Abstract
Interleukin-1 receptor (IL1R)-associated kinase 4 (IRAK4) is a central regulator of innate immune signaling, controlling IL1R and Toll-like receptor (TLR)-mediated responses and containing both scaffolding and kinase activities. Humans deficient in IRAK4 activity have autosomal recessive primary immune deficiency (PID). Here, we characterized the molecular mechanism of dysfunction of two IRAK4 PID variants, G298D and the compound variant R12C (R12C/R391H/T458I). Using these variants and the kinase-inactive D329A variant to delineate the contributions of IRAK4's scaffolding and kinase activities to IL1R signaling, we found that the G298D variant is kinase-inactive and expressed at extremely low levels, acting functionally as a null mutation. The R12C compound variant possessed WT kinase activity, but could not interact with myeloid differentiation primary response 88 (MyD88) and IRAK1, causing impairment of IL-1–induced signaling and cytokine production. Quantitation of IL-1 signaling in IRAK4-deficient cells complemented with either WT or the R12C or D329A variant indicated that the loss of MyD88 interaction had a greater impact on IL-1–induced signaling and cytokine expression than the loss of IRAK4 kinase activity. Importantly, kinase-inactive IRAK4 exhibited a greater association with MyD88 and a weaker association with IRAK1 in IRAK4-deficient cells expressing kinase-inactive IRAK4 and in primary cells treated with a selective IRAK4 inhibitor. Loss of IRAK4 kinase activity only partially inhibited IL-1–induced cytokine and NF-κB signaling. Therefore, the IRAK4–MyD88 scaffolding function is essential for IL-1 signaling, but IRAK4 kinase activity can control IL-1 signal strength by modulating the association of IRAK4, MyD88, and IRAK1.
Keywords: interleukin 1 (IL-1), myeloid differentiation primary response gene 88 (MyD88), Toll/interleukin-1 receptor (TIR), protein kinase, inflammation, cytokine, cell signaling, innate immunity, interleukin-1 receptor-associated kinase 4 (IRAK4)
Introduction
Inherited defects in Toll-like receptor (TLR)6 signaling lead to primary immune deficiency. Recent work has characterized defects in components of the TLR signaling cascade, including MyD88, TIRAP, and the kinases IRAK1 and IRAK4 (1–3). Interleukin receptor–associated kinase 4 (IRAK4) is a central kinase in innate immunity that controls responses to viral and bacterial pathogens as well as products of sterile inflammation. Homozygous loss of IRAK4 or MyD88 expression leads to autosomal recessive primary immune deficiency (PID), a narrow set of Gram-negative bacterial infections but not viral or fungal infections in humans (1, 4). Both humans and mice lacking IRAK4 expression do not respond to challenge from ligands for Toll-like receptors or IL-1 and IL-18 and are resistant to septic shock. Heterozygotes are normal, indicating that a single copy of the WT allele is sufficient for activity.
IRAK4 has two well-characterized domains: an N-terminal death domain, which is a homotypic binding domain that binds to other death domain–containing proteins, and a C-terminal serine-threonine kinase domain. Ligation of Toll-like receptors or IL-1 family receptors by their cognate ligands induces receptor dimerization, which causes aggregation of the TIR domains located on the cytoplasmic side of the receptors. This TIR aggregation forms a platform for other adaptor proteins, chiefly MyD88. MyD88 contains an N-terminal TIR domain that interacts with other TIR domains and a C-terminal death domain. Clustering of MyD88 then recruits IRAK4 through death domain interactions to the receptor complex, known as the myddosome. The IRAK4–MyD88 complex then recruits the kinases IRAK1 and IRAK2 to the myddosome. IRAK1 subsequently becomes modified by phosphorylation and ubiquitination, which is readily observed by a mobility shift upon Western blotting (5). The three-dimensional crystal structure of the myddosome has been solved with the death domains of MyD88 and the IRAK4 and IRAK2 kinases showing an exquisite symmetric architecture that demonstrates the cooperativity of the complex and its role in potentiating signaling (6).
We have shown that IRAK4 trans-autophosphorylates to become a fully active kinase and that the death domain is important in regulating kinase activity in the cell (7). Subsequently, this was shown by crystallography (8). Thus, the formation of the myddosome controls the autophosphorylation of IRAK4. Therefore, it follows that the autophosphorylation of IRAK4 fully activates the kinase to phosphorylate downstream substrates.
The role of IRAK4 kinase activity in the control of TLR and IL-1 receptor–induced cytokines is the subject of much interest, as IRAK4 kinase inhibitors are potential therapeutics for inflammatory diseases and cancer (9). However, in vitro experiments show that overexpression of kinase-inactive IRAK4 can still reconstitute IL-1–induced NF-κB activation in IRAK4 kinase-deficient fibroblasts (10). Additionally, we have shown that whereas complete inhibition of IRAK4 autophosphorylation has minimal effects on IL-1–induced cytokines and MAPK and NF-κB signaling, it has profound effects on TLR-induced cytokines (7). Recently, we demonstrated that cytokines produced by TLR signaling in monocytes are controlled by the IRAK4 kinase-dependent activation of the transcription factor IRF5 (11). However, the precise role of IRAK4 in IL-1 signaling is still not completely understood.
Due to its central role in mediating inflammation, IL-1 has been the subject of investigation and therapeutic intervention for many years (12). Use of anti-IL-1 antibodies has been shown to be of therapeutic benefit in several diseases, notably cryopyrin-associated periodic syndromes, juvenile rheumatoid arthritis, and cardiovascular disease (12). The IL-1 receptor is expressed mainly on nonmyeloid cells, such as fibroblasts, epithelial cells, and endothelial cells. IL-1 is produced by monocytes and macrophages upon detection of pathogens through TLRs, which are mainly expressed on myeloid cells. Activation of the IL-1 receptor then causes the secretion of cytokines and the up-regulation of cell adhesion molecules that are essential to the inflammatory response in nonmyeloid cells. Due to the observed differences of the role of IRAK4 kinase activity in TLR and IL-1 signaling, we focus here on analyzing the role of IRAK4 in IL-1 signaling separately from TLR signaling.
To better understand the differential contributions of IRAK4 scaffolding and the kinase activity in IL-1 signaling, we characterized the expression and biochemical activities of two naturally occurring variants, G298D (4) and the compound mutant R12C/R391H/T458I (13). In both cases, the patients carry one null allele for IRAK4 and a mutated copy of the full-length protein. As one copy of IRAK4 is sufficient for normal innate immune function in humans, we initially sought to understand the molecular defects of these two variants that lead to innate immune deficiency. As both proteins contain mutations in the N-terminal kinase domain, we determined whether abnormal kinase activity was the reason for the phenotype of loss of signaling. Next, we examined the ability of the variants to form complexes in the cell with the components of the myddosome to determine whether loss of myddosome formation was the reason for loss of function. Further, we quantified the ability of these variants to reconstitute signaling and IL-1–induced cytokine production in IRAK4-deficient cells. Finally, we confirmed these findings in primary human fibroblasts using a selective IRAK4 inhibitor. We find that the IRAK4 kinase activity controls IL-1 signaling by regulating the formation and stability of the myddosome and that scaffolding function is essential for both assembly of the myddosome and IL-1 signaling.
Results
Functional characterization of WT, P15, and R12C variant cells
Human dermal fibroblasts from a healthy patient (WT), a homozygous IRAK4-null patient (P15) (14, 15), and a patient carrying one null allele for IRAK4 and one full-length allele carrying the R12C compound mutation (R12C) (13) were characterized for their expression of IRAK4 and their responses to IL-1β. As shown in Fig. 1A, the R12C dermal fibroblasts expressed an IRAK4 protein at the same molecular weight as that of the healthy (WT) control and at levels that, when quantitated and normalized to tubulin, were ∼10–20% that of the healthy control (Fig. 1B). No IRAK4 was detected in the P15 patient cells. Following stimulation by IL-1β, cytokine and phosphoprotein responses were only observed in the healthy dermal fibroblasts, and no cytokine or phosphoprotein responses were observed in either the P15 or R12C patient cells (Fig. 1, A and C). This indicates that the IRAK4 protein in the R12C patient cells is nonfunctional.
Figure 1.
Characterization of IRAK4 variant patient dermal fibroblasts. SV40-transformed human dermal fibroblasts were stimulated with 10 ng/ml IL-1β and assayed for phosphoprotein expression and cytokines (WT homozygote (WT); IRAK4-null homozygote (P15), and R12C.R391H.T458I/IRAK4-null heterozygote (R12C)). A, immunoblots of whole-cell lysates at the indicated times of IL-1β stimulation. The blot is representative of three independent experiments. B, quantitation of IRAK4 expression as determined by immunoblot in A and normalized to tubulin. C, measurement of secreted IL-8 following 4 h of IL-1β treatment. Data reflect the mean of three independent experiments, significance determined by one-way analysis of variance. NS, not significant. Error bars, S.D.
Functional characterization of IRAK4 variant proteins
To further characterize the IRAK4 protein expressed in the R12C patient cells, total RNA was extracted and made into ssDNA by reverse transcriptase. This library was then used to clone IRAK4 using primers to the 5′- and 3′-ends of the ORF. Following sequencing, two forms of IRAK4 were isolated, a full-length form containing three missense mutations, R12C/R391H/T458I (subsequently designated as the R12C compound mutation), and a form containing a premature stop codon at position 249 caused by a frameshift at position 240 that has been described previously (13). To characterize the G298D mutation in the kinase domain of IRAK4 described previously (16), we inserted this mutation into a full-length WT IRAK4 by PCR mutagenesis. All of the full-length constructs were cloned with a C-terminal FLAG tag for ease of expression and isolation of the protein.
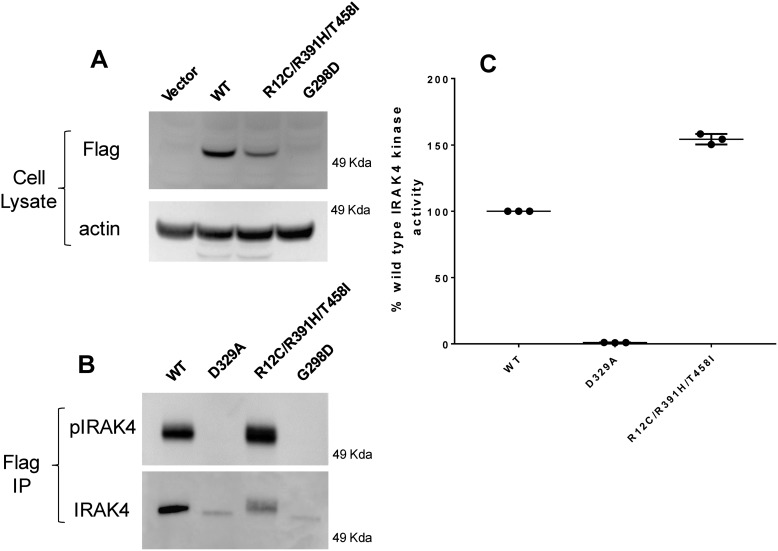
The R12C compound variant and G298D variant were overexpressed in COS7 cells along with WT and the kinase-inactive D329A variant. Proteins were purified by anti-FLAG antibody chromatography and assayed for expression via Western blotting and for kinase activity as described previously (7) and under “Experimental procedures.” When the constructs were transfected at identical concentrations of 5 μg of DNA per plate of COS7 cells, we observed that the R12C/R391H/T458I variant was expressed at about 30–50% of the WT protein when normalized to actin in the whole-cell lysate (Fig. 2A). The level of expression of the R12C variant correlates well with what is observed in the compound heterozygote R12C patient cell, which is 12% of the WT (Fig. 1B). These data indicate that the R12C compound mutation is slightly less stable the WT protein. Importantly, the G298D variant was not detected in the cell lysate. Western blots of FLAG-immunoprecipitated protein showed that the G298D protein was expressed, but detection of the protein required immunoprecipitation of significantly more cell lysate (>10-fold) than either WT or the R12C compound variant (Fig. 2B). This suggests that the G298D variant is highly unstable and is degraded rapidly.
Figure 2.
Expression and kinase activity of variants of IRAK4. A, Western blotting of cell lysates from COS7 cells overexpressing IRAK4 constructs. B, Western blotting of FLAG immunoprecipitated autophosphorylated IRAK4 constructs expressed in COS7 cells. C, kinase activity of purified IRAK4 constructs expressed in COS7 cells normalized to IRAK4 protein expression. Data reflect the mean of three independent experiments. IP, immunoprecipitation. Error bars, S.E.
We have previously shown that purified WT IRAK4 protein can undergo rapid autophosphorylation on its activation loop in the presence of 1 mm ATP and that these phosphorylation sites are easily detected by a phosphospecific antibody to pThr-345/pSer-346 of IRAK4 (7). When FLAG immunoprecipitates of overexpressed FLAG-tagged IRAK4 variants were incubated with 1 mm ATP for 1 h, neither the G298D nor the D329A kinase-inactive variant were able to autophosphorylate as detected by Western blotting of phosphorylated IRAK4 (Fig. 2B). However, both WT and the R12C compound variant robustly autophosphorylated in the presence of 1 mm ATP (Fig. 2B). These data demonstrate that the G298D variant, like the D329A variant, lacks kinase activity, whereas the R12C compound variant possesses WT kinase activity. To precisely determine the kinase activity of the variants, we overexpressed and purified the full-length R12C compound variant, the WT, and D329A variant from COS7 cells by FLAG immunoprecipitation. Kinase activity was determined by the DELFIA kinase assay described under “Experimental procedures” and previously (7). For each kinase assay, levels of IRAK4 protein were determined by Western blotting against a standard curve of recombinant IRAK4 to normalize kinase activity to protein concentration across experiments. In these assays, the R12C compound variant unexpectedly showed 50% higher activity than the WT protein (Fig. 2C). As expected, the D329A did not show any activity when assayed at levels equivalent to WT. We were unable to accurately determine the kinase activity of the G298D variant due to its low expression levels, but given its inability to autophosphorylate, it is likely that the lack of kinase activity is due to the protein being misfolded.
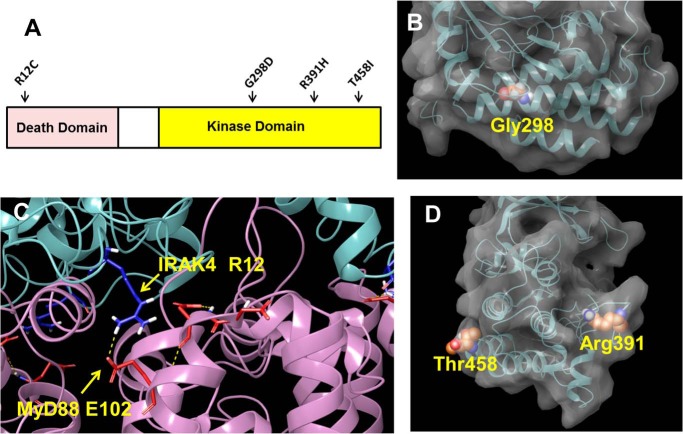
Taken together, these data demonstrate that the G298D IRAK4 variant is kinase-inactive, unstable, and likely unfolded and that the R12C compound variant is enzymatically active and properly folded, albeit with slightly reduced stability compared with the WT protein. Analysis of the crystal structure of the kinase domain explains the reason for the catastrophic nature of the G298D mutation and the relatively benign effects of the mutations R12C, R391H, and T458I. Fig. 3A shows the position of the R12C mutation in the N-terminal death domain and the mutations G298D, R391H, and T458I in the C-terminal kinase domain. The Gly-298 residue is deeply buried in a hydrophobic interface between two α-helices in the kinase domain (Fig. 3B). The substitution of a bulky and negatively charged aspartic acid for the neutral and smaller glycine would be expected to significantly disrupt the protein packing of the kinase domain. Yamamoto et al. (17) have also examined the G298D mutation and showed reduced expression. In contrast, the R12C, R391H, and T458I mutations occur near the surface of the protein and would not be expected to be highly destabilizing to the protein (Fig. 3, C and D). It is likely that the reason for the loss-of-function phenotype in the G298D patient is that the protein is misfolded and rapidly degraded, thus behaving as a complete null allele. The cause of the loss of function of the R12C compound mutation is less clear. Despite the apparent stability and WT kinase activity of this variant, the expression level is ∼10–20% of the WT protein.
Figure 3.
Position of mutations in IRAK4 structure. A, cartoon showing position of human mutations in IRAK4. B, space-fill representation of Gly-298 in the kinase domain of IRAK4 taken from PDB entry 2NRU. C, interaction of IRAK4 Arg-12 with Glu-102 of MyD88 taken from PDB entry 3MOP. D, space-fill representations of Thr-458 and Arg-391 in the kinase domain of IRAK4 taken from PDB entry 2NRU. Space-fill representations were created using the Maestro software program (Schrödinger LLC, New York).
Functional reconstitution of IRAK4 variants in IRAK4-deficient cells
To determine the ability of the R12C compound mutation to form an active myddosome, we performed reconstitution experiments of IRAK4 variants in IRAK4-null (P15) dermal fibroblasts. To control the relative expression levels of overexpressed IRAK4 in IRAK4-deficient cells, we created adenoviral constructs for the WT, D329A, and R12C/R391H/T458I IRAK4 variants and were able to quantitatively control protein expression by titrating multiplicity of infection (MOI) of the virus. IRAK4-null dermal fibroblasts were infected at multiple MOIs to test the ability of the different variants to reconstitute IL-1–induced cytokines, signaling, and the ability to interact with components of the myddosome. As shown in Fig. 4A, infection with increasing MOI of WT IRAK4 gave dose-dependent increases in IL-1–induced IL-8 production. Dose-dependent effects were also observed for the R12C compound mutation and D329A constructs, but at cytokine levels significantly lower than WT, indicating that both variants can reconstitute some degree of function at high levels of expression. A dose-dependent increase in protein expression levels with increasing MOI was verified and quantified by Western blotting (Fig. 4B). There was no observed effect of empty virus in the IRAK4-null cells when treated with IL-1β. When levels of cytokine induction were normalized for protein expression across multiple experiments, using IRAK4 expression levels quantitated from Fig. 4B, the kinase-inactive D329A variant reconstituted cytokine expression to ∼50% that of WT (Fig. 4C). The R12C compound variant was also able to reconstitute IL-1–induced IL-8 cytokine production to about 10–15% of the WT, indicating that despite the lack of activity observed in the R12C patient dermal fibroblasts, this variant still retains some activity at high levels of expression.
Figure 4.
IL-1–induced IL-8 production in IRAK4-null dermal fibroblasts reconstituted with IRAK4 variants. A, reconstitution of IL-1–induced IL-8 cytokine production. Human IRAK4-null dermal fibroblasts (P15 from Fig. 1A) were infected with the indicated MOIs of WT, R12C/R391H/T458I, and D329A IRAK4 adenovirus for 72 h and stimulated for 4 h with 10 ng/ml IL-1β. B, Western blotting of IRAK4 and tubulin from A. C, IL-8 levels measured in A normalized to IRAK4 expression levels determined in B and expressed as a percentage of WT activity. Data reflect the mean from four independent experiments. Error bars, S.D.
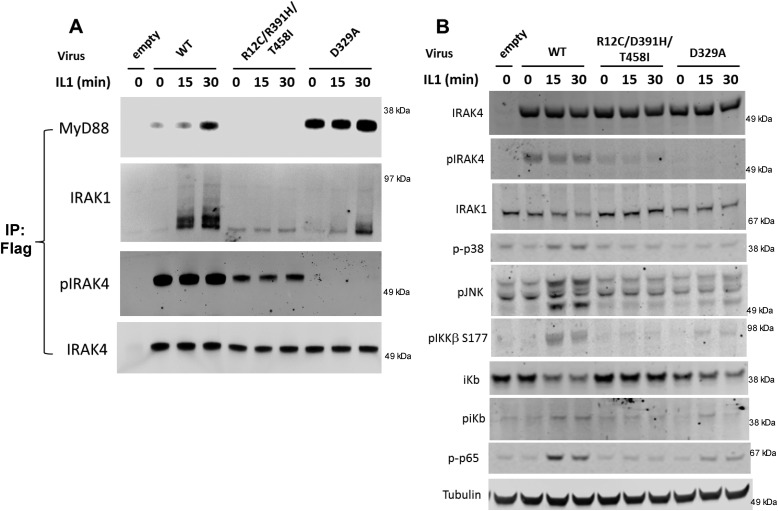
We examined the ability of the IRAK4 variants to associate with components of the myddosome, the adaptor protein MyD88 and the kinase IRAK1, in IRAK4-deficient cells by immunoprecipitation. We infected the IRAK4-deficient cells to give equivalent expression of all of the variant proteins. Following FLAG immunoprecipitation and immunoblotting, it was found that overexpressed WT IRAK4 was constitutively autophosphorylated in unstimulated cells, as described previously (7), and inducibly associated with both MyD88 and IRAK1 following 30 min of stimulation (Fig. 5A). The R12C compound variant was also constitutively autophosphorylated, albeit at a lower level than WT, but it did not associate with either MyD88 or IRAK1 either constitutively or following IL-1 stimulation. This behavior is likely due to the change of the Arg to Cys in the R12C mutation, the arginine of which forms a critical salt bridge interaction with residue Glu-102 in MyD88 (see Fig. 3B and Lin et al. (6). In support of this, Yamamoto et al. (17) demonstrated by analytical gel filtration and NMR titration that the R12C mutation in the IRAK4 death domain prevents association with MyD88. However, the fact that the R12C compound variant can still partially reconstitute IL-1–induced IL-8 at high levels of expression indicates that there is some residual interaction between the R12C compound variant and the myddosome that can drive a low level of IL-1–induced signaling. It is likely that the observed loss of function in the R12C compound variant patient is due to both the reduced ability to bind MyD88 and the reduced expression of the protein.
Figure 5.
Reconstitution of IRAK4-deficient dermal fibroblasts with IRAK4 mutations. Adenovirus containing WT, R12C/R391H/T458I, or D329A FLAG-tagged constructs of IRAK4 was transduced into IRAK4-deficient dermal fibroblasts for 72 h and stimulated with 10 ng/ml IL-1β for the indicated time. A, FLAG immunoprecipitation of cells followed by Western blotting with the indicated antibodies. B, whole-cell lysates from A probed with phosphospecific antibodies against the indicated proteins. Images represent one example of three independent experiments. IP, immunoprecipitation.
As expected, the D329A kinase-inactive variant did not autophosphorylate, as previously reported (7). Surprisingly, the D329A variant IRAK4 constitutively associated with MyD88 in the absence of IL-1β stimulation (Fig. 5A). However, the IL-1–induced association of the IRAK4 D329A variant with IRAK1 was significantly attenuated compared with WT (Fig. 5A). Furthermore, levels of modified IRAK1 were reduced by the kinase-inactive IRAK4, indicating that IRAK4 kinase activity is only partially responsible for IRAK1 modification and that IRAK4 scaffolding activity plays a major role in IRAK1 modification. These data demonstrate that IRAK4 kinase activity is important for modulating the interactions between MyD88, IRAK4, and IRAK1, with IRAK4 kinase activity strengthening the interaction between IRAK1 and IRAK4 and weakening the interaction between IRAK4 and MyD88.
We also analyzed the ability of the IRAK4 variants to reconstitute IL-1 signaling (Fig. 5B). Reconstitution of IRAK4-deficient cells with WT IRAK4 restored IL-1-induced activation of JNK, p38, and components of the NF-κB pathway. When expressed at approximately equivalent levels of IRAK4, as assessed by Western blotting, the R12C compound variant did not appear to reconstitute IL-1 signaling (Fig. 5B). However, it is possible that higher levels of expression of the protein might produce detectable signaling given that high levels of expression partially reconstitute IL-1–induced IL-8 activity (see Fig. 4). Expression of the kinase-inactive D329A variant partially reconstituted signaling through NF-κB and JNK compared with WT, consistent with the idea that the scaffolding function of IRAK4 alone can reconstitute some level of signaling, as shown in Fig. 4 and as observed previously using an IRAK4/1 dual inhibitor (7).
Inhibition of IRAK4 in primary human dermal fibroblasts using a selective IRAK4 inhibitor partially inhibits IL-1–induced cytokines and phosphoprotein signaling
To confirm that the observations in the heterologous expression system are relevant to primary cells and not due to artifacts of overexpression of the constructs, we treated primary human neonatal dermal fibroblasts with 2 ng/ml IL-1β and monitored phosphoprotein signaling and cytokine release in the presence of the highly selective and potent IRAK4 inhibitor PF-06650833 (compound 40 in Lee et al. (18)). A concentration of 200 nm PF-06650833, which completely suppresses IRAK4 autophosphorylation (see Fig. 6C), was used to determine the maximum level of inhibition of IL-1β–induced cytokines controlled by IRAK4 kinase activity. As is shown in Fig. 6, maximal inhibition of IL-1β–induced IL-6 and IL-8 cytokine (Fig. 6A) and transcript (Fig. 6B) is incomplete and is only suppressed by 50% of maximum induction. This replicates the observation in the IRAK4-null fibroblasts reconstituted with kinase-inactive IRAK4 (D329A), where maximal induction of IL-1–induced cytokine was approximately half of that induced by the WT protein (Fig. 4 and 5). In a previous publication (7), we reported that IL-1–induced cytokines were minimally impacted by an IRAK4 inhibitor at a concentration of 10 μm. However, the inhibitor used in that study had poor physical properties and did not allow us to completely test its pharmacology. The inhibitor PF-06650833 has superior cell potency, is highly selective for IRAK4, and maximally covers IRAK4 at a concentration of 200 nm with no inhibition of other kinases, including IRAK1 (18). Therefore, the data presented in this study substantiate our previous finding of partial inhibition of IL-1–mediated cytokine by an IRAK4 kinase inhibitor.
Figure 6.
Inhibition of IL-1–induced signaling and cytokine in primary dermal fibroblasts using an IRAK4 inhibitor. A, primary human neonatal dermal fibroblasts in the presence of either DMSO or 200 nm IRAK4 inhibitor PF-06650833 were stimulated with 2 ng/ml IL-1β overnight, after which IL-6 and IL-8 secretion was measured. B, IL-6 and IL-8 transcripts as determined following stimulation with 2 ng/ml IL-1β for 6 h in the presence of either DMSO or 200 nm IRAK4 inhibitor PF-06650833. C, primary human neonatal dermal fibroblasts were stimulated with 2 ng/ml IL-1β for the indicated times in the presence of DMSO or 200 nm PF-06650833 and lysed and immunoblotted for the indicated proteins. D, quantitation of phosphoproteins from C relative to parent protein (DMSO (black) or PF-06650833 (red)). Data represent the mean of three independent experiments.
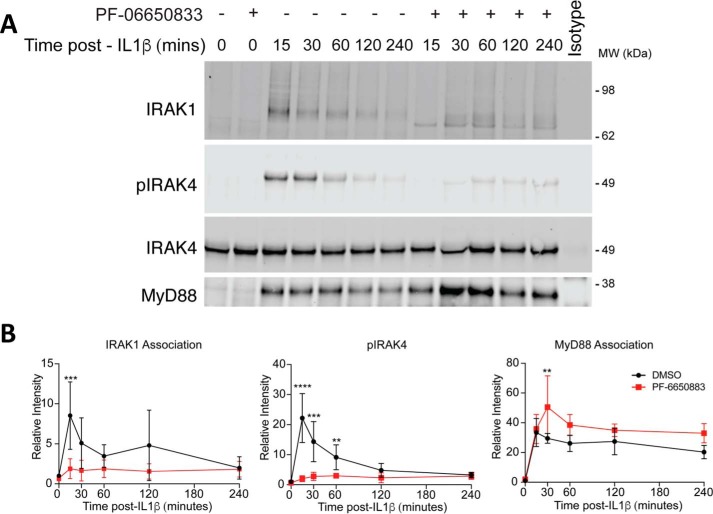
We tested the effects of 200 nm PF-06650833 on IL-1β–induced phosphoproteins over a time course of 1 h to better understand the effects of IRAK4 inhibition on signaling. As shown in Fig. 6C, the inhibitor completely blocked IL-1β–induced phospho-IRAK4 but had only minimal effects on the phosphorylation of p38, IKKβ, p65/RelA, JNK, and the modification of IRAK1. When levels of phosphorylation were quantified and normalized to total protein, the inhibitor appeared to slow the kinetics of phosphorylation and partially reduced levels of phosphorylation of IKKβ and p65 (Fig. 6D). The inhibitor did not appear to affect the phosphorylation of p38 or JNK kinases. We also note in Fig. 6C that the phosphorylation of p38, p65/RelA, IKKβ, and JNK precedes full phosphorylation of IRAK4. As we and others have described, IRAK4 becomes autophosphorylated following assembly of the myddosome by transphosphorylation by another IRAK4 molecule (7, 8). Thus, our data suggest that IRAK4 autophosphorylation is not required for early downstream signaling and that the early part of IL-1 signaling is largely IRAK4 kinase–independent and is likely mediated by the scaffold function of IRAK4. Thus, the reduction in cytokine observed in the presence of inhibitor may be due to the decrease in NF-κB signaling but not p38 or JNK signaling. Other mechanisms of cytokine control by IRAK4 kinase activity are the subject of current study by our group.
Inhibition of IRAK4 in primary dermal fibroblasts affects the dynamics of the myddosome
We sought to recapitulate the effects of increased MyD88-associated IRAK4 and decreased association of IRAK1 in kinase-inactive IRAK4 that we had observed in the reconstituted null IRAK4 cells in Fig. 5. We treated primary human dermal fibroblasts with 2 ng/ml IL-1β in the presence of 200 nm PF-06650833 and immunoprecipitated endogenous IRAK4. We then immunoblotted for the MyD88 and IRAK1, as shown in Fig. 7A, and observed that MyD88 increased its association with unphosphorylated IRAK4, whereas IRAK1 association with unphosphorylated IRAK4 decreased as compared with WT IRAK4. These changes in association can be more clearly seen when IRAK1 and MyD88 are quantitated and normalized to levels of immunoprecipitated IRAK4 (Fig. 7B). This behavior recapitulates the observation in the reconstituted IRAK4-null cells demonstrating that IRAK4 kinase activity controls the association with upstream and downstream proteins in the myddosome (Fig. 5). Therefore, the decreased association of IRAK1 and other downstream effectors with unphosphorylated IRAK4 is likely the cause of the decrease of IL-1–induced cytokines in the presence of the inhibitor. In this context, the scaffolding function of IRAK4 controls the early induction and maintenance of signaling, whereas the kinase activity controls the signal strength by stabilizing the association with IRAK1 and other signaling molecules.
Figure 7.
Immunoprecipitation of IRAK4-associated proteins from primary human dermal fibroblasts in the presence of the selective IRAK4 inhibitor PF-06650833. A, primary human neonatal dermal fibroblasts stimulated with 2 ng/ml IL-1β for the indicated time in the presence or absence of 200 nm PF-06650833 were lysed, and IRAK4 was immunoprecipitated and immunoblotted for the indicated proteins. The image represents one example of four independent experiments. B, quantitation of proteins from A relative to total IRAK4 (DMSO (black) or PF-06650833 (red)). Data represent the mean of four independent experiments. Error bars, S.D.
Discussion
IRAK4 controls IL-1 and TLR signaling based on both its scaffolding and kinase activities. IRAK4 forms a complex with its adaptor protein MyD88 that serves as a scaffolding platform to promote IL-1 signaling and the production of pro-inflammatory cytokines. In this study, we characterized the molecular mechanisms underpinning IRAK4 variants in primary human immune deficiency and, through this work, define the role of IRAK4 kinase and scaffolding activity in IL-1 signaling.
Individuals with one copy of functional IRAK4 are phenotypically normal, whereas those lacking both functional copies have primary immune deficiency. Therefore, we examined the functional activity of two reported variants of IRAK4 that cause primary immune deficiency but have point mutations that lead to a full-length transcript. We initially undertook this work in hopes of defining mechanisms that lead to loss of function in the full-length protein.
The variant G298D is shown to be expressed at extremely low levels and is therefore functionally inactive. The reason for this is likely due to the catastrophic nature of the mutation, which introduces a bulky, negatively charged residue, aspartic acid, in place of the smaller neutral glycine, into a highly hydrophobic pocket buried in the kinase domain. Such a substitution is likely to cause instability in the folding of the protein, and hence the partially folded protein is likely removed by the unfolded protein response network and degraded. The second variant examined was the compound variant R12C/R391H/T458I that was isolated from patient fibroblasts. This variant protein had kinase activity higher than the WT, demonstrating that the mechanism of dysfunction is not due to loss of kinase activity. However, when expression was assessed by Western blotting in patient dermal fibroblasts or transfected at equivalent levels to WT, the R12C compound variant protein was expressed at 30–50% that of the WT protein, indicating that the mutations reduced protein stability (Figs. 1 and 2). Therefore, the total expression of IRAK4 in the patient is about 25% of what would be expected in a WT individual. Dermal fibroblasts from this patient exhibited no cytokine or phosphoprotein response to IL-1β (Fig. 1). When reconstituted in IRAK4-null cells at high levels, the R12C compound variant was still able to partially reconstitute IL-1β–induced cytokine responses but did not reconstitute detectable phosphoprotein responses (Figs. 1 and 5), indicating that the protein is still functional but has drastically reduced activity when compared with WT or the kinase-inactive variant. When expressed at high levels, the R12C compound IRAK4 variant was unable to associate with MyD88 or IRAK1 following stimulation with IL-1. This defect is likely due to the loss of the interaction of the Arg-12 side chain of IRAK4 with the Glu-102 side chain of MyD88 (Fig. 3) (6, 17). Therefore, the loss of function for the R12C compound mutant is due to both loss of myddosome formation and low expression of the protein.
We also compared the ability of kinase-inactive IRAK4 (D329A) to form an IL-1–induced myddosome against both WT and the R12C/R391H/T458I variants in IRAK4-null dermal fibroblasts. Surprisingly, we found that the D329A kinase-inactive variant was significantly associated with MyD88 in both the unstimulated and stimulated human dermal fibroblasts, whereas interaction with IRAK1 was significantly reduced following stimulation with IL-1β when compared with WT protein (Fig. 5). We recapitulated these results in primary human dermal fibroblasts by immunoprecipitation of IRAK4 (Fig. 7), demonstrating that this behavior is not an artifact of overexpression of the protein. This result clearly demonstrates that IRAK4 kinase activity controls the dynamics of the myddosome, with nonphosphorylated IRAK4 associating preferentially with MyD88 and phosphorylated IRAK4 associating preferentially with IRAK1 and potentially other downstream effectors in IL-1 signaling. However, inhibition of IRAK4 kinase activity does not completely abrogate association of IRAK4 with MyD88 and IRAK1 because signaling and cytokine activity are both partially preserved in the absence of IRAK4 kinase activity (Fig. 7).
We have previously observed that IL-1–induced modification of IRAK1 is not entirely IRAK4 kinase-dependent (5, 7). Our data in this report confirm these observations and provide an explanation for the partial dependence on IRAK4 kinase activity. We propose that IRAK1 is not a direct substrate of IRAK4 and that IRAK1 modification is due primarily to the association with components of the myddosome. The kinase activity of IRAK4 increases the level of modification of IRAK1 by increasing the stability of the myddosome.
Interestingly, we observed a discrepancy between the amount of phosphorylated IRAK4 captured in the immunoprecipitate and the cell lysate. In the immunoprecipitate, phospho-IRAK reached a peak at 15 min and rapidly declined thereafter despite constant levels of total IRAK4 captured (Fig. 7). In the cell lysate, in contrast, the total level of phosphorylated IRAK4 reached a peak at 15 min and stayed constant over time (Fig. 6, B and C). This suggests that as phosphorylated IRAK4 aggregates in the myddosome, it becomes less able to be bound by the antibody because the epitope is being masked by other proteins in the complex. The time-dependent nature of this epitope masking also suggests that the formation of the myddosome is dynamic and changes over time.
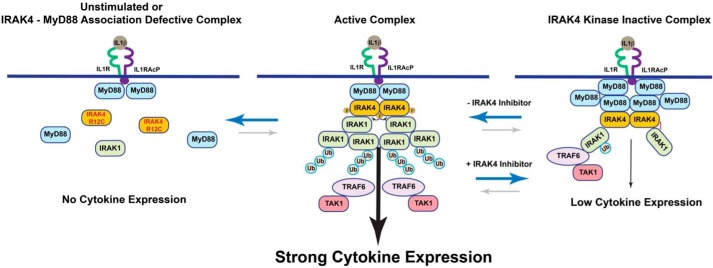
We propose a model for how IRAK4 kinase activity controls IL-1 signaling, as presented in Fig. 8. Upon engagement of the IL-1 receptor with its ligand, MyD88 forms a complex with the cytoplasmic domain of the receptor through the TIR domains. This scaffolding complex is then in the proper conformation to associate with the death domain of IRAK4 to form an early myddosome. This early myddosome does not form if the IL-1 receptor is not engaged by its ligand or, as in the case of the R12C compound variant, the death domain interaction between MyD88 and IRAK4 is disrupted. In this context, no signaling takes place. If the IRAK4 kinase activity is inhibited, a stronger complex forms between IRAK4 and MyD88, and a weaker complex forms between IRAK1 and IRAK4. The kinase-inactive complex is still able to signal, but the signal strength is reduced compared with the kinase-active complex due to the greater instability of the kinase-inactive complex. In support of this model, it has been shown that autophosphorylation of serine 8 in the death domain of IRAK4 can reduce association with MyD88, as this residue forms a critical interaction in the interface between the death domains of IRAK4 and MyD88 (2). Other phosphorylation-dependent interactions are likely necessary for enhancing the stability of downstream effectors with IRAK4. Our model explains the partial efficacy observed with selective IRAK4 kinase inhibitors or kinase-inactive knock-ins in IL-1 signaling and demonstrates that a function of IRAK4 kinase is to control phosphorylation-dependent equilibrium between components of the myddosome to regulate IL-1 signal strength.
Figure 8.
Scheme showing the role of kinase and scaffolding activity of IRAK4 in IL-1 signaling. In the unstimulated complex or the MyD88 interaction–incompetent R12C IRAK4 variant (left), no myddosome is formed, leading to complete loss of IL-1 signaling. In the active complex (center) a myddosome is formed with weak association of IRAK4 and MyD88 and strong association of IRAK4 and IRAK1 that potentiates robust ubiquitination and phosphorylation of IRAK1 and strong downstream signaling from the IL-1 receptor. In the IRAK4 kinase–inhibited complex (right), a myddosome is formed where IRAK4 is strongly associated with MyD88 but weakly associated with IRAK1, which reduces ubiquitination of IRAK1 and decreases IL-1–induced signaling and cytokine production.
Our hypothesis explains the observation that the kinase activity of IRAK4 is not essential for IL-1–induced signaling in human cells (10, 19). In the context of IL-1 signaling, IRAK4 kinase activity controls the stability of the myddosome and regulates signal strength. Clearly, loss of IRAK4 protein or inhibition of IRAK4 interaction with MyD88 has a more profound impact on IL-1–induced cytokines than loss of IRAK4 kinase activity.
In the presence of an IRAK4 inhibitor, both IL-1–induced cytokine transcript and protein levels are reduced equivalently by about half compared with DMSO control (Fig. 6, A and B). The kinetics and peak phosphorylation of p65/RelA and IKKβ are partially reduced in the presence of an IRAK4 inhibitor, but activation of p38 and JNK are not affected (Fig. 6D). It is possible that this attenuated NF-κB signaling due to inefficient myddosome formation is responsible for the partial reduction in cytokine production.
Whereas we restrict this study to IL-1 signaling, it is important to consider that IRAK4 plays a critical role in Toll-like receptor signaling. We have previously reported that IRAK4 inhibitors block TLR-induced cytokines in human monocytes with greater efficacy than they block IL-1–induced cytokines in human fibroblasts (7). This suggests that Toll receptor signaling is more dependent on IRAK4 kinase activity than is IL-1 signaling. The differences in the role of IRAK4 between Toll receptor and IL-1 receptor are not clear at this time and are the subject of active investigation by our group. A better understanding of the role of IRAK4 scaffolding versus kinase activity between different IRAK4-dependent receptors is critical for delineating the pharmacology of, and determining potential indications for, IRAK4 inhibitors.
Experimental procedures
Cloning of variants of IRAK4
Total RNA was purified from SV40-transformed human dermal fibroblasts from healthy (WT) and IRAK4-deficient patients (13, 15). Single-strand cDNA libraries were created from total RNA via reverse transcriptase using poly(dT) primers using the high-capacity cDNA reverse transcription kit from Thermo Fisher Scientific by according to the manufacturer's directions. IRAK4 was cloned via PCR using DNA primers containing AttB sites for the 5′- and 3′-ends of the ORF for human IRAK4 (NP_001107654). The 3′ primer also carried a FLAG tag sequence to facilitate immunoprecipitation detection and immunoprecipitation. PCR fragments were recombined into Gateway entry vector pDONR 201 (Invitrogen) according to the manufacturer's directions. Sequence-verified clones were further recombined into adenoviral or eukaryotic expression vectors via Gateway (Invitrogen) cloning for expression. The G298D and D329A mutations were created by PCR mutagenesis as described previously (7, 20).
Antibodies and Western blotting
The antibodies used were pIRAK4 (pThr-345/pSer-346) rabbit monoclonal (7), IRAK4 (mouse monoclonal, Abcam catalog no. 119942), p-p65 (rabbit, Cell Signaling Technologies catalog no. 3031), pIKKβ Ser-177 (rabbit, Cell Signaling Technologies catalog no. 2078), p-p38 (rabbit, Cell Signaling Technologies catalog no. 9211), tubulin (mouse monoclonal, LI-COR Biosciences (Lincoln, NE) catalog no. 926-42213), MyD88 (goat polyclonal R&D Systems catalog no. AF2928), actin (mouse monoclonal, Cell Signaling Technologies catalog no. 3700), IRAK1 (rabbit, Cell Signaling Technologies catalog no. 4359), FLAG tag (mouse monoclonal, Cell Signaling Technologies catalog no. 8146), p38 (rabbit, Cell Signaling Technologies catalog no. 9212), p-JNK (mouse monoclonal, Cell Signaling Technologies catalog no. 9255), JNK (rabbit, Cell Signaling Technologies catalog no. 9252), IKKβ (mouse monoclonal, Novus Biologicals, NB100–56509), p65 (mouse monoclonal, Cell Signaling Technologies catalog no. 6956). Antibodies were incubated with nitrocellulose membranes from Western transfer of SDS-PAGE gels (Thermo-Fisher Scientific) in Odyssey Blocking Buffer (LI-COR Biosciences) for 1 h at room temperature and then incubated overnight in primary antibody at 4 °C. Membranes were washed four times for 5 min each in 1× PBS-T, incubated in secondary antibody for 1 h at room temperature, washed again four times for 5 min each in PBS-T, rinsed in PBS, and imaged on an Odyssey CLx imager (LI-COR Biosciences). Protein levels were quantified using Image Studio version 4.0 (LI-COR Biosciences).
Expression and activity determination of IRAK4 variants
IRAK4 mutant DNA in the eukaryotic vector pcDNA3-DEST40 (Invitrogen) was transfected into COS7 cells at 5 μg of DNA/10-cm tissue culture plate using Lipofectamine 2000 (Invitrogen) and incubated for 72 h in Dulbecco's modified Eagle's medium (Invitrogen) containing 10% fetal bovine serum supplemented with glutamine as directed by the manufacturer and described previously (7). Cells were lysed in Triton lysis buffer (1% Triton X-100, 20 mm HEPES, pH 7.4, 100 mm NaCl) containing protease inhibitors (Sigma) at 4 ºC. Cell lysates were immunoprecipitated with anti-FLAG M2 beads (Sigma) washed extensively in lysis buffer and eluted with 200 μg/ml FLAG peptide (Sigma) in 10 mm HEPES, pH 7.4, with 150 mm NaCl. Eluates were dialyzed 1:1000 against 20 mm Tris-HCl, pH 7.5, 150 mm NaCl to remove FLAG peptide. Concentration of protein was determined via Western blotting against a standard curve of recombinant IRAK4. Kinase activity was determined by preincubating with 2 mm ATP in a kinase reaction buffer of 20 mm HEPES, pH 7.5, 5 mm MgCl2, 0.0025% Brij for 1 h at 25 ºC. The autophosphorylated IRAK4 protein was incubated with 200 nm peptide (biotinylated AGAGRDKYKTLRQIR, Tufts University) with 2 mm ATP for 1 h in reaction buffer. The reaction was stopped by the addition of 20 mm EDTA. 100 μl of reactions were transferred to a 96-well streptavidin-coated plate (R&D Systems) and allowed to bind for 30 min followed by extensive washing with 1× TBS containing 0.02% Tween. Plates were then incubated with anti-phospho-ezrin/radixin/moesin antibody (Cell Signaling Technology) diluted 1:1000 in blocking buffer (10 mm MOPS, pH 7.5, 150 mm NaCl, 3 mm NaN3, 0.025% Tween 20, 0.2% gelatin, 2% BSA) for 1 h followed by extensive washing with TBS/Tween. Plates were developed by incubation for 30 min with an anti-rabbit IgG–europium conjugate (PerkinElmer Life Sciences) diluted 1:5000 in blocking buffer followed by extensive washing in TBS/Tween and finally the addition of DELFIA enhancement solution (Perkin Elmer Life Sciences). Plates were read in an Envision reader (PerkinElmer Life Sciences) on a europium setting.
Adenoviral constructs and infection
IRAK4 constructs of the WT, D329A, and R12C compound variant containing C-terminal FLAG tag were cloned into pVQAd CMV K-NpA vector (Viraquest, North Liberty, IN). Adenovirus was produced under contract by Viraquest (North Liberty, IN). IRAK4-null human dermal fibroblasts were infected at various multiplicities of infection in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum for 72 h. Immunoprecipitation of FLAG-tagged overexpressed proteins and Western blotting of protein complexes were performed as described above and in previous publications (7, 11).
Immunoprecipitation of endogenous IRAK4
Primary human neonatal foreskin dermal fibroblasts were obtained from Zen-Bio and were cultured in dermal fibroblast growth medium (Zen-Bio catalog no. DF-1) and passaged every 2 days at a dilution of 1:5. Cells were stimulated for the indicated times with 2 ng/ml recombinant IL-1β (R&D Systems catalog no. 201-LB-005) and subsequently lysed in lysis buffer containing 1% Triton X-100, 20 mm Hepes, 100 mm NaCl. Lysates were precleared for 1 h with protein G–Sepharose beads (Sigma catalog no. P3296) and immunoprecipitated overnight with a sheep polyclonal anti-IRAK4 antibody (University of Dundee, catalog no. S522C) complexed with protein G–Sepharose beads (Sigma, catalog no. P3296). Beads were washed with lysis buffer three times, eluted by boiling in Laemmli sample buffer, and subjected to Western blotting as described above and previously (7, 11).
Quantification of IL-6 and CXCL8 (IL-8) transcripts
Primary neonatal dermal fibroblasts (Zen-Bio) were treated with either DMSO or 200 nm PF-06650833 and stimulated with 2 ng/ml IL-1β for 6 h. RNA was isolated using a Qiagen RNeasy minikit (Qiagen catalog no. 74106) as per Qiagen protocol. Briefly, 100 ng of RNA was utilized for a reverse transcriptase reaction and subsequent real-time quantitative PCR, using the Taqman RNA-to-Ct 1-step kit (Thermo Fisher Scientific catalog no. 4392656). Validated Taqman probes for RPLP0, IL-6, and IL-8 were obtained from Life Technologies. Ct values for IL-6 and IL-8 were normalized to RPLP0 and subsequently normalized to time 0.
Cytokine determination
IL-6 and IL-8 were determined from cell culture supernatants of human dermal fibroblasts treated with 2 ng/ml recombinant human IL-1β (R&D Systems, catalog no. 201-LB) for the indicated time. Supernatants were analyzed using the 4-plex pro-inflammatory II kit (Mesoscale Discovery) and developed according to the manufacturer's instructions. Statistical significance was determined by one-way analysis of variance (GraphPad Prism).
Author contributions
S. D. and V. R. R. conceptualization; S. D. and V. R. R. formal analysis; S. D., F. K., E. K., L. C., P. G., C. H., J.-L. C., A. P., and V. R. R. investigation; S. D., L.-L. L., and J.-L. C. writing-review and editing; F. K., E. K., and L. C. methodology; L.-L. L. project administration; V. R. R. writing-original draft.
Acknowledgment
We thank Dr. Frank Lovering for rendering the space-fill images of IRAK4 and MyD88 described in this work.
This work was supported by Pfizer Inc. S. D., F. K,. and V. R. R. are paid employees of Pfizer Inc. E. K., L. C., and L.-L. L. were paid employees of Pfizer Inc. at the time of this work.
- TLR
- Toll-like receptor
- IRAK
- interleukin-1 receptor–associated kinase
- MyD88
- myeloid differentiation primary response 88
- SV40
- simian virus 40
- PID
- primary immune deficiency
- IL
- interleukin
- JNK
- c-Jun N-terminal kinase
- PDB
- Protein Data Bank
- MOI
- multiplicity of infection
- p-
- phosphorylated.
References
- 1. Alsina L., Israelsson E., Altman M. C., Dang K. K., Ghandil P., Israel L., von Bernuth H., Baldwin N., Qin H., Jin Z., Banchereau R., Anguiano E., Ionan A., Abel L., Puel A., et al. (2014) A narrow repertoire of transcriptional modules responsive to pyogenic bacteria is impaired in patients carrying loss-of-function mutations in MYD88 or IRAK4. Nat. Immunol. 15, 1134–1142 10.1038/ni.3028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dossang A. C., Motshwene P. G., Yang Y., Symmons M. F., Bryant C. E., Borman S., George J., Weber A. N., and Gay N. J. (2016) The N-terminal loop of IRAK-4 death domain regulates ordered assembly of the myddosome signalling scaffold. Sci. Rep. 6, 37267 10.1038/srep37267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Israel L., Wang Y., Bulek K., Della Mina E., Zhang Z., Pedergnana V., Chrabieh M., Lemmens N. A., Sancho-Shimizu V., Descatoire M., Lasseau T., Israelsson E., Lorenzo L., Yun L., Belkadi A., et al. (2017) Human adaptive immunity rescues an inborn error of innate immunity. Cell 168, 789–800.e10 10.1016/j.cell.2017.01.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Picard C., Casanova J. L., and Puel A. (2011) Infectious diseases in patients with IRAK-4, MyD88, NEMO, or IκBα deficiency. Clin. Microbiol. Rev. 24, 490–497 10.1128/CMR.00001-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vollmer S., Strickson S., Zhang T., Gray N., Lee K. L., Rao V. R., and Cohen P. (2017) The mechanism of activation of IRAK1 and IRAK4 by interleukin-1 and Toll-like receptor agonists. Biochem. J. 474, 2027–2038 10.1042/BCJ20170097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lin S. C., Lo Y. C., and Wu H. (2010) Helical assembly in the MyD88-IRAK4-IRAK2 complex in TLR/IL-1R signalling. Nature 465, 885–890 10.1038/nature09121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cushing L., Stochaj W., Siegel M., Czerwinski R., Dower K., Wright Q., Hirschfield M., Casanova J. L., Picard C., Puel A., Lin L. L., and Rao V. R. (2014) Interleukin 1/Toll-like receptor-induced autophosphorylation activates interleukin 1 receptor-associated kinase 4 and controls cytokine induction in a cell type-specific manner. J. Biol. Chem. 289, 10865–10875 10.1074/jbc.M113.544809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ferrao R., Zhou H., Shan Y., Liu Q., Li Q., Shaw D. E., Li X., and Wu H. (2014) IRAK4 dimerization and trans-autophosphorylation are induced by myddosome assembly. Mol. Cell 55, 891–903 10.1016/j.molcel.2014.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chaudhary D., Robinson S., and Romero D. L. (2015) Recent advances in the discovery of small molecule inhibitors of interleukin-1 receptor-associated kinase 4 (IRAK4) as a therapeutic target for inflammation and oncology disorders. J. Med. Chem. 58, 96–110 10.1021/jm5016044 [DOI] [PubMed] [Google Scholar]
- 10. Qin J., Jiang Z., Qian Y., Casanova J. L., and Li X. (2004) IRAK4 kinase activity is redundant for interleukin-1 (IL-1) receptor-associated kinase phosphorylation and IL-1 responsiveness. J. Biol. Chem. 279, 26748–26753 10.1074/jbc.M400785200 [DOI] [PubMed] [Google Scholar]
- 11. Cushing L., Winkler A., Jelinsky S. A., Lee K., Korver W., Hawtin R., Rao V. R., Fleming M., and Lin L. L. (2017) IRAK4 kinase activity controls Toll-like receptor-induced inflammation through the transcription factor IRF5 in primary human monocytes. J. Biol. Chem. 292, 18689–18698 10.1074/jbc.M117.796912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schett G., Dayer J. M., and Manger B. (2016) Interleukin-1 function and role in rheumatic disease. Nat. Rev. Rheumatol. 12, 14–24 10.1038/nrrheum.2016.166 [DOI] [PubMed] [Google Scholar]
- 13. Hoarau C., Gérard B., Lescanne E., Henry D., François S., Lacapère J. J., El Benna J., Dang P. M., Grandchamp B., Lebranchu Y., Gougerot-Pocidalo M. A., and Elbim C. (2007) TLR9 activation induces normal neutrophil responses in a child with IRAK-4 deficiency: involvement of the direct PI3K pathway. J. Immunol. 179, 4754–4765 10.4049/jimmunol.179.7.4754 [DOI] [PubMed] [Google Scholar]
- 14. Ku C. L., von Bernuth H., Picard C., Zhang S. Y., Chang H. H., Yang K., Chrabieh M., Issekutz A. C., Cunningham C. K., Gallin J., Holland S. M., Roifman C., Ehl S., Smart J., Tang M., et al. (2007) Selective predisposition to bacterial infections in IRAK-4-deficient children: IRAK-4-dependent TLRs are otherwise redundant in protective immunity. J. Exp. Med. 204, 2407–2422 10.1084/jem.20070628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Picard C., Puel A., Bonnet M., Ku C. L., Bustamante J., Yang K., Soudais C., Dupuis S., Feinberg J., Fieschi C., Elbim C., Hitchcock R., Lammas D., Davies G., Al-Ghonaium A., et al. (2003) Pyogenic bacterial infections in humans with IRAK-4 deficiency. Science 299, 2076–2079 10.1126/science.1081902 [DOI] [PubMed] [Google Scholar]
- 16. Picard C., von Bernuth H., Ghandil P., Chrabieh M., Levy O., Arkwright P. D., McDonald D., Geha R. S., Takada H., Krause J. C., Creech C. B., Ku C. L., Ehl S., Maródi L., Al-Muhsen S., et al. (2010) Clinical features and outcome of patients with IRAK-4 and MyD88 deficiency. Medicine 89, 403–425 10.1097/MD.0b013e3181fd8ec3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yamamoto T., Tsutsumi N., Tochio H., Ohnishi H., Kubota K., Kato Z., Shirakawa M., and Kondo N. (2014) Functional assessment of the mutational effects of human IRAK4 and MyD88 genes. Mol. Immunol. 58, 66–76 10.1016/j.molimm.2013.11.008 [DOI] [PubMed] [Google Scholar]
- 18. Lee K. L., Ambler C. M., Anderson D. R., Boscoe B. P., Bree A. G., Brodfuehrer J. I., Chang J. S., Choi C., Chung S., Curran K. J., Day J. E., Dehnhardt C. M., Dower K., Drozda S. E., Frisbie R. K., et al. (2017) Discovery of clinical candidate 1-{[(2S,3S,4S)-3-ethyl-4-fluoro-5-oxopyrrolidin-2-yl]methoxy}-7-methoxyisoquinoline-6-carboxamide (PF-06650833), a potent, selective inhibitor of interleukin-1 receptor associated kinase 4 (IRAK4), by fragment-based drug design. J. Med. Chem. 60, 5521–5542 10.1021/acs.jmedchem.7b00231 [DOI] [PubMed] [Google Scholar]
- 19. Song K. W., Talamas F. X., Suttmann R. T., Olson P. S., Barnett J. W., Lee S. W., Thompson K. D., Jin S., Hekmat-Nejad M., Cai T. Z., Manning A. M., Hill R. J., and Wong B. R. (2009) The kinase activities of interleukin-1 receptor associated kinase (IRAK)-1 and 4 are redundant in the control of inflammatory cytokine expression in human cells. Mol. Immunol. 46, 1458–1466 10.1016/j.molimm.2008.12.012 [DOI] [PubMed] [Google Scholar]
- 20. Luciano B. S., Hsu S., Channavajhala P. L., Lin L. L., and Cuozzo J. W. (2004) Phosphorylation of threonine 290 in the activation loop of Tpl2/Cot is necessary but not sufficient for kinase activity. J. Biol. Chem. 279, 52117–52123 10.1074/jbc.M403716200 [DOI] [PubMed] [Google Scholar]