subjective criteria of assessment (see Supplemental Methods), and specific underlying disease findings in a given case. For instance, the core biopsy specimen’s role in confirming an acute leukemia diagnosis established on an aspirate sample is minimal, and no staging sample will be deemed insufficient regardless of its length when it is involved by tumor.2
QA is essential in clinical practice, but BMB/BMA specimen quality section was missing from pathology reports in 42% of centers and pre-CL was lacking from half of the reports in our cohort. Departmental BM procedure QA programs were rare and centers reporting such programs at the institutional level were the exception. This lack of sample monitoring and communication between pathologists and operators may perpetuate the sampling deficiency. Communication is only the first step and probably not sufficient.2,39
BM sample quality may be improved by providing feedback to the operator, through pathology bedside assistance, and by using a procedure checklist.37,58 Pathology reports should include CL measurement, a College of American Pathologists recommendation,59 and they ideally should document the evaluable marrow space length as well as BMA and BMB quality. Sampling may be enhanced by examination of sections from several levels performed on the core biopsy block by the pathologist, thus increasing the detection rate of focal disease.22
This retrospective study has several limitations. Community practice is not significantly represented in this cohort. The aspirate samples and their quality were not reviewed. Lymphoma subtype, individual operators for each procedure, and specific kit/needle or needle gauge used for each biopsy were not known.
In summary, we provide a comprehensive assessment of BM sampling practice and performance in tertiary care centers in the United States and Canada, as well as report on several practice trends noted both for the procedure operators and laboratory operations between 2001 and 2011. Overall, the core biopsy specimen length was shorter than recommended and varied with patient age and sex, clinical context, sample laterality, and center experience. On average, only two-thirds of a given core biopsy specimen contained evaluable marrow. In most cases, a diagnosis was rendered irrespective of the core length. While there was significant expansion in the utilization of ancillary studies, the physical characteristics of the BM specimens obtained did not change significantly over time. Implementing QA programs and adherence to existing standards may improve BM sample yield.
Supplementary Material
Acknowledgments
We thank Vishala Neppalli, MD, and George Deeb, MD, for their contributions to study design, validation set testing, and data collection; Ashley Sedelmeyer, MHA, Andrea Bruno, AA, and Camille Wicher, PhD, Esq, MSN, RN, for their contribution during protocol development, center enrollment, and coordination; Mathew B. Thompsen, MPH, for assistance with data collection; Mukund Seshadri, DDS, PhD, for technical assistance; and Seema Bhat, MD, L. Jeffrey Medeiros, MD, Philip McCarthy, MD, and Eunice Wang, MD, for review of the manuscript.
This work was supported by Roswell Park Comprehensive Cancer Center and National Cancer Institute (grant P30CA016056).
This work was presented in part as poster presentations at the American Society of Hematology annual meeting, San Francisco, CA, 2014; and at the United States and Canadian Academy of Pathology annual meeting, Seattle, WA, 2016.
References
- 1. Bishop PW, McNally K, Harris M. Audit of bone marrow trephines. J Clin Pathol. 1992;45:1105-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Reid MM, Roald B. Adequacy of bone marrow trephine biopsy specimens in children. J Clin Pathol. 1996;49:226-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lee SH, Erber WN, Porwit A, et al. ; International Council for Standardization in Hematology. ICSH guidelines for the standardization of bone marrow specimens and reports. Int J Lab Hematol. 2008;30:349-364. [DOI] [PubMed] [Google Scholar]
- 4. Arber D, Hasserjian RP. Introduction and overview of the classification of myeloid neoplasms. In: Swerdlow SH, Campo E, Harris NL, et al. eds. WHO Classification of Tumours. Lyon, France: IARC; 2017:16-27. [Google Scholar]
- 5. NCCN Clinical Practice Guidelines in Oncology. B-cell lymphomas. Version 3.2018. https://www.nccn.org/professionals/physician_gls/pdf/b-cell.pdf. Accessed April 13, 2018. [Google Scholar]
- 6. Thiele J, Kvasnicka HM, Facchetti F, et al. European consensus on grading bone marrow fibrosis and assessment of cellularity. Haematologica. 2005;90:1128-1132. [PubMed] [Google Scholar]
- 7. Cheson BD, Pfistner B, Juweid ME, et al. ; International Harmonization Project on Lymphoma. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579-586. [DOI] [PubMed] [Google Scholar]
- 8. Cheson BD, Fisher RI, Barrington SF, et al. ; Alliance, Australasian Leukaemia and Lymphoma Group; Eastern Cooperative Oncology Group; European Mantle Cell Lymphoma Consortium; Italian Lymphoma Foundation; European Organisation for Research; Treatment of Cancer/Dutch Hemato-Oncology Group; Grupo Español de Médula Ósea; German High-Grade Lymphoma Study Group; German Hodgkin’s Study Group; Japanese Lymphoma Study Group; Lymphoma Study Association; NCIC Clinical Trials Group; Nordic Lymphoma Study Group; Southwest Oncology Group; United Kingdom National Cancer Research Institute. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059-3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kvasnicka HM, Beham-Schmid C, Bob R, et al. Problems and pitfalls in grading of bone marrow fibrosis, collagen deposition and osteosclerosis—a consensus-based study. Histopathology. 2016;68:905-915. [DOI] [PubMed] [Google Scholar]
- 10. Pozdnyakova O, Wu K, Patki A, et al. High concordance in grading reticulin fibrosis and cellularity in patients with myeloproliferative neoplasms. Mod Pathol. 2014;27:1447-1454. [DOI] [PubMed] [Google Scholar]
- 11. Tefferi A, Barosi G, Mesa RA, et al. ; IWG for Myelofibrosis Research and Treatment (IWG-MRT). International Working Group (IWG) consensus criteria for treatment response in myelofibrosis with myeloid metaplasia, for the IWG for myelofibrosis research and treatment (IWG-MRT). Blood. 2006;108:1497-1503. [DOI] [PubMed] [Google Scholar]
- 12. Check W. watching the bone marrow story unfold. CAP Today. http://www.captodayonline.com/Archives/feature_stories/1107Bone.html. Accessed February 17, 2018. [Google Scholar]
- 13. Lawson S, Aston S, Baker L, et al. Trained nurses can obtain satisfactory bone marrow aspirates and trephine biopsies. J Clin Pathol. 1999;52:154-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Naegele M, Leppla L, Kiote-Schmidt C, et al. Trained clinical nurse specialists proficiently obtain bone marrow aspirates and trephine biopsies in a nearly painless procedure—a prospective evaluation study. Ann Hematol. 2015;94:1577-1584. [DOI] [PubMed] [Google Scholar]
- 15. Hostenkamp G, Lichtenberg FR. The impact of recent chemotherapy innovation on the longevity of myeloma patients: US and international evidence. Soc Sci Med. 2015;130:162-171. [DOI] [PubMed] [Google Scholar]
- 16. George TB, Natkunam FL, Geaghan Y, et al. Bone marrow biopsy for focal disease detection: evaluation of optimal biopsy length and comparison of bilateral to equivalent-length unilateral biopsies. Mod Pathol. 2004:248a.14657956 [Google Scholar]
- 17. Brunning RD, Bloomfield CD, McKenna RW, et al. Bilateral trephine bone marrow biopsies in lymphoma and other neoplastic diseases. Ann Intern Med. 1975;82:365-366. [DOI] [PubMed] [Google Scholar]
- 18. Barekman CL, Fair KP, Cotelingam JD. Comparative utility of diagnostic bone-marrow components: a 10-year study. Am J Hematol. 1997;56:37-41. [DOI] [PubMed] [Google Scholar]
- 19. Juneja SK, Wolf MM, Cooper IA. Value of bilateral bone marrow biopsy specimens in non-Hodgkin’s lymphoma. J Clin Pathol. 1990;43:630-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang J, Weiss LM, Chang KL, et al. Diagnostic utility of bilateral bone marrow examination: significance of morphologic and ancillary technique study in malignancy. Cancer. 2002;94:1522-1531. [DOI] [PubMed] [Google Scholar]
- 21. Roath S, Smith AG, Choudhury D. Bone marrow biopsy in non-Hodgkin’s lymphoma. J Clin Pathol. 1991;44:350-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Campbell JK, Matthews JP, Seymour JF, et al. ; Australasian Leukaemia Lymphoma Group. Optimum trephine length in the assessment of bone marrow involvement in patients with diffuse large cell lymphoma. Ann Oncol. 2003;14:273-276. [DOI] [PubMed] [Google Scholar]
- 23. Iancu D, Hao S, Lin P, et al. Follicular lymphoma in staging bone marrow specimens: correlation of histologic findings with the results of flow cytometry immunophenotypic analysis. Arch Pathol Lab Med. 2007;131:282-287. [DOI] [PubMed] [Google Scholar]
- 24. Brodeur GM, Pritchard J, Berthold F, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993;11:1466-1477. [DOI] [PubMed] [Google Scholar]
- 25. Burchill SA, Beiske K, Shimada H, et al. Recommendations for the standardization of bone marrow disease assessment and reporting in children with neuroblastoma on behalf of the International Neuroblastoma Response Criteria Bone Marrow Working Group. Cancer. 2017;123:1095-1105. [DOI] [PubMed] [Google Scholar]
- 26. Bearden JD, Ratkin GA, Coltman CA. Comparison of the diagnostic value of bone marrow biopsy and bone marrow aspiration in neoplastic disease. J Clin Pathol. 1974;27:738-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Charles KS, Winfield DA, Angel C, et al. Audit of bone marrow aspirates and trephine biopsies in multiple myeloma—a single centre study. Clin Lab Haematol. 2004;26:403-406. [DOI] [PubMed] [Google Scholar]
- 28. Joshi R, Horncastle D, Elderfield K, et al. Bone marrow trephine combined with immunohistochemistry is superior to bone marrow aspirate in follow-up of myeloma patients. J Clin Pathol. 2008;61:213-216. [DOI] [PubMed] [Google Scholar]
- 29. Tuzuner N, Cox C, Rowe JM, et al. Bone marrow cellularity in myeloid stem cell disorders: impact of age correction. Leuk Res. 1994;18:559-564. [DOI] [PubMed] [Google Scholar]
- 30. Kidd PG, Saminathan T, Drachtman RA, et al. Comparison of the cellularity and presence of residual leukemia in bone marrow aspirate and biopsy specimens in pediatric patients with acute lymphoblastic leukemia (ALL) at day 7-14 of chemotherapy. Med Pediatr Oncol. 1997;29:541-543. [DOI] [PubMed] [Google Scholar]
- 31. Al-Ibraheemi A, Pham T, Chen L, et al. Comparison between 1-needle technique versus 2-needle technique for bone marrow aspiration and biopsy procedures. Arch Pathol Lab Med. 2013;137:974-978. [DOI] [PubMed] [Google Scholar]
- 32. Duggan PR, Easton D, Luider J, et al. Bone marrow staging of patients with non-Hodgkin lymphoma by flow cytometry: correlation with morphology. Cancer. 2000;88:894-899. [PubMed] [Google Scholar]
- 33. Arber DA, George TI. Bone marrow biopsy involvement by non-Hodgkin’s lymphoma: frequency of lymphoma types, patterns, blood involvement, and discordance with other sites in 450 specimens. Am J Surg Pathol. 2005;29:1549-1557. [DOI] [PubMed] [Google Scholar]
- 34. Schmidt B, Kremer M, Götze K, et al. Bone marrow involvement in follicular lymphoma: comparison of histology and flow cytometry as staging procedures. Leuk Lymphoma. 2006;47:1857-1862. [DOI] [PubMed] [Google Scholar]
- 35. Boonstra H, Oosterhuis JW, Oosterhuis AM, et al. Cervical tissue shrinkage by formaldehyde fixation, paraffin wax embedding, section cutting and mounting. Virchows Arch A Pathol Anat Histopathol. 1983;402:195-201. [DOI] [PubMed] [Google Scholar]
- 36. Lane J, Rális ZA. Changes in dimensions of large cancellous bone specimens during histological preparation as measured on slabs from human femoral heads. Calcif Tissue Int. 1983;35:1-4. [DOI] [PubMed] [Google Scholar]
- 37. Odejide OO, Cronin AM, DeAngelo DJ, et al. Improving the quality of bone marrow assessment: impact of operator techniques and use of a specimen preparation checklist. Cancer. 2013;119:3472-3478. [DOI] [PubMed] [Google Scholar]
- 38. Rudzki Z, Partyła T, Okoń K, et al. Adequacy of trephine bone marrow biopsies: the doctor and the patient make a difference. Pol J Pathol. 2005;56:187-195. [PubMed] [Google Scholar]
- 39. Reid MM, Roald B. Deterioration in performance in obtaining bone marrow trephine biopsy cores from children. European Neuroblastoma Study Group. J Clin Pathol. 1999;52:851-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dayton VJ, Fink J, Linden MA, et al. Quality and adequacy of bone marrow samples obtained by the 2-needle technique: the Minnesota experience. Arch Pathol Lab Med. 2014;138:860-862. [DOI] [PubMed] [Google Scholar]
- 41. Rimmer EK, Houston DS, Roland K. Adequacy of bone marrow trephine biopsy in a tertiary care center. Blood. 2008;112:4702. [Google Scholar]
- 42. Bennett JM, Orazi A. Diagnostic criteria to distinguish hypocellular acute myeloid leukemia from hypocellular myelodysplastic syndromes and aplastic anemia: recommendations for a standardized approach. Haematologica. 2009;94:264-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Campbell J, Seymour JF, Matthews J, et al. The prognostic impact of bone marrow involvement in patients with diffuse large cell lymphoma varies according to the degree of infiltration and presence of discordant marrow involvement. Eur J Haematol. 2006;76:473-480. [DOI] [PubMed] [Google Scholar]
- 44. Sovani V, Harvey C, Haynes AP, et al. Bone marrow trephine biopsy involvement by lymphoma: review of histopathological features in 511 specimens and correlation with diagnostic biopsy, aspirate and peripheral blood findings. J Clin Pathol. 2014;67:389-395. [DOI] [PubMed] [Google Scholar]
- 45. Chung R, Lai R, Wei P, et al. Concordant but not discordant bone marrow involvement in diffuse large B-cell lymphoma predicts a poor clinical outcome independent of the international prognostic index. Blood. 2007;110:1278-1282. [DOI] [PubMed] [Google Scholar]
- 46. Sehn LH, Scott DW, Chhanabhai M, et al. Impact of concordant and discordant bone marrow involvement on outcome in diffuse large B-cell lymphoma treated with R-CHOP. J Clin Oncol. 2011;29:1452-1457. [DOI] [PubMed] [Google Scholar]
- 47. Chigrinova E, Mian M, Scandurra M, et al. Diffuse large B-cell lymphoma with concordant bone marrow involvement has peculiar genomic profile and poor clinical outcome. Hematol Oncol. 2011;29:38-41. [DOI] [PubMed] [Google Scholar]
- 48. Brudno J, Tadmor T, Pittaluga S, et al. Discordant bone marrow involvement in non-Hodgkin lymphoma. Blood. 2016;127:965-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Moid F, DePalma L. Comparison of relative value of bone marrow aspirates and bone marrow trephine biopsies in the diagnosis of solid tumor metastasis and Hodgkin lymphoma: institutional experience and literature review. Arch Pathol Lab Med. 2005;129:497-501. [DOI] [PubMed] [Google Scholar]
- 50. Maes B, Achten R, Demunter A, et al. Evaluation of B cell lymphoid infiltrates in bone marrow biopsies by morphology, immunohistochemistry, and molecular analysis. J Clin Pathol. 2000;53:835-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pittaluga S, Tierens A, Dodoo YL, et al. How reliable is histologic examination of bone marrow trephine biopsy specimens for the staging of non-Hodgkin lymphoma? A study of hairy cell leukemia and mantle cell lymphoma involvement of the bone marrow trephine specimen by histologic, immunohistochemical, and polymerase chain reaction techniques. Am J Clin Pathol. 1999;111:179-184. [DOI] [PubMed] [Google Scholar]
- 52. Coad JE, Olson DJ, Christensen DR, et al. Correlation of PCR-detected clonal gene rearrangements with bone marrow morphology in patients with B-lineage lymphomas. Am J Surg Pathol. 1997;21:1047-1056. [DOI] [PubMed] [Google Scholar]
- 53. Purz S, Mauz-Körholz C, Körholz D, et al. [18F]fluorodeoxyglucose positron emission tomography for detection of bone marrow involvement in children and adolescents with Hodgkin’s lymphoma. J Clin Oncol. 2011;29:3523-3528. [DOI] [PubMed] [Google Scholar]
- 54. Jackson AE, Smeltzer JP, Habermann TM, et al. The utility of restaging bone marrow biopsy in PET-negative patients with diffuse large B-cell lymphoma and baseline bone marrow involvement. Am J Hematol. 2014;89:865-867. [DOI] [PubMed] [Google Scholar]
- 55. Chen-Liang TH, Martin-Santos T, Jerez A, et al. The role of bone marrow biopsy and FDG-PET/CT in identifying bone marrow infiltration in the initial diagnosis of high grade non-Hodgkin B-cell lymphoma and Hodgkin lymphoma: accuracy in a multicenter series of 372 patients. Am J Hematol. 2015;90:686-690. [DOI] [PubMed] [Google Scholar]
- 56. Talaulikar D, Dahlstrom JE, Shadbolt B, et al. Role of immunohistochemistry in staging diffuse large B-cell lymphoma (DLBCL). J Histochem Cytochem. 2008;56:893-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Coller BS, Chabner BA, Gralnick HR. Frequencies and patterns of bone marrow involvement in non-Hodgkin lymphomas: observations on the value of bilateral biopsies. Am J Hematol. 1977;3:105-119. [DOI] [PubMed] [Google Scholar]
- 58. Loureiro G, Rizzatti EG, Sandes AF, et al. Quality control of bone marrow aspirates: additional steps toward a safer and more efficient procedure. Cancer. 2014;120:1441-1442. [DOI] [PubMed] [Google Scholar]
- 59. Hussong JW, Arber DA, Bradley KT, et al. Protocol for the examination of specimens from patients with hematopoietic neoplasms involving the bone marrow. 2013. http://www.cap.org/ShowProperty?nodePath=/UCMCon/Contribution%20Folders/WebContent/pdf/nonhodgkinlymph-13protocol-3200.pdf. Accessed January 28, 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.