Abstract
Objective
This study aimed to assess the relationship between BRCA1 gene methylation, PD-L1 protein expression, and the clinicopathologic features of sporadic ovarian cancer (OC).
Methods
Bisulfite pyrosequencing and immunohistochemistry were used to detect BRCA1 gene methylation and PD-L1 protein expression, respectively, in tumor tissues from 112 patients with sporadic OC. Their levels were analyzed against clinicopathologic characteristics and prognosis using standard statistical methods.
Results
Twenty percent (22/112) of the OC cases exhibited BRCA1 gene hypermethylation. The frequency of BRCA1 hypermethylation was significantly higher in serous OC (25%) than in nonserous OC (8%; P < 0.05). No significant correlations were discovered between BRCA1 hypermethylation and age, menstrual status, tumor location, stage, lymph node metastasis, and prognosis (P > 0.05). Among the 112 OC cases, 59% (66/112) cases were positive for PD-L1 protein expression. No significant difference existed between PD-L1 expression and age, menstrual status, histological type, tumor location, stage, lymph node metastasis, and prognosis (P > 0.05). Moreover, no correlation existed between BRCA1 methylation and PD-L1 expression (P > 0.05, r = 0.002).
Conclusions
This is the first study linking BRCA1 hypermethylation variability to PD-L1 protein expression and the clinicopathologic features of OC. The data demonstrated that an epigenetic alteration of BRCA1 was closely associated with serous OC. The expression of PD-L1 was unrelated to the clinicopathologic features or BRCA1 hypermethylation in sporadic OC.
Key Words: BRCA1, Promoter methylation, PD-L1, Ovarian cancer, Prognosis
Ovarian cancer (OC) is one of the most lethal gynecological cancers among women, with approximately 238,700 new cases and more than 150,000 deaths worldwide in the year of 2012.1 The standard and first-line treatment strategy for OC is cytoreductive surgery in combination with platinum-based and taxane-based chemotherapy. Although most patients initially achieve remission after primary chemotherapy, they relapse within 16 to 18 months and eventually die of the disease. To improve the prognosis of OC, studies have increasingly shifted their research focus to biological markers, such as genetic mutations, epigenetic alterations, and immune checkpoint proteins.
Breast cancer susceptibility gene 1 (BRCA1) has been under intense investigation since its identification in 1994. BRCA1 encodes a protein essential for double-stranded DNA break repair through a homologous recombination mechanism. The lifetime risk of OC is estimated to range from 35% to 59% for women with a BRCA1 mutation.2 Germline mutations within BRCA1 or BRCA2 account for approximately 25% of familial breast cancer–OC, which are clinically manifested as hereditary breast cancer and OC syndromes.3 The BRCA1 promoter hypermethylation is frequently detected in sporadic OC, whereas it has rarely been reported in samples from germline BRCA1 mutation cases.4 DNA methylation is mediated by DNA methyltransferase (DNMT) enzymes, which depend on the methyl donor S-adenosyl methionine. Within CpG dinucleotides, methyl is transferred to the 5′ carbon of the cytosine ring. When cytosines are methylated in the CpG island of a gene, the gene is silenced. This CpG island is termed “hypermethylated.” Studies have implied that DNA hypermethylation silences the gene that may be required for cancer development; therefore, the analysis of DNA hypermethylation is valuable for cancer prevention and treatment.
The current evidence clearly demonstrates that OCs are immunogenic malignancies. Tumors escape the host's immune attack through different pathways that constitute a cancer-immune escape system. The most crucial signaling pathway in the escape system is an immune checkpoint signal termed the programmed death-1 (PD-1) pathway. The PD-1 has 2 ligands, namely, PD-L1 and PD-L2.5 PD-L1 is regarded as the primary ligand of PD-1, although the interaction of PD-1 with PD-L2 has 2- to 6-fold higher affinity compared with the interaction of PD-1 with PD-L1.5 PD-L1 is not only expressed by activated cells including T cells, B cells, NK cells, dendritic cells, monocytes and macrophages, mesenchymal stem cells, and activated vascular endothelial cells, but also expressed in human carcinomas of the ovary, lung, and colon and in melanomas.6 Tumor-induced immune escape has been confirmed as a crucial factor in the promotion of tumor progression. Blocking PD-1 signaling is feasible and valid in several types of malignancies, such as melanoma, renal carcinoma, and non–small cell lung cancer. However, clinical trials on OC are rare, and the results have been limited and controversial.
One study demonstrated that BRCA1/2-mutated high-grade serous OC exhibited increased tumor-infiltrating lymphocyte (TIL) and PD-1/PD-L1 expression and that PD-1/PD-L1 inhibitors exerted remarkable efficacy against high-mutational-load cancers.7 An immune checkpoint blockade combined with BRCA1 inhibition induced protective antitumor immunity and considerable survival benefit in the BRCA1 mutated tumor model. Moreover, DNMT inhibitors (DNMTis) boosted tumor immunogenicity and immune response. To date, the correlations between clinicopathologic characteristics of sporadic OC and the status of BRCA1 methylation and PD-L1 expression have not been clearly clarified. In this study, we explored the correlation between BRCA1 and PD-L1 and their links to the clinicopathologic features of sporadic OC.
MATERIALS and METHODS
Patient Samples
A total of 112 cases were retrieved from clinical files at the Department of Obstetrics and Gynecology, Peking Union Medical College Hospital (PUMCH), the Chinese Academy of Medical Sciences, Beijing, China, during the period January 1, 2009, to December 31, 2010. All symptomatic patients presenting at PUMCH with OC were selected. The inclusion criteria were as follows: primary operable OC, no prior chemotherapies or radiotherapies before surgery, no family history of breast cancer or OC, and surgical specimens with sufficient tissue. Clinicopathologic information was available for all patients, namely, age, menstrual status, tumor location, histological type, grade, lymph node metastasis (LNM), International Federation of Gynecology and Obstetrics (FIGO) stage, and prognosis. This study was approved by the ethical committee of the institutional review board of PUMCH.
DNA Isolation, Extraction, and Pyrosequencing
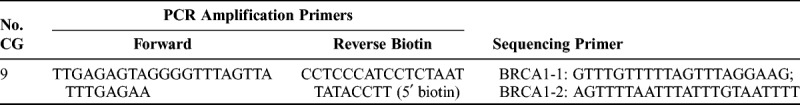
Genomic DNA was extracted from 112 formalin-fixed, paraffin-embedded (FFPE) OC tissue samples with a minimum of 75% malignant tumor cells. DNA isolation was performed according to the manufacturer's recommendations (QIAGEN, Suzhou, China). DNA concentration was determined using a Nanodrop8000 spectrophotometer (Thermo, Waltham, MA), and sodium bisulfite conversion of genomic DNA was performed using an Epitect Fast DNA Bisulfite Kit (QIAGEN). Quantitative DNA methylation detection was conducted through pyrosequencing. Segments of the BRCA1 gene were amplified and analyzed from bisulfite-converted DNA through polymerase chain reaction (PCR) using the primers described in Table 1 (QIAGEN). To isolate a single-stranded amplicon, the reverse primer was synthesized with a biotin moiety at the 5′ terminus in all PCR reactions. Polymerase chain reaction was performed in a 25-μL reaction volume and using a DNA Engine System (Bio-Rad, Hercules, CA). Nine CpG sites in BRCA1 (−1332 to −1268) were measured.
TABLE 1.
Primers of BRCA1 (breast cancer 1) used for pyrosequencing
After amplification, 10 μL of PCR product was mixed with Streptavidin Sepharose High Performance Beads (GE Healthcare, Chicago, IL) in a PyroMark binding buffer (QIAGEN) and diluted to a 70-μL total volume with ddH2O. Pyrosequencing was performed using a PyroMark Q24 real-time quantitative pyrophosphate sequence analyzer (QIAGEN). Nine CpG loci were detected for the BRCA1 gene, and the average methylation frequency at different sites was calculated to evaluate the methylation levels of the BRCA1 gene. Hypermethylation was determined when the index was 5% or greater.8
OC Tissue Immunohistochemistry Assays
The 112 FFPE OC samples were deparaffinized and then underwent antigen retrieval in a steam cooker for 1.5 minutes in edetate disodium at pH 9.0 (Maixin, Fuzhou, China). Immunohistochemistry (IHC) staining of 4-μm sections of FFPE tissue was performed with anti–PD-L1 (clone SP142; Zsbio, Beijing, China) primary monoclonal antibody. Universal secondary antibody (Agilent, Beijing, China) was applied for 15 minutes. Diaminobenzidine was used as a chromogen, and slides were counterstained with hematoxylin before mounting. Immunohistochemical evaluation of the PD-L1 in the OC specimens was based on relevant reports. Tumor samples were scored with 0 (no positive staining), 1+ (weak cytoplasmic or membranous staining in <10% of the positive cells), 2+ (weak to moderate cytoplasmic or membranous staining in ≥10% of the positive cells), or 3+ (strong cytoplasmic or membranous staining in ≥10% of the positive cells). Tumors were considered PD-L1 positive (2+ and 3+) when more than 10% of the tumor cells were positively stained in IHC (Fig. 1).9
FIGURE 1.
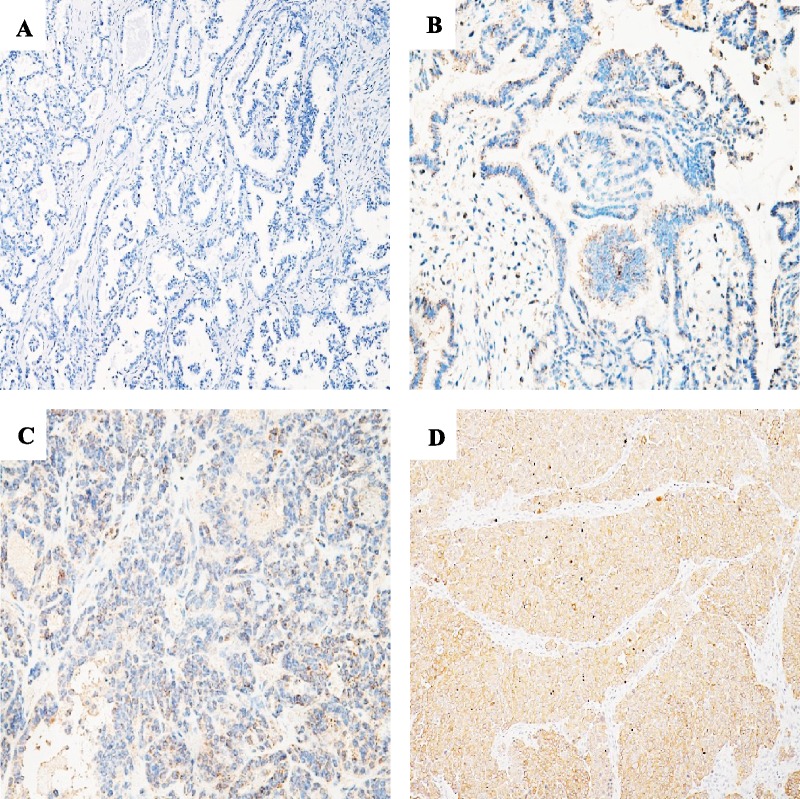
Images of IHC staining of PD-L1 in OC. A, PD-L1–negative expression in OC cells (0+; original magnification ×100). B, Score PD-L1 expression as 1+ (×200). C, Score PD-L1 expression as 2+ (×200). D, Score PD-L1 expression as 3+ (×200).
Statistical Methods
Data were analyzed using SPSS version 18.0 (SPSS Inc, Chicago, IL). To investigate the association between OC clinicopathologic parameters, BRCA1 promoter methylation status, and level of PD-L1 expression in OC cells, Pearson χ2 test was used. Disease-free survival (DFS) was defined as the time from diagnosis until the first recurrence or death due to OC, and overall survival (OS) was defined as time from diagnosis to date of death. Disease-free survival and OS were estimated using the Kaplan-Meier and multivariable Cox regression methods. The follow-up was defined as beginning on the day of operation. Pearson correlation analysis was performed to investigate the correlation of BRCA1 promoter methylation with PD-L1 expression in OC cells. A follow-up was conducted before July 1, 2017. All P values reported are 2-tailed, and the significance level is 5% (P < 0.05).
RESULTS
Clinicopathologic Characteristics of the 112 Sporadic OCs
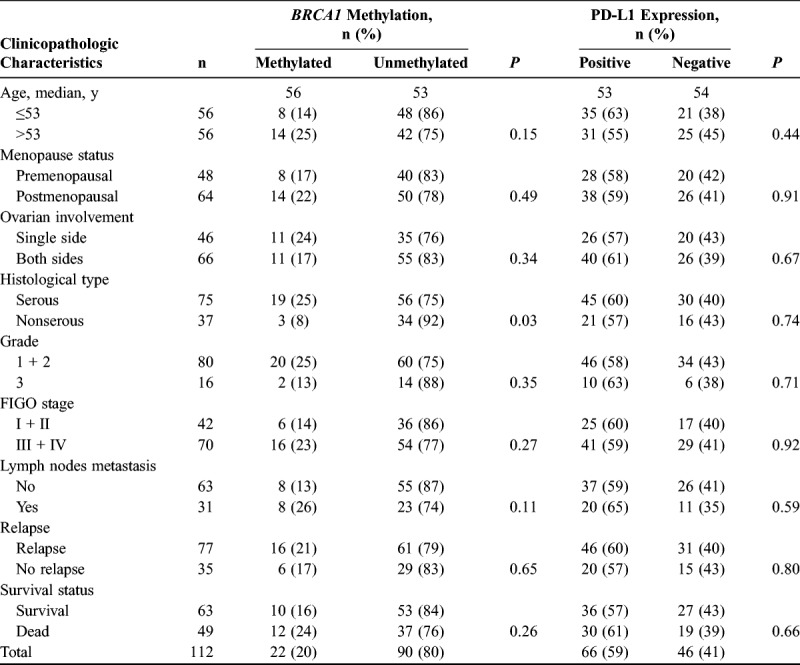
The clinicopathologic parameters, BRCA1 promoter methylation, and PD-L1 expression status of the 112 sporadic OC cases are summarized in Table 2. The median age of the patients was 53 years (range, 19–75 years). The average methylation frequency of 9 loci was 10%, 9%, 10%, 8%, 10%, 9%, 9%, 11%, and 8%, respectively. Twenty percent (22/112) of the sporadic OCs exhibited BRCA1 promoter methylation. Although no significant association existed between BRCA1 promoter methylation and patient age, menstruation status, tumor location, tumor grade, FIGO stage, LNM, recurrence, or survival status (P > 0.05), BRCA1 methylation was significantly correlated with serous cancer type (P = 0.03). BRCA1 promoter hypermethylation was 25% (19/75) in serous OC but only 8% (3/37) in nonserous OC. Among the 112 OC cases, 59% (66/112) were positive for PD-L1 protein expression and 41% (46/112) were negative. We did not observe a significant association between PD-L1 expression and patient age, menstruation status, tumor location, histological type, grade, FIGO stage, LNM, recurrence, or survival status (P > 0.05).
TABLE 2.
Tumor clinicopathologic characteristics, BRCA1 methylation, and PD-L1 expression in 112 consecutive sporadic OC samples
Survival Analyses
Survival data were most recently updated on July 1, 2017, and the median follow-up was 66 months (range, 0.5–101 months). Tumor location, histological type, grade, FIGO stage, and LNM were associated with DFS (P < 0.05). Cases with bilateral, serous, high-grade, and advanced FIGO stages and LNM were found to have a higher likelihood of relapse. Advanced FIGO stage and LNM were associated with shorter OS (P < 0.05). Among the 112 OC cases, the relapse rate was 69% (77/112). The relapse rate for BRCA1-methylated OC was 73% (16/22), which was slightly higher than that for nonmethylated OC (68%; 61/90); however, this difference was nonsignificant (P = 0.68). According to the Kaplan-Meier analysis indicated in Figure 2A, although the median OS time for BRCA1-methylated cases was shorter (47 months) than that for nonmethylated cases (67 months), no significant difference was discovered (P = 0.19). For PD-L1 protein expression, the median survival time was 67 months in the PD-L1–positive group, whereas the median survival time was 63 months in the negative group. No significant difference existed between the PD-L1–positive and PD-L1–negative groups regarding DFS and OS (P = 0.86 and P = 0.83, respectively).
FIGURE 2.
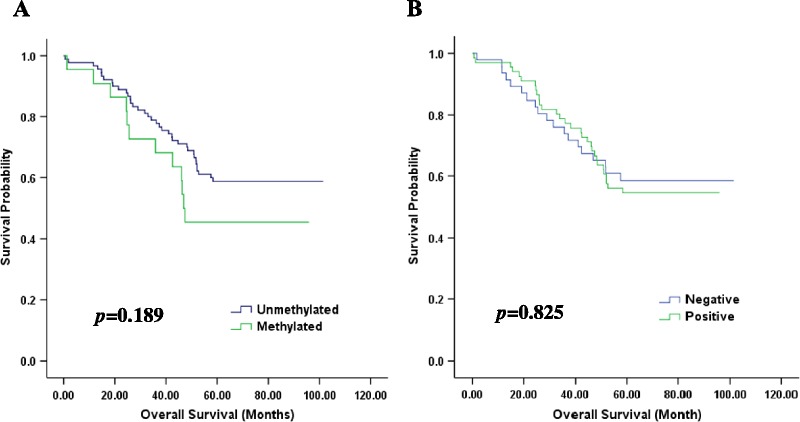
Kaplan-Meier curves of OS for sporadic OC patients with BRCA1 gene methylation (A) and PD-L1 protein expression (B).
Multivariate analyses revealed that FIGO stage was independently associated with DFS (P < 0.01; 95% confidence interval, 3.55–18.03) and OS (P < 0.01; 95% confidence interval 1.81–10.58). BRCA1 promoter methylation and PD-L1 expression in OC cells were not independent factors for DFS and OS (P > 0.05).
Correlation Between BRCA1 Promoter Methylation and PD-L1 Expression
To investigate the correlation between BRCA1 promoter methylation and PD-L1 expression in OC, Pearson correlation analysis was performed. No significant correlation was discovered between them (P = 0.99, r = 0.002).
DISCUSSION
The BRCA1 gene plays a major role in DNA double-strand breaks through the homologous dependent repair pathway, and its inactivation is linked to reduced BRCA1 protein expression and functionality, which eventually leads to a high risk of breast cancer or OC. BRCA1 mutation is known to be highly related to familial-BRCA1 cancers; however, BRCA1 promoter hypermethylation does not occur in patients with damaging germline or somatic mutations. These findings indicate that methylation is an alternate mechanism for BRCA1 silencing that has a similar role in ovarian tumorigenesis to that of BRCA1 mutation. The prognostic function of BRCA1 in sporadic OC is still in debate.
The hypermethylation rate of BRCA1 in OC was reported to be 5% to 89.9%. In our study, we adopted 5% as the methylation cutoff value according to existing studies, and the hypermethylation rate of BRCA1 was 20% (22/112). We speculate that cutoff value variation, histological heterogeneity of OCs, and differences in sample processing and assay design might account for the different DNA methylation frequencies obtained by studies. The frequency of BRCA1 hypermethylation was markedly higher in serous OC in this study, which is similar to the findings reported in previous publications.10,11 We did not observe a close relationship between BRCA1 promoter hypermethylation and prognosis (DFS and OS) of OC, which was also in accordance with a previous study.8
Immune evasion is a hallmark of tumorigenesis and cancer development. One of the most effective evasion tactics adopted by malignant tumors is to impair the potency of tumor immunity. As the fields of oncology and immunology have developed, it has been confirmed that OCs are immunogenic tumors. The number of CD8+ TILs was significantly associated with tumor grade and disease stage.12 Tumor-infiltrating lymphocytes were also related to the prognosis in OC which indicated that TILs might serve as a novel prognostic marker.12 Ovarian cancer cases with BRCA1 methylation had more intraepithelial TILs compared with those without BRCA1 abnormalities.13 Moreover, DNMTis boost tumor immunogenicity and immune response.14 To evaluate the correlation of immune checkpoints (PD-L1, PD-L2, PD-1, and CTLA-4) with DNMTi in patients with myeloid malignancies, a study evaluated the effect of the treatment of leukemia cells with decitabine (DAC) and revealed that DAC leads to dose-dependent upregulation of these 4 genes.15 Another study demonstrated that DNMTi reversed the repression of Th1-type chemokines CXCL9 and CXCL10, increased effector T-cell infiltration in the tumor site, and thus improved the therapeutic efficacy of adoptive T-cell transfusion and PD-L1 inhibitor in mice with OC.16 Moreover, mice treated with PD-L1 checkpoint blockade or DZNep (an inhibitor of S-adenosyl methionine–dependent enzymes) plus DAC exhibited decreased tumor size and increased CD8+ T lymphocyte and Th1-type chemokine expression.16 Therefore, the inhibition of DNA hypermethylation synergistically increased the therapeutic efficacy of anti–PD-L1 pathway therapy in malignant tumors. In the present study, we identified a correlation between BRCA1 methylation and the expression of PD-L1 in OC and thus indicated the possibility of a strategy combining DNMTis with PD-L1 inhibitors for OC therapy, particularly for drug-resistant OC. Several agents that block the PD-1 pathway have been approved by the Food and Drug Administration, such as pembrolizumab and nivolumab.
Because of the stability, sensitivity, specificity, and restriction to limited regions of DNA hypermethylation in comparison with DNA mutations, hypermethylation has potential as a biomarker. In our study, no significant difference was observed between BRCA1 hypermethylation and the clinicopathologic features of OC; we proposed that several factors should be considered before using OC hypermethylation in clinical practice. Ovarian cancer is a heterogeneous tumor, and the frequency of DNA methylation varies greatly with gene and tumor type. Moreover, the cutoff value is supposed to be standardized. Furthermore, compared with an individual gene, a panel of gene methylations could be explored in the future, which can increase the test's sensitivity and specificity. In addition, the characteristics of DNMTis such as low specificity, substantial toxicity, and poor bioavailability should not be neglected. Although the epigenetics of OC is still in its infancy, developments in oncologic epigenetics and immunology will move gene hypermethylation applications into new territory.
The predictive value of PD-L1 is inconsistent in patients with cancer. A study demonstrated that PD-L1 had no significant correlation with various clinicopathologic factors in OC, namely, age, primary tumor status, LNM, distant metastasis, histological type, residual tumor status, and chemotherapy.17 Other studies discovered that PD-L1 mRNA expression was associated with longer recurrence-free survival in breast cancer18 and that PD-L1–positive cases were significantly associated with poor prognosis in gastric cancer.19 In the present study, using a 10% threshold, positive tumor PD-L1 immunoreactivity was detected in 66 (59%) patients, and the remaining 46 (41%) patients had less than 10% tumor immunoreactivity, or complete absence or weak staining of PD-L1. No significant correlations were observed between PD-L1 expression and age, menstrual status, histological type, tumor location, stage, LNM, or prognosis (P > 0.05), which was similar to the finding of a previous study.20 Several reasons may account for these results. The scoring systems and cutoff values used to define PD-L1–positive expression were not the same. Moreover, different anti–PD-L1 antibodies were used with different staining platforms and protocols, which can cause different staining patterns. The population enrolled in the present study was relatively small, which could have limited statistical estimations. Because of our limited number of cases, a larger study to confirm the relationship between BRCA1 gene methylation, PD-L1 protein expression, TILs and the clinicopathologic features of sporadic OC is required in the future.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- 2.Eoh KJ, Park HS, Park JS, et al. Comparison of clinical outcomes of BRCA1/2 pathologic mutation, variants of unknown significance, or wild type epithelial ovarian cancer patients. Cancer Res Treat. 2017;49:408–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strumidło A, Skiba S, Scott RJ, et al. The potential role of miRNAs in therapy of breast and ovarian cancers associated with BRCA1 mutation. Hered Cancer Clin Pract. 2017;15:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bol GM, Suijkerbuijk KP, Bart J, et al. Methylation profiles of hereditary and sporadic ovarian cancer. Histopathology. 2010;57:363–370. [DOI] [PubMed] [Google Scholar]
- 5.Youngnak P, Kozono Y, Kozono H, et al. Differential binding properties of B7-H1 and B7-DC to programmed death-1. Biochem Biophys Res Commun. 2003;307:672–677. [DOI] [PubMed] [Google Scholar]
- 6.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. [DOI] [PubMed] [Google Scholar]
- 7.Strickland KC, Howitt BE, Shukla SA, et al. Association and prognostic significance of BRCA1/2-mutation status with neoantigen load, number of tumor-infiltrating lymphocytes and expression of PD-1/PD-L1 in high grade serous ovarian cancer. Oncotarget. 2016;7:13587–13598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruscito I, Dimitrova D, Vasconcelos I, et al. BRCA1 gene promoter methylation status in high-grade serous ovarian cancer patients—a study of the tumour Bank ovarian cancer (TOC) and ovarian cancer diagnosis consortium (OVCAD). Eur J Cancer. 2014;50:2090–2098. [DOI] [PubMed] [Google Scholar]
- 9.Guo L, Li W, Zhu X, et al. PD-L1 expression and CD274 gene alteration in triple-negative breast cancer: implication for prognostic biomarker. Springerplus. 2016;5:805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang HJ, Liu VW, Wang Y, et al. Differential DNA methylation profiles in gynecological cancers and correlation with clinico-pathological data. BMC Cancer. 2006;6:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Widschwendter M, Zikan M, Wahl B, et al. The potential of circulating tumor DNA methylation analysis for the early detection and management of ovarian cancer. Genome Med. 2017;9:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsushita N, Ghazizadeh M, Konishi H, et al. Association of ovarian tumor epithelium coexpressing HLA-DR and CA-125 antigens with tumor infiltrating cytotoxic T lymphocytes. J Nippon Med Sch. 2003;70:40–44. [DOI] [PubMed] [Google Scholar]
- 13.Clarke B, Tinker AV, Lee CH, et al. Intraepithelial T cells and prognosis in ovarian carcinoma: novel associations with stage, tumor type, and BRCA1 loss. Mod Pathol. 2009;22:393–402. [DOI] [PubMed] [Google Scholar]
- 14.Tomasi TB, Magner WJ, Khan AN. Epigenetic regulation of immune escape genes in cancer. Cancer Immunol Immunother. 2006;55:1159–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang H, Bueso-Ramos C, DiNardo C, et al. Expression of PD-L1, PD-L2, PD-1 and CTLA4 in myelodysplastic syndromes is enhanced by treatment with hypomethylating agents. Leukemia. 2014;28:1280–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peng D, Kryczek I, Nagarsheth N, et al. Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nature. 2015;527:249–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamanishi J, Mandai M, Iwasaki M, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007;104:3360–3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schalper KA, Velcheti V, Carvajal D, et al. In situ tumor PD-L1 mRNA expression is associated with increased TILs and better outcome in breast carcinomas. Clin Cancer Res. 2014;20:2773–2782. [DOI] [PubMed] [Google Scholar]
- 19.Qing Y, Li Q, Ren T, et al. Upregulation of PD-L1 and APE1 is associated with tumorigenesis and poor prognosis of gastric cancer. Drug Des Devel Ther. 2015;9:901–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li MJ, Li HR, Cheng X, et al. [Clinical significance of targeting drug-based molecular biomarkers expression in ovarian clear cell carcinoma]. Zhonghua Fu Chan Ke Za Zhi. 2017;52:835–843. [DOI] [PubMed] [Google Scholar]


