Supplemental Digital Content is available in the text.
Key Words: cone-beam CT, electromagnetic navigation bronchoscopy, augmented fluoroscopy, lung cancer
Abstract
Background:
Electromagnetic navigation bronchoscopy (ENB) has been widely adopted as a guidance technique for biopsy of peripheral lung nodules. However, ENB is limited by the lack of real-time confirmation of the biopsy devices. Intraprocedural cone-beam computed tomography (CBCT) imaging can be utilized to assess or confirm the location of biopsy devices. The aim of this study is to determine the safety and diagnostic yield (DY) of image fusion of intraprocedural CBCT data with live fluoroscopy (augmented fluoroscopy) during ENB-guided biopsy of peripheral lung nodules.
Methods:
Data from 75 consecutive patients who underwent biopsy with ENB was collected retrospectively. Patients underwent CBCT imaging while temporarily suspending mechanical ventilation. CBCT data were acquired and 3-dimensional segmentation of nodules was performed using commercially available software (OncoSuite). During ENB, the segmented lesions were projected and fused with live fluoroscopy enabling real-time 3-dimensional guidance.
Results:
A total of 93 lesions with a median size of 16.0 mm were biopsied in 75 consecutive patients. The overall DY by lesion was 83.7% (95% confidence interval, 74.8%-89.9%). Multivariate regression analysis showed no independent correlation between lesion size, lesion location, lesion visibility under standard fluoroscopy, and the presence of a bronchus sign with DY. Pneumothorax occurred in 3 patients (4%).
Conclusion:
Intraprocedural CBCT imaging with augmented fluoroscopy is feasible and effective and is associated with high DY during ENB-guided biopsies.
Bronchoscopic biopsy is commonly utilized for diagnosis of pulmonary nodules, with a well-accepted safety profile. The most common complication of this technique is pneumothorax, which occurs in 2% to 5% of all procedures.1,2 Traditionally fluoroscopy is utilized for navigation of biopsy devices towards peripheral lesions. Although fluoroscopy is effective for real-time imaging of radiopaque lesions, the identification of bronchial anatomy and localization of small or ground-glass lung nodules remains challenging. As a result, the reported diagnostic yield (DY) for traditional bronchoscopic transbronchial biopsy of small peripheral lesions can be as low as 14%.3
Computed tomography (CT)-guided transthoracic needle aspiration (TTNA) offers an alternative to achieve more controlled targeting of peripheral nodules with a reported DY of 50% to 95%, depending on lesion size.4–7 However, transthoracic needle punctures are associated with an increased incidence of pneumothorax (20% to 45%), which requires chest tube placement in 5% to 15% of patients.8–11 Complications of TTNA, such as pneumothorax and hemorrhage, are associated with prolonged hospital stays, blood transfusions, and increased rates of mechanical ventilation for respiratory failure.12 In addition, after TTNA, patients who require mediastinoscopy or endobronchial ultrasound (EBUS)-guided lymph node staging still need to be referred for additional diagnostic procedures.13,14 The importance of this is highlighted by a recent review demonstrating the false negative (FN) rate of positron emission tomography-CT scan for mediastinal staging in non–small-cell lung cancer is as high as 23% and concluded that this should not be used in isolation for accurate staging of the mediastinum.15 The FN rate of TTNA, particularly with small lesions, can be as high as 20% to 30% and may not be adequate to rule out cancer as a stand-alone procedure.16
More recently, the use of electromagnetic navigation bronchoscopy (ENB) has gained popularity as a complementary guidance tool during bronchoscopic biopsy of peripheral nodules. These systems utilize preoperative CT data and an electromagnetically tracked guide for procedure planning and guidance. Although several studies have demonstrated promising clinical efficacy and an acceptable safety profile, the DY of ENB remains inferior to TTNA (~70% for peripheral lung nodules).16–37 This is in contrast to the improved safety profile of ENB compared with TTNA. The ideal procedure would be one that combines the high DY of TTNA with the low complication rate of guided bronchoscopy.
Modern hybrid operating rooms (OR) equipped with flat-panel detector systems offer the possibility to acquire cone-beam CT (CBCT) data in addition to standard fluoroscopy. Recent developments have been pushing CBCT image quality close to that of conventional CT, enabling treatment planning and 3-dimensional (3D) fluoroscopic image guidance to be performed in the OR.38–41
In the current retrospective study, we investigate the feasibility and clinical utility of image fusion of intraprocedural CBCT data with live fluoroscopy [augmented fluoroscopy, (AF)] during ENB procedures. Our aim is to determine whether the addition of CBCT with AF to ENB may offer a safe procedure with high DY.
MATERIALS AND METHODS
This single-center retrospective study was approved by the Institutional Review Board and informed consent was waived. Data from 75 consecutive patients who underwent ENB-guided lung biopsy from September 2016 through May 2017 was collected retrospectively. Each patient had 1 bronchoscopic procedure (75 ENB procedures in total). A single pulmonologist performed all procedures under general anesthesia in a hybrid OR equipped with a C-arm system with CBCT capabilities (Allura Xper FD20; Philips). Lesion size, location, fluoroscopic visibility, and presence of a bronchus sign was determined from cross-sectional CT data. All lesions were characterized as peripheral nodules in that they were surrounded by normal aerated lung, none were visible endobronchially, and all were beyond the segmental bronchus so that all biopsies were transbronchial rather than endobronchial. CT data were imported into the electromagnetic navigation system (SuperDimension; Medtronic) and planning was performed as per manufacturer instructions.
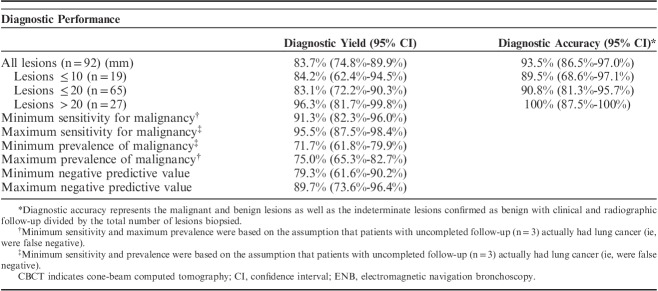
Subsequent to patient intubation, an 8-second scan was performed to obtain CBCT data. Lung nodules were highlighted by the physician using commercially available software (OncoSuite; Philips) during a process known as segmentation (Fig. 1). A bronchoscope (BF-1T180; Olympus) was introduced into the airway and then a curved steerable catheter (Edge Firm Tip; Medtronic) was inserted into the working channel and navigated to the lesion using the ENB system. Nodule segmentation was visualized in an overlay with live fluoroscopy (Fig. 2). Geometric correspondence of AF was maintained throughout the case while manipulating C-arm angulation, table position, and image-zoom settings. Final catheter position was then verified in multiple planes with AF. Additional CBCT scans were acquired when deemed necessary. Radial EBUS (r-EBUS) was not used in any case. Tissue samples were obtained using one or multiple biopsy tools, including a standard cytology brush, fine needle for aspiration (21 and 19 G), biopsy forceps, GenCut core biopsy tool (Medtronic), single and triple needle cytology brush, and bronchoalveolar lavage (BAL). Rapid on-site pathologic examination (ROSE), utilizing a cytotechnologist and cytopathologist present in the OR, was used for all procedures.
FIGURE 1.
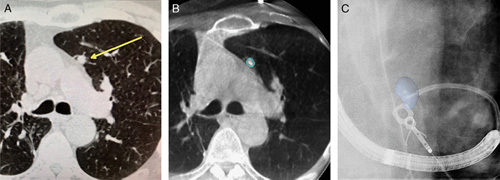
Illustration of the different imaging sources involved using CBCT with AF during ENB-guided biopsy procedures. CT data were acquired before the procedure (Yellow arrow shows the target nodule) (A). Intraoperative CBCT data were acquired and 3-dimensional nodule segmentation was performed (B). Three-dimensional nodule segmentation was visualized in overlay with live fluoroscopy: AF (C). AF indicates augmented fluoroscopy; CBCT, cone-beam computed tomography; ENB, electromagnetic navigation bronchoscopy.
FIGURE 2.
Comparison of standard fluoroscopy (A) and augmented fluoroscopy (B) for a fluoroscopically invisible nodule. The blue volume was segmented from cone-beam computed tomography data and automatically projected using dedicated software (OncoSuite; Philips).
The primary outcome was the DY of ENB using CBCT with AF for peripheral lesions. In line with prior literature,1 a bronchoscopy procedure was considered diagnostic if a specific malignant or benign diagnosis of the peripheral lesion was made. If only nonspecific findings were identified, such as inflammation, the procedure was considered nondiagnostic. These lesions were then labeled as indeterminate and followed with serial CT scans, if the patient was not suitable for a more invasive diagnostic procedure.
In a subset analysis, follow-up data were collected for subjects who had an indeterminate result on bronchoscopy to establish the true diagnosis. This was used to calculate the prevalence and sensitivity of ENB using CBCT with AF for malignancy. These cases were not used in the determination of DY, even if they subsequently resolved or were later found to be benign.
All bronchoscopic results that showed lung cancer were considered true positives (TPs). If initial bronchoscopy failed to reveal a specific diagnosis (ie, indeterminate), and follow-up data demonstrated that lung cancer was eventually diagnosed, or if the patient was sent for definitive treatment (ie, stereotactic body radiation therapy), the subject was considered a FN.
If an indeterminate lesion resolved, stayed stable or decreased in size on follow-up CT for at least 12 months after the index procedure then this was presumed benign and classified as a true negative (TN) for the purposes of calculating sensitivity and prevalence of malignancy, as well as for diagnostic accuracy. These cases were not used in the determination of DY. Diagnostic accuracy represents the malignant and benign lesions as well as the indeterminate lesions subsequently confirmed as benign with clinical and radiographic follow-up divided by the total number of lesions biopsied.
Sensitivity of ENB using CBCT with AF for malignancy was defined as TP/(TP+FN). Because some subjects did not have complete follow-up, we conducted a sensitivity analysis to determine the possible minimum and maximum diagnostic sensitivities. To determine the minimum sensitivity, subjects with incomplete follow-up were considered FN. To determine the maximum sensitivity, subjects with uncompleted follow-up were considered TN.42
Independent correlation of lesion size, location, visibility, and presence of a bronchus sign, with DY using a multivariate regression analysis was assessed. A P-value <0.05 was considered significant.
Radiation dose exposure was assessed in a representative subset of patients. Effective dose was estimated from dose area product measurements using a generalized conversion coefficient (E=0.16 mSv/Gy cm2), assuming an average x-ray spectra.43,44
Analyses were performed using R 2.15.0 (www.R-project.org).
RESULTS
A total of 93 suspicious pulmonary lung nodules were biopsied in 75 consecutive patients. Fifteen patients presented with multiple lesions, including 10 patients with bilateral lesions. Any patient with multiple lesions had all of these biopsied in a single ENB procedure. One patient was lost to follow-up and excluded from the performance analysis (Fig. 3).
FIGURE 3.
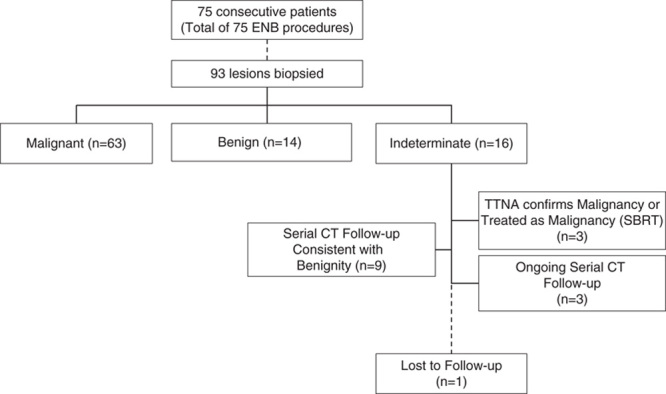
The flowchart of the study. Diagnostic yield per lesion was calculated by dividing the malignant lesions (n=63) and the benign lesions (n=14) by the total number of lesions (n=92, excluding one patient lost to follow-up), resulting in a diagnostic yield per lesion of 83.7% (95% CI, 74.8%-89.9%). CI indicates confidence interval; CT, computed tomography; ENB, electromagnetic navigation bronchoscopy; SBRT, stereotactic body radiation therapy.
Median lesion size was 16.0 mm (range, 7 to 55 mm). The majority of lesions were located in the middle or peripheral third of the lung (94.6%). A bronchus sign was visible on standard CT imaging in 39% of the cases, while 49% of the lesions were visible on standard fluoroscopy. Mean fluoroscopy time was 6.2±2.6 minutes (For complete information on patient, lesion, and procedural characteristics see Table 1).
TABLE 1.
Patient, Lesion, and Procedural Characteristics
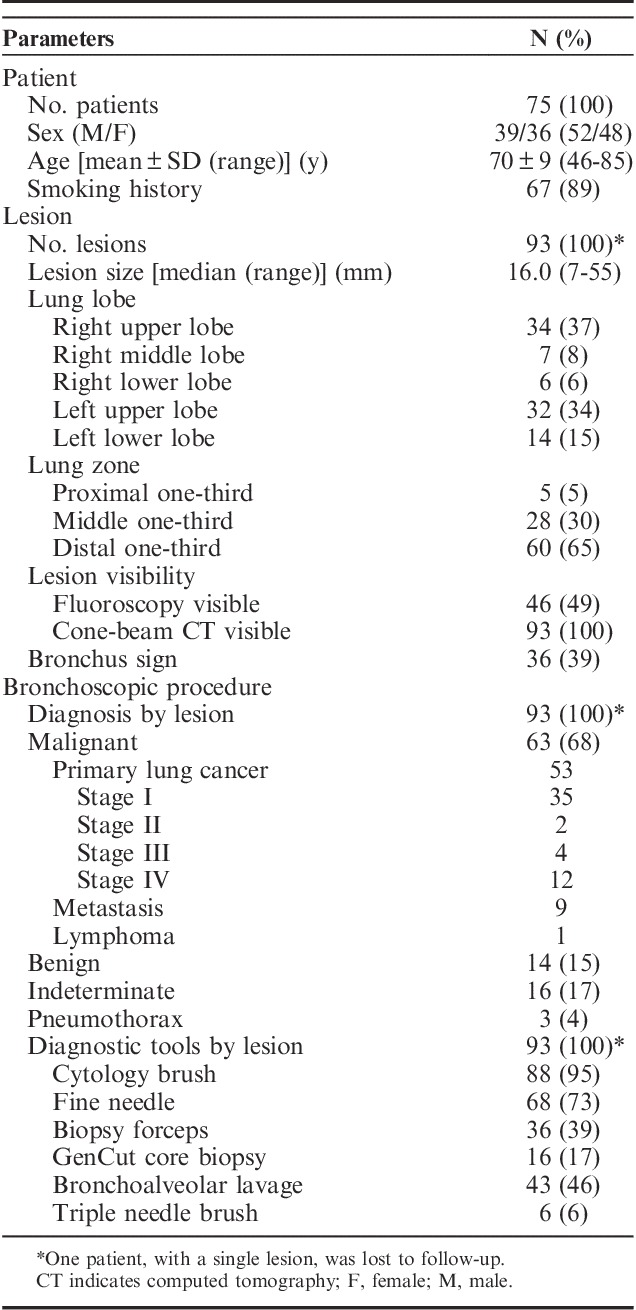
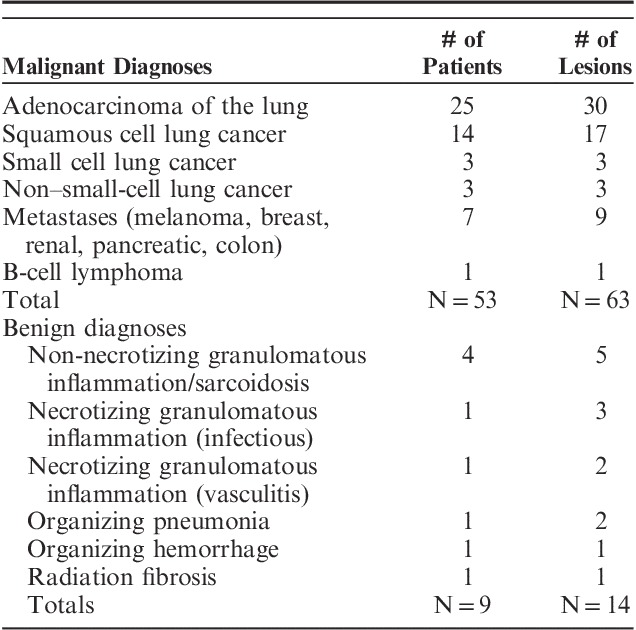
Overall proven malignancy rate was 68.5% (63/92 lesions). Primary lung cancer diagnosis was established in 57.6% (53/92) of the inspected lesions (Table 2). The majority of these patients were diagnosed at stage I (75%) or stage II (4%). Pneumothorax occurred in 3 patients (4%), out of which 2 received a chest tube. No significant bronchopulmonary hemorrhage or respiratory failure was reported.
TABLE 2.
Diagnostic Specifics for Malignant and Benign Diagnoses
The overall DY by lesion was calculated by dividing the malignant lesions (n=63) and the benign lesions (n=14) by the total number of lesions (n=92, excluding 1 lost to follow-up), resulting in a DY by lesion of 83.7% [95% confidence interval (CI), 74.8%-89.9%]. Diagnostic accuracy was 93.5%, which represents the malignant (n=63) and benign lesions (n=14) as well as the indeterminate lesions subsequently confirmed as benign with clinical and radiographic follow-up (n=9) divided by the total number of lesions biopsied (n=92).
The median number of diagnostic tools used per nodule was 3 (range, 1 to 5) and the median number of tool passes per lesion was 10 (range, 2 to 25). We also calculated the median number of passes with each diagnostic tool; cytology brush 4 (range, 1 to 14), transbronchial needle 3 (range, 1 to 9), forceps biopsy 3 (range, 1 to 10), GenCut core biopsy 2 (range, 1 to 4), triple needle brush 2 (range, 2 to 4), and BAL 1 (range, 1 to 2). Diagnostic tools with the highest yield in determining the diagnosis were biopsy forceps (80.6%) and transbronchial needle aspirates (66.2%). Other tools were also used but provided a diagnosis in a lower percentage of cases; cytology brush (54.5%), GenCut core biopsy (37.5%), BAL (25.6%), and a triple needle brush (0%). All diagnostic performance statistics can be found in Table 3.
TABLE 3.
Diagnostic Performance of ENB and CBCT With Augmented Fluoroscopy
If we assume that subjects with uncompleted follow-up (n=3) actually had lung cancer (ie, were FN), the prevalence of malignancy in our study was 71.7% (95% CI, 61.8%-79.9%) with a sensitivity of ENB using CBCT with AF for malignancy of 91.3% (95% CI, 82.3%-96.0%). Under the assumption that subjects with uncompleted follow-up did not have malignant disease (ie, were TNs), the minimum prevalence of malignancy in our study was 75.0% (95% CI, 65.3%-82.7%) with a maximum sensitivity of 95.5% (95% CI, 87.5%-98.4%). The minimum and maximum negative predictive value for malignancy was 79.3% (95% CI, 61.6%-90.2%) and 89.7% (95% CI, 73.6%-96.4%), respectively.
Multivariate regression analysis further showed no independent correlation between lesion size, lesion location, lesion visibility under standard fluoroscopy, and the presence of a bronchus sign with DY.
In a representative subset of 9 patients, the total dose area product per case was 31±16 Gycm2. The estimated effective dose per case related to fluoroscopy and exposure was 1.5±0.7 mSv and 3.0±1.4 mSv, respectively. With an average number of 1.5 CBCT scans per case, this leads to an average effective dose of 2.0 mSv per CBCT run.
DISCUSSION
This retrospective study of 93 lesions in 75 consecutive patients demonstrates the feasibility and effectiveness of CBCT with AF during ENB-guided biopsy procedures and highlights its potential in achieving high DY and low complication rates with an acceptable amount of radiation dose (comparable with CBCT-guided percutaneous needle interventions and considerably lower than conventional CT guidance).45 After exclusion of a single patient that was lost to follow-up the overall DY by lesion was 83.7% (95% CI, 74.8%-89.9%). This was found to be independent of lesion size, lesion location, presence of a bronchus sign, and visibility on fluoroscopy.
Direct comparison of our results with existing literature might be distorted due to heterogeneity (eg disease prevalence, lesion size). CBCT with AF combined with ENB does seem to provide a higher DY compared with earlier reports for ENB guidance or conventional bronchoscopic guidance with CBCT confirmation alone. A recent meta-analysis of DY using ENB showed a variable yield from 60% to 94%.36 In this meta-analysis, the median disease prevalence was 77.5%. This is similar to the prevalence of lung cancer in our study with a value of 75%. Hohenforst-Schmidt et al46 reported an overall yield of 70% when combining CBCT with conventional bronchoscopy, however the mean lesion size was considerably larger (25 mm) than that of this study. Park et al47 showed that CBCT confirmation of device positioning was the only factor associated with higher yield during transbronchial biopsy. Both authors postulated that the addition of CBCT might provide an increase in DY of conventional bronchoscopy that is equivalent to what has been reported for ENB alone.
We attribute our high DY in large part to the addition of CBCT with AF to ENB. This allowed us to avoid doing biopsies when the tip of the locatable guide was clearly not on target. In addition, the use of AF allowed us to limit the number of repeat CBCT scans, with the majority of cases only requiring a single scan, including cases with multiple or bilateral lesions. The ability to view AF in any plane was especially helpful, particularly the use of the lateral (90 degrees) position for anteriorly located lesions. The use of CBCT also offers the ability to adjust for factors like atelectasis and airway deformation by the bronchoscope that ENB is not able to, given its reliance on virtual spatial reconstruction based on preoperative CT scans. This has given us increased confidence in our negative results, many of which have been confirmed as TNs with additional radiographic and clinical follow-up. The use of CBCT with AF may give more confidence to physicians performing ENB procedure without the assistance of ROSE. The recent multicenter prospective NAVIGATE trial showed that only 66% of the sites use ROSE during ENB procedures.2
We noticed in our study a relatively low incidence of the presence of a bronchus sign, which previous studies have indicated as a strong determining factor for the yield of ENB.27 Our data did not show an independent relation between the presence of a bronchus sign and the overall DY. This may be attributed to the fact that small peripheral lesions are less likely to have a bronchus sign, the use of the variably curved steerable catheters, and the extensive experience of the user. The median lesion size in our study was only 16 mm and therefore most of these small peripheral lesions are randomly situated in the parenchyma and are not associated with a particular bronchus. On pathologic examination, even brushings usually contain small fragments of collapsed respiratory parenchyma (respiratory bronchioles and alveoli) indicating that we are not simply sampling the inside of a conducting bronchus or bronchiole. A bronchus sign can obviously simplify navigation to a lesion, reduce the distance of parenchyma that must be traversed and make access to a lesion easier with less pliable tools. Our reliance on multiple biopsy tools and the Edge catheters along with our willingness to make many attempts may be partially responsible for relatively high success rates for peripheral lesions without a bronchus sign. The use of CBCT with AF also enabled us to circumvent the usual pitfalls associated with nonbronchus sign lesions.
The 2 most recent meta-analyses of ENB procedures for peripheral nodules36,37 included studies published before the release of the variably curve-tipped, steerable Edge catheters. These curved-tip catheters are able to facilitate off-angle biopsies for lesions that do not have a direct airway to the lesion. We feel that the use of these catheters increased our success in targeting lesions and this may also be a reason why previous studies have shown comparatively lower DY.
This study also highlights improved accuracy in targeting small lesions. The median size lesion in the study is only 16 mm. To our knowledge this represents the smallest median lesion size in a study of ENB. In the most recent meta-analysis of ENB by Gex et al37 the median lesion size was 25 mm. Furthermore, we chose to use a more exclusive definition of DY, one in which only cases with a definitive malignant or benign diagnosis were counted towards the yield. This is the same definition used in the AQuIRE registry,1 which showed that ENB alone had a DY of only 38.5%. When ENB was combined with r-EBUS the yield increased to 47.1%. In that study, 46.8% of lesions were ≤20 mm. Similarly, in the NAVIGATE trial, 49.7% of lesions were <20 mm, whereas 70.7% of nodules in our study were ≤20 mm. Previous meta-analyses have clearly shown that lesion size effects of the DY.36,37 Multivariate regression analysis showed that lesion size did not affect the yield in our study. This is evidenced when looking at the DY for lesions ≤10 mm which was 84.2% (16/19 lesions).
This study has limitations. It is a retrospective analysis without a control arm. Moreover, having only one operator doing the procedures might hamper the generalizability of the results. However, given the promising results, these findings justify further confirmation in other centers, by different operators, and comparative studies against alternative technologies (ie, r-EBUS). Although the use of AF is an effective way to reduce the number of CBCT scans, the availability of ROSE may create a bias in the clinical workflow. Additional CBCT scans may be necessary for final confirmation in centers where ROSE is not available or for future applications such as bronchoscopic transbronchial tumor ablation.
Moreover, the effect of the learning curve on technology adoption and procedural time was not considered in this study. Involvement of supporting staff and anesthesia is of paramount importance to streamline workflow and achieve satisfactory CBCT image quality. It should also be considered that access to CBCT systems may be a roadblock for adoption of this technique. However, fixed C-arm systems capable of CBCT imaging are now standard of care in interventional cardiology and radiology, and fast growing with the increasing prevalence of hybrid ORs in surgery departments. Collaborative efforts focused around the lung cancer patient should be considered to initiate cross-discipline programs and open the doors of CBCT systems to more specialists.
Finally, one patient was lost to follow-up and was therefore excluded from all the performance calculations. A sensitivity analysis considering the missing outcome as either adding to the yield (DY, 78/93=83.9%) or not (DY, 77/93=82.8%) showed that excluding the patient had minimal impact on the reported DY.
As the field of bronchoscopy advances towards therapeutics there will be increased demand for high-yield bronchoscopic biopsy techniques. In addition, for anyone considering the future application of endobronchial microwave ablation, CBCT scanning will be required to confirm placement of the probe in the center of the lesion. Thus far, no virtual technology has been shown to be accurate enough to confidently apply ablative energy to a lung lesion. This will be a situation where the use of CBCT with AF will excel (Fig. 4, Supplemental Digital Content 1, http://links.lww.com/LBR/A169). In summary, intraprocedural CBCT imaging with AF is feasible and effective, and is associated with high DY and low complication rate during ENB-guided biopsy procedures.
Supplementary Material
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.bronchology.com.
ACKNOWLEDGMENTS
The authors wish to acknowledge the support of all OR staff, including anesthesia, radiology, pathology as well as our hospital administration. We would also like to acknowledge Kurt Muggli, R.T. (R) (CV) for his early support in our endeavors with CBCT. The authors are also appreciative of Alessandro Radaelli, PhD, for his consistent support for this project.
Footnotes
Presented in abstract form at CHEST 2017, Toronto, Canada.
Philips provided OncoSuite software package.
Disclosure: M.A.P. and C.C.S.: consulting/speaker fees for Medtronic. S.S., J.A.H.D.G., and I.V.D.B. are Philips employees.
REFERENCES
- 1.Ost DE, Ernst A, Lei X, et al. Diagnostic yield and complications of bronchoscopy for peripheral lung lesions. results of the AQuIRE registry. Am J Respir Crit Care Med. 2016;193:68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khandhar SJ, Bowling MR, Flandes J, et al. Electromagnetic navigation bronchoscopy to access lung lesions in 1,000 subjects: first results of the prospective, multicenter NAVIGATE study. BMC Pulm Med. 2017;17:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baaklini WA, Reinoso MA, Gorin AB, et al. Diagnostic yield of fiberoptic bronchoscopy in evaluating solitary pulmonary nodules. Chest. 2000;117:1049–1054. [DOI] [PubMed] [Google Scholar]
- 4.Swischuk JL, Castaneda F, Patel JC, et al. Percutaneous transthoracic needle biopsy of the lung: review of 612 lesions. J Vasc Interv Radiol. 1998;9:347–352. [DOI] [PubMed] [Google Scholar]
- 5.Laurent F, Latrabe V, Vergier B, et al. Percutaneous CT-guided biopsy of the lung: comparison between aspiration and automated cutting needles using a coaxial technique. Cardiovasc Intervent Radiol. 2000;23:266–272. [DOI] [PubMed] [Google Scholar]
- 6.Klein JS, Salomon G, Stewart EA. Transthoracic needle biopsy with a coaxially placed 20-gauge automated cutting needle: results in 122 patients. Radiology. 1996;198:715–720. [DOI] [PubMed] [Google Scholar]
- 7.Wallace MJ, Krishnamurthy S, Broemeling LD, et al. CT-guided percutaneous fine-needle aspiration biopsy of small (≤1-cm) pulmonary lesions. Radiology. 2002;225:823–828. [DOI] [PubMed] [Google Scholar]
- 8.Kazerooni EA, Lim FT, Mikhail A, et al. Risk of pneumothorax in CT-guided transthoracic needle aspiration biopsy of the lung. Radiology. 1996;198:371–375. [DOI] [PubMed] [Google Scholar]
- 9.Wu CC, Maher MM, Shepard JA. Complications of CT-guided percutaneous needle biopsy of the chest: prevention and management. AJR Am J Roentgenol. 2011;196:W678–W682. [DOI] [PubMed] [Google Scholar]
- 10.Geraghty PR, Kee ST, McFarlane G, et al. CT-guided transthoracic needle aspiration biopsy of pulmonary nodules: needle size and pneumothorax rate. Radiology. 2003;229:475–481. [DOI] [PubMed] [Google Scholar]
- 11.Cox JE, Chiles C, McManus CM, et al. Transthoracic needle aspiration biopsy: variables that affect risk of pneumothorax. Radiology. 1999;212:165–168. [DOI] [PubMed] [Google Scholar]
- 12.Wiener RS, Schwartz LM, Woloshin S, et al. Population-based risk for complications after transthoracic needle lung biopsy of a pulmonary nodule: an analysis of discharge records. Ann Intern Med. 2011;155:137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Almeida FA, Casal RF, Jimenez CA, et al. Quality gaps and comparative effectiveness in lung cancer staging: the impact of test sequencing on outcomes. Chest. 2013;144:1776–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ost DE, Jimenez CA, Lei X, et al. Quality-adjusted survival following treatment of malignant pleural effusions with indwelling pleural catheters. Chest. 2014;145:1347–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidt-Hansen M, Baldwin DR, Zamora J. FDG-PET/CT imaging for mediastinal staging in patients with potentially resectable non-small cell lung cancer. JAMA. 2015;313:1465–1466. [DOI] [PubMed] [Google Scholar]
- 16.Zarbo RJ, Fenoglio-Preiser CM. Interinstitutional database for comparison of performance in lung fine-needle aspiration cytology. A College of American Pathologists Q-Probe Study of 5264 cases with histologic correlation. Arch Pathol Lab Med. 1992;116:463–470. [PubMed] [Google Scholar]
- 17.Becker H, Herth F, Ernst A, et al. Bronchoscopic biopsy of peripheral lung lesions under electromagnetic guidance: a pilot study. J Bronchol Interv Pulmonol. 2005;12:9–13. [Google Scholar]
- 18.Hautmann H, Schneider A, Pinkau T, et al. Electromagnetic catheter navigation during bronchoscopy: validation of a novel method by conventional fluoroscopy. Chest. 2005;128:382–387. [DOI] [PubMed] [Google Scholar]
- 19.Schwarz Y, Greif J, Becker HD, et al. Real-time electromagnetic navigation bronchoscopy to peripheral lung lesions using overlaid CT images: the first human study. Chest. 2006;129:988–994. [DOI] [PubMed] [Google Scholar]
- 20.Gildea TR, Mazzone PJ, Karnak D, et al. Electromagnetic navigation diagnostic bronchoscopy: a prospective study. Am J Respir Crit Care Med. 2006;174:982–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Makris D, Scherpereel A, Leroy S, et al. Electromagnetic navigation diagnostic bronchoscopy for small peripheral lung lesions. Eur Respir J. 2007;29:1187–1192. [DOI] [PubMed] [Google Scholar]
- 22.Eberhardt R, Anantham D, Herth F, et al. Electromagnetic navigation diagnostic bronchoscopy in peripheral lung lesions. Chest. 2007;131:1800–1805. [DOI] [PubMed] [Google Scholar]
- 23.Wilson D, Bartlett R. Improved diagnostic yield of bronchoscopy in a community practice: combination of electromagnetic navigation system and rapid on-site evaluation. J Bronchol Intervent Pulmonol. 2007;14:227–232. [Google Scholar]
- 24.Lamprecht B, Porsch P, Pirich C, et al. Electromagnetic navigation bronchoscopy in combination with PET-CT and rapid on-site cytopathologic examination for diagnosis of peripheral lung lesions. Lung. 2009;187:55–59. [DOI] [PubMed] [Google Scholar]
- 25.Eberhardt R, Anantham D, Ernst A, et al. Multimodality bronchoscopic diagnosis of peripheral lung lesions: a randomized controlled trial. Am J Respir Crit Care Med. 2007;176:36–41. [DOI] [PubMed] [Google Scholar]
- 26.Bertoletti L, Robert A, Cottier M, et al. Accuracy and feasibility of electromagnetic navigated bronchoscopy under nitrous oxide sedation for pulmonary peripheral opacities: an outpatient study. Respiration. 2009;78:293–300. [DOI] [PubMed] [Google Scholar]
- 27.Seijo LM, de Torres JP, Lozano MD, et al. Diagnostic yield of electromagnetic navigation bronchoscopy is highly dependent on the presence of a Bronchus sign on CT imaging: results from a prospective study. Chest. 2010;138:1316–1321. [DOI] [PubMed] [Google Scholar]
- 28.Eberhardt R, Morgan RK, Ernst A, et al. Comparison of suction catheter versus forceps biopsy for sampling of solitary pulmonary nodules guided by electromagnetic navigational bronchoscopy. Respiration. 2010;79:54–60. [DOI] [PubMed] [Google Scholar]
- 29.Mahajan AK, Patel S, Hogarth DK, et al. Electromagnetic navigational bronchoscopy: an effective and safe approach to diagnose peripheral lung lesions unreachable by conventional bronchoscopy in high-risk patients. J Bronchol Interv Pulmonol. 2011;18:133–137. [DOI] [PubMed] [Google Scholar]
- 30.Brownback KR, Quijano F, Latham HE, et al. Electromagnetic navigational bronchoscopy in the diagnosis of lung lesions. J Bronchol Interv Pulmonol. 2012;19:91–97. [DOI] [PubMed] [Google Scholar]
- 31.Jensen KW, Hsia DW, Seijo LM, et al. Multicenter experience with electromagnetic navigation bronchoscopy for the diagnosis of pulmonary nodules. J Bronchol Interv Pulmonol. 2012;19:195–199. [DOI] [PubMed] [Google Scholar]
- 32.Lamprecht B, Porsch P, Wegleitner B, et al. Electromagnetic navigation bronchoscopy (ENB): Increasing diagnostic yield. Respir Med. 2012;106:710–715. [DOI] [PubMed] [Google Scholar]
- 33.Pearlstein DP, Quinn CC, Burtis CC, et al. Electromagnetic navigation bronchoscopy performed by thoracic surgeons: one center’s early success. Ann Thorac Surg. 2012;93:944–949; discussion 949–950. [DOI] [PubMed] [Google Scholar]
- 34.Loo FL, Halligan AM, Port JL, et al. The emerging technique of electromagnetic navigation bronchoscopy-guided fine-needle aspiration of peripheral lung lesions: promising results in 50 lesions. Cancer Cytopathol. 2014;122:191–199. [DOI] [PubMed] [Google Scholar]
- 35.Bowling MR, Kohan MW, Walker P, et al. The effect of general anesthesia versus intravenous sedation on diagnostic yield and success in electromagnetic navigation bronchoscopy. J Bronchol Interv Pulmonol. 2015;22:5–13. [DOI] [PubMed] [Google Scholar]
- 36.Wang Memoli JS, Nietert PJ, Silvestri GA. Meta-analysis of guided bronchoscopy for the evaluation of the pulmonary nodule. Chest. 2012;142:385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gex G, Pralong JA, Combescure C, et al. Diagnostic yield and safety of electromagnetic navigation bronchoscopy for lung nodules: a systematic review and meta-analysis. Respiration. 2014;87:165–176. [DOI] [PubMed] [Google Scholar]
- 38.Lee NK, Park CM, Kang CH, et al. CT-guided percutaneous transthoracic localization of pulmonary nodules prior to video-assisted thoracoscopic surgery using barium suspension. Korean J Radiol. 2012;13:694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rotolo N, Floridi C, Imperatori A, et al. Comparison of cone-beam CT-guided and CT fluoroscopy-guided transthoracic needle biopsy of lung nodules. Eur Radiol. 2016;26:381–389. [DOI] [PubMed] [Google Scholar]
- 40.Abi-Jaoudeh N, Fisher T, Jacobus J, et al. Prospective randomized trial for image-guided biopsy using cone-beam ct navigation compared with conventional CT. J Vasc Interv Radiol. 2016;27:1342–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pritchett M, Radaelli A, Schampaert S, et al. Cone Beam CT-guided endobronchial biopsy assisted by augmented fluoroscopy. Chest. 2017;152:A877; Meeting Abstract. [Google Scholar]
- 42.de Groot JA, Bossuyt PM, Reitsma JB, et al. Verification problems in diagnostic accuracy studies: consequences and solutions. BMJ. 2011;343:d4770. [DOI] [PubMed] [Google Scholar]
- 43.Hart D, Wall B. Radiation exposure of the UK population from medical and dental x-ray examinations. In. Vol W4. National Radiological Protection Board 2002. Available at: https://www.gov.uk/government/publications/medical-x-ray-examinations-doses-to-patients.
- 44.Wall B, Haylock R, Jansen J, et al. Radiation risks from medical x-ray examination as a function of age and sex of the patient. Health Protection Agency—Center for Radiation, Chemical and Environmental Hazards 2011. Available at: https://www.gov.uk/government/publications/medical-x-rays-radiation-risks-by-age-and-sex-of-patient.
- 45.Braak SJ, van Strijen MJ, van Es HW, et al. Effective dose during needle interventions: cone-beam CT guidance compared with conventional CT guidance. J Vasc Interv Radiol. 2011;22:455–461. [DOI] [PubMed] [Google Scholar]
- 46.Hohenforst-Schmidt W, Zarogoulidis P, Vogl T, et al. Cone beam computertomography (CBCT) in interventional chest medicine—high feasibility for endobronchial realtime navigation. J Cancer. 2014;5:231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park SC, Kim CJ, Han CH, et al. Factors associated with the diagnostic yield of computed tomography-guided transbronchial lung biopsy. Thorac Cancer. 2017;8:153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.bronchology.com.