Abstract
Significance: Cytokines released in and around pancreatic islets during islet inflammation are believed to contribute to impaired β cell function and β cell death during the development of diabetes. Nitric oxide, produced by β cells in response to cytokine exposure, controls many of the responses of β cells during islet inflammation.
Recent Advances: Although nitric oxide has been shown to inhibit insulin secretion and oxidative metabolism and induce DNA damage in β cells, it also activates protective pathways that promote recovery of insulin secretion and oxidative metabolism and repair of damaged DNA. Recent studies have identified a novel role for nitric oxide in selectively regulating the DNA damage response in β cells.
Critical Issues: Does nitric oxide mediate cytokine-induced β cell damage, or is nitric oxide produced by β cells in response to cytokines to protect β cells from damage?
Future Directions: β cells appear to be the only islet endocrine cell type capable of responding to proinflammatory cytokines with the production of nitric oxide, and these terminally differentiated cells have a limited capacity to regenerate. It is likely that there is a physiological purpose for this response, and understanding this could open new areas of study regarding the loss of functional β cell mass during diabetes development.
Keywords: : nitric oxide, diabetes, DNA/RNA damage and repair, cell survival and death
Introduction
In most cases, type-1 diabetes (T1D) is the result of autoimmune-mediated progressive destruction of the insulin-producing β cells found in the pancreatic islets of Langerhans and this results in a chronic deficiency of insulin in afflicted individuals (55). While genetic predisposition can contribute to the susceptibility for the development of T1D, it is not solely responsible for disease penetrance, as the concordance rate of diabetes development between monozygotic twins is only ∼40–60% (106, 107, 114). Because of this low concordance rate, environmental factors (such as viral infection) are hypothesized to initiate and contribute to disease onset (104). Viral infection is one of the most effective mechanisms to activate the immune system, and cytokines produced in response to infection may contribute to β cell damage (104). Nitric oxide is one effector molecule produced by β cells in response to proinflammatory cytokines (interleukin-1 [IL-1], tumor necrosis factor [TNF], and interferon [IFN]-γ) that has been shown to damage β cells (79, 105). Nitric oxide modifies a number of physiological β cell processes, including the inhibition of oxidative metabolism, inhibition of glucose-stimulated insulin secretion, changes in target gene expression, induction of endoplasmic reticulum (ER) stress, damage to DNA, and activation of a variety of signaling cascades that culminates in β cell death if exposure to nitric oxide is prolonged (14).
This review focuses on the mechanisms by which nitric oxide modulates signaling pathways that control β cell fate during cytokine exposure. Specific focus is placed on the ability of nitric oxide to regulate intracellular signaling cascades activated in response to DNA damage, such as the DNA damage response (DDR) of the double-strand break (DSB) repair pathway, and how nitric oxide plays a dual role in the regulation of this pathway.
Nitric Oxide Is the Mediator of Cytokine-Induced Damage
IL-1 and β cell damage
In 1985, Mandrup-Poulsen et al. found that the exposure of islets to cytokine-rich supernatants derived from activated monocytes resulted in an inhibition of insulin secretion and islet cell death (92). The cytokine IL-1 was identified as the primary damaging component of this conditioned supernatant (11, 91). IL-1 induces a time-dependent inhibition of insulin secretion that is maximal following 18 h of exposure (67). It is the ability of IL-1 to decrease oxidative metabolism that results in reduced levels of adenosine triphosphate (ATP) that are responsible for the inhibition of insulin secretion (40, 48). Macrophages have been identified as one potential intraislet source of IL-1. The activation of resident islet macrophages results in the generation of IL-1 in islets to levels sufficient to inhibit β cell function and cause islet destruction (9, 36, 79). While most studies support macrophages as the primary source of IL-1 in the islet, α cells and β cells have also been reported to be a potential source of this cytokine and may contribute to intraislet IL-1 during diabetes development (6, 22, 62). In support of local IL-1 release as a mediator of β cell damage, we have shown that the IL-1 receptor antagonist attenuates the damaging actions of intraislet macrophage action on the function and viability of human, rat, and mouse islets (8, 9, 36).
Nitric oxide as a mediator of IL-1-induced damage
Nitric oxide was first implicated in the pathogenesis of T1D in the early 1990s, when three groups discovered that the inhibitory effects of IL-1 on β cell function were dependent on the formation of this free radical (34, 129, 140) (Fig. 1). The stable metabolite of nitric oxide, nitrite, was detected in the supernatant of cytokine-treated islets, and inhibitors of nitric oxide synthase (NOS) attenuate the inhibitory actions of IL-1 on insulin secretion (34, 129, 140). Direct evidence to support nitric oxide production in islets came from the demonstration of iron–dinitrosyl complex formation in cytokine-treated rodent and human islets by electron paramagnetic resonance (34, 38). Three NOS isoforms can be found in islets (endothelial, neuronal, and inducible (10, 112), and in response to IL-1, it is the inducible isoform of NOS (iNOS) that is responsible for generating micromolar levels of nitric oxide (39, 40, 129, 140). Activation of the transcription factor nuclear factor kappa B (NF-κB) is required for the expression of iNOS in IL-1-treated rat islets (52, 76, 78, 119). While IL-1 alone is capable of stimulating iNOS expression in rat β cells, mouse and human β cells require IFNγ in addition to IL-1 for iNOS expression (38). In rat β cells, IFNγ primes the response to IL-1 and potentiates the response by decreasing the concentration of IL-1 required to stimulate iNOS expression and nitric oxide production by 10-fold (24, 63).
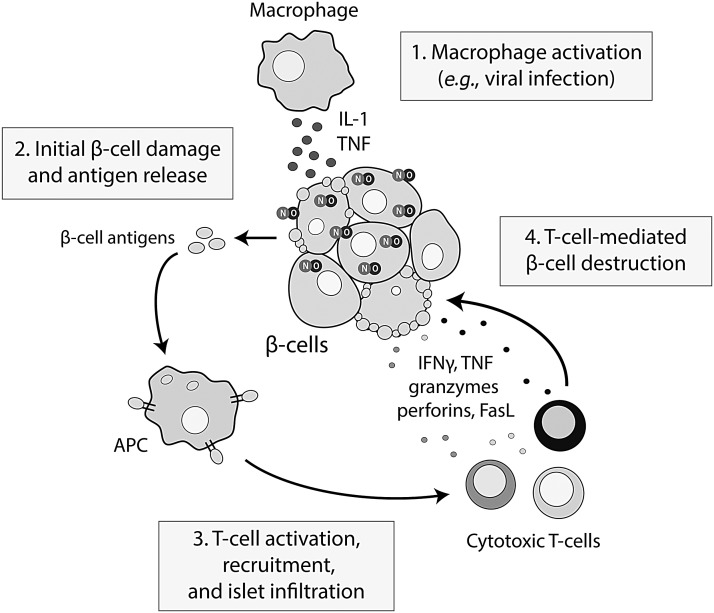
FIG. 1.
The role of IL-1 and nitric oxide in the precipitation of β cell destruction in type-1 diabetes. In response to an environmental trigger such as viral infection, activated macrophages release proinflammatory cytokines, including IL-1, leading to the stimulation of nitric oxide production within the β cell. Nitric oxide mediates the damaging effects of IL-1, and if IL-1 exposure persists, β cell death occurs, causing the release of β cell antigens, antigen presentation, T cell recruitment, and T cell-mediated destruction of remaining β cells. IFNγ, interferon-γ; IL-1, interleukin-1; TNFα, tumor necrosis factor-α.
Nitric oxide is the mediator of the inhibitory actions of IL-1 on insulin secretion. Inhibitors of NOS prevent the impairment in insulin secretion in cytokine-treated islets and purified β cells (34, 40, 129, 140), and nitric oxide donors inhibit insulin secretion from rat islets and insulinoma cell lines (43). The mechanism by which nitric oxide inhibits insulin secretion is through impairment of mitochondrial respiration (34, 40, 48, 129, 140). Nitric oxide inhibits mitochondrial aconitase through displacement of iron from the 4Fe-4S center contained in this enzyme (54). Nitric oxide also targets the electron transport chain by inhibiting complex I through Fe-S disruption or S-nitrosation (16) and reversibly inhibits complex IV by occupying the oxygen binding site in this complex (17, 29). The net effect is a fivefold decrease in cellular ATP levels (39) and a loss in the ability of glucose to stimulate the closure of ATP-sensitive potassium channels, membrane depolarization, calcium entry, and calcium-dependent secretion of insulin. The damaging effects of IL-1 are not limited to inhibition of insulin secretion, as β cells and islets exposed to IL-1 also experience an inhibition of protein synthesis and induction of DNA damage that occurs in a nitric oxide-dependent manner (105).
Reversibility of nitric oxide-induced damage
The cellular damage induced by nitric oxide during cytokine exposure is reversible, as β cells have a temporally limited capacity to recover from this damage (Fig. 2). Comens et al. first showed that the inhibitory actions of a 15-h incubation with IL-1 on insulin secretion can be reversed if the cytokine is removed and the islets are cultured in the absence of cytokine for 4 days (32). The time required to recover can be reduced from 4 days to 8 h by inhibiting iNOS (37). The addition of an NOS inhibitor to islets treated for 18 h with IL-1, followed by continued culture in the presence of IL-1 and the NOS inhibitor, results in the time-dependent recovery of islet secretory function that is maximal and complete after 8 h (32, 37). The recovery is not limited to insulin secretion, as oxidative metabolism and protein synthesis recover, and DNA is repaired, in a time-dependent manner that is similar to the recovery of insulin secretion (37, 68, 117, 122). The ability of β cells to recover from cytokine-induced damage is temporally limited, as exposures to IL-1 for 36 h or longer lead to an irreversible inhibition of insulin secretion, mitochondrial aconitase activity, protein synthesis, and DNA damage (37, 68, 122) (Fig. 2), and this irreversible damage correlates with a commitment of islets to degeneration (37, 68, 122). Caspase-3 cleavage and upregulation of several proapoptotic factors, such as p53 upregulated modulator of apoptosis (PUMA), death protein 5, the BH3-only sensitizer Bad, Bcl-2-interacting mediator of cell death (Bim), are associated with prolonged exposures to cytokines, suggesting that when recovery is no longer possible, apoptotic pathways are initiated (3, 58–60, 68, 87, 110) (Fig. 2).
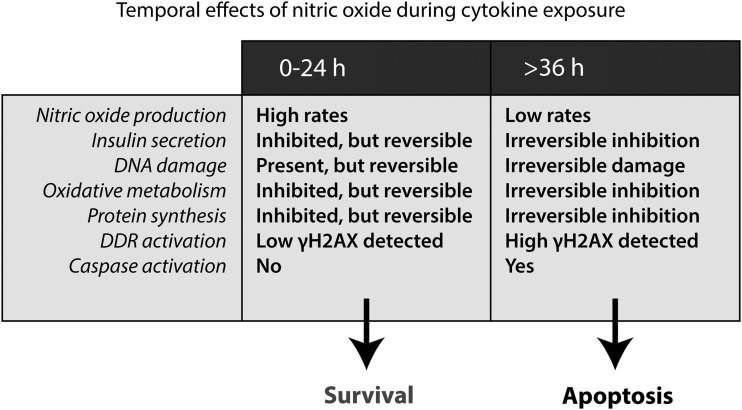
FIG. 2.
Temporal effects of cytokines on function and viability of β cells. Cytokines (IL-1 in rat, IL-1 + IFNγ in mouse and human) cause nitric oxide-dependent inhibition of insulin secretion, mitochondrial oxidative metabolism, protein synthesis, and damage to DNA. Cytokine-induced damage is reversible for up to 24 h of exposure if nitric oxide generation is prevented and β cells are allowed to repair and recover from this damage. After prolonged exposures of 36 h and longer, cytokine-induced damage becomes irreversible and β cells are committed to cell death by apoptosis. The molecular events that occur between 24 and 36 h of cytokine exposure and are responsible for “switch” from reversible to irreversible damage are currently unknown. H2AX, histone H2A.X.
Nitric oxide as the mediator of IL-1-induced cell death
Multiple studies suggest that nitric oxide production can lead to β cell death during cytokine exposure (2, 35, 37, 45, 49, 72, 86, 94, 122, 130, 133). A 6-day treatment of mouse islets with IL-1, IFNγ, and TNFα leads to an 88% decrease in viability in wild-type islets, yet iNOS−/− islets are completely protected from cytokine-induced cell death (86). Expression of iNOS under control of the insulin promoter leads to spontaneous insulin-dependent diabetes development in mice, and disease progression is delayed or prevented by administration of the NOS inhibitor aminoguanidine (133). Although the precise molecular events that trigger nitric oxide-induced β cell death are unknown, it is likely mediated by a combination of factors, including nitric oxide-dependent inhibition of mitochondrial metabolism and ATP generation, DNA damage, inhibition of protein synthesis, and the induction of ER stress (31, 47, 50, 68, 90, 103, 105, 117, 122). While there are a number of pathways involved in the β cell response to cytokines, the remainder of this review focuses on DNA damage and the pathways activated in response to this DNA damage that contribute to the regulation on β cell fate in response to cytokine treatment.
Nitric Oxide-Induced DNA Damage in β Cells
Nitric oxide-induced DNA damage and the role of DNA damage in cytokine-induced β cell death
Cytokines were first shown to induce islet cell DNA damage in a study by Delaney et al., who found that exposure of rat islets to IL-1 leads to the induction of DNA damage detected using the comet assay (47, 50). The DNA damage induced by IL-1 was completely prevented by inhibition of NOS (47, 50). Nitric oxide-induced DNA damage also takes place in human and rodent islet cells treated with cytokines or with nitric oxide donor compounds (46, 49, 50). Nitric oxide-induced DNA damage occurs in the form of oxidation and deamination of DNA bases, DNA strand breaks, or interstrand crosslinks (21, 134). Evidence suggests that DNA damage contributes to β cell death during IL-1 exposure (47, 50, 68, 103), as the induction of DNA damage in cytokine- and nitric oxide-treated β cells precedes cell lysis (50). While DNA damage contributes to β cell death, β cells also have a limited capacity to repair this damage (68, 117). Hughes et al. found that rat and human islets could repair cytokine-induced DNA damage for up to 24 h of exposure if nitric oxide production was inhibited using L-NG-monomethyl arginine, and the islets were cultured for 8 additional hours in the presence of the NOS inhibitor without removal of the cytokines (68). After 36 h of cytokine exposure, DNA damage becomes irreversible and apoptosis ensues, as evidenced by the activation of caspases (68, 125). Thus, when IL-1-induced DNA damage can no longer be repaired and β cells cannot recover, apoptotic pathways are activated (68).
Mechanisms by which β cells repair damaged DNA
Base excision repair (BER) appears to be a primary pathway used to repair cytokine- and nitric oxide-induced DNA damage in β cells (69, 125). In this pathway, growth arrest and DNA damage (GADD) 45α interacts with proliferating cell nuclear antigen, p21, polymerase beta, and apurinic/apyrimidinic endonuclease 1/redox factor 1 (69, 71). This complex then binds to damaged chromatin to facilitate BER (71). We have shown that GADD45α plays an essential role in the repair of damaged β cell DNA (69). In a nitric oxide-dependent manner, cytokines stimulate GADD45α mRNA accumulation, and siRNA knockdown of this factor inhibits the repair of nitric oxide-induced DNA damage in β cells (69). The signaling cascade by which nitric oxide induces GADD45α expression requires c-Jun N-terminal kinase (JNK) activation, as pharmacological inhibition of JNK prevents both GADD45α expression and DNA repair following nitric oxide exposure (69). These findings describe a protective role for JNK, contrary to several reports, suggesting that this mitogen-activated protein kinase promotes β cell apoptosis during cytokine exposure (1, 4, 18, 19, 60). JNK may play a dual role in the response to cytokines, regulating the induction of pathways leading to the repair of nitric oxide-induced damage, and, when this damage is no longer repairable, stimulating apoptosis.
The transcription factor forkhead box O1 (FOXO1) also participates in the repair of nitric oxide-induced DNA damage through regulation of GADD45α expression (70). Under basal conditions, FOXO1 is phosphorylated by Akt and sequestered in the cytosol (66). Nitric oxide decreases Akt activity as evidenced by decreased Akt and FOXO1 phosphorylation, allowing FOXO1 to translocate to the nucleus to control gene expression in β cells (70). Overexpression of nonfunctional mutants of FOXO1 results in an inhibition in nitric oxide-stimulated GADD45α expression and DNA repair in INS832/13 cells (70). The transcriptional activity of FOXO1 is controlled by the actions of sirtuins, a family of NAD+-dependent deacetylases. Inhibitors of SIRT1 attenuate the repair of damaged DNA, while the sirtuin activator resveratrol accelerates DNA repair in β cells (70). Consistent with the role of sirtuins in β cell protection, Lee et al. have shown that cytokine-induced RINm5F insulinoma cell and rat islet death is attenuated by SIRT1 overexpression and resveratrol treatment (82). While it is yet to be fully elucidated, it is likely that sirtuin activity regulates DNA repair in β cells by controlling the acetylation status of FOXO1 (70). When deacetylated, FOXO1 directs a transcriptional program that is associated with enhanced expression of free radical scavenging enzymes and DNA repair genes such as GADD45α (70). When in the acetylated state, FOXO1 instead directs a proapoptotic program that results in the expression of PUMA, phorbol-12-myristate-13-acetate-induced protein 1, and other factors that contribute to apoptotic cell death (70).
In addition to the pathways known to participate in the β cell response to nitric oxide-induced DNA damage, a number of known DNA repair pathways do not participate in the repair of cytokine-induced DNA damage in β cells. The tumor suppressor p53 is known to regulate GADD45α expression and stimulate BER pathways (111, 127), but in response to cytokines or nitric oxide, p53 expression is not stimulated (69). Furthermore, knockdown of p53 does not modify the β cell responses to cytokines nor does it affect DNA repair (69). Early studies suggested that cytokine-induced β cell death is mediated by protein poly(ADP-ribose) polymerase (PARP) overactivation due to peroxynitrite production in islets (20, 53). PARP is a component of the BER that is activated in response to DNA damage (88). Once active, PARP catalyzes the NAD+-dependent ADP-ribosylation of proteins near DNA damage to facilitate opening of damaged chromatin for repair (88). Overactivation of PARP results in the depletion of cellular levels of NAD+ and ATP leading to PARP-dependent necrosis (61). This process was proposed by Okamato in the 1980s to explain how β cells might be killed during T1D development (27, 141). However, PARP overactivation does not occur in cytokine-treated β cells and does not play a role in β cell death following exposure to nitric oxide (5, 97).
The DDR
The DDR is the collective network of signaling cascades that coordinate cellular responses to DNA damage (Fig. 3) (28). DSBs are the most severe type of DNA lesion and can arise from overlapping single-strand breaks, strand breaks generated during DNA repair or cell division, and can be induced by genotoxic agents (64). Following formation of a DSB, chromatin remodeling allows access of DDR sensor complexes, such as Mre11-Rad50-Nbs1 (MRN), ataxia telangiectasia, and Rad3-related protein (ATR)-interacting protein, or Ku70/80 heterodimers, to the site of the DNA lesion (23). Active sensor complexes recruit apical DDR kinases [e.g., ataxia telangiectasia mutated (ATM) by the MRN complex (81)] leading to DDR kinase autophosphorylation and activation (126). ATM is a primary DDR kinase that, when active, phosphorylates an array of substrates, estimated to include more than 1000 proteins (126). The fundamental objective of the DDR is to arrest cell cycle and promote pathways responsible for DNA repair (28). Under conditions where DNA repair fails or DNA damage is too extensive for repair, pathways that result in cellular senescence or programmed cell death are activated (116). Histone variant H2A.X (H2AX) is one DDR substrate that undergoes rapid phosphorylation within minutes of DSB lesion formation (109, 115). H2AX is phosphorylated by ATM (and by related kinases, ataxia telangiectasia and Rad3-related protein [ATR] or DNA-dependent protein kinase [DNA-PK]) on Ser139, and when H2AX is phosphorylated on this residue it is termed γH2AX (115). The phosphorylation of H2AX initiates a positive feedback loop, leading to the spreading and amplification of the γH2AX signal to promote recruitment and retention of downstream repair factors to the site of DNA strand breaks and to facilitate DNA repair (108, 115). It is due to the rapid and amplifying nature of γH2AX formation that this signaling event is regarded as one of the most sensitive markers of DDR activation and thus is commonly used as experimental evidence to indicate DDR activation and DSB formation (115, 118).
FIG. 3.
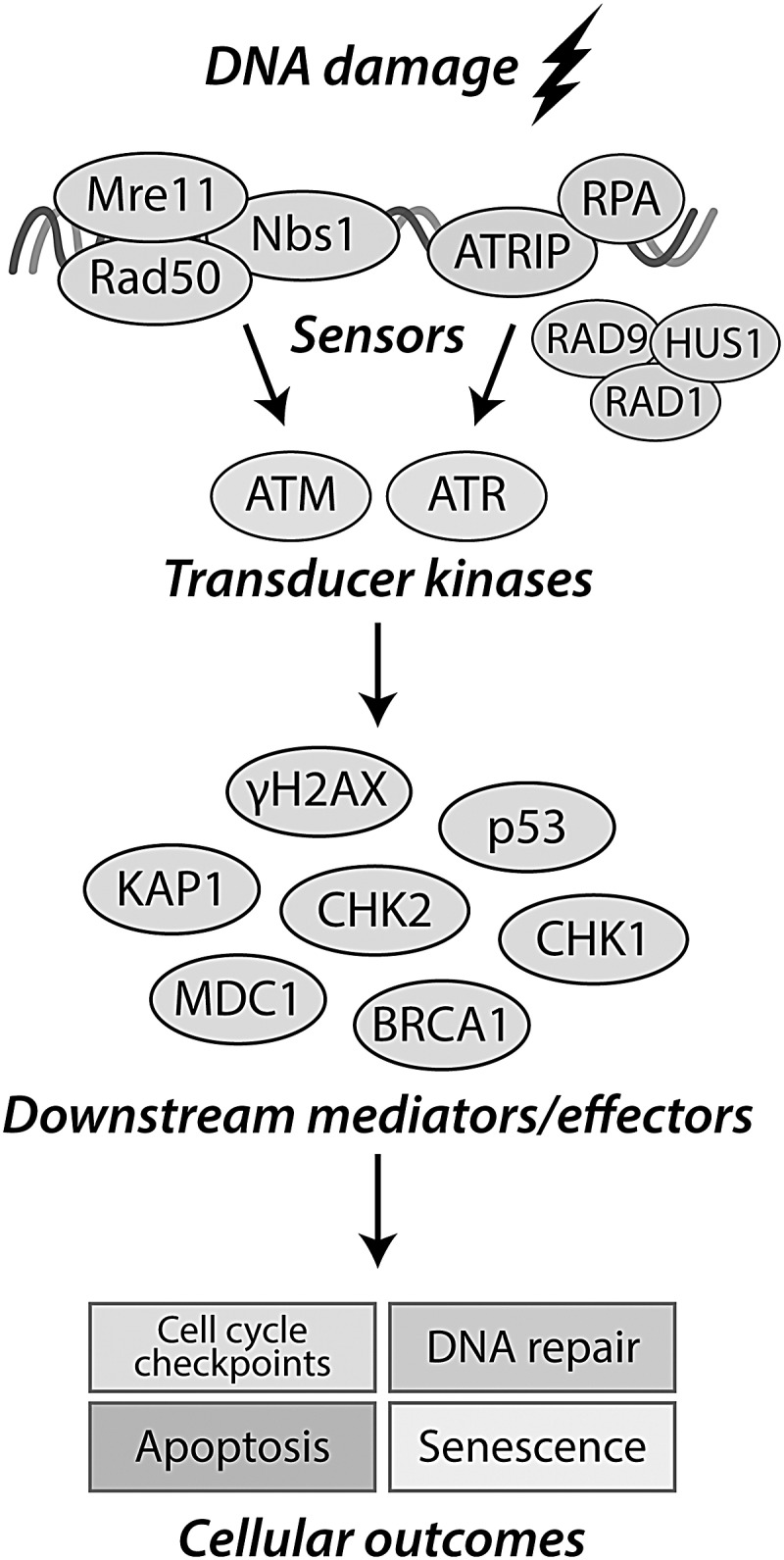
The DDR. DNA damage is detected by the DNA damage sensor complexes MRN (double-strand break detection) or by a complex comprising ATRIP and RPA (ssDNA associated with replication stress). The transducer kinases ATM, ATR, and DNA-PK are activated and localize to the site of the assembled sensor complexes. Activated ATM, ATR, and DNA-PK then phosphorylate many downstream mediators to promote a variety of cellular outcomes, including cell cycle arrest and activation of DNA repair mechanisms. If DNA damage is not able to be repaired, the DDR initiates programs promoting cell senescence or apoptosis. 53BP1, p53-binding protein 1; ATM, ataxia telangiectasia mutated; ATR, ataxia telangiectasia and Rad3-related protein; ATRIP, ataxia-telangiectasia-and-RAD3-related-ATR-interacting-protein; BRCA1, breast cancer type 1 susceptibility protein; CHK1/2, checkpoint kinase-1/2; DDR, DNA damage response; DNA-PK, DNA-dependent protein kinase; KAP1, KRAB-associated protein-1; MDC1, mediator of DNA damage checkpoint 1; MRN, MRE11-Rad50-Nbs1; RPA, replication protein A.
Potential significance of DDR proteins in diabetes
While few studies have examined the role of DDR in β cell, there is evidence from animal models suggesting that defects in DDR signaling may contribute to diabetes development. There is an increased incidence of diabetes in patients with ataxia telangiectasia, a disease caused by mutation in and subsequent loss of function of ATM (100). Miles et al. found that young ATM−/− mice had impaired insulin secretion before the onset of spontaneous diabetes, suggesting that ATM may be important for proper regulation of β cell insulin secretion (98). Schneider et al. found that a number of features of metabolic syndrome are more severe in mice heterozygous or deficient in ATM, although β cell function specifically was not examined in this study (124). Defects in ATM substrates have been associated with diabetes as well. Mice with a p53 Ser15 mutation, a site phosphorylated by multiple kinases, including ATM, were found to have impaired glucose tolerance and insulin resistance (7). Accumulation of DNA damage has been shown to lead to β cell death and spontaneous induction of diabetes due to the loss of insulin-producing β cells (136). Islets from mice deficient in DNA ligase IV, a crucial component of the nonhomologous end-joining pathway, show a progressive accumulation of DNA damage and accumulation of p53 and p21 (136). When these mice also contain a hypomorphic mutation in p53 that selectively prevents p53-dependent apoptosis, the accumulated DNA damage drives β cells into senescence, ultimately leading to a decrease in β cell mass and induction of diabetes (136).
Dual Role of Nitric Oxide in the Regulation of DDR
Nitric oxide-induced DNA damage and activation of DDR
While nitric oxide is not considered a direct inducer of DSBs, it is likely that single-strand breaks induced by nitric oxide accumulate over time and eventually lead to DSB formation when they are in close proximity to one another (21). Indeed, accumulation of γH2AX has been documented in several cell types exposed to nitric oxide (30, 101, 135, 142). In rat islets, IL-1 and IFNγ exposure leads to formation of γH2AX that is prevented by inhibitors of NOS, indicating that cytokines stimulate DSB formation in a nitric oxide-dependent manner (103). Cytokine-induced γH2AX formation occurs exclusively in insulin-containing cells and is not observed in other islet endocrine or nonendocrine cells (103), a finding consistent with β cells as the islet cellular source of iNOS in response to cytokine treatment (36, 40). In addition, nitric oxide donor compounds Diethylamine NONOate (DEA/NO) and Dipropylenetriamine NONOate (DPTA/NO) stimulate γH2AX in rat islets and insulinoma cell lines (103). ATM appears to be the primary kinase responsible for the formation of γH2AX in nitric oxide-treated β cells (103). Pharmacological inhibition and siRNA knockdown of ATM attenuate nitric oxide-induced γH2AX (103), and islets isolated from ATM−/−mice do not accumulate γH2AX in response to a nitric oxide donor (103). These findings are consistent with other studies reporting ATM activation following nitric oxide exposure (56, 65, 95, 135, 137). Despite the classical role for ATM in DNA repair, β cells do not require this kinase for the repair of nitric oxide-induced DNA damage (103). Cytokine-induced DNA damage in rat islets is repaired in the presence of an ATM inhibitor, and ATM inhibition does not modify JNK activation or GADD45α expression in response to nitric oxide (103). As described above, JNK and GADD45α are two factors required for the repair of nitric oxide-induced DNA damage in β cell (69).
Several observations indicate that the primary role of ATM in cytokine-treated β cells is the activation of apoptotic pathways. In cytokine-treated islets, ATM activation (as measured by γH2AX formation) is a late event with maximal activation occurring following exposure lengths of 36 h or longer (103). Incubation of islets for 36 h or longer with IL-1 results in the irreversible inhibition of oxidative metabolism, insulin secretion, and DNA damage, correlating with caspase-3 cleavage activation (103). ATM inhibitors prevent cytokine-induced caspase-3 cleavage following this 36-h exposure to IL-1 (103). Furthermore, the pan-nuclear localization pattern of γH2AX, observed in cytokine-treated β cells following 36-h exposure (103), has been reported to occur during apoptosis and functions as a preapoptotic signal (44). These findings suggest that DSB formation in cytokine-treated β cells may function as an initiating event committing β cells to apoptotic cell death. Temporally, γH2AX formation is a late event that occurs when the inhibition of islet function and DNA damage become irreversible (Fig. 2) (103). While these findings describe a role for ATM in the regulation of cytokine-induced apoptosis, the pathways activated downstream of ATM that mediate this apoptotic signaling are unknown. The tumor suppressor protein p53 has been shown to mediate ATM-regulated apoptosis p53 (116); however, it is not likely that the p53-dependent pathway participates in cytokine-induced β cell apoptosis. The ATM-dependent phosphorylation of p53 at Ser15 in response to DNA damage (26) is considered a priming modification that promotes the proapoptotic signaling of p53 (138). Despite the presence of DNA damage and stabilization of total p53, Ser15 is not phosphorylated in insulinoma cell lines or rat islets during cytokine exposure (69). Also, cytokine-induced caspase-3 cleavage and β cell death can occur under these conditions in the absence of p53 phosphorylation (69). Thus, ATM-dependent apoptosis in a cytokine-treated β cell likely occurs via a process independent of p53 activation.
Nitric oxide as an inhibitor of the DDR
Although DNA damage in response to nitric oxide is sufficient to lead to DSB formation and DDR activation in β cells, we have recently shown that nitric oxide, when present at micromolar levels, is an effective inhibitor of the DDR (Fig. 4) (102). The phosphorylation of H2AX, p53, and the ATM substrate KRAB-associated protein-1 (KAP1) in rat islets and β cell lines treated with genotoxic agents such as camptothecin or hydrogen peroxide is prevented by nitric oxide supplied by chemical donors or produced endogenously following cytokine-induced iNOS expression (102). These findings temporally dissociate nitric oxide-induced DNA damage from DDR activation, raising the possibility that the production of nitric oxide by β cells may serve to inhibit DDR signaling and attenuate DDR-induced apoptosis (102). This interpretation is consistent with our observations that there is a sixfold decrease in the rates of IL-1-induced nitric oxide production by β cells between 24 and 36 h of incubation, such that when β cells are making micromolar levels of nitric oxide (24-h IL-1 exposure), the damaging actions of this free radical are reversible (68). In contrast, when nitric oxide production is diminished (after 36-h IL-1 exposure), islet function is irreversibly damage and the β cells are committed to death by apoptosis (68). Even though DDR signaling is inhibited by nitric oxide, the extent of DNA damage is unaffected, indicating that nitric oxide does not prevent induction of DNA damage but uncouples the signaling response from the damage (102).
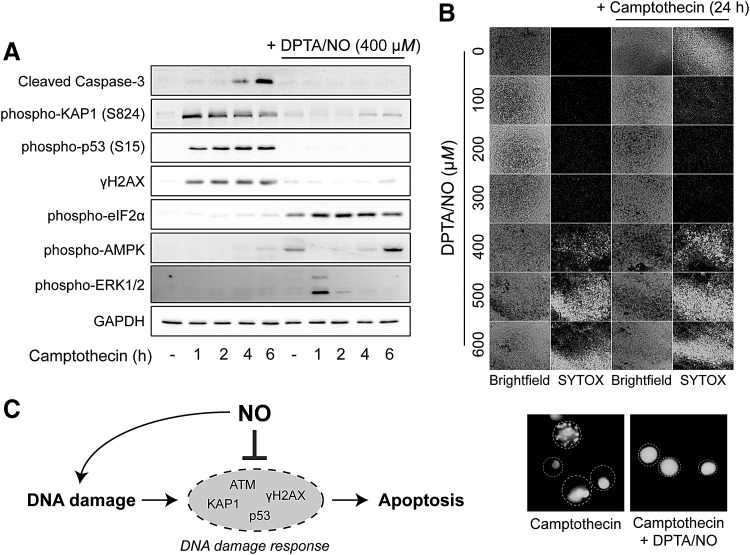
FIG. 4.
Nitric oxide prevents camptothecin-induced cell death. (A) Camptothecin-treatment of INS 832/13 cells leads to the rapid phosphorylation of DDR substrates KAP1, p53, and H2AX and caspase-3 cleavage at later time points. In the presence of the nitric oxide donor DPTA/NO, the activation of these signaling processes is prevented. (B) Camptothecin-induced cell death (measured by SYTOX fluorescence) following camptothecin treatment is prevented by DPTA/NO in a concentration-dependent manner, with maximal protection afforded at 300 μM. Nitric oxide alone becomes toxic at higher donor concentrations. Morphology of SYTOX-positive cells in camptothecin ± DPTA/NO (400 μM) conditions is shown in 40 × fluorescent images in the lower portion of (B), showing the loss of morphological changes consistent with apoptosis in the presence of DPTA/NO. Cell borders are denoted by the dashed circles. (C). Schematic depicting the dual role of nitric oxide in the regulation of DDR in β cells. Reprinted with permission from Oleson et al. (102). AMPK, AMP-activated protein kinase; DPTA/NO, Dipropylenetriamine NONOate.
The inhibitory actions of nitric oxide on DDR signaling appear to be restricted to the DSB response. Under conditions in which nitric oxide attenuates the phosphorylation of multiple ATM substrates, including H2AX, p53, and KAP1, nitric oxide-stimulated phosphorylation of eukaryotic translation initiation factor 2 alpha, AMP-activated protein kinase (AMPK), and extracellular signal-regulated kinases 1/2 is not effected (102). These findings suggest that the inhibitory actions of nitric oxide are selective for DDR signaling, and are not a consequence of reduced cell viability or global attenuation in cell signaling (102). In addition to ATM substrates, nitric oxide also inhibits signaling from other phosphatidylinositol 3-kinase-related kinase signaling cascades that include Akt phosphorylation in β cells (70), and the ATR substrate checkpoint kinase 1 in β cells treated with the replication stress inducer hydroxyurea (BJO and JAC, unpublished observations). In addition, the ability of nitric oxide, but not ATM inhibitors, to completely prevent H2AX phosphorylation in response to camptothecin indicates that kinases in addition to ATM are activated under these conditions, and that nitric oxide can suppress signaling from these kinases (BJO and JAC, unpublished observation). These findings indicate that nitric oxide has broad inhibitory effects on signaling from ATM, ATR, and DNA-PK in the DSB response.
Inhibition of the DDR by nitric oxide is a protective response that attenuates DNA damage-dependent apoptotic signaling in β cells (102). Camptothecin, a topoisomerase inhibitor that induces apoptotic cell death through the induction of DSBs (113, 128), induces a rapid activation of the DDR that is followed by caspase activation and β cell death after 6–12 h of exposure. Nitric oxide not only inhibits the rapid, initial DDR signaling but also attenuates downstream caspase-3 cleavage and β cell death resulting from DNA damage [Fig. 4, (102)]. Importantly, camptothecin induces morphological changes that are consistent with β cell apoptosis, including condensation of nuclei and formation of apoptotic bodies (77). While nitric oxide attenuates the development of these morphological changes consistent with apoptosis, DNA damage remains and the morphology of these cells appears to be more consistent with necrosis [(77), Fig. 4B, lower]. This protective action of nitric oxide appears to be selective for apoptosis resulting from DNA damage, as PARP-dependent β cell death in response to hydrogen peroxide exposure is not modified in the presence of nitric oxide (102). In addition to other antiapoptotic actions of nitric oxide, such as direct suppression of caspase activity by S-nitrosation (75, 85, 99), these exciting findings describe a new mechanism by which nitric oxide can attenuate apoptosis through inhibition of DDR activation (102).
β cell selectivity of nitric oxide-induced DDR inhibition
The ability of nitric oxide to suppress DDR signaling does not occur in all cell types, and to date has only been observed in pancreatic β cells (102). Nitric oxide does not inhibit camptothecin-induced p53, KAP1, and H2AX phosphorylation in RAW264.7 macrophages, mouse embryonic fibroblasts, HepG2 hepatocytes, HEK293 cells, or SH-SY5Y neuroblastoma cells [(102) and unpublished observations]. Given that β cells are terminally differentiated with a limited capacity to divide (41), it is tempting to speculate that DNA damage may be an ideal mechanism to control the β cell response to inflammation, such that when DNA damage is too extensive and DSB formation occurs, DDR-dependent apoptosis is triggered. Under these conditions, nitric oxide affords protection to β cells by activating pathways that promote repair of damage (e.g., GADD45α for damaged DNA) and to limit DDR activation and thereby attenuate induction of an ATM- and caspase-dependent proapoptotic cascade (103). If DNA damage is too extensive and nitric oxide production diminishes, ATM becomes active and triggers an apoptotic cascade. In this context, the response of β cells to cytokines in vivo may be protective. Similar to DNA damage, nitric oxide inhibits insulin secretion and oxidative metabolism, while also activating protective pathways to repair this damage. However, when damage is too extensive and nitric oxide is no longer produced at levels sufficient to attenuate apoptotic signaling via the DDR, the DDR-induced apoptotic cascade is activated to remove the damaged β cell by apoptosis, potentially avoiding islet inflammation and thereby protecting remaining β cells in islet from further damage (Fig. 5).
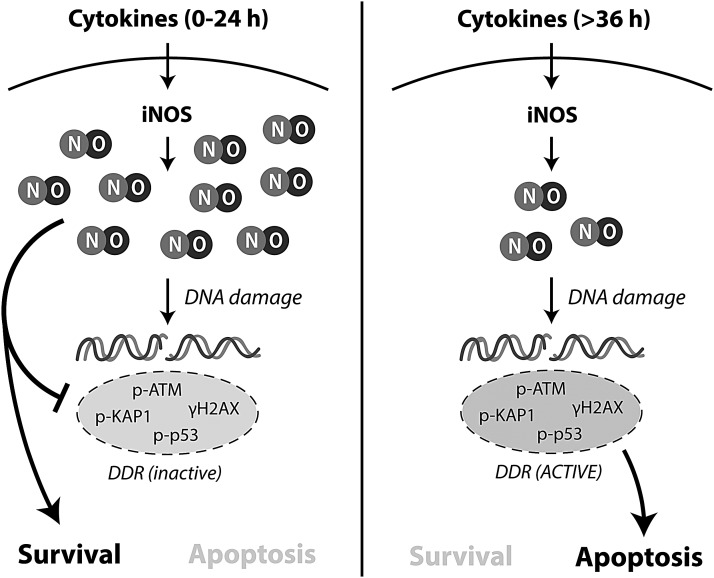
FIG. 5.
Proposed model for the dual regulation of DDR by nitric oxide during cytokine exposure. During short exposure to cytokines (0–24 h), the high rates of nitric oxide production suppress DDR signaling despite causing DNA damage. Under these conditions, the DDR cannot initiate apoptosis and β cells are able to recover and survive. When cytokine exposure lengthens (>36 h) and cellular damage increases, the rates of nitric oxide production decrease by sixfold and allow for DDR activation and DDR-dependent apoptosis. iNOS, isoform of nitric oxide synthase.
β cell resistance to peroxynitrite
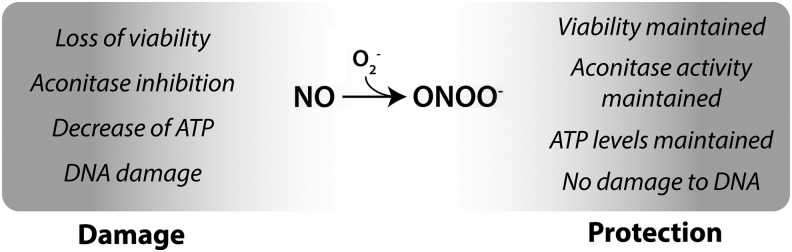
While the damaging effects of cytokines are clearly dependent on iNOS activity and nitric oxide production, there has been much debate on the identity, or chemical form, of the reactive nitrogen species responsible for cytokine-induced β cell damage (13, 15, 57, 80, 83, 131). Many consider peroxynitrite, a highly reactive product of the diffusion-controlled reaction of nitric oxide and superoxide, to be the reactive species responsible for mediating the damage in cells generating nitric oxide, including β cells (21, 80, 131, 132). Furthermore, β cells have been regarded as being particularly susceptible to reactive oxygen and nitrogen species due to the relatively low levels of antioxidant enzymes superoxide dismutase, glutathione peroxidase, and catalase when compared with the levels expressed in the liver (83,84). Recently, we have shown that β cells are markedly resistant to peroxynitrite, and instead of inducing damage through the production of peroxynitrite, superoxide scavenges nitric oxide and protects against nitric oxide-mediated damage [Fig. 6, (13)]. Broniowska et al. showed that peroxynitrite is not generated in cytokine-treated β cells due to an absence of superoxide production (13, 15). When chemically produced in β cells, using nitric oxide and superoxide generating systems, or when supplied using the donor SIN-1, peroxynitrite does not induce β cell damage (13, 15), even though the same conditions are highly toxic to other cell types such as endothelial cells (12). In β cells, superoxide effectively neutralizes nitric oxide and thereby prevents the inhibitory effects of nitric oxide on aconitase activity, the reductions in ATP levels, and the loss of cell viability (13, 15). Superoxide also attenuates the inhibitory actions of nitric oxide on the DDR and the protective actions of nitric oxide on DNA damage-induced apoptosis (102). Collectively, these findings challenge a number of hypotheses regarding reactive oxygen and nitrogen species and cytokine-induced β cell damage. First, β cells do not produce superoxide when treated with cytokines and as such cytokine induced damage cannot be attributed to the formation of this radical (13). Second, β cells are resistant to peroxynitrite, and peroxynitrite formation in β cells is associated with a loss of the inhibitory effects of nitric oxide (13, 15). Third, while nitric oxide inhibits oxidative metabolism and induces DNA damage, it also stimulates repair pathways that are associated with reconstitution of oxidative metabolism (AMPK) and repair of DNA damage (JNK and GADD45α) (69, 96). Taken together, the resistance to peroxynitrite, the lack of superoxide production, and the ability of superoxide to scavenge nitric oxide and modify nitric oxide signaling suggest that β cells have developed pathways to limit toxicity to reactive species other than nitric oxide.
FIG. 6.
Peroxynitrite formation in β cells and protection from nitric oxide. β cells do not generate superoxide (O2−) and thus peroxynitrite (ONOO−) during cytokine treatment. Chemical generation of superoxide leads to the scavenging of nitric oxide, formation of peroxynitrite, and loss of nitric oxide-dependent effects. Thus, β cells are resistant to peroxynitrite, and under conditions where peroxynitrite is formed, β cells are protected from nitric oxide-induced damage. ATP, adenosine triphosphate.
Is It Time to Reconsider the Role of Nitric Oxide in Cytokine-Mediated β Cell Damage?
Protective actions of nitric oxide in β cells
Since the initial studies showing that nitric oxide mediates cytokine-induced β cell damage, the generation of this free radical during cytokine exposure has been thought of as a pathway that causes β cell damage (Fig. 2). Conversely, nitric oxide has many protective functions that promote β cell health and survival. These pathways include the following: (i) AMPK, which functions to augment mitochondrial oxidative metabolism (69, 96), (ii) JNK, which is required for the recovery of aconitase activity and the expression of DNA repair gene GADD45α (69, 123), (iii) peroxisome proliferator-activated receptor gamma coactivator-1α expression, which promotes the expression of enzymes involved in mitochondrial oxidative metabolism (74, 96), (iv) unfolded protein response activation, a protective pathway designed to resolve and limit ER stress (25), and (v) the induction of the heat shock response, which limits cytokine signaling when active (89, 121, 139). Nitric oxide is also an effective inhibitor of caspase activity through direct S-nitrosation of the active site cysteine (75, 85, 99), and suppresses ATM activation and thereby limits ATM-dependent β cell apoptosis in response to DNA damage (68, 102). These findings suggest that nitric oxide functions to enhance protective pathways leading to restoration of metabolic function and insulin secretion, while at the same time opposing apoptotic signaling to delay and attenuate β cell death during cytokine exposure.
Why do β cells respond to cytokines with the production of nitric oxide?
When considering that the actions of cytokines on islets are selective for β cells (8, 36), and that the product of IL-1 (or IL-1 + IFNγ in mouse and human islets) actions includes iNOS expression and nitric oxide production, it is tempting to speculate on why β cells produce nitric oxide in response to cytokines. Indeed, other endocrine cells in islets do not respond to IL-1 and do not generate nitric oxide; it is only the β cell that responds to cytokines such as IL-1 and this results in the generation of micromolar levels of nitric oxide (33). Since β cells are terminally differentiated with a limited capacity to regenerate (41), the ability of β cells to respond to IL-1 and produce high levels of nitric oxide likely serves a physiological purpose. Could it be that the damage associated with the generation of nitric oxide following cytokine stimulation is collateral and a consequence of the activation of protective pathways that are designed to limit damage from more serious threats, such as infection with a pathogen? Indeed, several studies have shown that nitric oxide attenuates the ability of pathogens, such as viruses, to replicate (42, 51, 73, 93, 120). Few studies have examined the role of IL-1 and nitric oxide in the response of β cells to a viral infection where IL-1 production in islet would be anticipated. However, under this type of condition, nitric oxide produced in islets may cause temporary inhibition of β cell function and cellular damage, but may also provide a beneficial and protective function by maintaining the viability of β cells in the infected islet. It is possible that prolonged elevation of IL-1 levels for multiple days may result in direct β cell damage due to extended production of nitric oxide and diabetes could develop [as evidenced in mice expressing iNOS under control of the insulin promoter, (133)], although this is an extreme case. Under most infection conditions, the ability of β cells to respond to cytokines likely plays a physiologically relevant role in host defense and metabolic control.
Conclusions
This review highlights the damaging and protective actions of nitric oxide in the β cell. This free radical is produced by cytokine-treated β cells in all species tested to date (105). While there has been speculation that human β cells respond differently to cytokines than rodent islets, many reports have shown similar response with the only difference being the concentrations of cytokines required to stimulate iNOS by β cells (105). Nitric oxide is the primary mediator of cytokine-induced changes in gene expression, protein synthesis, oxidative metabolism, DNA damage, and ER stress in β cells (14). While many of these responses have been described as damaging, nitric oxide plays numerous protective roles, and the ability of nitric oxide to inhibit DDR-dependent apoptotic pathways in response to DNA damage highlighted in this review is one example. Based on these protective responses, it may be time to rethink the role of cytokines as potential mediators of β cell damage in the context of diabetes development, and begin to consider the physiological roles played by β cells when they respond to cytokines to produce nitric oxide. We look forward to continuing to identify and characterize the mechanisms by which nitric oxide controls pathways that limit damage and protect β cells from damaging insults.
Abbreviations Used
- AMPK
AMP-activated protein kinase
- ATM
ataxia telangiectasia mutated
- ATP
adenosine triphosphate
- ATR
ataxia telangiectasia and Rad3-related protein
- ATRIP
ataxia telangiectasia-and-RAD3-related-ATR-interacting protein
- Bad
Bcl-2-associated death promoter
- BER
base excision repair
- Bim
Bcl-2-like protein 11
- CHK1
checkpoint kinase-1
- DDR
DNA damage response
- DEA/NO
Diethylamine NONOate
- DNA-PK
DNA-dependent protein kinase
- DPTA/NO
Dipropylenetriamine NONOate
- DSB
double-strand break
- ER
endoplasmic reticulum
- FOXO1
forkhead box O1
- GADD45α
growth arrest and DNA damage 45α
- H2AX
histone H2A.X
- IFNγ
interferon-γ
- IL-1
interleukin-1
- iNOS
isoform of nitric oxide synthase
- JNK
c-Jun N-terminal kinase
- KAP1
KRAB-associated protein-1
- MRN
MRE11-Rad50-Nbs1
- NF-κB
nuclear factor kappa B
- NOS
nitric oxide synthase
- PARP
poly ADP-ribose polymerase
- PUMA
p53-upregulated modulator of apoptosis
- RPA
replication protein A
- T1D
type-1 diabetes
- TNFα
tumor necrosis factor-α
References
- 1.Abdelli S, Abderrahmani A, Hering BJ, Beckmann JS, and Bonny C. The c-Jun N-terminal kinase JNK participates in cytokine- and isolation stress-induced rat pancreatic islet apoptosis. Diabetologia 50: 1660–1669, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Adeghate E. and Parvez SH. Nitric oxide and neuronal and pancreatic beta cell death. Toxicology 153: 143–156, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Allagnat F, Fukaya M, Nogueira TC, Delaroche D, Welsh N, Marselli L, Marchetti P, Haefliger JA, Eizirik DL, and Cardozo AK. C/EBP homologous protein contributes to cytokine-induced pro-inflammatory responses and apoptosis in beta-cells. Cell Death Differ 19: 1836–1846, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ammendrup A, Maillard A, Nielsen K, Aabenhus Andersen N, Serup P, Dragsbaek Madsen O, Mandrup-Poulsen T, and Bonny C. The c-Jun amino-terminal kinase pathway is preferentially activated by interleukin-1 and controls apoptosis in differentiating pancreatic beta-cells. Diabetes 49: 1468–1476, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Andreone T, Meares GP, Hughes KJ, Hansen PA, and Corbett JA. Cytokine-mediated beta-cell damage in PARP-1-deficient islets. Am J Physiol Endocrinol Metab 303: E172–E179, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anquetil F, Sabouri S, Thivolet C, Rodriguez-Calvo T, Zapardiel-Gonzalo J, Amirian N, Schneider D, Castillo E, Lajevardi Y, and von Herrath MG. Alpha cells, the main source of IL-1beta in human pancreas. J Autoimmun 81: 68–73, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armata HL, Golebiowski D, Jung DY, Ko HJ, Kim JK, and Sluss HK. Requirement of the ATM/p53 tumor suppressor pathway for glucose homeostasis. Mol Cell Biol 30: 5787–5794, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnush M, Heitmeier MR, Scarim AL, Marino MH, Manning PT, and Corbett JA. IL-1 produced and released endogenously within human islets inhibits beta cell function. J Clin Invest 102: 516–526, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arnush M, Scarim AL, Heitmeier MR, Kelly CB, and Corbett JA. Potential role of resident islet macrophage activation in the initiation of autoimmune diabetes. J Immunol 160: 2684–2691, 1998 [PubMed] [Google Scholar]
- 10.Bedoya FJ, Salguero-Aranda C, Cahuana GM, Tapia-Limonchi R, Soria B, and Tejedo JR. Regulation of pancreatic beta-cell survival by nitric oxide: clinical relevance. Islets 4: 108–118, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Bendtzen K, Mandrup-Poulsen T, Nerup J, Nielsen JH, Dinarello CA, and Svenson M. Cytotoxicity of human pI 7 interleukin-1 for pancreatic islets of Langerhans. Science 232: 1545–1547, 1986 [DOI] [PubMed] [Google Scholar]
- 12.Broniowska KA, Diers AR, Corbett JA, and Hogg N. Effect of nitric oxide on naphthoquinone toxicity in endothelial cells: role of bioenergetic dysfunction and poly (ADP-ribose) polymerase activation. Biochemistry 52: 4364–4372, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Broniowska KA, Mathews CE, and Corbett JA. Do beta-cells generate peroxynitrite in response to cytokine treatment? J Biol Chem 288: 36567–36578, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Broniowska KA, Oleson BJ, and Corbett JA. beta-Cell responses to nitric oxide. Vitam Horm 95: 299–322, 2014 [DOI] [PubMed] [Google Scholar]
- 15.Broniowska KA, Oleson BJ, McGraw J, Naatz A, Mathews CE, and Corbett JA. How the location of superoxide generation influences the beta-cell response to nitric oxide. J Biol Chem 290: 7952–7960, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown GC. and Borutaite V. Inhibition of mitochondrial respiratory complex I by nitric oxide, peroxynitrite and S-nitrosothiols. Biochim Biophys Acta 1658: 44–49, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Brown GC. and Cooper CE. Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase. FEBS Lett 356: 295–298, 1994 [DOI] [PubMed] [Google Scholar]
- 18.Brozzi F, Gerlo S, Grieco FA, Nardelli TR, Lievens S, Gysemans C, Marselli L, Marchetti P, Mathieu C, Tavernier J, and Eizirik DL. A combined “omics” approach identifies N-Myc interactor as a novel cytokine-induced regulator of IRE1 protein and c-Jun N-terminal kinase in pancreatic beta cells. J Biol Chem 289: 20677–20693, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brozzi F, Nardelli TR, Lopes M, Millard I, Barthson J, Igoillo-Esteve M, Grieco FA, Villate O, Oliveira JM, Casimir M, Bugliani M, Engin F, Hotamisligil GS, Marchetti P, and Eizirik DL. Cytokines induce endoplasmic reticulum stress in human, rat and mouse beta cells via different mechanisms. Diabetologia 58: 2307–2316, 2015 [DOI] [PubMed] [Google Scholar]
- 20.Burkart V, Wang ZQ, Radons J, Heller B, Herceg Z, Stingl L, Wagner EF, and Kolb H. Mice lacking the poly(ADP-ribose) polymerase gene are resistant to pancreatic beta-cell destruction and diabetes development induced by streptozocin. Nature Medicine 5: 314–319, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Burney S, Caulfield JL, Niles JC, Wishnok JS, and Tannenbaum SR. The chemistry of DNA damage from nitric oxide and peroxynitrite. Mutat Res 424: 37–49, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Cavallo MG, Baroni MG, Toto A, Gearing AJ, Forsey T, Andreani D, Thorpe R, and Pozzilli P. Viral infection induces cytokine release by beta islet cells. Immunology 75: 664–668, 1992 [PMC free article] [PubMed] [Google Scholar]
- 23.Ceccaldi R, Rondinelli B, and D'Andrea AD. Repair pathway choices and Consequences at the double-strand break. Trends Cell Biol 26: 52–64, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cetkovic-Cvrlje M. and Eizirik DL. TNF-alpha and IFN-gamma potentiate the deleterious effects of IL-1 beta on mouse pancreatic islets mainly via generation of nitric oxide. Cytokine 6: 399–406, 1994 [DOI] [PubMed] [Google Scholar]
- 25.Chambers KT, Unverferth JA, Weber SM, Wek RC, Urano F, and Corbett JA. The role of nitric oxide and the unfolded protein response in cytokine-induced beta-cell death. Diabetes 57: 124–132, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Chao C, Saito S, Anderson CW, Appella E, and Xu Y. Phosphorylation of murine p53 at ser-18 regulates the p53 responses to DNA damage. Proc Natl Acad Sci U S A 97: 11936–11941, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Charron MJ. and Bonner-Weir S. Implicating PARP and NAD+ depletion in type I diabetes. Nat Med 5: 269–270, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Ciccia A. and Elledge SJ. The DNA damage response: making it safe to play with knives. Mol Cell 40: 179–204, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cleeter MW, Cooper JM, Darley-Usmar VM, Moncada S, and Schapira AH. Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide. Implications for neurodegenerative diseases. FEBS Lett 345: 50–54, 1994 [DOI] [PubMed] [Google Scholar]
- 30.Clemons NJ, McColl KE, and Fitzgerald RC. Nitric oxide and acid induce double-strand DNA breaks in Barrett's esophagus carcinogenesis via distinct mechanisms. Gastroenterology 133: 1198–1209, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Cnop M, Welsh N, Jonas JC, Jorns A, Lenzen S, and Eizirik DL. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes 54 Suppl 2: S97–S107, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Comens PG, Wolf BA, Unanue ER, Lacy PE, and McDaniel ML. Interleukin 1 is potent modulator of insulin secretion from isolated rat islets of Langerhans. Diabetes 36: 963–970, 1987 [DOI] [PubMed] [Google Scholar]
- 33.Corbett JA, Kwon G, Misko TP, Rodi CP, and McDaniel ML. Tyrosine kinase involvement in IL-1 beta-induced expression of iNOS by beta-cells purified from islets of Langerhans. Am J Physiol 267: C48–C54, 1994 [DOI] [PubMed] [Google Scholar]
- 34.Corbett JA, Lancaster JR, Jr., Sweetland MA, and McDaniel ML. Interleukin-1 beta-induced formation of EPR-detectable iron-nitrosyl complexes in islets of Langerhans. Role of nitric oxide in interleukin-1 beta-induced inhibition of insulin secretion. J Biol Chem 266: 21351–21354, 1991 [PubMed] [Google Scholar]
- 35.Corbett JA. and McDaniel ML. Does nitric oxide mediate autoimmune destruction of beta-cells? Possible therapeutic interventions in IDDM. Diabetes 41: 897–903, 1992 [DOI] [PubMed] [Google Scholar]
- 36.Corbett JA. and McDaniel ML. Intraislet release of interleukin 1 inhibits beta cell function by inducing beta cell expression of inducible nitric oxide synthase. J Exp Med 181: 559–568, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Corbett JA. and McDaniel ML. Reversibility of interleukin-1 beta-induced islet destruction and dysfunction by the inhibition of nitric oxide synthase. Biochem J 299: 719–724, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corbett JA, Sweetland MA, Wang JL, Lancaster JR, Jr., and McDaniel ML. Nitric oxide mediates cytokine-induced inhibition of insulin secretion by human islets of Langerhans. Proc Natl Acad Sci U S A 90: 1731–1735, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Corbett JA, Wang JL, Hughes JH, Wolf BA, Sweetland MA, Lancaster JR, Jr., and McDaniel ML. Nitric oxide and cyclic GMP formation induced by interleukin 1 beta in islets of Langerhans. Evidence for an effector role of nitric oxide in islet dysfunction. Biochem J 287 (Pt 1): 229–235, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Corbett JA, Wang JL, Sweetland MA, Lancaster JR, Jr., and McDaniel ML. Interleukin 1 beta induces the formation of nitric oxide by beta-cells purified from rodent islets of Langerhans. Evidence for the beta-cell as a source and site of action of nitric oxide. J Clin Invest 90: 2384–2391, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cozar-Castellano I, Fiaschi-Taesch N, Bigatel TA, Takane KK, Garcia-Ocana A, Vasavada R, and Stewart AF. Molecular control of cell cycle progression in the pancreatic beta-cell. Endocr Rev 27: 356–370, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Croen KD. Evidence for antiviral effect of nitric oxide. Inhibition of herpes simplex virus type 1 replication. J Clin Invest 91: 2446–2452, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cunningham JM, Mabley JG, Delaney CA, and Green IC. The effect of nitric oxide donors on insulin secretion, cyclic GMP and cyclic AMP in rat islets of Langerhans and the insulin-secreting cell lines HIT-T15 and RINm5F. Mol Cell Endocrinol 102: 23–29, 1994 [DOI] [PubMed] [Google Scholar]
- 44.de Feraudy S, Revet I, Bezrookove V, Feeney L, and Cleaver JE. A minority of foci or pan-nuclear apoptotic staining of gammaH2AX in the S phase after UV damage contain DNA double-strand breaks. Proc Natl Acad Sci U S A 107: 6870–6875, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Delaney CA. and Eizirik DL. Intracellular targets for nitric oxide toxicity to pancreatic beta-cells. Braz J Med Biol Res 29: 569–579, 1996 [PubMed] [Google Scholar]
- 46.Delaney CA, Green IC, Lowe JE, Cunningham JM, Butler AR, Renton L, D'Costa I, and Green MH. Use of the comet assay to investigate possible interactions of nitric oxide and reactive oxygen species in the induction of DNA damage and inhibition of function in an insulin-secreting cell line. Mutat Res 375: 137–146, 1997 [DOI] [PubMed] [Google Scholar]
- 47.Delaney CA, Green MH, Lowe JE, and Green IC. Endogenous nitric oxide induced by interleukin-1 beta in rat islets of Langerhans and HIT-T15 cells causes significant DNA damage as measured by the “comet” assay. FEBS Lett 333, 29 1–295, 1993 [DOI] [PubMed] [Google Scholar]
- 48.Eizirik DL. Interleukin-1 induced impairment in pancreatic islet oxidative metabolism of glucose is potentiated by tumor necrosis factor. Acta Endocrinol (Copenh) 119: 321–325, 1988 [DOI] [PubMed] [Google Scholar]
- 49.Eizirik DL, Delaney CA, Green MH, Cunningham JM, Thorpe JR, Pipeleers DG, Hellerstrom C, and Green IC. Nitric oxide donors decrease the function and survival of human pancreatic islets. Mol Cell Endocrinol 118: 71–83, 1996 [DOI] [PubMed] [Google Scholar]
- 50.Fehsel K, Jalowy A, Qi S, Burkart V, Hartmann B, and Kolb H. Islet cell DNA is a target of inflammatory attack by nitric oxide. Diabetes 42: 496–500, 1993 [DOI] [PubMed] [Google Scholar]
- 51.Flodstrom M, Horwitz MS, Maday A, Balakrishna D, Rodriguez E, and Sarvetnick N. A critical role for inducible nitric oxide synthase in host survival following coxsackievirus B4 infection. Virology 281: 205–215, 2001 [DOI] [PubMed] [Google Scholar]
- 52.Flodstrom M, Welsh N, and Eizirik DL. Cytokines activate the nuclear factor kappa B (NF-kappa B) and induce nitric oxide production in human pancreatic islets. FEBS Lett 385: 4–6, 1996 [DOI] [PubMed] [Google Scholar]
- 53.Gale EA. Molecular mechanisms of beta-cell destruction in IDDM: the role of nicotinamide. Horm Res 45 Suppl 1: 39–43, 1996 [PubMed] [Google Scholar]
- 54.Gardner PR, Costantino G, Szabo C, and Salzman AL. Nitric oxide sensitivity of the aconitases. J Biol Chem 272: 25071–25076, 1997 [DOI] [PubMed] [Google Scholar]
- 55.Gepts W. Pathologic anatomy of the pancreas in juvenile diabetes mellitus. Diabetes 14: 619–633, 1965 [DOI] [PubMed] [Google Scholar]
- 56.Goodman JE, Hofseth LJ, Hussain SP, and Harris CC. Nitric oxide and p53 in cancer-prone chronic inflammation and oxyradical overload disease. Environ Mol Mutagen 44: 3–9, 2004 [DOI] [PubMed] [Google Scholar]
- 57.Gurgul-Convey E, Mehmeti I, Lortz S, and Lenzen S. Cytokine toxicity in insulin-producing cells is mediated by nitro-oxidative stress-induced hydroxyl radical formation in mitochondria. J Mol Med (Berlin, Germany) 89: 785–798, 2011 [DOI] [PubMed] [Google Scholar]
- 58.Gurzov EN. and Eizirik DL. Bcl-2 proteins in diabetes: mitochondrial pathways of beta-cell death and dysfunction. Trends Cell Biol 21: 424–431, 2011 [DOI] [PubMed] [Google Scholar]
- 59.Gurzov EN, Germano CM, Cunha DA, Ortis F, Vanderwinden JM, Marchetti P, Zhang L, and Eizirik DL. p53 up-regulated modulator of apoptosis (PUMA) activation contributes to pancreatic beta-cell apoptosis induced by proinflammatory cytokines and endoplasmic reticulum stress. J Biol Chem 285: 19910–19920, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gurzov EN, Ortis F, Cunha DA, Gosset G, Li M, Cardozo AK, and Eizirik DL. Signaling by IL-1beta+IFN-gamma and ER stress converge on DP5/Hrk activation: a novel mechanism for pancreatic beta-cell apoptosis. Cell Death Differ 16: 1539–1550, 2009 [DOI] [PubMed] [Google Scholar]
- 61.Ha HC. and Snyder SH. Poly(ADP-ribose) polymerase is a mediator of necrotic cell death by ATP depletion. Proc Natl Acad Sci U S A 96: 13978–13982, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Heitmeier MR, Arnush M, Scarim AL, and Corbett JA. Pancreatic beta-cell damage mediated by beta-cell production of interleukin-1. A novel mechanism for virus-induced diabetes. J Biol Chem 276: 11151–11158, 2001 [DOI] [PubMed] [Google Scholar]
- 63.Heitmeier MR, Scarim AL, and Corbett JA. Interferon-gamma increases the sensitivity of islets of Langerhans for inducible nitric-oxide synthase expression induced by interleukin 1. J Biol Chem 272: 13697–13704, 1997 [DOI] [PubMed] [Google Scholar]
- 64.Helleday T, Eshtad S, and Nik-Zainal S. Mechanisms underlying mutational signatures in human cancers. Nat Rev Genet 15: 585–598, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hofseth LJ, Saito S, Hussain SP, Espey MG, Miranda KM, Araki Y, Jhappan C, Higashimoto Y, He P, Linke SP, Quezado MM, Zurer I, Rotter V, Wink DA, Appella E, and Harris CC. Nitric oxide-induced cellular stress and p53 activation in chronic inflammation. Proc Natl Acad Sci U S A 100: 143–148, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang H. and Tindall DJ. Dynamic FoxO transcription factors. J Cell Sci 120: 2479–2487, 2007 [DOI] [PubMed] [Google Scholar]
- 67.Hughes JH, Colca JR, Easom RA, Turk J, and McDaniel ML. Interleukin 1 inhibits insulin secretion from isolated rat pancreatic islets by a process that requires gene transcription and mRNA translation. J Clin Invest 86: 856–863, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hughes KJ, Chambers KT, Meares GP, and Corbett JA. Nitric oxides mediates a shift from early necrosis to late apoptosis in cytokine-treated beta-cells that is associated with irreversible DNA damage. Am J Physiol Endocrinol Metab 297: E1187–E1196, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hughes KJ, Meares GP, Chambers KT, and Corbett JA. Repair of nitric oxide-damaged DNA in beta-cells requires JNK-dependent GADD45alpha expression. J Biol Chem 284: 27402–27408, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hughes KJ, Meares GP, Hansen PA, and Corbett JA. FoxO1 and SIRT1 regulate beta-cell responses to nitric oxide. J Biol Chem 286: 8338–8348, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jung HJ, Kim EH, Mun JY, Park S, Smith ML, Han SS, and Seo YR. Base excision DNA repair defect in GADD45a-deficient cells. Oncogene 26: 7517–7525, 2007 [DOI] [PubMed] [Google Scholar]
- 72.Kaneto H, Fujii J, Seo HG, Suzuki K, Matsuoka T, Nakamura M, Tatsumi H, Yamasaki Y, Kamada T, and Taniguchi N. Apoptotic cell death triggered by nitric oxide in pancreatic beta-cells. Diabetes 44: 733–738, 1995 [DOI] [PubMed] [Google Scholar]
- 73.Karupiah G, Xie QW, Buller RM, Nathan C, Duarte C, and MacMicking JD. Inhibition of viral replication by interferon-gamma-induced nitric oxide synthase. Science 261: 1445–1448, 1993 [DOI] [PubMed] [Google Scholar]
- 74.Kaufman BA, Li C, and Soleimanpour SA. Mitochondrial regulation of beta-cell function: maintaining the momentum for insulin release. Mol Aspects Med 42: 91–104, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim YM, Talanian RV, and Billiar TR. Nitric oxide inhibits apoptosis by preventing increases in caspase-3-like activity via two distinct mechanisms. J Biol Chem 272: 31138–31148, 1997 [DOI] [PubMed] [Google Scholar]
- 76.Knowles RG. and Moncada S. Nitric oxide synthases in mammals. Biochem J 298 (Pt 2): 249–258, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke EH, Blagosklonny MV, El-Deiry WS, Golstein P, Green DR, Hengartner M, Knight RA, Kumar S, Lipton SA, Malorni W, Nunez G, Peter ME, Tschopp J, Yuan J, Piacentini M, Zhivotovsky B, Melino G, and Nomenclature Committee on Cell Death. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ 16: 3–11, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kwon G, Corbett JA, Rodi CP, Sullivan P, and McDaniel ML. Interleukin-1 beta-induced nitric oxide synthase expression by rat pancreatic beta-cells: evidence for the involvement of nuclear factor kappa B in the signaling mechanism. Endocrinology 136: 4790–4795, 1995 [DOI] [PubMed] [Google Scholar]
- 79.Lacy PE. The intraislet macrophage and type I diabetes. Mt Sinai J Med 61: 170–174, 1994 [PubMed] [Google Scholar]
- 80.Lakey JR, Suarez-Pinzon WL, Strynadka K, Korbutt GS, Rajotte RV, Mabley JG, Szabo C, and Rabinovitch A. Peroxynitrite is a mediator of cytokine-induced destruction of human pancreatic islet beta cells. Lab Invest 81: 1683–1692, 2001 [DOI] [PubMed] [Google Scholar]
- 81.Lee JH. and Paull TT. ATM activation by DNA double-strand breaks through the Mre11-Rad50-Nbs1 complex. Science 308: 551–554, 2005 [DOI] [PubMed] [Google Scholar]
- 82.Lee JH, Song MY, Song EK, Kim EK, Moon WS, Han MK, Park JW, Kwon KB, and Park BH. Overexpression of SIRT1 protects pancreatic beta-cells against cytokine toxicity by suppressing the nuclear factor-kappaB signaling pathway. Diabetes 58: 344–351, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lenzen S. Oxidative stress: the vulnerable beta-cell. Biochem Soc Trans 36: 343–347, 2008 [DOI] [PubMed] [Google Scholar]
- 84.Lenzen S, Drinkgern J, and Tiedge M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic Biol Med 20: 463–466, 1996 [DOI] [PubMed] [Google Scholar]
- 85.Li J, Billiar TR, Talanian RV, and Kim YM. Nitric oxide reversibly inhibits seven members of the caspase family via S-nitrosylation. Biochem Biophys Res Commun 240: 419–424, 1997 [DOI] [PubMed] [Google Scholar]
- 86.Liu D, Pavlovic D, Chen MC, Flodstrom M, Sandler S, and Eizirik DL. Cytokines induce apoptosis in beta-cells isolated from mice lacking the inducible isoform of nitric oxide synthase (iNOS-/-). Diabetes 49: 1116–1122, 2000 [DOI] [PubMed] [Google Scholar]
- 87.Lopes M, Kutlu B, Miani M, Bang-Berthelsen CH, Storling J, Pociot F, Goodman N, Hood L, Welsh N, Bontempi G, and Eizirik DL. Temporal profiling of cytokine-induced genes in pancreatic beta-cells by meta-analysis and network inference. Genomics 103: 264–275, 2014 [DOI] [PubMed] [Google Scholar]
- 88.Luo X. and Kraus WL. On PAR with PARP: cellular stress signaling through poly(ADP-ribose) and PARP-1. Genes Dev 26: 417–432, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Maggi LB, Jr., Sadeghi H, Weigand C, Scarim AL, Heitmeier MR, and Corbett JA. Anti-inflammatory actions of 15-deoxy-delta 12,14-prostaglandin J2 and troglitazone: evidence for heat shock-dependent and -independent inhibition of cytokine-induced inducible nitric oxide synthase expression. Diabetes 49: 346–355, 2000 [DOI] [PubMed] [Google Scholar]
- 90.Mandrup-Poulsen T. beta-cell apoptosis: stimuli and signaling. Diabetes 50 Suppl 1: S58–S63, 2001 [DOI] [PubMed] [Google Scholar]
- 91.Mandrup-Poulsen T, Bendtzen K, Nerup J, Dinarello CA, Svenson M, and Nielsen JH. Affinity-purified human interleukin I is cytotoxic to isolated islets of Langerhans. Diabetologia 29: 63–67, 1986 [DOI] [PubMed] [Google Scholar]
- 92.Mandrup-Poulsen T, Bendtzen K, Nielsen JH, Bendixen G, and Nerup J. Cytokines cause functional and structural damage to isolated islets of Langerhans. Allergy 40: 424–429, 1985 [DOI] [PubMed] [Google Scholar]
- 93.Mannick JB. The antiviral role of nitric oxide. Res Immunol 146: 693–697, 1995 [DOI] [PubMed] [Google Scholar]
- 94.Mathews CE, Suarez-Pinzon WL, Baust JJ, Strynadka K, Leiter EH, and Rabinovitch A. Mechanisms underlying resistance of pancreatic islets from ALR/Lt mice to cytokine-induced destruction. J Immunol 175: 1248–1256, 2005 [DOI] [PubMed] [Google Scholar]
- 95.McLaughlin LM. and Demple B. Nitric oxide-induced apoptosis in lymphoblastoid and fibroblast cells dependent on the phosphorylation and activation of p53. Cancer Res 65: 6097–6104, 2005 [DOI] [PubMed] [Google Scholar]
- 96.Meares G, Hughes K, Jaimes K, Salvatori A, Rhodes C, and Corbett J. AMP-activated protein kinase attenuates nitric oxide induced {beta}-cell death. J Biol Chem 285: 3191–200, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Meares GP, Fontanilla D, Broniowska KA, Andreone T, Lancaster JR, Jr., and Corbett JA. Differential responses of pancreatic beta-cells to ROS and RNS. Am J Physiol Endocrinol Metab 304: E614–E622, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Miles PD, Treuner K, Latronica M, Olefsky JM, and Barlow C. Impaired insulin secretion in a mouse model of ataxia telangiectasia. Am J Physiol Endocrinol Metab 293: E70–E74, 2007 [DOI] [PubMed] [Google Scholar]
- 99.Mohr S, Zech B, Lapetina EG, and Brune B. Inhibition of caspase-3 by S-nitrosation and oxidation caused by nitric oxide. Biochem Biophys Res Commun 238: 387–391, 1997 [DOI] [PubMed] [Google Scholar]
- 100.Morrell D, Chase CL, Kupper LL, and Swift M. Diabetes mellitus in ataxia-telangiectasia, Fanconi anemia, xeroderma pigmentosum, common variable immune deficiency, and severe combined immune deficiency families. Diabetes 35: 143–147, 1986 [DOI] [PubMed] [Google Scholar]
- 101.Murata M, Thanan R, Ma N, and Kawanishi S. Role of nitrative and oxidative DNA damage in inflammation-related carcinogenesis. J Biomed Biotechnol 2012: 623019, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Oleson BJ, Broniowska KA, Naatz A, Hogg N, Tarakanova VL, and Corbett JA. Nitric oxide suppresses beta-cell apoptosis by inhibiting the DNA damage response. Mol Cell Biol 36: 2067–2077, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Oleson BJ, Broniowska KA, Schreiber KH, Tarakanova VL, and Corbett JA. Nitric oxide induces ataxia telangiectasia mutated (ATM) protein-dependent gammaH2AX protein formation in pancreatic beta cells. J Biol Chem 289: 11454–11464, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Op de Beeck A and Eizirik DL. Viral infections in type 1 diabetes mellitus—why the beta cells? Nat Rev Endocrinol 12: 263–273, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Padgett LE, Broniowska KA, Hansen PA, Corbett JA, and Tse HM. The role of reactive oxygen species and proinflammatory cytokines in type 1 diabetes pathogenesis. Ann N Y Acad Sci 1281: 16–35, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Patterson CC, Dahlquist GG, Gyurus E, Green A, Soltesz G, and Group ES. Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–2020: a multicentre prospective registration study. Lancet 373: 2027–2033, 2009 [DOI] [PubMed] [Google Scholar]
- 107.Patterson CC, Gyurus E, Rosenbauer J, Cinek O, Neu A, Schober E, Parslow RC, Joner G, Svensson J, Castell C, Bingley PJ, Schoenle E, Jarosz-Chobot P, Urbonaite B, Rothe U, Krzisnik C, Ionescu-Tirgoviste C, Weets I, Kocova M, Stipancic G, Samardzic M, de Beaufort CE, Green A, Dahlquist GG, and Soltesz G. Trends in childhood type 1 diabetes incidence in Europe during 1989–2008: evidence of non-uniformity over time in rates of increase. Diabetologia 55: 2142–2147, 2012 [DOI] [PubMed] [Google Scholar]
- 108.Paull TT, Rogakou EP, Yamazaki V, Kirchgessner CU, Gellert M, and Bonner WM. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr Biol 10: 886–895, 2000 [DOI] [PubMed] [Google Scholar]
- 109.Pinto DM. and Flaus A. Structure and function of histone H2AX. Subcell Biochem 50: 55–78, 2010 [DOI] [PubMed] [Google Scholar]
- 110.Pirot P, Cardozo AK, and Eizirik DL. Mediators and mechanisms of pancreatic beta-cell death in type 1 diabetes. Arq Bras Endocrinol Metabol 52: 156–165, 2008 [DOI] [PubMed] [Google Scholar]
- 111.Poletto M, Legrand AJ, Fletcher SC, and Dianov GL. p53 coordinates base excision repair to prevent genomic instability. Nucleic Acids Res 44: 3165–3175, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pollock JS, Forstermann U, Tracey WR, and Nakane M. Nitric oxide synthase isozymes antibodies. Histochem J 27: 738–744, 1995 [PubMed] [Google Scholar]
- 113.Pommier Y. Topoisomerase I inhibitors: camptothecins and beyond. Nat Rev Cancer 6: 789–802, 2006 [DOI] [PubMed] [Google Scholar]
- 114.Redondo MJ, Jeffrey J, Fain PR, Eisenbarth GS, and Orban T. Concordance for islet autoimmunity among monozygotic twins. N Engl J Med 359: 2849–2850, 2008 [DOI] [PubMed] [Google Scholar]
- 115.Rogakou EP, Pilch DR, Orr AH, Ivanova VS, and Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem 273: 5858–5868, 1998 [DOI] [PubMed] [Google Scholar]
- 116.Roos WP. and Kaina B. DNA damage-induced cell death: from specific DNA lesions to the DNA damage response and apoptosis. Cancer Lett 332: 237–248, 2013 [DOI] [PubMed] [Google Scholar]
- 117.Rosales AL, Cunningham JM, Bone AJ, Green IC, and Green MH. Repair of cytokine-induced DNA damage in cultured rat islets of Langerhans. Free Radic Res 38: 665–674, 2004 [DOI] [PubMed] [Google Scholar]
- 118.Rothkamm K, Barnard S, Moquet J, Ellender M, Rana Z, and Burdak-Rothkamm S. DNA damage foci: meaning and significance. Environ Mol Mutagen 56: 491–504, 2015 [DOI] [PubMed] [Google Scholar]
- 119.Saldeen J. and Welsh N. Interleukin-1 beta induced activation of NF-kappa B in insulin producing RINm5F cells is prevented by the protease inhibitor N alpha-p-tosyl-L-lysine chloromethylketone. Biochem Biophys Res Commun 203: 149–155, 1994 [DOI] [PubMed] [Google Scholar]
- 120.Sanders SP, Siekierski ES, Porter JD, Richards SM, and Proud D. Nitric oxide inhibits rhinovirus-induced cytokine production and viral replication in a human respiratory epithelial cell line. J Virol 72: 934–942, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Scarim AL, Heitmeier MR, and Corbett JA. Heat shock inhibits cytokine-induced nitric oxide synthase expression by rat and human islets. Endocrinology 139: 5050–5057, 1998 [DOI] [PubMed] [Google Scholar]
- 122.Scarim AL, Heitmeier MR, and Corbett JA. Irreversible inhibition of metabolic function and islet destruction after a 36-hour exposure to interleukin-1beta. Endocrinology 138: 5301–5307, 1997 [DOI] [PubMed] [Google Scholar]
- 123.Scarim AL, Nishimoto SY, Weber SM, and Corbett JA. Role for c-Jun N-terminal kinase in beta-cell recovery from nitric oxide-mediated damage. Endocrinology 144: 3415–3422, 2003 [DOI] [PubMed] [Google Scholar]
- 124.Schneider JG, Finck BN, Ren J, Standley KN, Takagi M, Maclean KH, Bernal-Mizrachi C, Muslin AJ, Kastan MB, and Semenkovich CF. ATM-dependent suppression of stress signaling reduces vascular disease in metabolic syndrome. Cell Metab 4: 377–389, 2006 [DOI] [PubMed] [Google Scholar]
- 125.Seeberg E, Eide L, and Bjoras M. The base excision repair pathway. Trends Biochem Sci 20: 391–397, 1995 [DOI] [PubMed] [Google Scholar]
- 126.Shiloh Y. and Ziv Y. The ATM protein kinase: regulating the cellular response to genotoxic stress, and more. Nat Rev Mol Cell Biol 14: 197–210, 2013 [PubMed] [Google Scholar]
- 127.Smith ML. and Seo YR. p53 regulation of DNA excision repair pathways. Mutagenesis 17: 149–156, 2002 [DOI] [PubMed] [Google Scholar]
- 128.Sordet O, Khan QA, Kohn KW, and Pommier Y. Apoptosis induced by topoisomerase inhibitors. Curr Med Chem Anticancer Agents 3: 271–290, 2003 [DOI] [PubMed] [Google Scholar]
- 129.Southern C, Schulster D, and Green IC. Inhibition of insulin secretion by interleukin-1 beta and tumour necrosis factor-alpha via an L-arginine-dependent nitric oxide generating mechanism. FEBS Lett 276: 42–44, 1990 [DOI] [PubMed] [Google Scholar]
- 130.Steer SA, Scarim AL, Chambers KT, and Corbett JA. Interleukin-1 stimulates beta-cell necrosis and release of the immunological adjuvant HMGB1. PLoS Med 3: e17, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Suarez-Pinzon WL, Szabo C, and Rabinovitch A. Development of autoimmune diabetes in NOD mice is associated with the formation of peroxynitrite in pancreatic islet beta-cells. Diabetes 46: 907–911, 1997 [DOI] [PubMed] [Google Scholar]
- 132.Szabo C, Ischiropoulos H, and Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov 6: 662–680, 2007 [DOI] [PubMed] [Google Scholar]
- 133.Takamura T, Kato I, Kimura N, Nakazawa T, Yonekura H, Takasawa S, and Okamoto H. Transgenic mice overexpressing type 2 nitric-oxide synthase in pancreatic beta cells develop insulin-dependent diabetes without insulitis. J Biol Chem 273: 2493–2496, 1998 [DOI] [PubMed] [Google Scholar]
- 134.Tamir S, Burney S, and Tannenbaum SR. DNA damage by nitric oxide. Chem Res Toxicol 9: 821–827, 1996 [DOI] [PubMed] [Google Scholar]
- 135.Tanaka T, Kurose A, Halicka HD, Huang X, Traganos F, and Darzynkiewicz Z. Nitrogen oxide-releasing aspirin induces histone H2AX phosphorylation, ATM activation and apoptosis preferentially in S-phase cells: involvement of reactive oxygen species. Cell Cycle 5: 1669–1674, 2006 [DOI] [PubMed] [Google Scholar]
- 136.Tavana O, Puebla-Osorio N, Sang M, and Zhu C. Absence of p53-dependent apoptosis combined with nonhomologous end-joining deficiency leads to a severe diabetic phenotype in mice. Diabetes 59: 135–142, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tripathi DN, Chowdhury R, Trudel LJ, Tee AR, Slack RS, Walker CL, and Wogan GN. Reactive nitrogen species regulate autophagy through ATM-AMPK-TSC2-mediated suppression of mTORC1. Proc Natl Acad Sci U S A 110: E2950–E2957, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Unger T, Sionov RV, Moallem E, Yee CL, Howley PM, Oren M, and Haupt Y. Mutations in serines 15 and 20 of human p53 impair its apoptotic activity. Oncogene 18: 3205–3212, 1999 [DOI] [PubMed] [Google Scholar]
- 139.Weber SM, Chambers KT, Bensch KG, Scarim AL, and Corbett JA. PPARgamma ligands induce ER stress in pancreatic beta-cells: ER stress activation results in attenuation of cytokine signaling. Am J Physiol Endocrinol Metab 287: E1171–E1177, 2004 [DOI] [PubMed] [Google Scholar]
- 140.Welsh N, Eizirik DL, Bendtzen K, and Sandler S. Interleukin-1 beta-induced nitric oxide production in isolated rat pancreatic islets requires gene transcription and may lead to inhibition of the Krebs cycle enzyme aconitase. Endocrinology 129: 3167–3173, 1991 [DOI] [PubMed] [Google Scholar]
- 141.Yamamoto H, Uchigata Y, and Okamoto H. Streptozotocin and alloxan induce DNA strand breaks and poly(ADP-ribose) synthetase in pancreatic islets. Nature 294: 284–286, 1981 [DOI] [PubMed] [Google Scholar]
- 142.Yang YC, Chou HY, Shen TL, Chang WJ, Tai PH, and Li TK. Topoisomerase II-mediated DNA cleavage and mutagenesis activated by nitric oxide underlie the inflammation-associated tumorigenesis. Antioxid Redox Signal 18: 1129–1140, 2013 [DOI] [PubMed] [Google Scholar]