Abstract
Significance: The complex etiology of type 1 diabetes (T1D) is the outcome of failures in regulating immunity in combination with beta cell perturbations. Mitochondrial dysfunction in beta cells and immune cells may be involved in T1D pathogenesis. Mitochondrial energy production is essential for the major task of beta cells (the secretion of insulin in response to glucose). Mitochondria are a major site of reactive oxygen species (ROS) production. Under immune attack, mitochondrial ROS (mtROS) participate in beta cell damage. Similarly, T cell fate during immune responses is tightly regulated by mitochondrial physiology, morphology, and metabolism. Production of mtROS is essential for signaling in antigen-specific T cell activation. Mitochondrial dysfunction in T cells has been noted as a feature of some human autoimmune diseases.
Recent Advances: Preclinical and clinical studies indicate that mitochondrial dysfunction in beta cells sensitizes these cells to immune-mediated destruction via direct or indirect mechanisms. Sensitivity of beta cells to mtROS is associated with genetic T1D risk loci in human and the T1D-prone nonobese diabetic (NOD) mouse. Mitochondrial dysfunction and altered metabolism have also been observed in immune cells of NOD mice and patients with T1D. This immune cell mitochondrial dysfunction has been linked to deleterious functional changes.
Critical Issues: It remains unclear how mitochondria control T cell receptor signaling and downstream events, including calcium flux and activation of transcription factors during autoimmunity.
Future Directions: Mechanistic studies are needed to investigate the mitochondrial pathways involved in autoimmunity, including T1D. These studies should seek to identify the role of mitochondria in regulating innate and adaptive immune cell activity and beta cell failure.
Keywords: : type 1 diabetes, mitochondria, reactive oxygen species, beta cell, T cell, ROS
Insulin-producing pancreatic beta cells are the target of an autoimmune attack during the development of type 1 diabetes (T1D). Evidence from animal models and human organ donor samples points to T cells as a mediator of the beta cell damage and destruction (9, 14, 17, 45). Dysfunction and loss of beta cells lead to insulin deficiency and hyperglycemia, which along with autoantibodies are clinical tools used to diagnose T1D. T1D currently has no cure. Patients with T1D rely on external insulin and a disciplined diet to maintain proper plasma glucose levels. Deviation from this regimen increases the risk of hypoglycemia, which can be fatal, or hyperglycemia, and develops long-term complications including retinopathy, nephropathy, and cardiovascular diseases. Therefore, diabetes can be viewed as a disease of multiple stages, at both the preclinical and postdiagnosis periods.
Mitochondria have long been viewed as a participant in many of the normal and pathogenic features of diabetes. These essential organelles produce energy, reducing equivalents and reactive oxygen species (ROS). In addition, they are critical metabolic centers important in regulating cellular homeostasis and signal transduction. The production of ROS and other oxidants by mitochondria has implicated these organelles in the pathogenesis of various diseases, including T1D. Oxidants are considered to be a pathogenic contributor in most forms of diabetes, but the cellular sources and types of oxidants remain controversial. In this review, we discuss the specific role(s) of mitochondria in pancreatic beta and immune cells during the pathogenesis of autoimmune T1D.
Mitochondrial Function in Beta Cells and T1D
In the postprandial state, beta cells respond to absorbed nutrients by releasing insulin into the blood stream. Secretion of insulin can be induced by sugars and amino acids with potentiation by fatty acids. With the exception of positively charged amino acids (which induce secretion through an electrogenic mechanism), nutrients require mitochondria for the induction of insulin release. Therefore, the role of mitochondria in pancreatic beta cell physiological function has long been recognized and widely studied.
Under normal conditions, beta cells sense glucose levels and transport glucose via glucose transporters. The transported glucose molecules are subsequently phosphorylated and converted to glucose-6-phosphate by glucokinase (the beta cell glucose sensor). Glucose-6-phosphate is metabolized via glycolysis to produce pyruvate and then acetyl coenzyme A (acetyl-CoA). Acetyl-CoA enters the mitochondrial tricarboxylic acid (TCA) cycle to facilitate adenosine triphosphate (ATP) generation by oxidative phosphorylation (OXPHOS). The production of ATP by mitochondria as a result of rising circulating nutrient concentrations is a key and essential physiological function of these organelles in beta cells.
ATP exchange for cytoplasmic adenosine diphosphate (ADP) by adenine nucleotide translocases increases the cytoplasmic ATP/ADP ratio allowing for ATP to displace ADP bound to the Kir6.2 subunit of the ATP-sensitive K+ channel, an inward-rectifier potassium ion channel (62). ATP binding inhibits this channel, triggering plasma membrane depolarization, opening of L-type voltage-dependent calcium channels, and the influx of calcium. Increased intracellular calcium directly triggers fusion of insulin granules and insulin exocytosis. The increase in cytosolic calcium also enhances both mitochondrial metabolism and mitochondrial ATP production. As such, secretion of insulin is tightly regulated by mitochondrial function, specifically through ATP production and regulation of intracellular calcium concentrations (54, 94, 95, 97). The strong requirement for mitochondria during beta cell function has also been extended to roles during cell survival and death (14, 30, 31). This concept will be discussed in detail later.
During the pathogenesis of T1D, pancreatic beta cells are targeted and destroyed by an autoimmune attack by islet infiltrating beta cell antigen-specific autoreactive T cells (59, 67). Preclinical models including the nonobese diabetic (NOD) mouse and biobreeding–diabetes-prone (BB-DP) rat [reviewed by Pearson et al. (68)] have provided significant information on the kinetics of cellular infiltration during the progression of insulitis. Although it was initially observed that macrophages and/or dendritic cells were the first immune cell types to infiltrate the islets, the more recent thought is that these are tissue-resident macrophages (11, 26). These tissue-resident antigen-presenting cells (APCs) take on inflammatory characteristics early (3–4 weeks of age in NOD females) and produce chemokines that recruit lymphocytes (CD4+ and CD8+ T cells as well as B cells) into the islets (3). The signals that induce the islet APCs to mature and become inflammatory remain unknown; however, long-term depletion of these cells results in protection from T1D, highlighting the essential nature of macrophages in T1D pathogenesis (8, 43, 85). T lymphocytes (both CD4+ and CD8+ cells) are also required for T1D initiation (70). The cellular components of insulitis that are essential for T1D onset have provided clues as to the effector mechanisms that induce beta cell death. However, the experimental models used to identify these mechanisms remain controversial. Early knowledge of cellular component and patterns of insulitis has been from animal models, including NOD mice (43, 102, 103), BB rats (37), and transgenic animals (1, 80). With the increased availability of human pancreas samples for research in recent years, it is evidenced that animal models do not represent human insulitis (9, 10, 40, 41).
A long held notion in T1D is that macrophages within the islet produce ROS and proinflammatory cytokines, creating a beta cytotoxic environment (64). Activated proinflammatory macrophages can destroy islets in coculture systems (78). Historically, the proinflammatory cytokine combinations of interleukin 1 (IL-1), interferon gamma (IFNγ), and tumor necrosis factor alpha (TNFα) have been used in vitro to model this system. These inflammatory cytokines are produced from macrophages and CD4+ T cells and result in the activation of the inducible nitric oxide synthase (iNOS) (19, 38) through NF-κB [nuclear factor kappa-light-chain enhancer of activated B cells (24, 58)]-dependent pathways. Nitric oxide production within the beta cell results in reversible inhibition of mitochondrial OXPHOS. These inhibitions take place at cytochrome c oxidase (Complex IV) (75, 76), NADH dehydrogenase (Complex I) (72, 73), and inhibition of the TCA cycle enzyme aconitase (18). Inhibition of iNOS via pharmacological or genetic means prevents beta cell death by this cytokine combination in human and murine islets (52, 86). The major effector cytokine in this combination is IL-1; however, a number of recent studies have failed to support a role of IL-1 in the pathogenesis of human or mouse T1D (2, 29, 87). Accordingly, in human T1D, support for a pathogenic role of iNOS production and function within the beta cell has yet to materialize.
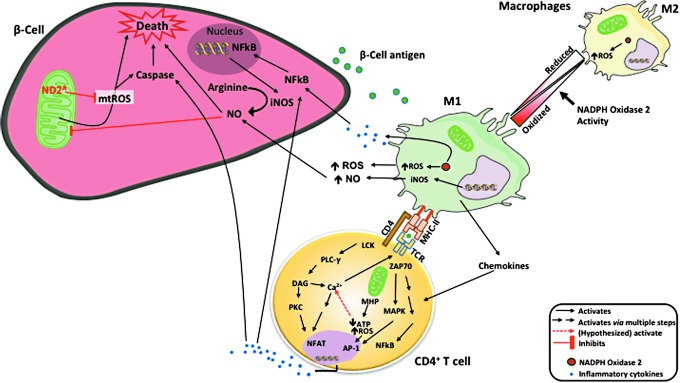
Other cells within the islet could be a source of ROS and reactive nitrogen species that damage beta cells during T1D pathogenesis. Indeed, activated proinflammatory macrophages can destroy islets in coculture systems (78). M1 macrophages produce ROS (64), which plays an important role in the recruitment of CD4+ and progression to T1D (Fig. 1). However, ROS are also critical for the regulation of M1 phenotypic macrophages (84). A lack of ROS production by nicotinamide adenine dinucleotide phosphate (NADPH) oxidase 2 (NOX2) in mice causes an increase in macrophage polarization toward an M2 phenotype (65). In addition, adoptive transfer systems have demonstrated that macrophage production of ROS via NOX2 is essential for T1D onset (85). Thus, a decrease in M1 macrophages highlights the essential role that ROS have in the islet microenvironment and is key to T1D pathogenesis
FIG. 1.
Autoantigens released by beta cells are collected by intraislet APCs, processed, and presented by MHC molecules. ROS produced through NADPH oxidase 2 plays a role in macrophage phenotype switching from regulatory M2 to inflammatory M1 macrophages that are essential for T1D pathogenesis. M1 macrophages secrete chemokines and recruit CD4+ T cells. Macrophages also present beta cell antigens to CD4+ T cells to restimulate these cells within the islet microenvironment. The combination of inflammatory cytokines from CD4+ T cells and M1 macrophages (IFNγ, TNFα, and IL-1β), in addition to macrophage-generated ROS and NO, can destroy or functionally impair beta cells. This potential impairment could be attributed to extracellular and intracellular ROS and NO levels within the beta cells, causing mitochondrial dysfunction. mt-ND2A allele protects beta cells by a lower level of mtROS generation. APCs, antigen-presenting cells; IFNγ, interferon gamma; IL-1, interleukin 1; iNOS, inducible nitric oxide synthase; MHC, major histocompatibility complex; mt-Nd2, mitochondrially encoded NADH:ubiquinone oxidoreductase core subunit 2; mtROS, mitochondrial reactive oxygen species; NADPH, nicotinamide adenine dinucleotide phosphate; NO, nitric oxide; ROS, reactive oxygen species; T1D, type 1 diabetes; TNFα, tumor necrosis factor alpha.
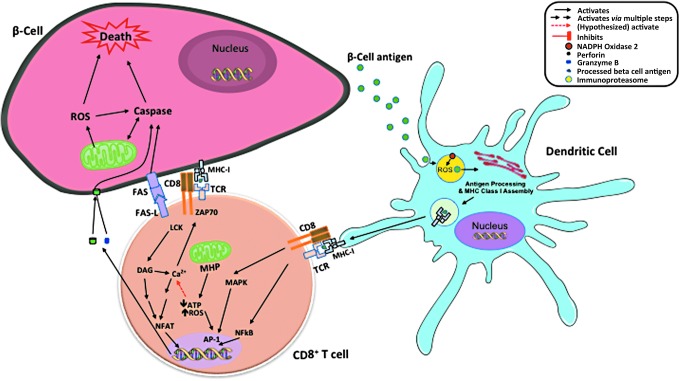
T cell induction of beta cell death has been linked to intrinsic apoptosis with involvement of mitochondria. CD8+ T cells recognize beta cell antigens in the context of major histocompatibility complex (MHC) class I and directly kill beta cells by the release of perforin and granzymes (23). Perforin and granzymes mediate cleavage of BH3 Interacting Domain Death Agonist (BID), resulting in mitochondrial permeability transition, release of cytochrome C, and eventually beta cell death (25). FAS-FAS ligand has been linked to mechanisms by which both CD4+ and CD8+ T cells mediate beta cell killing (20, 23, 91). These death receptors signal activation of caspases and mitochondrial apoptotic pathway. Therefore, mitochondrial function, particularly ROS production, plays an important role in regulating beta cell death under autoimmune attack [Reviewed by Padgett et al. (64)]. In addition, ROS produced by NOX2 within dendritic cells are essential for maintenance of a neutral pH within lysosomes where autoantigens are processed and assembled for cross-presenting to CD8+ T cells (Fig. 2).
FIG. 2.
Role of ROS in dendritic cell function and cross-presentation of autoantigen. Dendritic cells pick up beta cell antigen, process in phagosome and lysosomes, assembly with MHC class I in lysosome, and cross-present to CD8. ROS from NADPH oxidase keep the neutral environment in lysosome and are important for enzyme activities in antigen processing and cross-presentation. CD8+ T cells recognize beta cell antigens presented by MHC class I. MHP in CD8+ T cells also leads to lower ATP production and increased ROS generation. ROS further stimulate calcium flux and facilitate TCR signaling, which results in activation of transcription factors and production of inflammatory cytokines, perforin, granzyme B, and FASL that are all cytotoxic to beta cells. ATP, adenosine triphosphate; MHP, mitochondrial inner membrane hyperpolarization; TCR, T cell receptor.
Free radicals generated within the mitochondria are also players in the process of immune-mediated beta cell destruction (32). Beta cells are particularly sensitive to ROS/oxidant damage due to lower antioxidant enzyme expression in islets (49, 56, 58). In vitro studies have shown improvements in mitochondrial antioxidant defenses of beta cell lines or primary islets with the overexpression of antioxidant enzymes protecting these cells against inflammatory cytokines or oxidative stress (4, 16, 31, 53, 54). Beta cell susceptibility to cytokine-induced mitochondrial dysfunction pathways has been linked to T1D-genetic risk loci. Gli-similar 3 (GLIS3), a candidate gene for both T1D and type 2 diabetes (T2D) susceptibility, is reported to sensitize beta cells to cytokine-induced death through mitochondrial apoptosis pathway (63). Another T1D-associated candidate gene, CLEC16A (35), affects beta cell survival by changing mitochondrial morphology, function, and mitophagy (81). Downregulation of C-type lectin domain containing 16A (CLEC16A) leads to reduction of Nrdp1 (neuregulin receptor degradation protein-1), an E3 ubiqutin ligase (81). Nrdp1 regulates proteasomal degradation of Parkin (104), a key regulator of mitophagy (42). Mitochondrial ROS have been shown to initiate parkin RBR E3 ubiquitin protein ligase (PARK2)/Parkin-dependent mitophagy (93).
Studies using the T1D-prone NOD mouse, and a closely related diabetes-resistant strain, alloxan resistant (ALR), mapped a single nucleotide polymorphism (SNP), C4738A, to mitochondrial DNA (mtDNA) (57). This SNP is within the NADH dehydrogenase subunit 2 (mt-Nd2) gene and is associated with protection against both spontaneous T1D and chemically induced diabetes (16, 55, 57). To study this SNP, conplastic NOD.mtALR mice were created by crossing female ALR mice with males of the NOD strain, followed by backcrossing with male NOD for 10 generations. This process resulted in mice harboring mt-Nd2a on the NOD genetic background (34). Reciprocal ALR.mtNOD mice were also created. The protective allotype (mt-Nd2a) has been demonstrated to be associated with lower ROS production within isolated mitochondria (33, 34). We have generated beta cell lines harboring either mt-Nd2a or mt-Nd2c on the NOD genetic background (14). Beta cells with the T1D-resistant mt-Nd2a allele display resistance to alloxan, inflammatory cytokines, and autoreactive CD8+ cytotoxic T cell-mediated lysis (14). The protection has been associated with lower mitochondrial ROS production under inflammatory conditions. We have also investigated the mechanism of protection from mt-Nd2a in vivo by comparing spontaneous T1D incidence between NOD and NOD.mtALR mice. Not surprisingly, as a complex disease under the influence of multiple genetic and environmental factors, the incidence of spontaneous T1D was unaltered by this single mitochondrial SNP. Yet, mice with mt-Nd2a were resistant to induction of diabetes after the transfer of highly pathogenic beta cell antigen responsive T cells. Mice were completely protected from transfer of T1D by the diabetogenic CD4+ T cell clone BDC2.5 (14). Induction of T1D by BDC2.5 CD4+ T cells requires macrophages to mediate beta cell death. After adoptive transfer to recipients, the CD4+ T cells recruit macrophages (8) and activate the latter to release ROS and cytokines (8, 85). BDC2.5-mediated destruction of beta cells is also FAS-FAS ligand dependent (20, 91). Since BDC2.5 T cells attack beta cells through a cytokine-mediated ROS-dependent pathway (64, 69), the protection provided by mt-Nd2a is through enhancing the ability of these cells against ROS-mediated damage. In contrast, mice harboring mt-Nd2a are also protected to a certain degree against monoclonal diabetogenic CD8+ T cell-induced diabetes (14). As already mentioned, mitochondria participate in CD8+ killing of beta cells. Decreased mitochondrial ROS from mt-Nd2a beta cells are, therefore, considered to lower sensitivity of these beta cells to CD8+ T cell-induced activation of caspases and contribute to the partial protection.
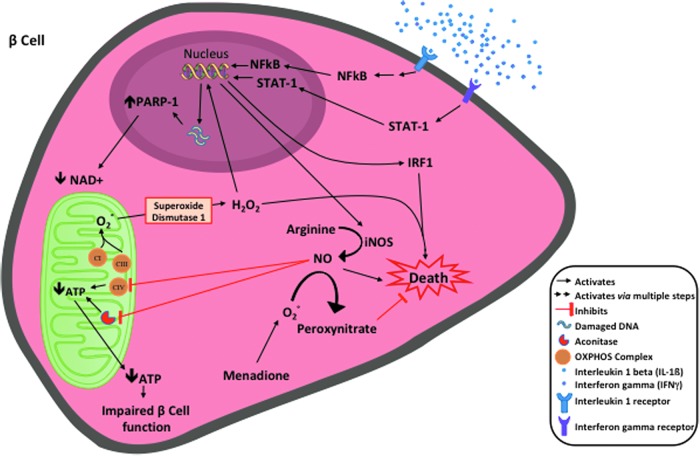
A corresponding SNP exists in the mtDNA of humans within NADH dehydrogenase subunit 2 gene, mt-ND2. This SNP, C5178A, results in an identical amino acid substitution when compared with mouse (leucine to methionine). The protective mt-ND2A of the mtDNA haplogroup D is associated with lower prevalence of both T1D and T2D in certain populations (51, 88), as well as a series of diseases and conditions related with oxidative stresses, including cardiovascular disease, plasma lipid levels, and longevity (39, 46, 47, 83, 100). Using a human beta cell line BetaLox5 (21, 36), we were able remove the mitochondrial genome and repopulate mtDNA from platelets of donors harboring mtDNA haplotypes with either mt-ND2A or mt-ND2C. These cytoplasmic hybrid, or cybrid, cells were used to understand the influence of mt-ND2 alleles on the interaction of beta cells with autoimmune mechanisms. These cybrid cells have identical nuclear genetic backgrounds and only differ in the mitochondrial genome. When compared with cells with the mt-ND2C allele, human beta cells encoding an mtDNA haplotype with mt-ND2A exhibited resistance against inflammatory cytokine-induced damage, which is associated with lower mitochondrial ROS production. Inflammatory cytokines damage beta cells and impair their function through induction of nitric oxide (NO) and hydrogen peroxide (H2O2) production (Fig. 3). These cells are also resistant to diabetogenic autoreactive CD8+ T cell attack in vitro [(50) and our unpublished observation] possibly via a reduction in NO and H2O2 production. These data suggest that protective variants of this mitochondrial OXPHOS subunit exert protection at the beta cell level and potentially impact both T1D and T2D by reducing stress when beta cells encounter inflammatory conditions.
FIG. 3.
Inflammatory cytokines damage beta cells and impair their function through induction of NO and H2O2 production. At higher concentrations, both NO and H2O2 cause beta cell death. At lower concentrations that do not affect beta cell viability, NO and H2O2 inhibit beta cell function through different pathways. NO inhibits mitochondrial aconitase and results in reduction of ATP. H2O2 breaks double stranded DNA, with the resulting DNA repair process of this breakage inducing hyperactivation of PARP-1, which consumes NAD+ and leads to depletion of cellular NAD+ pool, further resulting in decreased ATP production. Chemically induced intracellular production of superoxide (O2−) scavenges NO to produce peroxynitrate and attenuate NO-induced increased damage in beta cells. H2O2, hydrogen peroxide; NO, nitric oxide; PARP-1, poly [ADP-ribose] polymerase 1.
Mitochondrial Function in T Cells and T1D
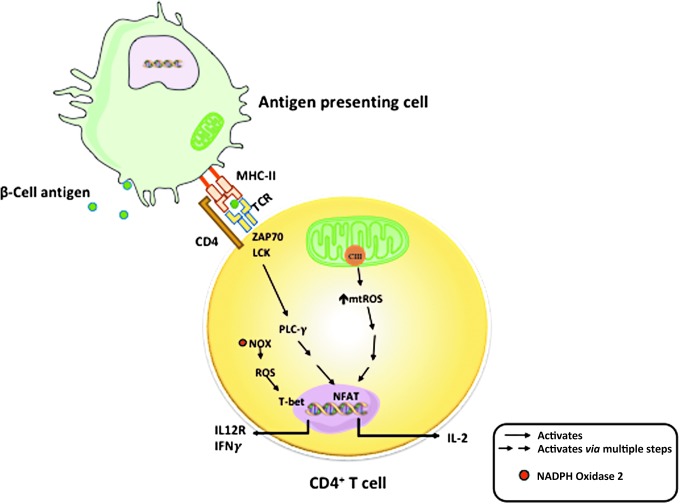
The essential role of mitochondria in T cell activity has drawn great attention in recent years (7, 89). Upon T cell activation, mitochondria are relocated to the immune synapse, where, through balanced fission and fusion processes, mitochondria regulate the local concentrations of ATP, calcium, and free radicals (5, 29, 71, 77). T cell activation and function are regulated by substrate metabolism and mitochondrial function. Naive and memory cells depend on the more efficient mitochondrial OXPHOS process for energy source. When activated, both OXPHOS and aerobic glycolysis are increased in these T cells. The increase in aerobic glycolysis is to fulfill energy demand, that is, Warburg effect (90). This increase in glycolysis allows fast ATP generation from glucose and TCA cycle intermediates to be utilized for biosynthesis of materials that are required for cell proliferation. Evidence from studies using mouse CD4+ T cells suggests that OXPHOS is needed for T cell activation, whereas activated T cells can use either OXPHOS or glycolysis for proliferation (48). T cell receptor (TCR) signaling activates ADP-dependent glucokinase (ADPGK) that catalyzes the phosphorylation of glucose to glucose-6-phosphate using ADP as the phosphate donor. ADPGK is responsible for switching between glycolysis and enhanced mitochondrial ROS generation (44). Mitochondrial ROS, specifically ROS produced at complex III, are essential for antigen-specific activation of T cells and effector function (Fig. 4) (79). In a study wherein mouse cells were differentiated and activated in vitro, it was demonstrated that mitochondrial fission and fusion activities closely control T cell development toward memory or effector phenotypes (6).
FIG. 4.
Role of ROS in T cell activation. Mitochondria in T cells have key roles in activation and regulation of antigen-specific responses. ROS produced from mitochondrial electron transport chain complex III are essential for antigen-specific T cell activation through activation of nuclear factor of activated T-cells (NFAT) and downstream production of IL-2. Cytoplasmic ROS produced by NADPH oxidases are important for effector responses, including upregulation of T-box transcription factor TBX21 (T-bet), expression of IL-12R, and production of interferon gamma.
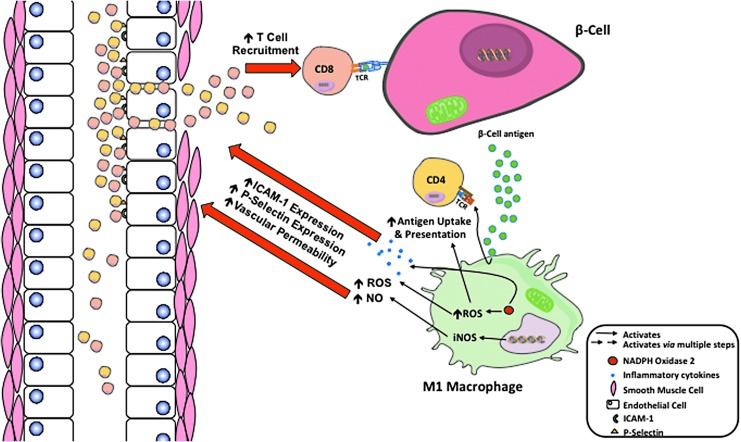
The final barrier that prevents autoimmune T cells from invading islets is vascular walls, particularly the vascular endothelium. During the development of T1D, islet microenvironment becomes altered due to the increased production of inflammatory cytokines (IFNγ, TNFα, and IL-1β), ROS, and NO by M1 macrophages. This increased production of cytokines, ROS, and NO causes the vascular endothelium to become more permeable, allowing enhanced movement of CD4+ and CD8+ T cells to move into the extravascular space (60). In addition, ROS and NO increase the expression of intracellular adhesion molecule 1 (ICAM-1) and P-selectin on vascular endothelium (60, 82). P-selectin is responsible for the adhesion and rolling of T cells, with ICAM-1 binding to membrane proteins causing cellular arrest and allowing extravasation to occur (82). This combination increases the extravasation of CD4+ and CD8+ T cells exacerbating the inflammatory microenvironment, leading to further destruction of beta cells and T1D progression (Fig. 5).
FIG. 5.
ROS and NO increase T cells extravasation from the blood into the islets. The production and secretion of inflammatory cytokines (IFNγ, TNFα, and IL-1β), ROS, and NO by M1 macrophages alter the vascular architecture. ROS and NO increase the expression of ICAM-1 and P-selectin on vascular endothelium. These adhesion molecules allow for increased T cell rolling, adhesion, and diapedesis. ROS also enhance vascular permeability, allowing for increased amounts of CD4+ and CD8+ T cells to move into the islet microenvironment. This combination of elevated adhesion molecules and permeability increases the extravasation of CD4+ and CD8+ T cells exacerbating the inflammatory microenvironment, leading to autoantigen-targeted destruction of beta cells and T1D progression. ICAM-1, intracellular adhesion molecule 1.
Given the important role of mitochondria in T cell function, it is not surprising that T cell mitochondrial dysfunction has been associated with autoimmune disease in humans. T cells from patients with systemic lupus erythematosus (SLE) exhibits mitochondrial hyperpolarization (28), which is thought to be the consequence of abnormal nitric oxide production from monocytes (61), leading to ATP depletion, increased ROS production, and necrotic death of T cells (28). Animal models of SLE identified that CD4+ T cells have elevated glycolysis and mitochondrial oxidative metabolism when compared with those in control mice (101). Using these mouse models, potential therapeutic approaches have been discovered linking inhibition of the mitochondrial oxidation and glycolytic rate in CD4+ T cells that may normalize their metabolism and potentially reduce the risk of SLE with targeted therapy (101). CD4+ T cells from autoimmune rheumatoid arthritis also displayed ATP depletion, elevated autophagy, and impaired reduction–oxidation status (98, 99). In autoimmune multiple sclerosis (MS), mitochondrial dysfunction in the nervous system has been long known (74, 96). CD4+ T cells from MS patients exhibit significant increases in the mitochondrial inner membrane lipid cardiolipin (92). Changes in mitochondrial enzyme activities and bioenergetic profiles are also detected. CD4+ T cells from patients with MS showed decreased respiratory control ratio, decreased mitochondrial complex I and complex IV activities, increased activities of hexokinase and phosphofructokinase, increased expression of glucose transporter 1 (GLUT1), and decreased activities of antioxidant enzymes superoxide dismutase and glutathione peroxidase (22).
Early research and identification of mitochondria in T cells participating in T1D pathogenesis came from animal studies. Disruption of a mitochondrial outer membrane protein GTPase, IMAP family member 5 (GIMAP5) was determined to account for the lymphopenia that has been described as a major feature of T1D onset in the diabetes-prone BB-DP rats. The mutation in GIMAP5 regulates T cell apoptosis (66). BDC2.5 CD4+ T cell behavior was followed in fetal thymus organ culture (FTOC) of NOD and major histocompatibility complex congenic C57BL/6.NODc17-H2 g7 mice. BDC2.5 FTOC was challenged by peptide in culture and the resulting induction of genes was analyzed by hybridization of messenger RNA from sorted double-positive thymocytes to gene expression arrays (105). Data revealed that the NOD background promotes a significantly increased expression of antiapoptotic gene Bcl2 (B cell lymphoma 2) (105), which is thought to be attributed to failed central tolerance induction in NOD. In a separate study, gene array results showed that islet-specific glucose 6 phosphatase catalytic subunit-related protein (IGRP)-reactive CD8+ T cells from NOD mice failed to induce increased expression of genes associated with OXPHOS pathways, which were detected in nonautoreactive CD8+ T cells, which have otherwise the same phenotype (27). As in the case of the SLE mouse model treated with mitochondrial metabolism inhibitor 2-deoxy-d-glucose (2DG) (101), short-term treatment of the T1D model NOD mice with 2DG resulted in reduction of autoreactive CD8+ T cells in pancreatic lymph nodes and spleen, a reduction in insulitis, and improved beta cell granularity (27).
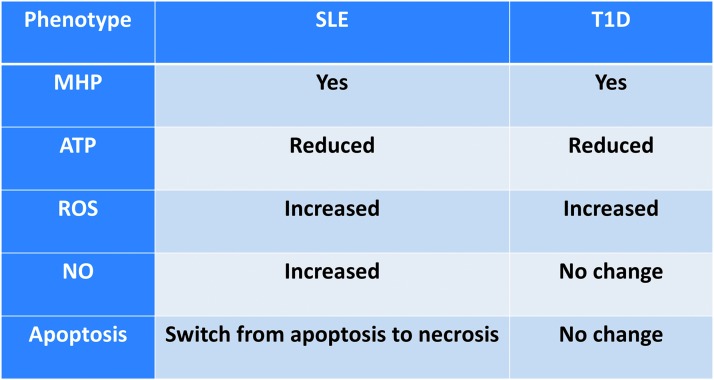
In accord with results from other autoimmune diseases, T cells from patients with T1D are characterized by mitochondrial abnormalities, specifically, mitochondrial inner membrane hyperpolarization (MHP) (13). This phenomenon is similar in some ways to the observations from T cells of autoimmune SLE patients (28), shown in Figure 6. However, key differences are present when comparing MHP T cells in T1D and SLE. T1D T cells are not necrosis prone (our observation). T cell MHP in T1D is associated with functional changes of both CD4+ and CD8+ subsets. CD4+ T cells from T1D patients that display MHP exhibit a higher IFNγ production upon activation in vitro. Activation-induced IFNγ production is further correlated with increased mitochondrial reactive oxygen species (mtROS) (12). Human CD8+ T cells from donors with MHP also showed an enhanced antigen-specific cytotoxicity when assayed using human beta cells as targets (12). We have observed a decreased mitochondrial spare respiratory capacity in enriched total T cells from patients with T1D (15). T cell mitochondrial dysfunction is not correlated with patients' hemoglobin A1c (HbA1c) level or T1D disease duration (12). Furthermore, MHP is not detected in T cells from T2D patients. Therefore, this T cell mitochondrial defect is not a consequence of abnormal metabolic control but rather intrinsic to these T cells. In addition, genetic linkages of human T1D-risk SNPs have been detected and are associated with these abnormal T cell mitochondrial metabolisms in T1D (12). These data indicate the important role of T cell mitochondrial function in the pathogenesis of human T1D.
FIG. 6.
Comparison of mitochondrial functional changes in T cell in two human autoimmune diseases, SLE and T1D. Although T cells in patients with both diseases exhibit MHP, the phenotypes associated with MHP in SLE and T1D are significantly different. SLE, systemic lupus erythematosus
In summary, during the development of T1D, mitochondria are involved in both sides of the autoimmune attack: in the victim (target beta cells) as well as in the attacker (effector T cells). Within the target beta cells, mitochondria are required for nutrient-induced insulin secretion. Perturbations to the mitochondria from metabolic or autoimmune stress may enhance susceptibility to autoimmunity through enhanced production of mtROS. On the effector side, mitochondria affect T cell autoreactivity through regulating T cell metabolic activities. In addition to mtROS, cytoplasmic ROS generated from NADPH oxidase also participate in macrophage phenotype switching, dendritic cell antigen cross-presentation, effector T cell response, and impaired vascular endothelium integrity. Taken together, ROS and mitochondrial function play important roles in the pathogenesis of T1D. It remains to be clarified how mitochondrial metabolism and substrate utilization affect immune balance during the development of T1D. This is possibly occurring via changes in development and function of different subsets of T cells, including, but not limited to, effector, memory, naive, and regulatory T cells. During the development of T1D, how mitochondrial function affects each T cell subset differently also remains inconclusive. Owing to their known role in intracellular calcium modulation, mitochondria are implicated in TCR signaling. Nevertheless, a major unmet need is to understand how mitochondrial dysfunction affects intracellular TCR signaling. Dysfunction of mitochondria could result from impaired calcium flux or ROS production, improper endoplasmic reticulum interactions, and/or aberrant downstream transcription factor regulation. These mechanisms of TCR and mitochondrial communication require further investigation to compile a comprehensive understanding of the intracellular pathways that lead to autoimmunity in T1D. Furthermore, studies are necessary to explore how mitochondria regulate the innate immune system and antigen presentation in the context of human T1D.
Abbreviations Used
- 2DG
2-deoxy-d-glucose
- acetyl-CoA
acetyl-coenzyme A
- ADP
adenosine diphosphate
- ADPGK
ADP-dependent glucokinase
- ALR
alloxan resistant
- APCs
antigen-presenting cells
- ATP
adenosine triphosphate
- BB-DP
biobreeding-diabetes prone
- CLEC16A
C-type lectin domain containing 16A
- FTOC
fetal thymus organ culture
- GIMAP5
GTPase, IMAP family member 5
- H2O2
hydrogen peroxide
- ICAM1
intracellular adhesion molecule 1
- IFNγ
interferon gamma
- IL-1
interleukin 1
- iNOS
inducible nitric oxide synthase
- MHC
major histocompatibility complex
- MHP
mitochondrial inner membrane hyperpolarization
- MS
multiple sclerosis
- mtDNA
mitochondrial DNA
- mt-Nd2
mitochondrially encoded NADH:ubiquinone oxidoreductase core subunit 2
- mtROS
mitochondrial reactive oxygen species
- NADPH
nicotinamide adenine dinucleotide phosphate
- NO
nitric oxide
- NOD
nonobese diabetic
- NOX2
NADPH oxidase 2
- Nrdp1
neuregulin receptor degradation protein-1
- OXPHOS
oxidative phosphorylation
- ROS
reactive oxygen species
- SLE
systemic lupus erythematosus
- SNP
single nucleotide polymorphism
- T1D
type 1 diabetes
- T2D
type 2 diabetes
- TCA
tricarboxylic acid cycle
- TCR
T cell receptor
- TNFα
tumor necrosis factor alpha
Acknowledgments
The authors gratefully acknowledge funding from the National Institutes of Health, R01 DK074656 (C.E.M.), P01 AI042288 (C.E.M.), and UC4 DK104194 (C.E.M.), as well as funding from the JDRF (J.C.) and American Diabetes Association (J.C.).
Author Contributions
J.C. and C.E.M. conceived the overall idea for this article, contributed to discussion, and wrote/reviewed/edited the article. G.A.F.B. and S.E.S. contributed to discussion and wrote/reviewed/edited the article.
References
- 1.Amrani A, Verdaguer J, Anderson B, Utsugi T, Bou S, and Santamaria P. Perforin-independent beta-cell destruction by diabetogenic CD8(+) T lymphocytes in transgenic nonobese diabetic mice. J Clin Invest 103: 1201–1209, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anquetil F, Sabouri S, Thivolet C, Rodriguez-Calvo T, Zapardiel-Gonzalo J, Amirian N, Schneider D, Castillo E, Lajevardi Y, von Herrath MG. Alpha cells, the main source of IL-1β in human pancreas. J Autoimmune 8: 68–73, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnush M, Scarim AL, Heitmeier MR, Kelly CB, and Corbett JA. Potential role of resident islet macrophage activation in the initiation of autoimmune diabetes. J Immunol 160: 2684–2691, 1998 [PubMed] [Google Scholar]
- 4.Azevedo-Martins AK, Lortz S, Lenzen S, Curi R, Eizirik DL, and Tiedge M. Improvement of the mitochondrial antioxidant defense status prevents cytokine-induced nuclear factor-kappaB activation in insulin-producing cells. Diabetes 52: 93–101, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Baixauli F, Martin-Cofreces NB, Morlino G, Carrasco YR, Calabia-Linares C, Veiga E, Serrador JM, and Sanchez-Madrid F. The mitochondrial fission factor dynamin-related protein 1 modulates T-cell receptor signalling at the immune synapse. EMBO J 30: 1238–1250, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buck MD, O'Sullivan D, Klein Geltink RI, Curtis JD, Chang CH, Sanin DE, Qiu J, Kretz O, Braas D, van der Windt GJ, Chen Q, Huang SC, O'Neill CM, Edelson BT, Pearce EJ, Sesaki H, Huber TB, Rambold AS, and Pearce EL. Mitochondrial dynamics controls T cell fate through metabolic programming. Cell 166: 63–76, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buck MD, O'Sullivan D, and Pearce EL. T cell metabolism drives immunity. J Exp Med 212: 1345–1360, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calderon B, Suri A, and Unanue ER. In CD4+ T-cell-induced diabetes, macrophages are the final effector cells that mediate islet beta-cell killing: studies from an acute model. Am J Pathol 169: 2137–2147, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell-Thompson M, Fu A, Kaddis JS, Wasserfall C, Schatz DA, Pugliese A, and Atkinson MA. Insulitis and beta-cell mass in the natural history of type 1 diabetes. Diabetes 65: 719–731, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campbell-Thompson ML, Atkinson MA, Butler AE, Chapman NM, Frisk G, Gianani R, Giepmans BN, von Herrath MG, Hyoty H, Kay TW, Korsgren O, Morgan NG, Powers AC, Pugliese A, Richardson SJ, Rowe PA, Tracy S, and In't Veld PA. The diagnosis of insulitis in human type 1 diabetes. Diabetologia 56: 2541–2543, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Carrero JA, Ferris ST, and Unanue ER. Macrophages and dendritic cells in islets of Langerhans in diabetic autoimmunity: a lesson on cell interactions in a mini-organ. Curr Opin Immunol 43: 54–59, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen J, Chernatynskaya A, Kimbrell M, Li J-W, Newby B, Johnston A, and Mathews CE. T cell mitochondrial dysfunction in human type 1 diabetes. Diabetes 65: A98, 2016 [Google Scholar]
- 13.Chen J, Chernatynskaya AV, Li JW, Kimbrell MR, Cassidy RJ, Perry DJ, Muir AB, Atkinson MA, Brusko TM, and Mathews CE. T cells display mitochondria hyperpolarization in human type 1 diabetes. Sci Rep 7: 10835, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen J, Gusdon AM, Piganelli J, Leiter EH, and Mathews CE. mt-Nd2(a) Modifies resistance against autoimmune type 1 diabetes in NOD mice at the level of the pancreatic beta-cell. Diabetes 60: 355–359, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen J, Li JW, Perry DJ, Brusko TM, and Mathews CE. Patients and individuals at-risk for developing type 1 diabetes exhibit peripheral t-cell mitochondrial inner membrane hyperpolarization. Diabetes 62: A440-A440, 2013 [Google Scholar]
- 16.Chen J, Lu Y, Lee CH, Li R, Leiter EH, and Mathews CE. Commonalities of genetic resistance to spontaneous autoimmune and free radical—mediated diabetes. Free Radic Biol Med 45: 1263–1270, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christianson SW, Shultz LD, and Leiter EH. Adoptive transfer of diabetes into immunodeficient NOD-scid/scid mice. Relative contributions of CD4+ and CD8+ T-cells from diabetic versus prediabetic NOD.NON-Thy-1a donors. Diabetes 42: 44–55, 1993 [DOI] [PubMed] [Google Scholar]
- 18.Corbett JA. and McDaniel ML. Reversibility of interleukin-1 beta-induced islet destruction and dysfunction by the inhibition of nitric oxide synthase. Biochem J 299 (Pt 3): 719–724, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corbett JA, Wang JL, Sweetland MA, Lancaster JR, Jr, and McDaniel ML. Interleukin 1 beta induces the formation of nitric oxide by beta-cells purified from rodent islets of Langerhans. Evidence for the beta-cell as a source and site of action of nitric oxide. J Clin Invest 90: 2384–2391, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Darwiche R, Chong MM, Santamaria P, Thomas HE, and Kay TW. Fas is detectable on beta cells in accelerated, but not spontaneous, diabetes in nonobese diabetic mice. J Immunol 170: 6292–6297, 2003 [DOI] [PubMed] [Google Scholar]
- 21.de la Tour D, Halvorsen T, Demeterco C, Tyrberg B, Itkin-Ansari P, Loy M, Yoo SJ, Hao E, Bossie S, and Levine F. Beta-cell differentiation from a human pancreatic cell line in vitro and in vivo. Mol Endocrinol 15: 476–483, 2001 [DOI] [PubMed] [Google Scholar]
- 22.De Riccardis L, Rizzello A, Ferramosca A, Urso E, De Robertis F, Danieli A, Giudetti AM, Trianni G, Zara V, and Maffia M. Bioenergetics profile of CD4(+) T cells in relapsing remitting multiple sclerosis subjects. J Biotechnol 202: 31–39, 2015 [DOI] [PubMed] [Google Scholar]
- 23.Dudek NL, Thomas HE, Mariana L, Sutherland RM, Allison J, Estella E, Angstetra E, Trapani JA, Santamaria P, Lew AM, and Kay TW. Cytotoxic T-cells from T-cell receptor transgenic NOD8.3 mice destroy beta-cells via the perforin and Fas pathways. Diabetes 55: 2412–2418, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Eizirik DL, Moore F, Flamez D, and Ortis F. Use of a systems biology approach to understand pancreatic beta-cell death in Type 1 diabetes. Biochem Soc Trans 36: 321–327, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Estella E, McKenzie MD, Catterall T, Sutton VR, Bird PI, Trapani JA, Kay TW, and Thomas HE. Granzyme B-mediated death of pancreatic beta-cells requires the proapoptotic BH3-only molecule bid. Diabetes 55: 2212–2219, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Ferris ST, Zakharov PN, Wan X, Calderon B, Artyomov MN, Unanue ER, and Carrero JA. The islet-resident macrophage is in an inflammatory state and senses microbial products in blood. J Exp Med 214: 2369–2385, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garyu JW, Uduman M, Stewart A, Rui J, Deng S, Shenson J, Staron MM, Kaech SM, Kleinstein SH, and Herold KC. Characterization of Diabetogenic CD8+ T cells: immune therapy with metabolic blockade. J Biol Chem 291: 11230–11240, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gergely P, Jr., Grossman C, Niland B, Puskas F, Neupane H, Allam F, Banki K, Phillips PE, and Perl A. Mitochondrial hyperpolarization and ATP depletion in patients with systemic lupus erythematosus. Arthritis Rheum 46: 175–190, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gill T. and Levine AD. Mitochondria-derived hydrogen peroxide selectively enhances T cell receptor-initiated signal transduction. J Biol Chem 288: 26246–26255, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grunnet LG, Aikin R, Tonnesen MF, Paraskevas S, Blaabjerg L, Storling J, Rosenberg L, Billestrup N, Maysinger D, and Mandrup-Poulsen T. Proinflammatory cytokines activate the intrinsic apoptotic pathway in beta-cells. Diabetes 58: 1807–1815, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gurgul E, Lortz S, Tiedge M, Jorns A, and Lenzen S. Mitochondrial catalase overexpression protects insulin-producing cells against toxicity of reactive oxygen species and proinflammatory cytokines. Diabetes 53: 2271–2280, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Gurgul-Convey E, Mehmeti I, Lortz S, and Lenzen S. Cytokine toxicity in insulin-producing cells is mediated by nitro-oxidative stress-induced hydroxyl radical formation in mitochondria. J Mol Med (Berl) 89: 785–798, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Gusdon AM, Votyakova TV, and Mathews CE. mt-Nd2a suppresses reactive oxygen species production by mitochondrial complexes I and III. J Biol Chem 283: 10690–10697, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gusdon AM, Votyakova TV, Reynolds IJ, and Mathews CE. Nuclear and mitochondrial interaction involving mt-Nd2 leads to increased mitochondrial reactive oxygen species production. J Biol Chem 282: 5171–5179, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Hakonarson H, Grant SF, Bradfield JP, Marchand L, Kim CE, Glessner JT, Grabs R, Casalunovo T, Taback SP, Frackelton EC, Lawson ML, Robinson LJ, Skraban R, Lu Y, Chiavacci RM, Stanley CA, Kirsch SE, Rappaport EF, Orange JS, Monos DS, Devoto M, Qu HQ, and Polychronakos C. A genome-wide association study identifies KIAA0350 as a type 1 diabetes gene. Nature 448: 591–594, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Halvorsen TL, Leibowitz G, and Levine F. Telomerase activity is sufficient to allow transformed cells to escape from crisis. Mol Cell Biol 19: 1864–1870, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hanenberg H, Kolb-Bachofen V, Kantwerk-Funke G, and Kolb H. Macrophage infiltration precedes and is a prerequisite for lymphocytic insulitis in pancreatic islets of pre-diabetic BB rats. Diabetologia 32: 126–134, 1989 [DOI] [PubMed] [Google Scholar]
- 38.Heitmeier MR, Scarim AL, and Corbett JA. Prolonged STAT1 activation is associated with interferon-gamma priming for interleukin-1-induced inducible nitric-oxide synthase expression by islets of Langerhans. J Biol Chem 274: 29266–29273, 1999 [DOI] [PubMed] [Google Scholar]
- 39.Honmyo R, Kokaze A, Karita K, Yoshida M, Ishikawa M, and Ohno H. Influence of mitochondrial DNA 5178 C/A polymorphism on serum cholesterol changes: a short-term follow-up in middle-aged Japanese men. Environ Health Prev Med 17: 401–407, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.In't Veld P. Insulitis in human type 1 diabetes: a comparison between patients and animal models. Semin Immunopathol 36: 569–579, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.In't Veld P, Lievens D, De Grijse J, Ling Z, Van der Auwera B, Pipeleers-Marichal M, Gorus F, and Pipeleers D. Screening for insulitis in adult autoantibody-positive organ donors. Diabetes 56: 2400–2404, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Jin SM. and Youle RJ. PINK1- and Parkin-mediated mitophagy at a glance. J Cell Sci 125: 795–799, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jun HS, Yoon CS, Zbytnuik L, van Rooijen N, and Yoon JW. The role of macrophages in T cell-mediated autoimmune diabetes in nonobese diabetic mice. J Exp Med 189: 347–358, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaminski MM, Sauer SW, Kaminski M, Opp S, Ruppert T, Grigaravicius P, Grudnik P, Grone HJ, Krammer PH, and Gulow K. T cell activation is driven by an ADP-dependent glucokinase linking enhanced glycolysis with mitochondrial reactive oxygen species generation. Cell Rep 2: 1300–1315, 2012 [DOI] [PubMed] [Google Scholar]
- 45.Kay TW, Chaplin HL, Parker JL, Stephens LA, and Thomas HE. CD4+ and CD8+ T lymphocytes: clarification of their pathogenic roles in diabetes in the NOD mouse. Res Immunol 148: 320–327, 1997 [DOI] [PubMed] [Google Scholar]
- 46.Kokaze A, Ishikawa M, Matsunaga N, Yoshida M, Sekine Y, Sekiguchi K, Harada M, Satoh M, Teruya K, Takeda N, Fukazawa S, Uchida Y, and Takashima Y. Longevity-associated mitochondrial DNA 5178 A/C polymorphism and blood pressure in the Japanese population. J Hum Hypertens 18: 41–45, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Kokaze A, Ishikawa M, Matsunaga N, Yoshida M, Sekine Y, Teruya K, Takeda N, Sumiya Y, Uchida Y, and Takashima Y. Association of the mitochondrial DNA 5178 A/C polymorphism with serum lipid levels in the Japanese population. Hum Genet 109: 521–525, 2001 [DOI] [PubMed] [Google Scholar]
- 48.Kolev M, Dimeloe S, Le Friec G, Navarini A, Arbore G, Povoleri GA, Fischer M, Belle R, Loeliger J, Develioglu L, Bantug GR, Watson J, Couzi L, Afzali B, Lavender P, Hess C, and Kemper C. Complement Regulates Nutrient Influx and Metabolic Reprogramming during Th1 Cell Responses. Immunity 42: 1033–1047, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lenzen S, Drinkgern J, and Tiedge M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic Biol Med 20: 463–466, 1996 [DOI] [PubMed] [Google Scholar]
- 50.Lightfoot YL, Chen J, and Mathews CE. Role of the mitochondria in immune-mediated apoptotic death of the human pancreatic beta cell line betaLox5. PLoS One 6: e20617, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liou CW, Chen JB, Tiao MM, Weng SW, Huang TL, Chuang JH, Chen SD, Chuang YC, Lee WC, Lin TK, and Wang PW. Mitochondrial DNA coding and control region variants as genetic risk factors for type 2 diabetes. Diabetes 61: 2642–2651, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu D, Pavlovic D, Chen MC, Flodstrom M, Sandler S, and Eizirik DL. Cytokines induce apoptosis in beta-cells isolated from mice lacking the inducible isoform of nitric oxide synthase (iNOS-/-). Diabetes 49: 1116–1122, 2000 [DOI] [PubMed] [Google Scholar]
- 53.Lortz S, Tiedge M, Nachtwey T, Karlsen AE, Nerup J, and Lenzen S. Protection of insulin-producing RINm5F cells against cytokine-mediated toxicity through overexpression of antioxidant enzymes. Diabetes 49: 1123–1130, 2000 [DOI] [PubMed] [Google Scholar]
- 54.Maechler P. and Wollheim CB. Mitochondrial function in normal and diabetic beta-cells. Nature 414: 807–812, 2001 [DOI] [PubMed] [Google Scholar]
- 55.Mathews CE, Graser RT, Bagley RJ, Caldwell JW, Li R, Churchill GA, Serreze DV, and Leiter EH. Genetic analysis of resistance to Type-1 Diabetes in ALR/Lt mice, a NOD-related strain with defenses against autoimmune-mediated diabetogenic stress. Immunogenetics 55: 491–496, 2003 [DOI] [PubMed] [Google Scholar]
- 56.Mathews CE. and Leiter EH. Resistance of ALR/Lt islets to free radical-mediated diabetogenic stress is inherited as a dominant trait. Diabetes 48: 2189–2196, 1999 [DOI] [PubMed] [Google Scholar]
- 57.Mathews CE, Leiter EH, Spirina O, Bykhovskaya Y, Gusdon AM, Ringquist S, and Fischel-Ghodsian N. mt-Nd2 Allele of the ALR/Lt mouse confers resistance against both chemically induced and autoimmune diabetes. Diabetologia 48: 261–267, 2005 [DOI] [PubMed] [Google Scholar]
- 58.Mathews CE, Suarez-Pinzon WL, Baust JJ, Strynadka K, Leiter EH, and Rabinovitch A. Mechanisms underlying resistance of pancreatic islets from ALR/Lt mice to cytokine-induced destruction. J Immunol 175: 1248–1256, 2005 [DOI] [PubMed] [Google Scholar]
- 59.Michels AW, Landry LG, McDaniel KA, Yu L, Campbell-Thompson M, Kwok WW, Jones KL, Gottlieb PA, Kappler JW, Tang Q, Roep BO, Atkinson MA, Mathews CE, and Nakayama M. Islet-derived CD4 T cells targeting proinsulin in human autoimmune diabetes. Diabetes 66: 722–734, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mittal M, Siddiqui MR, Tran K, Reddy SP, and Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal 20: 1126–1167, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nagy G, Koncz A, and Perl A. T cell activation-induced mitochondrial hyperpolarization is mediated by Ca2+- and redox-dependent production of nitric oxide. J Immunol 171: 5188–5197, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nichols CG, Shyng SL, Nestorowicz A, Glaser B, Clement JPT, Gonzalez G, Aguilar-Bryan L, Permutt MA, and Bryan J. Adenosine diphosphate as an intracellular regulator of insulin secretion. Science 272: 1785–1787, 1996 [DOI] [PubMed] [Google Scholar]
- 63.Nogueira TC, Paula FM, Villate O, Colli ML, Moura RF, Cunha DA, Marselli L, Marchetti P, Cnop M, Julier C, and Eizirik DL. GLIS3, a susceptibility gene for type 1 and type 2 diabetes, modulates pancreatic beta cell apoptosis via regulation of a splice variant of the BH3-only protein Bim. PLoS Genet 9: e1003532, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Padgett LE, Broniowska KA, Hansen PA, Corbett JA, and Tse HM. The role of reactive oxygen species and proinflammatory cytokines in type 1 diabetes pathogenesis. Ann N Y Acad Sci 1281: 16–35, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Padgett LE, Burg AR, Lei W, and Tse HM. Loss of NADPH oxidase-derived superoxide skews macrophage phenotypes to delay type 1 diabetes. Diabetes 64: 937–946, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pandarpurkar M, Wilson-Fritch L, Corvera S, Markholst H, Hornum L, Greiner DL, Mordes JP, Rossini AA, and Bortell R. Ian4 is required for mitochondrial integrity and T cell survival. Proc Natl Acad Sci U S A 100: 10382–10387, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pathiraja V, Kuehlich JP, Campbell PD, Krishnamurthy B, Loudovaris T, Coates PT, Brodnicki TC, O'Connell PJ, Kedzierska K, Rodda C, Bergman P, Hill E, Purcell AW, Dudek NL, Thomas HE, Kay TW, and Mannering SI. Proinsulin-specific, HLA-DQ8, and HLA-DQ8-transdimer-restricted CD4+ T cells infiltrate islets in type 1 diabetes. Diabetes 64: 172–182, 2015 [DOI] [PubMed] [Google Scholar]
- 68.Pearson JA, Wong FS, and Wen L. The importance of the Non Obese Diabetic (NOD) mouse model in autoimmune diabetes. J Autoimmun 66: 76–88, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peterson JD, Berg R, Piganelli JD, Poulin M, and Haskins K. Analysis of leukocytes recruited to the pancreas by diabetogenic T cell clones. Cell Immunol 189: 92–98, 1998 [DOI] [PubMed] [Google Scholar]
- 70.Phillips JM, Parish NM, Raine T, Bland C, Sawyer Y, De La Pena H, and Cooke A. Type 1 diabetes development requires both CD4+ and CD8+ T cells and can be reversed by non-depleting antibodies targeting both T cell populations. Rev Diabet Stud 6: 97–103, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Quintana A, Schwindling C, Wenning AS, Becherer U, Rettig J, Schwarz EC, and Hoth M. T cell activation requires mitochondrial translocation to the immunological synapse. Proc Natl Acad Sci U S A 104: 14418–14423, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Radi R, Rodriguez M, Castro L, and Telleri R. Inhibition of mitochondrial electron transport by peroxynitrite. Arch Biochem Biophys 308: 89–95, 1994 [DOI] [PubMed] [Google Scholar]
- 73.Riobo NA, Clementi E, Melani M, Boveris A, Cadenas E, Moncada S, and Poderoso JJ. Nitric oxide inhibits mitochondrial NADH:ubiquinone reductase activity through peroxynitrite formation. Biochem J 359: 139–145, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sadeghian M, Mastrolia V, Rezaei Haddad A, Mosley A, Mullali G, Schiza D, Sajic M, Hargreaves I, Heales S, Duchen MR, and Smith KJ. Mitochondrial dysfunction is an important cause of neurological deficits in an inflammatory model of multiple sclerosis. Sci Rep 6: 33249, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sarti P, Forte E, Mastronicola D, Giuffre A, and Arese M. Cytochrome c oxidase and nitric oxide in action: molecular mechanisms and pathophysiological implications. Biochim Biophys Acta 1817: 610–619, 2012 [DOI] [PubMed] [Google Scholar]
- 76.Scarim AL, Heitmeier MR, and Corbett JA. Irreversible inhibition of metabolic function and islet destruction after a 36-hour exposure to interleukin-1beta. Endocrinology 138: 5301–5307, 1997 [DOI] [PubMed] [Google Scholar]
- 77.Schwindling C, Quintana A, Krause E, and Hoth M. Mitochondria positioning controls local calcium influx in T cells. J Immunol 184: 184–190, 2010 [DOI] [PubMed] [Google Scholar]
- 78.Schwizer RW, Leiter EH, and Evans R. Macrophage-mediated cytotoxicity against cultured pancreatic islet cells. Transplantation 37: 539–544, 1984 [DOI] [PubMed] [Google Scholar]
- 79.Sena LA, Li S, Jairaman A, Prakriya M, Ezponda T, Hildeman DA, Wang CR, Schumacker PT, Licht JD, Perlman H, Bryce PJ, and Chandel NS. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity 38: 225–236, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Serreze DV, Chapman HD, Varnum DS, Hanson MS, Reifsnyder PC, Richard SD, Fleming SA, Leiter EH, and Shultz LD. B lymphocytes are essential for the initiation of T cell-mediated autoimmune diabetes: analysis of a new “speed congenic” stock of NOD.Ig mu null mice. J Exp Med 184: 2049–2053, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Soleimanpour SA, Gupta A, Bakay M, Ferrari AM, Groff DN, Fadista J, Spruce LA, Kushner JA, Groop L, Seeholzer SH, Kaufman BA, Hakonarson H, and Stoffers DA. The diabetes susceptibility gene Clec16a regulates mitophagy. Cell 157: 1577–1590, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Su Y, Lei X, Wu L, and Liu L. The role of endothelial cell adhesion molecules P-selectin, E-selectin and intercellular adhesion molecule-1 in leucocyte recruitment induced by exogenous methylglyoxal. Immunology 137: 65–79, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Takagi K, Yamada Y, Gong JS, Sone T, Yokota M, and Tanaka M. Association of a 5178C—>A (Leu237Met) polymorphism in the mitochondrial DNA with a low prevalence of myocardial infarction in Japanese individuals. Atherosclerosis 175: 281–286, 2004 [DOI] [PubMed] [Google Scholar]
- 84.Tan HY, Wang N, Li S, Hong M, Wang X, and Feng Y. The reactive oxygen species in macrophage polarization: reflecting its dual role in progression and treatment of human diseases. Oxid Med Cell Longev 2016: 2795090, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Thayer TC, Delano M, Liu C, Chen J, Padgett LE, Tse HM, Annamali M, Piganelli JD, Moldawer LL, and Mathews CE. Superoxide production by macrophages and T cells is critical for the induction of autoreactivity and type 1 diabetes. Diabetes 60: 2144–2151, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Thomas HE, Darwiche R, Corbett JA, and Kay TW. Interleukin-1 plus gamma-interferon-induced pancreatic beta-cell dysfunction is mediated by beta-cell nitric oxide production. Diabetes 51: 311–316, 2002 [DOI] [PubMed] [Google Scholar]
- 87.Thomas HE, Irawaty W, Darwiche R, Brodnicki TC, Santamaria P, Allison J, and Kay TW. IL-1 receptor deficiency slows progression to diabetes in the NOD mouse. Diabetes 53: 113–121, 2004 [DOI] [PubMed] [Google Scholar]
- 88.Uchigata Y, Okada T, Gong JS, Yamada Y, Iwamoto Y, and Tanaka M. A mitochondrial genotype associated with the development of autoimmune-related type 1 diabetes. Diabetes Care 25: 2106, 2002 [DOI] [PubMed] [Google Scholar]
- 89.van der Windt GJ. and Pearce EL. Metabolic switching and fuel choice during T-cell differentiation and memory development. Immunol Rev 249: 27–42, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vander Heiden MG, Cantley LC, and Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324: 1029–1033, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vence L, Benoist C, and Mathis D. Fas deficiency prevents type 1 diabetes by inducing hyporesponsiveness in islet beta-cell-reactive T-cells. Diabetes 53: 2797–2803, 2004 [DOI] [PubMed] [Google Scholar]
- 92.Vergara D, D'Alessandro M, Rizzello A, De Riccardis L, Lunetti P, Del Boccio P, De Robertis F, Trianni G, Maffia M, and Giudetti AM. A lipidomic approach to the study of human CD4(+) T lymphocytes in multiple sclerosis. BMC Neurosci 16: 46, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang Y, Nartiss Y, Steipe B, McQuibban GA, and Kim PK. ROS-induced mitochondrial depolarization initiates PARK2/PARKIN-dependent mitochondrial degradation by autophagy. Autophagy 8: 1462–1476, 2012 [DOI] [PubMed] [Google Scholar]
- 94.Wiederkehr A. and Wollheim CB. Impact of mitochondrial calcium on the coupling of metabolism to insulin secretion in the pancreatic beta-cell. Cell Calcium 44: 64–76, 2008 [DOI] [PubMed] [Google Scholar]
- 95.Wiederkehr A. and Wollheim CB. Mitochondrial signals drive insulin secretion in the pancreatic beta-cell. Mol Cell Endocrinol 353: 128–137, 2012 [DOI] [PubMed] [Google Scholar]
- 96.Witte ME, Bo L, Rodenburg RJ, Belien JA, Musters R, Hazes T, Wintjes LT, Smeitink JA, Geurts JJ, De Vries HE, van der Valk P, and van Horssen J. Enhanced number and activity of mitochondria in multiple sclerosis lesions. J Pathol 219: 193–204, 2009 [DOI] [PubMed] [Google Scholar]
- 97.Wollheim CB. Beta-cell mitochondria in the regulation of insulin secretion: a new culprit in type II diabetes. Diabetologia 43: 265–277, 2000 [DOI] [PubMed] [Google Scholar]
- 98.Yang Z, Fujii H, Mohan SV, Goronzy JJ, and Weyand CM. Phosphofructokinase deficiency impairs ATP generation, autophagy, and redox balance in rheumatoid arthritis T cells. J Exp Med 210: 2119–2134, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yang Z, Shen Y, Oishi H, Matteson EL, Tian L, Goronzy JJ, and Weyand CM. Restoring oxidant signaling suppresses proarthritogenic T cell effector functions in rheumatoid arthritis. Sci Transl Med 8: 331ra38, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yao YG, Kong QP, and Zhang YP. Mitochondrial DNA 5178A polymorphism and longevity. Hum Genet 111: 462–463, 2002 [DOI] [PubMed] [Google Scholar]
- 101.Yin Y, Choi SC, Xu Z, Perry DJ, Seay H, Croker BP, Sobel ES, Brusko TM, and Morel L. Normalization of CD4+ T cell metabolism reverses lupus. Sci Transl Med 7: 274ra18, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yoon JW, Jun HS, and Santamaria P. Cellular and molecular mechanisms for the initiation and progression of beta cell destruction resulting from the collaboration between macrophages and T cells. Autoimmunity 27: 109–122, 1998 [DOI] [PubMed] [Google Scholar]
- 103.Zhao LJ, Wang W, Ren H, and Qi ZT. ERK signaling is triggered by hepatitis C virus E2 protein through DC-SIGN. Cell Stress Chaperones 18: 495–501, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhong L, Tan Y, Zhou A, Yu Q, and Zhou J. RING finger ubiquitin-protein isopeptide ligase Nrdp1/FLRF regulates parkin stability and activity. J Biol Chem 280: 9425–9430, 2005 [DOI] [PubMed] [Google Scholar]
- 105.Zucchelli S, Holler P, Yamagata T, Roy M, Benoist C, and Mathis D. Defective central tolerance induction in NOD mice: genomics and genetics. Immunity 22: 385–396, 2005 [DOI] [PubMed] [Google Scholar]