Supplemental Digital Content is available in the text.
Keywords: angiogenesis, endothelial cells, ischemia, pericytes, peripheral vascular diseases
Abstract
Objective—
Angiogenesis, entire step from endothelial cells (ECs) sprouts to vascular maturation, is a critical response to ischemia. To form functional mature vessels, interactions between ECs and pericytes are essential. Ninj1 (ninjurin1) is an adhesion molecule that contributes to the pathogenesis of neuroinflammation. We recently demonstrated that Ninj1 is expressed in pericytes during angiogenesis. However, the role of Ninj1 in angiogenesis under pathophysiological ischemic conditions has not yet been elucidated.
Approach and Results—
Ninj1 was detected in microvessels, and its expression was enhanced in ischemic tissues after mouse hindlimb ischemia. Knockdown of Ninj1 was performed by injection of biodegradable microspheres releasing Ninj1-small interfering RNA into muscle tissues. Alternatively, pericyte-specific Ninj1 knockout was induced by tamoxifen treatment of NG2-CreERT/Ninj1-flox mice. Ninj1 knockdown/knockout reduced the formation of blood-circulating functional vessels among total CD31+ microvessels within ischemic tissues and subsequently attenuated color Doppler–assessed blood flow recovery. Ninj1 overexpression enhanced expression of Anpt (angiopoietin) 1, whereas Ninj1 knockdown enhanced the endogenous Anpt1 antagonist, Anpt2 expression in pericytes and inhibited the association of pericytes with ECs and subsequent formation of capillary-like structure, that is, EC tube surrounded with pericytes in 3-dimensional gel culture.
Conclusions—
Our data demonstrate that Ninj1 is involved in the formation of functional matured vessels through the association between pericytes and ECs, resulting in blood flow recovery from ischemia. These findings further the current our understanding of vascular maturation and may support the development of therapeutics for ischemic diseases.
Ischemic cardiovascular and cerebrovascular disorders are leading causes of mortality worldwide, representing a major economic and resource burden on health and public health systems. Preclinical and clinical trials of angiogenic therapies for cardiovascular ischemic diseases produced unfulfilled promises.1 Clinical designs for these trials have not always considered the entire mechanism of angiogenesis, including sprouting of endothelial cells (ECs) and further vascular maturation. For therapeutic angiogenesis, appropriate angiogenic processes, not only during the early step of angiogenesis, that is, EC sprouting, but also vascular maturation should be considered for the establishment of functional vasculature in ischemic tissues.
Ischemia prompts angiogenesis, the formation of new microvessels from the walls of existing vessels, via an initial process termed EC sprouting. Mature vessels are structurally stable, resistant to regression, and functional for the regulation of blood circulation depending on the circumstances.2,3 Pericytes play a pivotal role in the entire angiogenic process from initial EC sprouting to later vascular maturation.4 For the initial step of angiogenesis, pericytes are detached from existing vessel walls, and the resultant plasticity window in the vascular walls allows EC sprouting. Free detached pericytes are located at the growing front of EC sprouts and participate in angiogenesis by guiding newly formed vessels through tissue invasion and release of EC growth factors.5,6 In contrast, the later stage of angiogenesis is vascular maturation, which is strongly associated with the recruitment of pericytes to the immature vessels. The recruited pericytes suppress the growth and migration of ECs, thereby inducing vascular stabilization and maturation.2,3 Therefore, the dynamic interaction between pericytes and ECs regulates angiogenesis from the initial step to later vascular maturation. Although several pathways mediating the angiogenic effects of pericytes have been documented,3,7 the mechanisms that regulate their actions in vascular maturation and stabilization are not completely defined.
Nerve injury–induced protein (Ninj1 [ninjurin 1]) was originally identified as a membrane protein expressed in neuronal cells in response to nerve injury and contributes to nerve regeneration, for example, promoting neurite outgrowth from the dorsal root ganglion.8–10 To date, Ninj1 is known to be widely expressed in several types of tissues or cells, including the epithelia and blood cells, and is involved in diverse pathophysiological conditions, including neuronal regeneration and inflammation.11–14 Being located at the cell surface, Ninj1 mediates homophilic cellular adhesion,8,9 contributes to the trafficking and transmigration of inflammatory cells across the blood-brain barrier, and emphasizes the recruitment of myeloid cells to neuroinflammatory lesions.12,13,15 Recently, Ninj1 was reported to also contribute to angiogenesis. Ninj1 expressed in macrophages contributes to the regression of hyaloid blood vessels through interaction between macrophages and vascular ECs during early ocular development.11 In a diabetic mouse model, enhanced expression of Ninj1 reduces angiogenesis in the penile tissues and induces erectile dysfunction.14 These findings suggest that Ninj1 is a negative regulator of angiogenesis. However, the role of Ninj1 in angiogenesis under pathophysiological conditions in peripheral tissues, excluding specific organs such as the penile tissue, has not been elucidated.
We recently showed that Ninj1 is a novel factor that modulates pericyte function in angiogenesis.16 Pericytes have trophic effects on angiogenesis, that is, induce the production of growth factors and stimulate EC growth/sprout from vessel walls. Through in vitro angiogenesis experiments, we demonstrated that Ninj1 negatively regulates the formation of EC tubes by reducing the trophic effects of pericytes. This finding is consistent with previous reports stating that Ninj1 negatively regulates angiogenesis.14 However, inhibition of EC growth by pericytes may not always induce the regression of vessels and is also associated with vascular maturation to form functional vessels. Accordingly, in this study, we examined the role of Ninj1 in angiogenesis under pathological peripheral ischemia using a pericyte-specific Ninj1-knockout murine model of hindlimb ischemia (HLI).
Materials and Methods
The authors declare that all supporting data are available within the article and its online-only Data Supplement, including Major Resources Table.
Animals
All experiments involving animals were performed according to protocols approved by the Animal Care and Use Committee of Asahikawa Medical University. Animals were maintained in a temperature- and light-controlled facility and were fed normal chow. Male C57BL/6 mice aged 12 weeks were used for the in vivo experiments. Only male mice were used to exclude any effects of female hormones, such as estrogen, in this study. To generate tamoxifen (Tm)-inducible Ninj1 knockout mice, Ninj1loxp mice (Ninj1(tm1a/KOMP)Wts, KOMP Repository) were crossed with NG2 (also known as CSPG4)-CreER transgenic mice (Jackson Laboratories), which express CreER under the control of NG2 promoter.17 To induce Cre recombination, adult NG2-CreER/Ninj1loxP mice (12–18 weeks of age) were treated with Tm (Sigma-Aldrich) by a single intraperitoneal injection daily for 7 consecutive days at a dosage of 50 mg/kg per day. Tm was dissolved in 10% ethanol and 90% corn oil (Sigma-Aldrich) to prepare a 20-mg/mL solution that was stored in the dark until usage. The corresponding vehicle-treated NG2-CreER/Ninj1loxP or Tm-treated NG2-CreER mice were prepared simultaneously as controls 1 and 2, respectively. After Tm or its vehicle administration, mice were housed individually for 1 week just before HLI operation. For confirmation of pericyte-specific knockout of Ninj1 gene, Ninj1expression in NG2+ cells was estimated by real-time polymerase chain reaction.16,18
HLI Model
Unilateral HLI models were established by ligation and excision of the femoral artery and vein as previously described.19 These models successfully reduced individual differences and severe HLI by removing the subcutaneous fatty tissues from the lower limbs. For further consistency in measurement, imaging was performed after mice had been placed in a heating cage at 37°C to minimize temperature variation. The calculated recovery of blood flow (RBF) was expressed as the ratio of the left (ischemic) to the right (normal) limb. To reduce the expression of Ninj1 in skeletal muscles, bioabsorbable microparticles (microsphere E50, MedGEL) releasing Ninj1-small interfering RNA (siRNA) were injected into muscle tissues just before the HLI operation.20 Briefly, 10 µL siRNA (50 µmol/L; either Ninj1-siRNA or its scramble-siRNA as control) were added to 1 mg of microsphere and incubated at 37°C for 1 hour. The microsphere was resuspended with saline at a concentration of 30 mg/mL, and 100 µL of microsphere solution were injected at 5 to 7 sites in the hindlimb. For confirmation of Ninj1 expression in hind limbs, the gastrocnemius muscle tissues at each time before and after HLI operation, Ninj1 expressions (gene expression and protein level) were measured by real-time polymerase chain reaction or Western blot analyses.
Immunostaining Analysis
The histological assessment of ischemic limb tissue was performed at 14 days after HLI surgery. Functional vessels were stained by injecting 300 µL PBS containing fluorescein isothiocyanate– or rhodamine-labeled Griffonia simplicifolia lectin I (100 µg/mL, Vector Laboratories) via the tail vein. After 5 minutes, the mice were euthanized and perfused through the heart with PBS followed by 4% paraformaldehyde in PBS. The gastrocnemius muscle was dissected and embedded in Tissue-Tek optimal cutting temperature compound. Target proteins in the cross-sections (10-µm thickness) or decolorized tissues were detected by immunohistochemical analysis using anti-CD31 (Abcam, ab7388, 1 μg/mL), anti-Ninj1 (Bioss Antibodies, bs-11105R, 10 μg/mL), anti-NG2 (Miltenyi Biotec, 130-097-455, 1 μg/mL), anti–platelet-derived growth factor receptor (PDGFR) β (Cell Signaling, no. 4564, 0.4 µg/mL), and Alexa 488-, 594-, and 647-conjugated secondary antibodies (Invitrogen, 1:1000). Nuclei were counterstained with Hoechst 33258 (Lonza). Fluorescence images were observed under a confocal fluorescence microscope (FV1000D Olympus, and BZ-X700 Keyence). To confirmation of specificities of each antigen-targeted antibody, nonimmune control IgG from identical host animal instead of primary antibody was used (Figure I in the.
For estimation of functional microvessels, in each animal, 5 randomly selected fields (≈0.35 mm2 area, ×200 magnitude) in the short-axis view of skeletal muscle cross-section were blindly analyzed. Total microvessels were detected by immunostaining of ECs-specific CD31. The number of microvessels was expressed per mm2 of tissue section. Blood-circulating vessels were stained by injection of rhodamine-conjugated lectin via the tail vein. Functional vessels were defined as a percentage (%) of lectin-stained vessels in total micorvessels per field. To quantitatively evaluate microvessels covered with pericytes, CD31-positive ECs colocalized with PDGFRβ-positive pericytes were detected in the tissue sections. Pericyte-associated microvesels were expressed as a percentage of PDGFRβ-positive cells–localized vessels in total microvessels per field.
Cell Preparation
Capillary derived immortalized ECs/pericytes or primary pericytes isolated form GFP (green fluorescent protein)–expressing mouse were used for in vitro experiments as described previously.18 Briefly, skeletal muscle stroma cells were prepared from gastrocnemius muscle tissues of Ninj1-knockout mouse (as described above) or GFP-expressing mice, and NG2+ pericytes were isolated from skeletal muscle stroma cells by using a fluorescence magnetic activated cell sorting or magnetic activated cell sorting systems with anti-NG2 antibody–conjugated microbeads. For label the living cells, the immortalized cells were infected with a retrovirus harboring the GFP or DsRed gene.
Gain- and loss-of-function experiments were performed as described previously.3 For downregulation of target genes, siRNA against mouse Ninj1, Anpt (angiopoietin) 1, 2, and control scramble-siRNA (Nippon Gene Material) were used. For Ninj1 overexpression, the complete sequence of mouse Ninj1 was purchased from GenScript, and an expression vector was constructed by using pcDNA3 (Invitrogen) as described previously.16 At 48 hours after transfection, cells were analyzed for gene expression and then used in in vitro angiogenesis assays. We confirmed that Ninj1 or Anpts expression is reduced or increased by transfection of Ninj1 or Anpt-siRNA or Ninj1 expression vector, respectively, by quantitative reverse transcription polymerase chain reaction as previously described.16
In Vitro 3-Dimensional Gel Angiogenesis Assay
The formation of capillary-like structures was performed as described previously.18,21 DsRed-expressing ECs and GFP-expressing pericytes were incubated in culture medium for 10 minutes at 37°C and mixed in growth factor–reduced Matrigel (BD Biosciences). Then, the cells in the gel were seeded on multiwell plates and incubated in a CO2 incubator along with endothelial basal medium-2 (PromoCell) containing 2% fetal bovine serum and 10 ng/mL vascular endothelial growth factor. The assay was performed 3 days after incubation. Images of tube formation in the gels were obtained by using a fluorescence microscope (BZ-X700, Keyence), and the length DsRed-EC tubes wrapped with or without GFP-pericytes in each well was measured at a screen with 40 magnification. The total length of the tubes formed (EC tubes wrapped with and without pericytes) and the ratio of the mature capillary-like tubes (EC tubes wrapped with pericytes) to the total tube length were calculated by using an angiogenesis image analysis software (KURABO).
Cell Cluster Assay
Cell cluster assay was performed as described previously8,9 with slight modification. Briefly, isolated cells were resuspended in complete DMEM containing 10% fetal bovine serum and an antibiotics cocktail,22 followed by incubation in ultralow attachment multiwell plates (Corning) for 2 hours with gentle agitation at 37°C. The cells were collected by centrifugation and resuspended in methylcellulose gels (MethoCult, StemCell Technologies) by gentle pipetting through a 1-mL tip. The cells/methylcellulose gels were applied to each well of the multiwell plate to form a 1-mm thin gel layer, and the cellular clusters formed were observed by phase-contrast microscopy (BZ-X700, Keyence). The size and number of cellular clusters were analyzed by the software attached to the microscope (BZ-H3A, Keyence). The cellular clusters were determined as the range of 900 to 20 000 μm2 (Figure IV in the online-only Data Supplement). According to Ninj1 dependency, the cellular clusters were classified as cluster I (Ninj1-independent clusters; 900–4000 μm2), cluster II (Ninj1-dependent pericytes clusters; 4001–11 000), and cluster III (Ninj1-dependent ECs/pericytes complexes 11 001–20 000; Figure IV in the online-only Data Supplement).
Cellular Function Assays
Cell viability was assessed through quantification of cell proliferation using WST1 reagent (Roche) as described previously.23 Cell migration activity was estimated by the in vitro scratch assay.24 Briefly, cells were grown until they formed a layer on the collagen-coated dish. They were then mechanically scratched with a pin array. The migrated cells in the wound healing field after scratching were visualized by 4′,6-diamidino-2-phenylindole fluorescence labeling 24 hours after scratching, and the number of migrated cells was counted.
Western Blot Analysis
Skeletal muscle tissues (the gastrocnemius muscle) were homogenized in lysis buffer (20 mmol/L Tris/HCl pH 7.5, 150 mmol/L NaCl, 1 mmol/L EDTA, 1 mmol/L EGTA, 1% Triton, 2.5 mmol/L Na pyrophosphate, 1 mmol/L β-glycerophsophate, 1 mg/mL leupeptin, 1 mmol/L phenylmethylsulfonyl fluoride) and solubilized by boiling in Laemmli buffer, fractionated by SDS-PAGE, and transferred to polyvinylidene fluoride membranes. The blots were incubated with the indicated antibodies, anti-Ninj1 (Santa Cruz, sc136295, 400 ng/mL), anti-βtubulin (Cell Signaling, no. 2128, 6 ng/mL) antibodies, followed to horseradish peroxidase–conjugated second antibodies. The reactive bands were visualized by use of Western Lightning Ultra (Perkin Elmer, NEL112001EA). The luminescent signals were determined using ImageQuant LAS500 (GE Healthcare Life Science).
Statistical Analysis
Normally distributed data and homogeneity of variance of each data were confirmed by F test and Bartlett test, respectively. A Student t test was used in 2-group comparisons. For comparisons of >2 groups, 1-way ANOVA was used for normal distributions. Blood flow recovery in the ischemic hindlimb was compared between the 2 groups by 2-way repeated measures ANOVA, followed by Turkey-Kramer analyses. P<0.05 was considered as statistically significant.
Results
Ninj1 Is Necessary for Blood Flow Recovery in HLI
To clarify the role of Ninj1 on angiogenesis in vivo, we prepared the HLI mouse model to induce skeletal muscle ischemia. Ninj1 was expressed mostly in the microvessels in normal and ischemic skeletal muscle tissues. Especially, Ninj1 was expressed dominantly in pericytes in ischemic muscle tissues (Figure 1A and 1B). Ninj1 gene expression in skeletal muscles was determined at the indicated times before and after HLI operation. Ninj1 gene expression level increased and reached a maximum level at 7 days after surgery and gradually decreased thereafter (Figure 1E). Next, Ninj1 expression was knocked down within the tissues during the HLI period (at least 2 weeks) by injection of bioabsorbable microparticles releasing Ninj1-specific siRNA into the muscle tissues. At 7 days after HLI operation, the immunostaining of Ninj1 in ischemic tissues was reduced compared with that in controls (Figure 1C and 1D). The protein expression of Ninj1 was enhanced in skeletal muscle tissues and reduced by injection of Ninj1-siRNA microparticles at 7 days after HLI operation (Figure II in the online-only Data Supplement). RBF in ischemic limbs was determined by laser Doppler imaging as described previously.19 Interestingly, RBF was significantly attenuated in the Ninj1 knockdown (Ninj1KD) group compared with that in control (Figure 1F and 1G).
Figure 1.
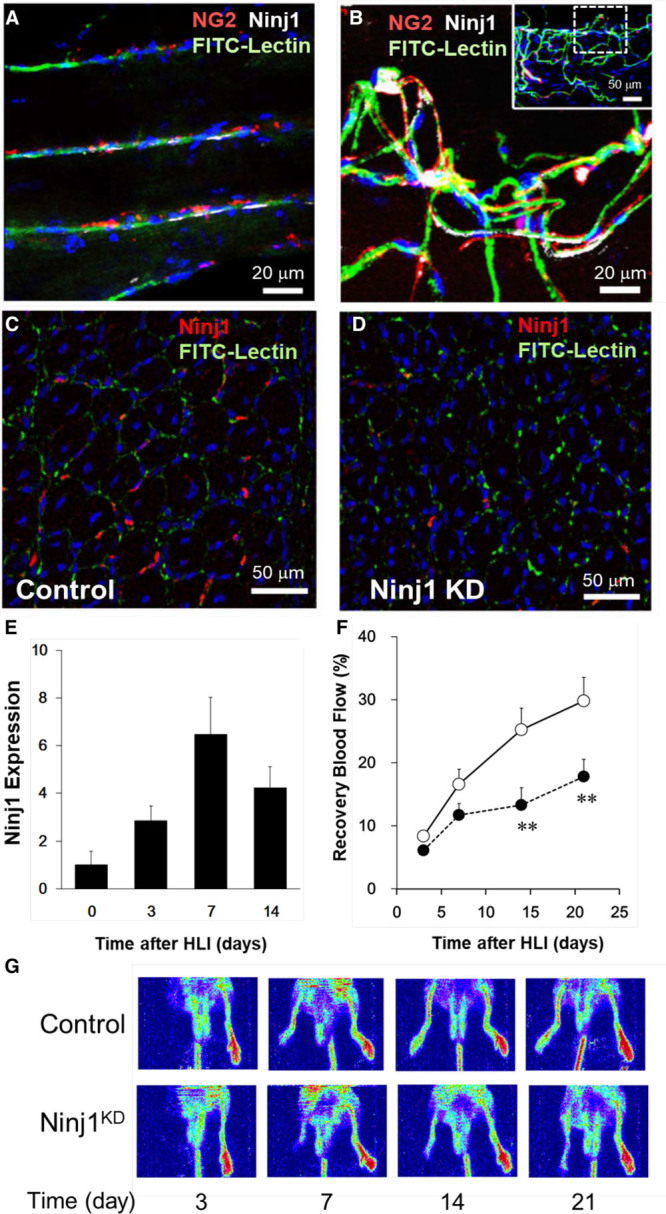
Small interfering RNA (siRNA)–mediated Ninj1 (ninjurin1) knockdown (KD) in skeletal muscle attenuates blood flow recovery in a murine model of hindlimb ischemia (HLI). Confocal fluorescence image of decolorized skeletal muscles of NG2promoter-DsRed mouse before (A) and after HLI (B). Vessel endothelium and pericytes around skeletal muscle fibers were stained by fluorescein isothiocyanate (FITC)-lectin (green) and anti-NG2 antibodies (red). Immunostaining signals of Ninj1 (white) are shown. C and D, The microspheres releasing Ninj1-siRNA (Ninj1KD) or scramble-siRNA (control) were injected into muscle tissues 1 d before the HLI operation. Then, CD31+microvessels (green) and Ninj1 (red) were immunostained in skeletal muscle sections at 7 d after HLI. Nuclei were counterstained with Hoechst 33258 (blue). E, Ninj1 mRNA level in skeletal muscles was estimated by quantitative reverse transcription polymerase chain reaction at each time point after the HLI operation. F, Ninj1KD ( ) and its control (
) and its control ( ) mice were subjected to HLI operation, and then peripheral blood flow (BF) was estimated by laser Doppler flow imaging. The ischemic to nonischemic BF ratio was calculated as recovery blood flow. Mean±SEM (n=8–10, **P<0.01 vs control at each time, 2-way repeated measures ANOVA and Turkey-Kramer analyses). G, Representative laser Doppler BF images at indicated times after HLI are shown.
) mice were subjected to HLI operation, and then peripheral blood flow (BF) was estimated by laser Doppler flow imaging. The ischemic to nonischemic BF ratio was calculated as recovery blood flow. Mean±SEM (n=8–10, **P<0.01 vs control at each time, 2-way repeated measures ANOVA and Turkey-Kramer analyses). G, Representative laser Doppler BF images at indicated times after HLI are shown.
Ninj1 Stimulates the Formation of Functional Vessels in Ischemic Tissues
To elucidate the mechanism by which Ninj1 rescues the tissues from ischemia, vessel formation within the ischemic skeletal muscle was observed at 14 days after HLI. The number of CD31-positive microvessels was significantly increased in the ischemic tissues compared with that in nonischemic tissues, and no significant difference was observed between the Ninj1KD and control groups (Figure 2A and 2B). In nonischemic tissues, ≈80% of the total CD31-positive vessels were lectin-positive functional vessels (Figure 2A and 2C). At 14 days after HLI, the growing neovessels partially formed functional matured vessels, and the ratio of functional vessels was relatively low at ≈40% in control ischemic tissues. The ratio of functional vessels in the Ninj1KD group was significantly decreased compared with that in the controls (Figure 2C).
Figure 2.
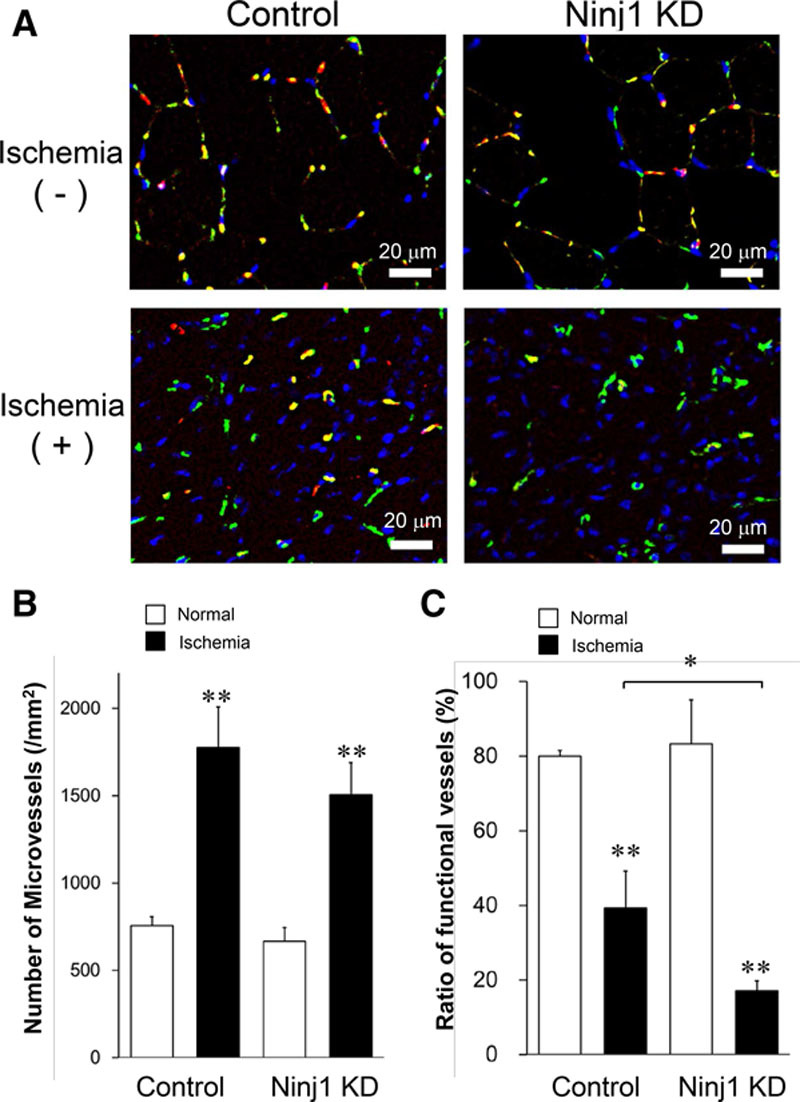
Ninj1 (ninjurin1) knockdown (KD) reduces the formation of functional vessels in ischemic muscles. A, Immunostaining of skeletal muscle tissue sections of ischemic and nonischemic limbs at 14 d after hindlimb ischemia in Ninj1KD and control mice. Microvessels were determined by CD31 staining (green), and functional microvessels were stained by intravenous injection of rhodamine-lectin (red). Nuclei were counterstained with Hoechst 33258 (blue). B, The total number of CD31+ microvessels on each tissue section was counted. C, The ratio of the number of functional vessels to total microvessels was calculated. Mean±SEM (n=8–10, *P<0.05, **P<0.01 vs white bar except indication, t test analysis).
Pericyte-Specific Knockout of Ninj1 Attenuates Blood Flow Recovery in HLI
Although Ninj1 was detected mostly in the microvessels of the skeletal muscles (Figure 1A and 1B), the possibility that injected Ninj1-siRNA affects other Ninj1-expressing cells, such as inflammatory cells,12,25 to modify angiogenesis in vivo remains. To address this concern, we prepared a Tm-induced NG2-CreER/Ninj1loxp mouse model, wherein Ninj1 gene was specifically deleted in pericytes. NG2- or PDGFRβ-positive cells were observed as pericytes of microvessels in skeletal muscle tissues (Figures 3B and 4B). Ninj1 was detected in the NG2-positive pericytes of skeletal muscle tissues in Tm-untreated NG2-CreER/Ninj1loxP mice but not in Tm-treated mice (Figure 3A). To confirm specific reduction of Ninj1 expression in pericytes, NG2-positive pericytes and -negative other cells were isolated from skeletal muscle tissues (Figure 3B and 3C). Ninj1 expression was significantly reduced in pericytes of Tm-treated, but not in Tm-untreated, NG2-CreER/Ninj1loxP, whereas Ninj1 expression in NG2-negative cells was not altered in the 2 groups (Figure 3D).
Figure 3.
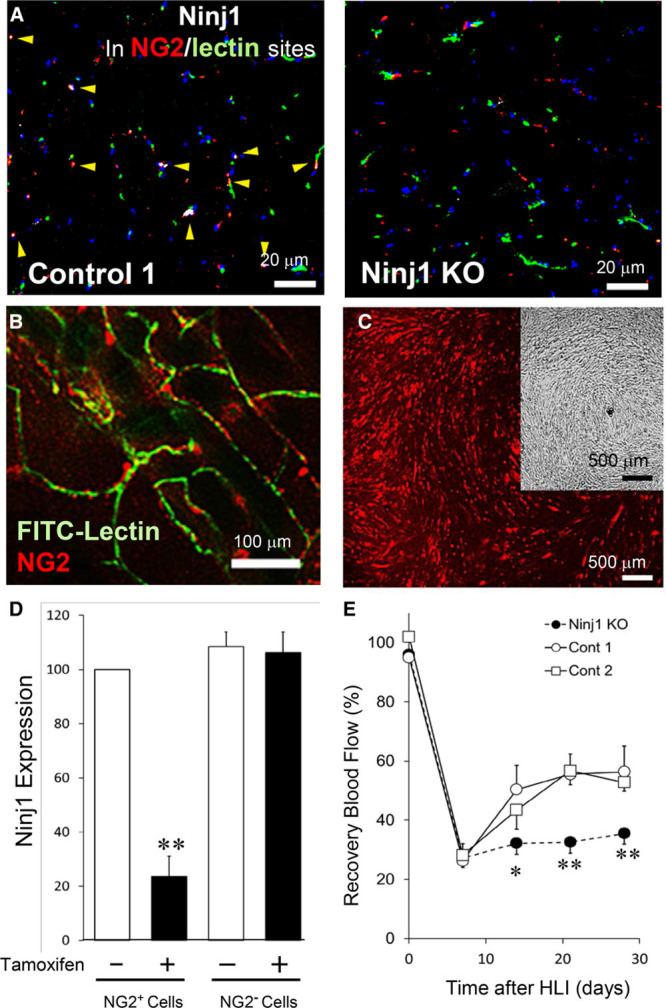
Pericytes-specific deletion of Ninj1 (ninjurin1) gene decreases blood flow recovery in hindlimb ischemia (HLI). A, Tamoxifen (Tm) treatment induced the knockout (KO) of Ninj1 gene in pericytes in NG2-CreER/Ninj1loxP mice. Vehicle-treated NG2-CreER/Ninj1loxP or Tm-treated NG2-CreER were used simultaneously as controls 1 and 2, respectively. Immunostaining of skeletal muscle sections of Ninj1 KO and control 1 mice. Microvessels were stained with fluorescein isothiocyanate (FITC)-lectin (green), and pericytes were stained with anti-NG2 antibody (red). Ninj1 expression was determined by anti–Ninj1 antibody (white, arrow head). Nuclei were counterstained with Hoechst 33258 (blue). B, Microvessels of decolorized skeletal muscle fibers of NG2promoter-DsRed mice were stained with FITC-lectin (green). Fluorescence image by confocal microscopy show that NG2+pericytes (red) were located at perivascular sites of microvessels. C, NG2+ pericytes (red) were isolated by magnetic bead sorting and incubated in culture dish. Phase-contrast view of cultured cells (insertion). D, NG2+ or NG2- cells were isolated from skeletal muscle tissues of Tm-treated (Ninj1KO) or nontreated (control 1, Cont1) NG2-CreER/Ninj1loxP mice, and the level of Ninj1 mRNA in each cellular group was estimated by quantitative reverse transcription polymerase chain reaction. The ratio of gene expression of Ninj-KO (black bar) to control 1 (white bar) was shown. Mean±SEM (n=4, **P<0.01 vs white bar, t test analysis). E, Ninj1KO, control 1, and control 2 (Tm-treated NG2-CreER) mice were prepared. Before and after the HLI operation of Ninj1 KO, control 1, or 2 mice, peripheral blood flow (BF) was estimated by laser Doppler flow imaging. The recovery of BF in ischemic limb was calculated at the indicated time. Mean±SEM (n=6, *P<0.05, **P<0.01 vs control at each time, 2-way repeated measures ANOVA and Turkey-Kramer analysis).
Figure 4.
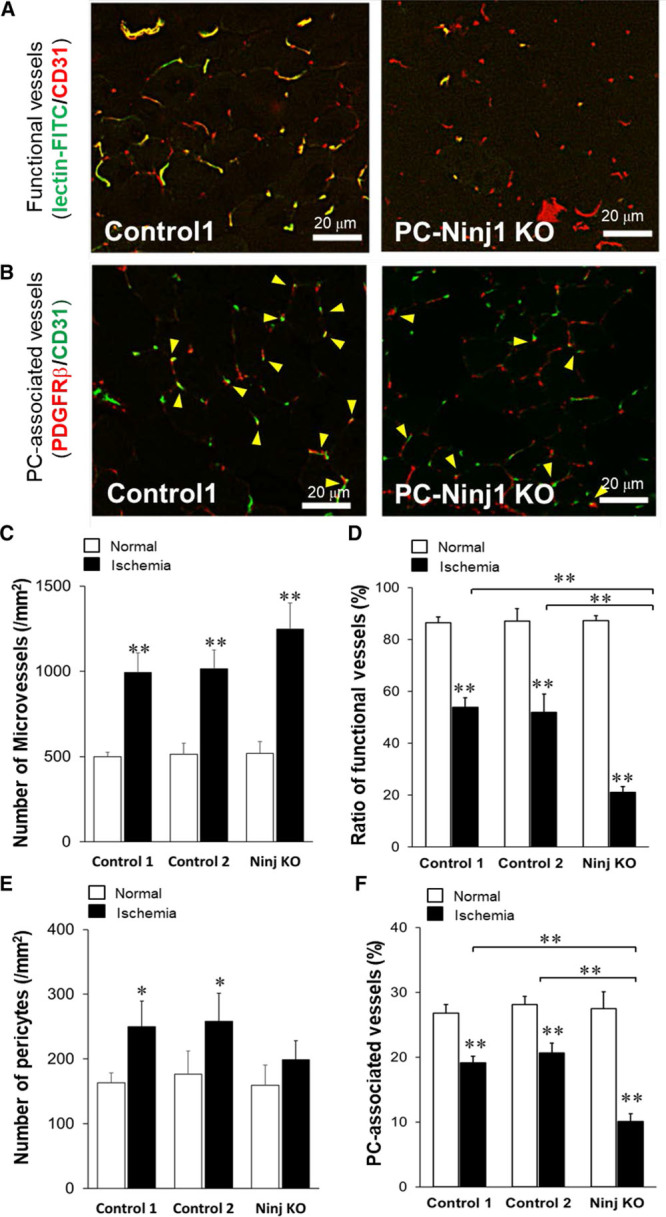
Ninj1 (ninjurin1)-knockout (KO) in pericytes (PCs) reduces the formation of functional matured vessels in ischemic tissues. A, Immunostaining of skeletal muscle tissue sections of PC-specific Ninj1KO and control 1 mice. Microvessels were determined by CD31 staining, and functional microvessels were stained by intravenous injection of fluorescein isothiocyanate (FITC)-lectin. B, PCs were also determined by platelet-derived growth factor receptor (PDGFR) β staining, and the number of CD31+microvessels and PDGFRβ+PCs-associated microvessels were blindly determined (arrow head). C, The total number of CD31+microvessels on each tissue section was counted. D, The ratios of lectin-stained functional vessels to CD31+ total microvessels were calculated. E, The number of PDGFRβ-stained PCs on each section was determined. F, PC-associated CD31+vessels to total microvessels were calculated. Mean±SEM (n=6, *P<0.05, **P<0.01 vs white bar except indication, ANOVA).
Using the Tm-inducible pericyte-specific Ninj1 knockout (PCNinj1 KO) mouse model, we prepared the HLI model and estimated RBF of ischemic tissues and performed further experiments. As shown in Figure 3E, RBF was significantly reduced in the PCNinj1 KO mice compared with that in the control groups, either Tm-untreated NG2-CreER/ Ninj1loxP (control 1) or Tm-treated NG2-CreER (control 2), whereas the level of blood flow before HLI operation was mostly identical among these groups.
Ninj1 in Pericytes Is Crucial for the Formation of Functional Vessels in Ischemic Tissues
Consistent with HLI experiment using Ninj1-siRNA, at 14 days after HLI, the total numbers of CD31-positive microvessels within skeletal muscles increased in all groups in response to ischemia (Figure 4C). Most of microvessels (≈85%) were functional in nonischemic tissues. The functional vessels were relatively decreased in all groups at 2 weeks after HLI operation; however, the formation of functional vessels was significantly reduced in PCNinj1 KO compared with that in control 1 or 2 (Figure 4A and 4D). The number of PDGFRβ-positive pericytes in controls were increased in response to ischemia but not significantly in PCNinj1 KO group (Figure 4E). To quantitatively evaluate microvessels covered with pericytes, CD31-positive ECs colocalized with pericytes were detected in the short-axis view of sections (Figure 4F). Approximately 28% of ECs were similarly associated with pericytes in nonischemic tissues of 3 groups. In parallel with the reduced ratio of functional vessels in ischemic tissues, the ratio of pericyte-associated ECs was also decreased. However, the ratio of pericyte-associated ECs in ischemic tissues was significantly attenuated in PCNinj1 KO compared with the 2 controls (Figure 4B and 4F).
Ninj1 Mediates the Adhesion Properties of Pericytes
Pericytes are mesenchymal cells that partially grown in multiple layers at confluency. When Ninj1 was overexpressed by transfection of a Ninj1-expressing plasmid vector,16 most cells grew into a multiple layer with hill and valley-like appearances as control pericytes. In contrast, when endogenous Ninj1 expression was knocked down by Ninj1-siRNA, pericytes easily detached from the culture dish, decreasing the number of adhesive cells (Figure III in the online-only Data Supplement). Ninj1 knockdown significantly increased the migration of pericytes, whereas cell viability as assessed by cell proliferation water soluble tetrazolium assay was not altered (Figure IIIB and IIIC in the online-only Data Supplement). Thus, these data demonstrate that Ninj1 is critical for extra- or intercellular properties of pericyte adhesion.
To investigate whether Ninj1 mediates homologous pericyte-pericyte or EC-EC adhesion, we performed a cell cluster assay. When isolated cells were incubated in ultralow attachment culture dishes for 2 hours with gentle agitation, cells formed cell clusters (Figure 5A). The clusters of pericytes were recognized mainly as ranging from 900 to 11 000 μm2 in size (clusters I and 2II; Figure IV in the online-only Data Supplement). Although ECs also formed clusters, the number of clusters was lower, and their size was relatively small (900–4000 μm2 in size, cluster I) compared with pericytes (Figure IVC and IVD in the online-only Data Supplement). The formation of pericytes cluster II (4001–11 000 μm2) was reduced by Ninj1 knockdown. The average size of clusters was significantly reduced by Ninj1 knockdown in pericytes, but the formation of small ECs cluster I was not affected (Figure 5B). These data indicated that formations of cellular clusters of pericytes or ECs were specific, and Ninj1 mediated the homologous pericyte-pericyte adhesion but not EC-EC adhesion to form cellular clusters.
Figure 5.
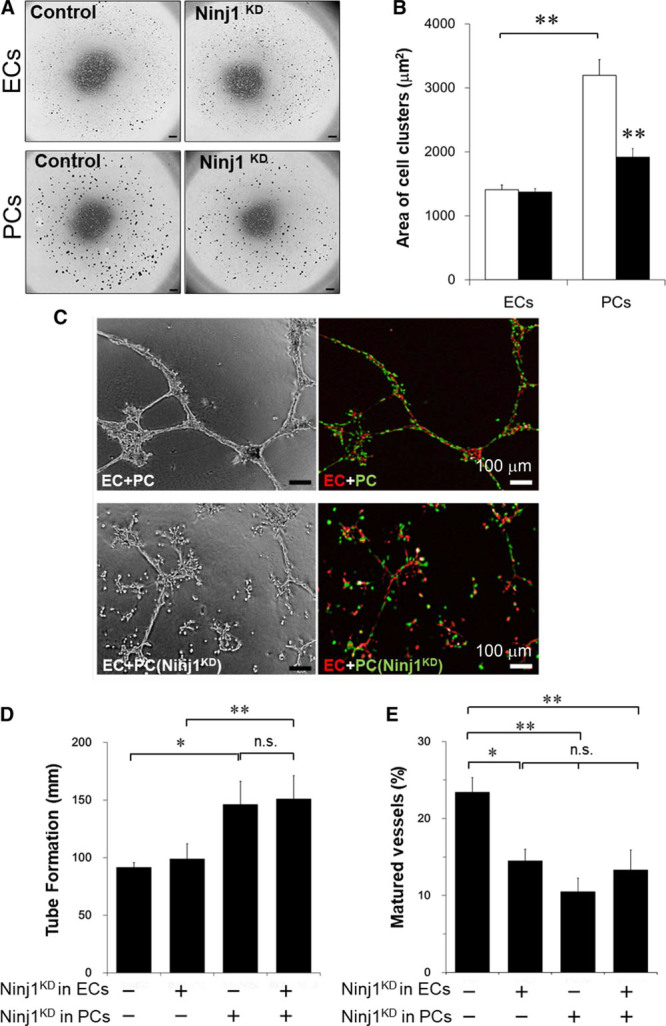
Ninj1 (ninjurin1) knockdown (KD) blocks the pericytes (PC) association and formation of capillary-like microvessels. A, At 48 h after transfection with Ninj1-small interfering RNA (siRNA; Ninj1KD) or scramble-siRNA (control), endothelial cells (ECs) or PCs were incubated in floating culture to form cell clusters. Cell clusters were fixed with methylcellulose gel in multiwell plate. Phase-contrast view of each wells is shown. Scale bar, 500 μm. B, The area size of each cell cluster was measured, and the average size of cellular clusters of PCs or ECs was calculated. White bar, control; black bar, Ninj1KD. Mean±SEM (n=8, **P<0.01, t test analysis). C, After transfection of Ninj1-siRNA (Ninj1KD) or scramble-siRNA (control), DsRed-ECs (red) and GFP (green fluorescent protein)-PCs (green) were mixed and incubated in Matrigel with angiogenesis medium. The mixture of ECs and PCs formed capillary-like tubes (mature vessels), whereas ECs alone formed thin EC tube structures. Phase-contrast and fluorescence images are shown. D, The total lengths of formed tubes (EC tubes+capillary-like tubes) in the gels were measured. E, The ratio of matured vessels to total length of formed tubes is demonstrated. Mean±SEM (n=6, *P<0.05, **P<0.01, n.s. indicates not significant, ANOVA).
Ninj1 Induces Heterogeneous Pericyte-EC Interaction
Next, we examined whether Ninj1 affects heterologous adhesion of pericytes and ECs. When pericytes were mixed with ECs (ECs+pericytes), larger cellular clusters (11 001–20 000 μm2; cluster III) were observed (Figure V in the online-only Data Supplement). When Ninj1 expression was attenuated in pericytes (ECs+pericytes Ninj1KD), the formation of pericyte-EC complexes (cluster III) and homologous pericyte clusters (cluster II) was attenuated (Figure VA in the online-only Data Supplement). Conversely, the number of small cluster I and nonclustered cells (<900 μm2) increased. When Ninj1 in ECs was reduced (ECs Ninj1KD+pericytes), the number of cluster III (pericyte-EC complexes) was reduced, and the number of clusters I and II increased (Figure VB in the online-only Data Supplement). Similar to ECs+pericytes Ninj1KD, when Ninj1 was reduced in both ECs and pericytes (ECs Ninj1KD+PCs Ninj1KD), the numbers of cluster II and III were reduced, and most cells shifted to cluster I and the noncluster range (Figure VC in the online-only Data Supplement). The number of cluster II in (ECs+PCs Ninj1KD) was specifically reduced compared with that in (ECs Ninj1KD+pericytes; Figure VD in the online-only Data Supplement). These data show that Ninj1 in both ECs and pericytes is critical to mediate EC-pericyte adhesion.
Ninj1 Mediates the Formation of Capillary-Like Structure
To examine the role of Ninj1 in the formation of microvessels, we performed in vitro angiogenesis assay.18 When ECs and pericytes were coincubated in a 3-dimensional gel, ECs formed tube-like structures on their own, namely EC tubes, and some of the EC tubes were associated with pericytes (24.3%±7% of total length of EC tubes), resulting in the formation of capillary-like tubes (Figure 5C). The formation of EC tubes was not affected by siRNA-mediated Ninj1 knockdown in ECs (ECs Ninj1KD). When ECs were coincubated with PCs Ninj1KD, the total length of tubes (EC tubes and capillary-like tubes) significantly increased (Figure 5C and 5D). In contrast to the total length of tube formation, the ratio of capillary-like tubes significantly decreased (Figure 5D). Interestingly, ECs Ninj1KD also attenuated the formation of capillary-like tubes, regardless of Ninj1 expression in pericytes (Figure 5D). These data indicate that Ninj1 in both pericytes and ECs is required for the formation of mature microvessel through the association between pericytes and EC tubes.
Ninj1 Mediates the Expression of Vascular Maturation–Related Factors
The role of several molecules including PDGF and Anpt is well documented for vascular maturation through the interaction of pericytes and ECs.3,7 Therefore, we analyzed the effect of Ninj1 on the expression of these factors in pericytes in which Ninj1 expression was overexpressed or knocked down. Ninj1 overexpressed enhanced Anpt1 expression in pericytes, whereas the expression of Anpt2, an endogenous antagonist of Anpt1, was enhanced in Ninj1 knockdown cells (Figure 6A). However, the expressions of PDGFRβ and angiogenic growth factors, such as vascular endothelial growth factor, were not affected by the expression of Ninj1(Figure 6A).
Figure 6.
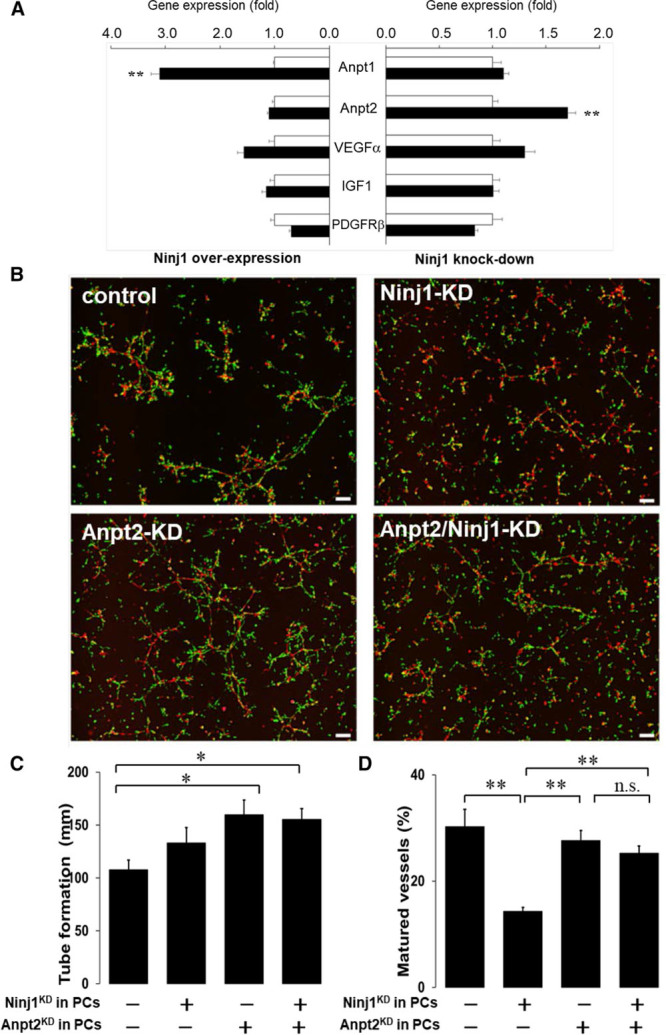
Role of angiopoietin in Ninj1 (ninjurin1)–mediated vascular formation. A, Expression of angiogenesis-related genes in primary cultured pericytes (PCs). Ninj1 expression was increased (overexpression) or decreased (knockdown) by transfection with Ninj1-expressiong vectors or Ninj1-specific small interfering RNA (siRNA), respectively (black bars), and their controls (white bars). After 2 d incubation, indicated gene expression was determined by quantitative polymerase chain reaction. Changes in mRNA after Ninj1 overexpression or knockdown were calculated. Mean±SEM (n=6, **P<0.01 vs white bar, t test analysis). B, After transfection of Ninj1- and Anpt (angiopoietin) 2-siRNA (Ninj1 knockdown [KD], Anpt2 KD, respectively) or their scramble-siRNA (control), DsRed-endothelial cells (red) and primary PCs isolated from GFP transgenic mice (green) were mixed and incubated in Matrigel. Fluorescence images are shown. C, The total lengths of formed tubes in the gels were measured. D, The ratio of matured vessels to total length of formed tubes is demonstrated. Mean±SEM (n=6, *P<0.05, **P<0.01, n.s. indicates not significant, ANOVA).
To assess the impact of Anpt1 and 2 in pericytes harboring Ninj1 deficiency, we examined the effects of Anpt/Ninj1-knockdown in pericytes on in vitro angiogenesis assay. Anpt2 knockdown enhanced the tube formation, but the ratio of capillary-like mature vessels was not changed (this means that total length of mature vessels was increased; Figure 6B and 6C). Anpt2 knockdown reduced the effects of Ninj1 knockdown, that is, attenuated mature vessels ratio in Ninj knockdown was partially recovered by Anpt2 knockdown (Figure 6B and 6C). The effects of Anpt2 knockdown, that is, enhanced matured vessel formation was blocked by Anpt1 knockdown, indicate that effects of Anpt2 knockdown might be through the enhancement of endogenous Anpt1 in pericytes (Figure VI in the online-only Data Supplement). Therefore, these data suggest that angiogenic effects of Ninj1 in pericytes are, in part, mediated by Anpt 1/2 signals.
Discussion
In this study, we describe a novel role of Ninj1 in the formation of mature functional microvessels and blood perfusion recovery from ischemia using siRNA-mediated Ninj1-knockdown and inducible pericyte-specific Ninj1 knockout mouse models. Typically, the capillary density in tissues and functional measures of angiogenesis, such as blood perfusion, change in parallel. Surprisingly, reduction in Ninj1 expression significantly attenuated blood perfusion recovery although capillary density in ischemic tissues was not altered or tended to increase in ischemic tissues. Our unique pericyte-specific Ninj1 knockout mouse model reveals that changes in capillary density (a quantitative measure of angiogenesis) and blood-circulating functional neovessels/blood perfusion (a qualitative measure of angiogenesis) can be separated. This finding suggests that the entire angiogenesis process, not only EC growth and sprouting but also vascular maturation, must be considered for functional vasculature in ischemic tissues.
Ninj1 is located in the plasma membrane, consisting of 2 transmembrane domains and extracellular N- and C-termini, and acts as an adhesion molecule. Ninj1 mediates cell-to-cell association through homophilic interactions.8,9 In our previous16 and present studies (Figure 1), we demonstrated that Ninj1 is expressed within microvessels, dominantly in pericytes of ischemic tissues. Although the cellular cluster formation of ECs or pericytes is a nonphysiological phenomenon, the assay demonstrated that Ninj1 on pericytes mediates both homogenous pericyte-pericyte and also heterogenous pericyte-EC associations. In addition to this experiment, we observed capillary-like tube formation, that is, EC tubes surrounded with pericytes as a consequence of the association between EC and pericytes in a 3-dimensional gel angiogenesis assay (Figure 5). In contrast, Ninj1-knockdown in either pericytes or ECs reduced the ratio of capillary-like tubes. These data indicate that Ninj1 mediates the cellular association between pericytes and ECs and, subsequently, the formation of matured vessels.
Pericytes produce angiogenic growth factors and enhance the growth and sprouts of ECs. In contrast, for vascular stabilization/maturation processes, pericytes also suppress the growth and migration of ECs through both pericyte-EC contact-dependent as well as independent mechanisms.4,26,27 As previously reported,16 the expression of Ninj1 and angiogenic factors, such as vascular endothelial growth factor and insulin growth factor, is upregulated in pericytes while neovessels are sprouting. Meanwhile, Ninj1 attenuated the production of these angiogenic factors, indicating that Ninj1 acts as a negative regulator for EC growth.16 In the present study, however, gain- and loss-of-function studies demonstrated that Ninj1 did not affect the production of these angiogenic factors in primary pericytes isolated from skeletal muscles (Figure 6A). Furthermore, deletion of Ninj1 genes using either siRNA-mediated knockdown or Cre/loxp knockout system did not increase the formation of microvessels within ischemic skeletal muscles (Figures 2 and 4). The discrepancy of the effect of Ninj1 on the production of angiogenic factors might be because of different source and cell type of pericytes, that is, immortalized pericyte lines derived from femoral artery and primary pericytes isolated from skeletal muscle tissues. We examined the effects of Ninj1 on in vitro angiogenesis assay using primary pericytes (Figure 6), in addition to immortalized pericytes (Figure 5). Importantly, the results were similar, as regards to pericyte-EC interaction to form mature vessels. Pericyte accumulated around EC tubes to form capillary-like structure, and Ninj1-knockdown in pericytes reduced the attachment to EC tubes and subsequently decreased the formation of capillary-like structure.
We examined whether the effect of Ninj1, that is, neovessel maturation through the association of pericytes to ECs contributes to the phenotype of Ninj1-knockout or -knockdown mouse models in HLI. Immunostaining of CD31-positive total neovessels and blood-circulating functional vessels reveals that some neovessels that formed in ischemic tissues had not functionally matured within 1 to 2 weeks after HLI operation (Figures 2C and 4C). Pericytes-specific Ninj1-knockdown attenuated the formation of functional microvessels (Figure 4B and 4D). Pericyte-specific Ninj1 deletion decreased the ratio of pericyte-associated vessels in ischemic tissues compared with that in controls (Figure 4F). A long-axis view of microvessels around isolated skeletal muscle fibers showed that pericytes are not continually covered around microvessels (Figure 3B). It is ideal to observe microvessels in long-axis view within tissues to estimate pericyte coverage in microvessels. For further study, advanced techniques would be used to estimate 3-dimensional view of microvessels within the entire tissues or organs, including skeletal muscle tissues.28 Recent studies demonstrated that pericytes contribute to functional regulation of microcirculation. Cerebral blood flow and thereby neuronal activity are regulated by microvascular capillary dilation through pericytes contractility without arteriolar change.29 We, therefore, propose that Ninj1 mediates the association of pericytes to neovessels and contributes to the formation of functional mature neovessels, resulting in blood flow recovery.
Arteriogenesis is referred to as the enlargement of preexisting arteriole-to-arteriole anastomoses (collateral circulation), which is also an important determinant in addition to microvessel maturation for protection against ischemic tissue injury. In this study, we prepare a relatively severe mouse HLI model by deletion of subcutaneous fatty tissues around limb in addition to the femoral vessels.19 In this method, preexisting vessels within subcutaneous fatty tissues act as collateral roots after femoral vessel deletion. The role of Ninj1 on collateral formation, that is, maturation of relatively large vessels, remains to be determined.
Ninj1 is expressed in vascular cells within skeletal muscle tissues even under nonischemic conditions. However, Ninj1 may not contribute to the maintenance of vasculature in steady conditions because pericyte-specific Ninj1 knockout did not affect the vascular flow in nonischemic limbs (Figure 3E). In addition, there is no apparent phenotype alteration in traditional Ninj1-knockout mice.14 We previously reported that Ninj1 expression level in pericytes is enhanced by hypoxia and inflammatory cytokines, such as tumor necrosis factor α.16 In response to HLI, Ninj1expression within skeletal muscles would be temporally enhanced through similar stimuli (Figure 1E). Yin et al14 reported that Ninj1 expression in penile tissue is enhanced under diabetes mellitus, and neutralized Ninj1 antibody improves angiogenesis/neuronal penile function in parallel with restoring Anpt1 expression. The discrepancy as regards to the angiogenic effect of Ninj1 might be, in part, because of different condition of Ninj1 expression, that is, chronic excess expression of Ninj1 under diabetes mellitus condition versus temporal expression of Ninj1 in ischemic tissues. Importantly, strategies to decrease the function and expression of Ninj1 would be therapeutically effective in certain condition, for example, penile dysfunction in diabetes mellitus.14 However, our present finding suggests that inhibition of Ninj1 expression may induce unfavorable effect in patients with certain pathophysiological conditions, such as ischemia.
It has been reported that the perivascular nerve is involved in neovessels formation in postnatal physiological condition.30 Peripheral nerves provide template that determines the organotypic pattern of vessel branching and arterial differentiation.31,32 We previously reported that perivascular innervation occurs during angiogenesis and contributes to stabilization and maturation of microvessels growing in adventitia of injured vasculature.21 Ninj1 is originally identified as membrane protein induced by nerve injury and contributes to nerve regeneration.8 It has been reported that some of pericytes have multipotency, such as mesenchymal stem cells and neural stem cells.18,33–35 To date, characteristics of multipotent pericytes have not been fully understood because of lack of specific markers of the specific cell population. It would be interesting to examine the possibility that Ninj1 mediates the neurogenic effects of pericytes and contributes to perivascular innervation to form functional matured vessels.
In this study, we report for the first time that Ninj1 is a novel molecule for formation of functional mature vessels and contributes blood flow recovery in ischemic tissues. Angiogenesis and maturation of the resulting vessels contribute to several physiological processes, including tissue injury, ischemia followed by wound healing, and tissue regeneration.36 It has been recognized that inadequate maturation or abnormal microvessel structure is closely associated with pathogenesis in several intractable diseases.36 Abnormalities in microvasculature, vasa vasorum in adventitial or perivascular fat of relatively bigger vessels including coronary arteries may contribute to atherosclerosis and functional spastic disorder.37,38 Microvascular dysfunction in cardiac tissues also critically contributes to transition from compensative cardiac hypertrophy to heart failure.39 It is well documented that vascular immaturation in tumor is associated with pathogenesis of tumor, including their growth and metastasis, and abnormalities in pericytes and their interaction with tumor ECs contribute to tumor vascular immaturation.40,41 Thus, vascular normalization could be an emerging strategy to enhance antitumor therapy through the distribution of antitumor agents or immune cells within tumor tissues.42 Therefore, role of Ninj1 in pericytes in pathogenesis of these cardiovascular diseases would be investigated for the development of therapeutics for these chronic intractable diseases.
Acknowledgments
We thank K. Kanno, S. Takahashi, Y. Horikawa, S. Tazawa, M. Kudo, A. Oda, E. Shinokawa, and M. Kusakabe for laboratory assistance, Y. Segawa for secretarial assistance, and medical students; S. Baba, A. Masuo, Y. Matsuo, and K. Asai for technical support.
Sources of Funding
This study was supported by JSPS KAKENHI Grant-in-Aid for Scientific Research, 22590820, 25461121, 17H04170, 17K19368 (J.-i Kawabe), and 15K19359 (M. Kabara), Japan Science and Technology Agency (J.-i Kawabe), a grant from the Akiyama Life Science Foundation, and the Mitsubishi Pharma Research Foundation.
Disclosures
None.
Supplementary Material
Nonstandard Abbreviations and Acronyms
- Anpt
- angiopoietin
- EC
- endothelial cell
- GFP
- green fluorescent protein
- HLI
- hindlimb ischemia
- Ninj
- nerve injury–induced protein, ninjurin
- RBF
- recovery of blood flow
- siRNA
- small interfering RNA
- Tm
- tamoxifen
- PDGFR
- platelet-derived growth factor receptor
The online-only Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/ATVBAHA.118.311375.
Highlights.
The maturation of the resulting neovessels contributes to several physiological processes, including organogenesis and embryonic development, and is also a key process in pathological conditions, such as myocardial infarction and other ischemic diseases.
Our results show the increase of adhesion molecule ninjurin1 expression in microvasculature in ischemic hind limbs.
Pericyte-specific deletion of ninjurin1 reduces the formation of functional matured microvessels and attenuated blood recovery in hindlimb ischemia.
Ninjurin1 is involved in the association of endothelial cells and pericytes for formation of matured vessels, in part, through angipoietin 1/2.
Our study proposes that Ninjurin1 is an attractive target for ischemic diseases.
References
- 1.Cooke JP, Losordo DW. Modulating the vascular response to limb ischemia: angiogenic and cell therapies. Circ Res. 2015;116:1561–1578. doi: 10.1161/CIRCRESAHA.115.303565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–693. doi: 10.1038/nm0603-685. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 3.Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97:512–523. doi: 10.1161/01.RES.0000182903.16652.d7. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- 4.Díaz-Flores L, Gutiérrez R, Madrid JF, Varela H, Valladares F, Acosta E, Martín-Vasallo P, Díaz-Flores L., Jr. Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histol Histopathol. 2009;24:909–969. doi: 10.14670/HH-24.909. doi: 10.14670/HH-24.909. [DOI] [PubMed] [Google Scholar]
- 5.Hoffmann J, Feng Y, vom Hagen F, et al. Endothelial survival factors and spatial completion, but not pericyte coverage of retinal capillaries determine vessel plasticity. FASEB J. 2005;19:2035–2036. doi: 10.1096/fj.04-2109fje. doi: 10.1096/fj.04-2109fje. [DOI] [PubMed] [Google Scholar]
- 6.Anghelina M, Krishnan P, Moldovan L, Moldovan NI. Monocytes/macrophages cooperate with progenitor cells during neovascularization and tissue repair: conversion of cell columns into fibrovascular bundles. Am J Pathol. 2006;168:529–541. doi: 10.2353/ajpath.2006.050255. doi: 10.2353/ajpath.2006.050255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaengel K, Genové G, Armulik A, Betsholtz C. Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler Thromb Vasc Biol. 2009;29:630–638. doi: 10.1161/ATVBAHA.107.161521. doi: 10.1161/ATVBAHA.107.161521. [DOI] [PubMed] [Google Scholar]
- 8.Araki T, Milbrandt J. Ninjurin, a novel adhesion molecule, is induced by nerve injury and promotes axonal growth. Neuron. 1996;17:353–361. doi: 10.1016/s0896-6273(00)80166-x. [DOI] [PubMed] [Google Scholar]
- 9.Araki T, Zimonjic DB, Popescu NC, Milbrandt J. Mechanism of homophilic binding mediated by ninjurin, a novel widely expressed adhesion molecule. J Biol Chem. 1997;272:21373–21380. doi: 10.1074/jbc.272.34.21373. [DOI] [PubMed] [Google Scholar]
- 10.Kubo T, Yamashita T, Yamaguchi A, Hosokawa K, Tohyama M. Analysis of genes induced in peripheral nerve after axotomy using cDNA microarrays. J Neurochem. 2002;82:1129–1136. doi: 10.1046/j.1471-4159.2002.01060.x. [DOI] [PubMed] [Google Scholar]
- 11.Lee HJ, Ahn BJ, Shin MW, Jeong JW, Kim JH, Kim KW. Ninjurin1 mediates macrophage-induced programmed cell death during early ocular development. Cell Death Differ. 2009;16:1395–1407. doi: 10.1038/cdd.2009.78. doi: 10.1038/cdd.2009.78. [DOI] [PubMed] [Google Scholar]
- 12.Ifergan I, Kebir H, Terouz S, et al. Role of Ninjurin-1 in the migration of myeloid cells to central nervous system inflammatory lesions. Ann Neurol. 2011;70:751–763. doi: 10.1002/ana.22519. doi: 10.1002/ana.22519. [DOI] [PubMed] [Google Scholar]
- 13.Odoardi F, Sie C, Streyl K, et al. T cells become licensed in the lung to enter the central nervous system. Nature. 2012;488:675–679. doi: 10.1038/nature11337. doi: 10.1038/nature11337. [DOI] [PubMed] [Google Scholar]
- 14.Yin GN, Choi MJ, Kim WJ, Kwon MH, Song KM, Park JM, Das ND, Kwon KD, Batbold D, Oh GT, Koh GY, Kim KW, Ryu JK, Suh JK. Inhibition of Ninjurin 1 restores erectile function through dual angiogenic and neurotrophic effects in the diabetic mouse. Proc Natl Acad Sci USA. 2014;111:E2731–E2740. doi: 10.1073/pnas.1403471111. doi: 10.1073/pnas.1403471111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahn BJ, Le H, Shin MW, et al. Ninjurin1 deficiency attenuates susceptibility of experimental autoimmune encephalomyelitis in mice. J Biol Chem. 2014;289:3328–3338. doi: 10.1074/jbc.M113.498212. doi: 10.1074/jbc.M113.498212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsuki M, Kabara M, Saito Y, Shimamura K, Minoshima A, Nishimura M, Aonuma T, Takehara N, Hasebe N, Kawabe J. Ninjurin1 is a novel factor to regulate angiogenesis through the function of pericytes. Circ J. 2015;79:1363–1371. doi: 10.1253/circj.CJ-14-1376. doi: 10.1253/circj.CJ-14-1376. [DOI] [PubMed] [Google Scholar]
- 17.Zhu X, Bergles DE, Nishiyama A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development. 2008;135:145–157. doi: 10.1242/dev.004895. doi: 10.1242/dev.004895. [DOI] [PubMed] [Google Scholar]
- 18.Kabara M, Kawabe J, Matsuki M, Hira Y, Minoshima A, Shimamura K, Yamauchi A, Aonuma T, Nishimura M, Saito Y, Takehara N, Hasebe N. Immortalized multipotent pericytes derived from the vasa vasorum in the injured vasculature. A cellular tool for studies of vascular remodeling and regeneration. Lab Invest. 2014;94:1340–1354. doi: 10.1038/labinvest.2014.121. doi: 10.1038/labinvest.2014.121. [DOI] [PubMed] [Google Scholar]
- 19.Aburakawa Y, Kawabe J, Okada M, Yamauchi A, Asanome A, Kabara M, Matsuki M, Takehara N, Nakagawa N, Okumura S, Minami Y, Mizukami Y, Yuhki K, Ushikubi F, Hasebe N. Prostacyclin stimulated integrin-dependent angiogenic effects of endothelial progenitor cells and mediated potent circulation recovery in ischemic hind limb model. Circ J. 2013;77:1053–1062. doi: 10.1253/circj.cj-12-0897. [DOI] [PubMed] [Google Scholar]
- 20.Xia Z, Abe K, Furusu A, Miyazaki M, Obata Y, Tabata Y, Koji T, Kohno S. Suppression of renal tubulointerstitial fibrosis by small interfering RNA targeting heat shock protein 47. Am J Nephrol. 2008;28:34–46. doi: 10.1159/000108759. doi: 10.1159/000108759. [DOI] [PubMed] [Google Scholar]
- 21.Asanome A, Kawabe J, Matsuki M, Kabara M, Hira Y, Bochimoto H, Yamauchi A, Aonuma T, Takehara N, Watanabe T, Hasebe N. Nerve growth factor stimulates regeneration of perivascular nerve, and induces the maturation of microvessels around the injured artery. Biochem Biophys Res Commun. 2014;443:150–155. doi: 10.1016/j.bbrc.2013.11.070. doi: 10.1016/j.bbrc.2013.11.070. [DOI] [PubMed] [Google Scholar]
- 22.Kawabe J, Yuhki K, Okada M, Kanno T, Yamauchi A, Tashiro N, Sasaki T, Okumura S, Nakagawa N, Aburakawa Y, Takehara N, Fujino T, Hasebe N, Narumiya S, Ushikubi F. Prostaglandin I2 promotes recruitment of endothelial progenitor cells and limits vascular remodeling. Arterioscler Thromb Vasc Biol. 2010;30:464–470. doi: 10.1161/ATVBAHA.109.193730. doi: 10.1161/ATVBAHA.109.193730. [DOI] [PubMed] [Google Scholar]
- 23.Yamauchi A, Kawabe J, Kabara M, Matsuki M, Asanome A, Aonuma T, Ohta H, Takehara N, Kitagawa T, Hasebe N. Apurinic/apyrimidinic endonucelase 1 maintains adhesion of endothelial progenitor cells and reduces neointima formation. Am J Physiol Heart Circ Physiol. 2013;305:H1158–H1167. doi: 10.1152/ajpheart.00965.2012. doi: 10.1152/ajpheart.00965.2012. [DOI] [PubMed] [Google Scholar]
- 24.Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2:329–333. doi: 10.1038/nprot.2007.30. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- 25.Jennewein C, Sowa R, Faber AC, Dildey M, von Knethen A, Meybohm P, Scheller B, Dröse S, Zacharowski K. Contribution of ninjurin1 to Toll-like receptor 4 signaling and systemic inflammation. Am J Respir Cell Mol Biol. 2015;53:656–663. doi: 10.1165/rcmb.2014-0354OC. doi: 10.1165/rcmb.2014-0354OC. [DOI] [PubMed] [Google Scholar]
- 26.Orlidge A, D’Amore PA. Inhibition of capillary endothelial cell growth by pericytes and smooth muscle cells. J Cell Biol. 1987;105:1455–1462. doi: 10.1083/jcb.105.3.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sato Y, Rifkin DB. Inhibition of endothelial cell movement by pericytes and smooth muscle cells: activation of a latent transforming growth factor-beta 1-like molecule by plasmin during co-culture. J Cell Biol. 1989;109:309–315. doi: 10.1083/jcb.109.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tainaka K, Kubota SI, Suyama TQ, Susaki EA, Perrin D, Ukai-Tadenuma M, Ukai H, Ueda HR. Whole-body imaging with single-cell resolution by tissue decolorization. Cell. 2014;159:911–924. doi: 10.1016/j.cell.2014.10.034. doi: 10.1016/j.cell.2014.10.034. [DOI] [PubMed] [Google Scholar]
- 29.Kisler K, Nelson AR, Rege SV, Ramanathan A, Wang Y, Ahuja A, Lazic D, Tsai PS, Zhao Z, Zhou Y, Boas DA, Sakadžić S, Zlokovic BV. Pericyte degeneration leads to neurovascular uncoupling and limits oxygen supply to brain. Nat Neurosci. 2017;20:406–416. doi: 10.1038/nn.4489. doi: 10.1038/nn.4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carmeliet P, Tessier-Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature. 2005;436:193–200. doi: 10.1038/nature03875. doi: 10.1038/nature03875. [DOI] [PubMed] [Google Scholar]
- 31.Mukouyama YS, Shin D, Britsch S, Taniguchi M, Anderson DJ. Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell. 2002;109:693–705. doi: 10.1016/s0092-8674(02)00757-2. [DOI] [PubMed] [Google Scholar]
- 32.Mukouyama YS, Gerber HP, Ferrara N, Gu C, Anderson DJ. Peripheral nerve-derived VEGF promotes arterial differentiation via neuropilin 1-mediated positive feedback. Development. 2005;132:941–952. doi: 10.1242/dev.01675. doi: 10.1242/dev.01675. [DOI] [PubMed] [Google Scholar]
- 33.Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 34.Feng J, Mantesso A, De Bari C, Nishiyama A, Sharpe PT. Dual origin of mesenchymal stem cells contributing to organ growth and repair. Proc Natl Acad Sci USA. 2011;108:6503–6508. doi: 10.1073/pnas.1015449108. doi: 10.1073/pnas.1015449108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dore-Duffy P, Katychev A, Wang X, Van Buren E. CNS microvascular pericytes exhibit multipotential stem cell activity. J Cereb Blood Flow Metab. 2006;26:613–624. doi: 10.1038/sj.jcbfm.9600272. doi: 10.1038/sj.jcbfm.9600272. [DOI] [PubMed] [Google Scholar]
- 36.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 37.Kawabe J, Hasebe N. Role of the vasa vasorum and vascular resident stem cells in atherosclerosis. Biomed Res Int. 2014;2014:701571. doi: 10.1155/2014/701571. doi: 10.1155/2014/701571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nishimiya K, Matsumoto Y, Shindo T, Hanawa K, Hasebe Y, Tsuburaya R, Shiroto T, Takahashi J, Ito K, Ishibashi-Ueda H, Yasuda S, Shimokawa H. Association of adventitial vasa vasorum and inflammation with coronary hyperconstriction after drug-eluting stent implantation in pigs in vivo. Circ J. 2015;79:1787–1798. doi: 10.1253/circj.CJ-15-0149. doi: 10.1253/circj.CJ-15-0149. [DOI] [PubMed] [Google Scholar]
- 39.Oka T, Akazawa H, Naito AT, Komuro I. Angiogenesis and cardiac hypertrophy: maintenance of cardiac function and causative roles in heart failure. Circ Res. 2014;114:565–571. doi: 10.1161/CIRCRESAHA.114.300507. doi: 10.1161/CIRCRESAHA.114.300507. [DOI] [PubMed] [Google Scholar]
- 40.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 41.Minami Y, Sasaki T, Bochimoto H, Kawabe J, Endo S, Hira Y, Watanabe T, Okumura S, Hasebe N, Ohsaki Y. Prostaglandin I2 analog suppresses lung metastasis by recruiting pericytes in tumor angiogenesis. Int J Oncol. 2015;46:548–554. doi: 10.3892/ijo.2014.2783. doi: 10.3892/ijo.2014.2783. [DOI] [PubMed] [Google Scholar]
- 42.Goel S, Duda DG, Xu L, Munn LL, Boucher Y, Fukumura D, Jain RK. Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev. 2011;91:1071–1121. doi: 10.1152/physrev.00038.2010. doi: 10.1152/physrev.00038.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]


