Abstract
Human papilloma virus (HPV) has been recently recognised as a carcinogenic factor for a subset of head and neck cancers (HNC). In Europe, France has one of the highest incidence rates of HNC. The aim of this study is to explore changes in HNC incidence in France, potentially in relation with infection by HPV.
HNC were classified into two anatomical groups: potentially HPV-related and HPV-unrelated. Trends over the period 1980-2012 were analysed by an age-period-cohort model based on data from eleven French cancer registries.
Among men, the age-standardised incidence rate (ASR) of HNC decreased in both groups, but less so for HPV-related sites as compared to unrelated sites, especially in recent years (annual percentage change (APC) over the period 2005-2012 : -3.5% versus -5.4%). Among women, the ASR increased in both groups, but more rapidly for HPV-related as compared to unrelated sites (APC over the period 2005-2012: +1.9% versus -0.4%). This preferential growth of HPV-related versus unrelated HNC was observed in the cohorts born from 1930-1935.
The differences in trends between possible HPV-related and HPV-unrelated sites suggest an increasing incidence of HNC due to HPV infection. The difference was less marked in men as compared to women, most likely because of a higher contamination in the HPV-related group by cancers due to tobacco or alcohol consumption. The pattern observed is consistent with observations made in other countries, with studies of HPV prevalence in HNC and the evolution of sexual behaviour in France.
Keywords: Head and neck cancer, Human papilloma virus, Incidence, Cancer registry, France, Epidemiology
Introduction
In Europe, head and neck cancers (HNC) (encompassing oral cavity, pharynx and larynx cancers) are the seventh most common group of cancers, with an estimated 139 500 cases diagnosed in 2012 and an estimated 63 500 deaths1.
HNC incidence trends are usually correlated with the level of tobacco and alcohol consumption, the main well-known risk factors, which act synergistically. Other observed or suspected risk factors include diets low in fruit and vegetables, poor dental care, professional exposure to carcinogens, or viral infection with Epstein-Barr virus or human immunodeficiency virus2, 3. But in recent decades, a lot of countries have observed changes in the epidemiological pattern of a subset of these cancers: the United States4, 5, Canada6 and Scandinavian countries7–9 have seen an increase in the incidence of oropharyngeal cancers, whereas the incidence of other HNC is decreasing or constant. For example, from Canadian Cancer Registry data, Forte et al. showed a significant increase of oropharynx cancers from 1992 to 2009 (annual percentage change-APC=2.7, p<0.001), in contrast, HNC overall incidence decreased between 1992 and1998 (APC=-3.0, p<0.01), then remained stable 6. This increase is linked by the researchers to increased infections by human papillomavirus (HPV) which has been recognised since 2007 by the International Agency for Research against Cancer (IARC) as a carcinogenic agent for the oral cavity and oropharynx. The evidence is strongest for squamous cell tumours arising from the tonsils, base of tongue and oropharynx10.
The prevalence of HPV in oropharyngeal cancer varies from 40 to 80% in the US and from 20% (countries where alcohol and tobacco consumption is frequent) to 90% (Sweden) in Europe10. The relative contribution of this risk factor in HNC incidence may be evolving rapidly: Swedish hospital studies have shown that the prevalence of HPV (detection of HPV DNA by polymerase chain reaction (PCR)) in tonsil cancer progressed from 68% in 2000-2002 to 93% in 2006-200711 and in base of the tongue cancer from 58% in 1998-2001 to 84% in 2004-20078.
These epidemiological changes should receive attention because patients with HPV-positive oropharynx cancers have a better sensitivity to radio- and chemotherapy than HPV-negative patients and a better overall survival and progression-free survival 10, 12, 13.
Changes in sexual behaviour may be associated with the increased prevalence of HPV-infection in HNC 5, 10.
France has one of the highest incidence rates of HNC in Europe1. Incidence is steadily decreasing in men and increasing in women14, 15. Studies have reported an overall HPV prevalence in oropharyngeal cancer (PCR) comprised between 30.4% and 65.4% 13, 16, 17. However, the potential impact of HPV infection on incidence trends is unknown. The aim of this study is to explore changes in HNC incidence in France, potentially in relation with infection by HPV: we assessed differences in incidence between possible HPV-related and HPV-unrelated HNC for the period 1980 to 2012.
Material and methods
Incidence data
Incidence data were provided by the eleven French general cancer registries of the Départements Bas-Rhin, Calvados, Doubs, Haut-Rhin, Hérault, Isère, Loire-Atlantique, Manche, Somme, Tarn and Vendée, which covered 8.7 million inhabitants. The database contains information on date of diagnosis and birthdate, gender, topography and morphology for each patient. The cases of HNC included in the study were invasive tumours diagnosed between the beginning of the registry (the earliest being 1975 for the Bas-Rhin cancer registry and the latest 1998 for the Loire-Atlantique cancer registry) and 2011 and were limited to squamous cell carcinomas, defined as International Classification of Disease for Oncology version 3 (ICDO-3) histology codes 8050-8076, 8078, 8083, 8084, 8094, 8123.
Because information regarding the presence of HPV within the tumours is rarely available in medical records, we classified the HNC into two anatomical groups in accordance with molecular and epidemiologic data 18 and following previous studies 6, 19–21: potentially HPV-related (HPV-R) cancers and HPV-unrelated (HPV-Unr) cancers.
HPV-R cancers included the following sites (ICDO-3): Base of tongue (C01.9), Tonsil (C02.4, C09), Oropharynx (C10) and Waldeyer ring (C14.2).
HPV-Unr cancers included the following topography codes (ICDO-3): Lip (C00), Tongue (C02 except C02.4), Gum (C03), Floor of mouth (C04), Palate (C05), Other and unspecified parts of mouth (C06), Pyriform sinus (C12), Hypopharynx (C13), Other and ill-defined sites (C14 except C14.2), Larynx (C32).
Population data
Population data were obtained from the Institut National de la Statistique et des Etudes Economiques (INSEE) tabulated by Département, sex, annual age and year from 1975 to 2013, in order to estimate person-years up to 2012.
Statistical analyses
Incidence data were tabulated using 1-year intervals for age, period and cohort. Age-period-cohort models were used to estimate national incidence trends for each sex and HPV group (related or unrelated), based on observed incidence data from the area covered by registries up to 2011, with a short-term projection to estimate incidence in 2012. The area covered by the registries was assumed to be representative of France.
The following model was fitted: Log(Ka,c,d/ PYa,c,d)= αd+ s1(a)+ s2(c)+p2, where Ka,c,d is the number of cases diagnosed at age a from cohort c in the département d, PYa,c,d is the corresponding person-years, p is the period of diagnosis (year), and αd represents a categorical effect of the département. Effects of age and cohort were modelled using smoothing splines, denoted by s1 and s2. The second-order period term p2 was introduced into the model only when it was statistically significant (likelihood ratio test, α = 1%). Note that introducing p2 is equivalent to introducing a linear age-cohort interaction.
Incidence rates were age-standardised on the worldstandard population (ASR). The annual percentage change (APC) of the ASR over the period 1980-2012 was estimated using the formula λ2012= λ1980x(1+APC)32, where λy is the ASR estimate for the year y. APC over the period 2005-2012 was estimated similarly.
In addition, trends in the net probability of developing cancer (i.e. assuming that there are no competing causes of death) between 0 and 74 years by birth cohort were estimated. This indicator was estimated from the age-period-cohort model, with an extrapolation up to 74 years for the recent cohorts.
Analyses were performed using S-PLUS software, version 6.2
Results
Model selected
For HPV-R HNC, the model retained was a simple Age-Cohort model for both men and women, while an Age-Cohort model with a second-order period term p2 was selected for HPV-Unr HNC in both men and women.
Trends according to year
In France, the estimated number of all cases of HNC was 12 972 in 2012, with 10 319 new HNC in men and 2 653 in women (Table 1).
Table 1.
Trends of age-standardised (world standard) incidence, number of cases and median age at diagnosis for head and neck cancers according to potential human papilloma virus association and sex, 1980-2012, France
| Years |
Annual percentage change (%) |
||||||
|---|---|---|---|---|---|---|---|
| 1980 | 1990 | 2000 | 2005 | 2012 | 1980-2012 | 2005-2012 | |
| HPV-related HNC | |||||||
| Age-standardised incidence | |||||||
| Men | 11.9 | 10.6 | 9 | 7.9 | 6.2 | -2 | -3.5 |
| Women | 0.6 | 0.8 | 1.2 | 1.4 | 1.6 | 3 | 1.9 |
| Number of cases | |||||||
| Men | 3663 | 3622 | 3545 | 3435 | 3042 | ||
| Women | 229 | 321 | 506 | 646 | 834 | ||
| Age (median) | |||||||
| Men | 56 | 58 | 58 | 58 | 61 | ||
| Women | 60 | 60 | 57 | 57 | 60 | ||
| HPV-unrelated HNC | |||||||
| Age-standardised incidence | |||||||
| Men | 37.6 | 34.0 | 26.0 | 21.1 | 14.3 | -3 | -5.4 |
| Women | 1.9 | 2.4 | 2.9 | 3.0 | 2.9 | 1.4 | -0.4 |
| Number of cases | |||||||
| Men | 11786 | 11873 | 10551 | 9436 | 7277 | ||
| Women | 773 | 1113 | 1472 | 1661 | 1819 | ||
| Age (median) | |||||||
| Men | 58 | 60 | 61 | 60 | 62 | ||
| Women | 65 | 65 | 65 | 63 | 64 | ||
HPV : Human papilloma virus ; HNC : Head and neck cancer
Incidence of both HPV-R and HPV-Unr HNC was higher among men than women.
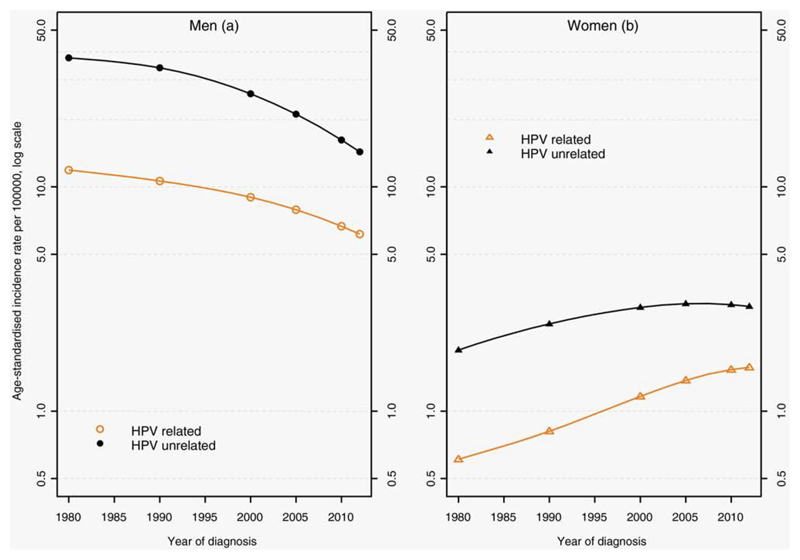
Among men, the age-standardised incidence rate of all HNC decreased but less so for HPV-R sites (Figure 1). Overall, the APC was higher for HPV-R compared to HPV-Unr HNC (Table 1). The sharpest incidence decrease in both groups was seen in the most recent period (2005-2012).
Figure 1.
Human papilloma virus-related and -unrelated head and neck cancers age-standardised incidence rates by sex: trends from 1980 to 2012, France (logarithmic scale)
Among women, the age-standardised incidence rate of all HNC increased but more rapidly for HPV-R sites. Overall, the APC was higher for HPV-R compared to HPV-Unr HNC. For the recent period (2005-2012), the APC increased for HPV-R HNC while it remained stable for HPV-Unr HNC.
During the period, the incidence sex ratio decreased from 19.8 to 3.9 for HPV-R HNC and from 19.8 to 4.9 for HPV-Unr. Among men, the median age at diagnosis increased for HPV-R and HPV-Unr sites from 1980 to 2012. Among women, the median age at diagnosis remained stable. Men with HPV-Unr HNC were diagnosed at younger ages than women. Patients with HPV-R sites were diagnosed at younger ages than HPV-Unr sites, especially among women.
Among HNC, the proportion of HPV-R cancer cases increased from 23.7 % in 1980 to 29.5% in 2012 in men and from 22.9% to 31.4% in women. The percentage increased from the beginning of the nineties. It became higher in women compared to men at the end of the nineties.
Trends according to birth cohort
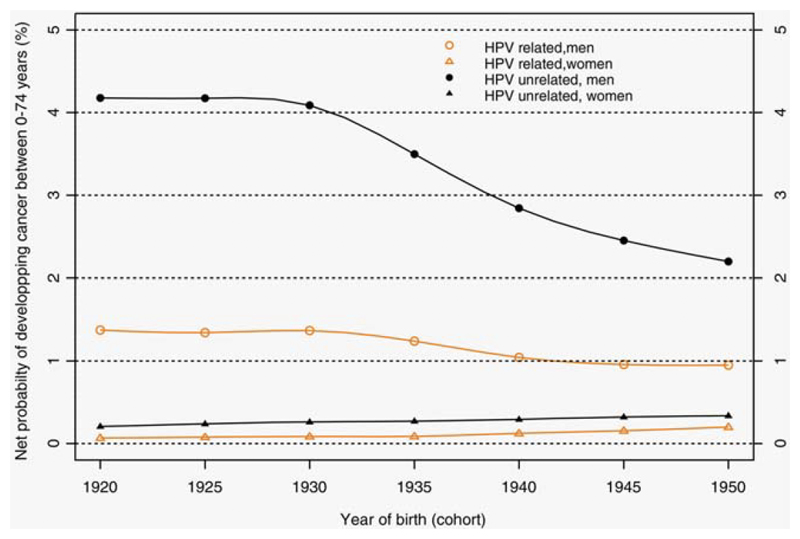
Regarding the probability of developing cancer between 0 and 74 years (Figure 2) by birth cohort (1920 to 1950), it has decreased in men by 30.7% (1.37% to 0.95%) for HPV-R HNC and by 47.4% for HPV-Unr HNC (4.18% to 2.20%). In women, the probability has tripled for HPV-R HNC (from 0.07% to 0.20%) and increased by 61.9% for HPV-Unr HNC (0.21% to 0.34%).
Figure 2.
Net probability of developing cancer between 0 and 74 years according to birth cohort for human papilloma virus(HPV)-related head and neck cancers and HPV-unrelated and by sex
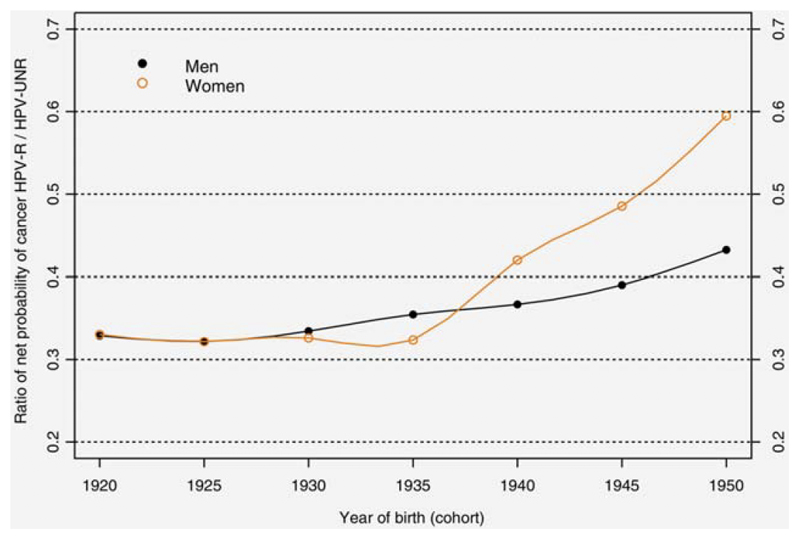
The ratio of net probability of developing cancer between 0 and 74 years for HPV-R vs HPV-Unr HNC (Figure 3) has increased for both sexes, indicating an increasingly greater proportion of HPV-R cancers in relation to HPV-Unr cancers. Among men, the cumulative risk for HPV-R decreased less quickly than that for HPV-Unr HNC starting from the 1930 cohort. Among women, starting from birth cohort 1935, the cumulative risk of HPV-R cancers increased more quickly than that of HPV-Unr cancers.
Figure 3.
Ratio of net probability of developing cancer between 0 and 74 years for human papilloma virus (HPV)-related head and neck cancers / HPV-unrelated by birth cohort and sex
Trends according to birth cohort and age
The evolution of incidence rates by birth cohort (Figure 4) shows an overall decrease in incidence rate among men, whatever their age. However, incidence rates of HPV-R sites fell less rapidly than those of HPV-Unr sites in all age groups.
Figure 4.
Head and neck cancer incidence rates according to age by birth cohort and sex: Human papilloma virus(HPV)-related cancers and men (A), HPV-related cancers and women (B), HPV-unrelated cancers and men (C), HPV-unrelated cancers and women (D)
Among women, an increase in incidence rate was observed in the oldest birth cohorts at all ages for HPV-R sites (Figure 4). With regard to HPV-Unr sites, incidence rate went up for the oldest birth cohorts then remained stable for the most recent cohorts at ages 60, 70 and 80 years. For the most recent cohorts, incidence rate at 50 years has decreased slightly.
Discussion
Differences in incidence trends in HNC according to their potential association with HPV are being observed for both sexes, more markedly among women. Among women, the overall incidence of HNC was increasing but, for a recent calendar period, HPV-Unr HNC incidence remained stable whereas HPV-R HNC incidence was still increasing. In consequence, the proportion of HNC potentially related to HPV increased from 1980 to 2012, especially after 1990. Among men, the overall incidence of HNC was decreasing, but less for HPV-R than HPV-Unr sites, leading to a slight increase in the proportion of HPV-R cancers. In both men and women, the main epidemiological changes concerning HPV-R cancers started in birth cohorts from1930-1935.
Our study has several important strengths. To our knowledge, there has not been a previous study of the French population using cancer registry data to examine the incidence of HNC, taking into account a potential role of HPV. Furthermore, our study covered a long period (32 years). The modelling adopted provides smooth estimations and enables the estimation of the net probability of developing cancer, particularly by birth cohort. This indicator is especially valuable in providing synthetic information regarding incidence trends by birth cohort.
The main limitation of the present study is the difficulty in determining which cancers were associated with HPV. HPV has been recognised only since 2007 by the IARC as a risk factor for tonsil and pharynx cancers. Its detection is not recommended in France; therefore, actual HPV status is not known. Our classification of HNC subsites relied on the potential of being related to HPV, based on etiologic evidence and previous study. HPV can’t be thus totally excluded from the carcinogenicity of the HPV-Unr HNC : the number of cancers attributable to HPV is estimated to 4.3% for the oral cavity cancer (base of tongue excluded) and 4.6% for the cancer of the larynx22. Similarly, due to the high prevalence, in France, of the two main risk factors of HNC i.e. tobacco and alcohol, the HPV-R group is actually highly contaminated by cancers unrelated to HPV. This would explain for instance, the decrease in HPV-R cancers observed in men. Yet it is possible to comment and try to interpret the differences in trends between the two groups, with respect to our knowledge regarding trends in other risk factors. Tobacco consumption and alcohol drinking habits are not collected by cancer registries because these data are not available from the medical records. The distribution of these main risk factors for each HNC is unknown; however, changes in their consumption in France may suggest the role of another risk factor in the epidemiological pattern observed.
Another limitation of the study is the extension of the results to all of France: we used observed data issued from 11 general cancers registries which cover approximately 14% of the French metropolitan population. These registries do not cover major urban centres, where the incidence trends may be quite different. They meet high-quality criteria and are certified every four years by the National Institute of Health and Medical Research (INSERM), the French Institute for Public Health Surveillance (Santé Publique France) and the French National Cancer Institute (INCa, Institut National du Cancer).
To check results, a sensitivity analysis has been done by forcing the model on the HPV-R groups (for both men and women) to include a period term p2 (thus using the same model as for the HPV-Unr HNC groups) and then computed the ratio of net probability of developing cancer between 0 and 74 years for HPV-R vs HPV-Unr HNC in both sex. The results of this sensitivity were almost identical to those depicted on Figure 3, reinforcing the robustness of our findings.
Trends of incidence of HPV-Unr HNC
The procedures for diagnosis, notification process and HNC recording did not change during the study period, so it is unlikely that the observed trends are a result of period effect. These trends call for an examination of the main factors associated with HNC; namely, alcohol and tobacco, whose consumption has regularly decreased.
The decrease in alcohol consumption has been observed in both sexes since the thirties-forties but men’s consumption is 3 fold higher than women’s consumption 23. The mean consumption in France in 1961 was 26 litres of pure alcohol per person per year24; which was extremely high compared to alcohol consumption in other countries at the same period2. That consumption had decreased to 11.6 litres in 201324. In 2014, 14.6% of men and 4.9% of women were daily alcohol consumers25.
Concerning tobacco, the prevalence of regular smoking in the general population has been regularly decreasing in men: 72% in 195326 vs. 32% in 201027. This prevalence has been increasing in women (17% in 195326 vs. 26% in 201027), with a period of stabilization or decrease between 1990 and 200527, 28.
These decreases in alcohol and tobacco consumption may explain the decrease of HNC incidence observed among men since the cohort born in 1930.
The rise in tobacco consumption amongst women is a factor in the increased incidence of HNC. However, the incidence of HNC in women has not reached the same rate as that observed in men, as tobacco and alcohol consumption has nevertheless remained inferior to that of men.
Tobacco consumption in women probably cannot explain the more rapid rise in HPV-R cancers in relation to HPV-Unr cancers, particularly as, according to certain authors, HNC sites preferentially associated with tobacco consumption are the floor of the mouth 29, 30, hypopharynx 29 and larynx 29, 31.
Among women, incidence rates of HPV-Unr HNC remained stable over the most recent period. This result needs to be confirmed in the coming years because the same phenomenon has not been observed for other cancers with the same risk factors. These include lung cancer and oesophageal cancer, for which the APC for 2005-2012 were +5.4% and +1.1% respectively 15.
Trends of incidence of HPV-R HNC
Some studies have shown an increase in the incidence of HPV-R HNC in both sexes and especially for patients aged less than 60 years, whereas the incidence of other HNC is decreasing or constant.
Thus, Chaturvedi et al. 19 showed with data from the SEER program that patients with HPV-R oral squamous cell carcinomas (OSCC) were younger and more likely to be men. The increase in HPV-R incidence was accompanied by a decrease in HPV-Unr OSCC. The increase in incidence was observed among the age groups 40-49 (period 1975-2004) and 50-59 years (period 2000-2004) and in recent birth cohorts. Forte et al. 6 showed with data obtained from the Canadian Cancer Registry that HPV-R oropharyngeal cancers increased among men and women, whereas overall HNC decreased among men and remained stable among women. The increase in HPV-R oropharyngeal cancers was observed for all age groups and was highest for patients aged 50-59 from 1997 to 2009. Blomberg et al. 21 showed with data from the Danish Cancer Registry an increasing incidence in men (more marked for patients <60 years) for HPV-associated and potentially HPV-associated HNC, while the incidence of HPV-Unr HNC decreased during the study period (1978-2007). In women, a significant increase of HPV-associated HNC was observed, while potentially HPV-associated and HPV-Unr HNC showed a slight increase.
Our study shows epidemiological trends consistent with these papers. However, we did not see any increase in the incidence of HPV-R HNC in men, probably because incidence rates in France were considerably higher during the eighties than in those countries where an epidemiological evolution in HNC linked to HPV has been demonstrated 32. Thus, the evolution of incidence rate in France is still very much linked to alcohol and tobacco consumption, which could be masking the impact of HPV.
In parallel, the prevalence of HPV in HNC is not yet very high in France. A study reported an overall HPV prevalence of 46.5% (PCR) in oropharyngeal cancer (53.7%-tonsil, 30.2%-base of tongue) for the period 2000-200916. A second and a third studies reported an HPV prevalence in oropharyngeal cancer of 50% (detection of p16 expression using immunohistochemistry)-65% (PCR) for the period 2007-200913 and of 30.4% for the period 2007-201017. By contrast, in Sweden, the prevalence of HPV (PCR) in tonsil cancer was evaluated at 93% in 2006–200711 and at 84% in 2004–20078 in base of the tongue cancer.
In our study, there was a difference in the trend between the two groups of cancers, which was more marked among women. This result is compatible with studies of prevalence of HPV in HNC in France: in the period 2000-2009, the prevalence of the virus (PCR) was significantly higher in women compared with men, 80% vs 52% 33 and 63.5% vs 42.2% 16 respectively for tonsils and oropharynx.
A positive association exists between the risk of HPV-16-positive oropharyngeal cancer and lifetime number of vaginal and/or oral sex partners, engagement in casual sex, early age at first intercourse, and infrequent use of condoms 34–36. In France, changes in sexual behaviour between 1970 and 2006 matched the increased risk of HNC linked to HPV 37 : the median age of first intercourse went down respectively in men and women from 18.8 years and 20.6 years in 1950 to 17.2 years and 17.6 years in 2000. The average lifetime number of sexual partners among men has remained stable (11.6) but has increased among women (1.8 to 4.4), with a more notable rise in women in the 30-49 year age group (1.9 to 5.1). Engaging in oral sex at least once during a lifetime went up to over 80% in 2006 amongst men and women, whereas in 1970 it was less than 60%.
However, the prevalence of HPV 16 in the cervix is lower in western Europe (1.6%) compared with North America (4.1%) and northern Europe (3.0%) 38.
Apart from its association with oropharyngeal cancers, HPV is a risk factor for anal canal cancers, the incidence of which is increasing in France39, with a greater rise during the period 2005-2012 in men (APC:+2.6%) and in women (APC:+3.4%).
Thus, the observed changes in the epidemiology of HNC show that at least some of these cancers are very probably linked to HPV. This is consistent with observations made in other countries, with the few French studies on prevalence of HPV in HNC and also with the evolution of sexual behaviour in France.
Conclusion
The differences in trends between possible HPV-related and HPV-unrelated HNC suggest an increasing incidence of HNC due to HPV infection in France. Among men, differences are less marked, probably because of a higher contamination of tobacco and alcohol-related cancers in the HPV-related group. Future epidemiological patterns of these cancers should also be discussed by taking into account the sexual behaviour during the AIDS epidemic, “binge-drinking” alcohol consumption and the impact of the vaccination program for the prevention of cervical cancer.
Novelty and Impact.
This study showed, for the first time in France, differences in incidence trends between possible Human papilloma virus (HPV)-related and HPV-unrelated head and neck cancers. This suggests an increasing incidence of head and neck cancers due to HPV infection. The difference was less marked in men as compared to women, most likely because of a higher contamination in the HPV-related group by cancers due to tobacco or alcohol consumption.
Acknowledgement
The authors thank the French Assurance Maladie and all participant cancer centers, hospitals, private clinics, and pathologists for providing case identification and data collection. They also thank Mrs Gillian Cadier for the translation of the manuscript.
This study was funded by French Institut national du cancer (INCa) and Santé publique France.
Role of the funding source
Funders have no role in the design or interpretation or presentation of the study.
Abbreviations
- APC
Annual percentage change
- ASR
Age standardized rates
- HNC
Head and neck cancer
- HPV
Human papilloma virus
- HPV-R
Human papilloma virus-related
- HPV-Unr
Human papilloma virus-unrelated
- ICD-O
International classification of diseases for oncology
- OSCC
Oral squamous cell carcinoma
Footnotes
Competing interests
The authors declare that they have no competing interest.
Authors’ contribution
KL conceived the study, participated to its design and coordination and drafted the manuscript. ZU, AB, NB, GL, AVG participated to the design of the study and helped in drafting the manuscript. ZU and AB performed the statistical analyses. FRANCIM provided the data. All authors read and approved the final manuscript.
References
- 1.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D, Bray F. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. European journal of cancer. 2013;49:1374–403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 2.Auperin A, Hill C. [Epidemiology of head and neck carcinomas] Cancer radiotherapie : journal de la Societe francaise de radiotherapie oncologique. 2005;9:1–7. doi: 10.1016/j.canrad.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Righini CA, Karkas A, Morel N, Soriano E, Reyt E. [Risk factors for cancers of the oral cavity, pharynx (cavity excluded) and larynx] Presse medicale. 2008;37:1229–40. doi: 10.1016/j.lpm.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 4.Shiboski CH, Schmidt BL, Jordan RC. Tongue and tonsil carcinoma: increasing trends in the U.S. population ages 20-44 years. Cancer. 2005;103:1843–9. doi: 10.1002/cncr.20998. [DOI] [PubMed] [Google Scholar]
- 5.Sturgis EM, Cinciripini PM. Trends in head and neck cancer incidence in relation to smoking prevalence: an emerging epidemic of human papillomavirus-associated cancers? Cancer. 2007;110:1429–35. doi: 10.1002/cncr.22963. [DOI] [PubMed] [Google Scholar]
- 6.Forte T, Niu J, Lockwood GA, Bryant HE. Incidence trends in head and neck cancers and human papillomavirus (HPV)-associated oropharyngeal cancer in Canada, 1992-2009. Cancer causes & control : CCC. 2012;23:1343–8. doi: 10.1007/s10552-012-0013-z. [DOI] [PubMed] [Google Scholar]
- 7.Hammarstedt L, Dahlstrand H, Lindquist D, Onelov L, Ryott M, Luo J, Dalianis T, Ye W, Munck-Wikland E. The incidence of tonsillar cancer in Sweden is increasing. Acta oto-laryngologica. 2007;127:988–92. doi: 10.1080/00016480601110170. [DOI] [PubMed] [Google Scholar]
- 8.Attner P, Du J, Nasman A, Hammarstedt L, Ramqvist T, Lindholm J, Marklund L, Dalianis T, Munck-Wikland E. The role of human papillomavirus in the increased incidence of base of tongue cancer. International journal of cancer Journal international du cancer. 2010;126:2879–84. doi: 10.1002/ijc.24994. [DOI] [PubMed] [Google Scholar]
- 9.Annertz K, Anderson H, Palmer K, Wennerberg J. The increase in incidence of cancer of the tongue in the Nordic countries continues into the twenty-first century. Acta oto-laryngologica. 2012;132:552–7. doi: 10.3109/00016489.2011.649146. [DOI] [PubMed] [Google Scholar]
- 10.Marur S, D'Souza G, Westra WH, Forastiere AA. HPV-associated head and neck cancer: a virus-related cancer epidemic. The Lancet Oncology. 2010;11:781–9. doi: 10.1016/S1470-2045(10)70017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nasman A, Attner P, Hammarstedt L, Du J, Eriksson M, Giraud G, Ahrlund-Richter S, Marklund L, Romanitan M, Lindquist D, Ramqvist T, et al. Incidence of human papillomavirus (HPV) positive tonsillar carcinoma in Stockholm, Sweden: an epidemic of viral-induced carcinoma? International journal of cancer Journal international du cancer. 2009;125:362–6. doi: 10.1002/ijc.24339. [DOI] [PubMed] [Google Scholar]
- 12.Chung CH, Gillison ML. Human papillomavirus in head and neck cancer: its role in pathogenesis and clinical implications. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:6758–62. doi: 10.1158/1078-0432.CCR-09-0784. [DOI] [PubMed] [Google Scholar]
- 13.Melkane AE, Auperin A, Saulnier P, Lacroix L, Vielh P, Casiraghi O, Msakni I, Drusch F, Temam S. Human papillomavirus prevalence and prognostic implication in oropharyngeal squamous cell carcinomas. Head & neck. 2014;36:257–65. doi: 10.1002/hed.23302. [DOI] [PubMed] [Google Scholar]
- 14.Ligier K, Belot A, Launoy G, Velten M, Bossard N, Iwaz J, Righini CA, Delafosse P, Guizard AV, network F. Descriptive epidemiology of upper aerodigestive tract cancers in France: incidence over 1980-2005 and projection to 2010. Oral oncology. 2011;47:302–7. doi: 10.1016/j.oraloncology.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 15.Binder-Foucard F, Bossard N, Delafosse P, Belot A, Woronoff AS, Remontet L, French network of cancer r Cancer incidence and mortality in France over the 1980-2012 period: solid tumors. Revue d'epidemiologie et de sante publique. 2014;62:95–108. doi: 10.1016/j.respe.2013.11.073. [DOI] [PubMed] [Google Scholar]
- 16.Guily JL, Jacquard AC, Pretet JL, Haesebaert J, Beby-Defaux A, Clavel C, Agius G, Birembaut P, Okais C, Leocmach Y, Soubeyrand B, et al. Human papillomavirus genotype distribution in oropharynx and oral cavity cancer in France--The EDiTH VI study. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2011;51:100–4. doi: 10.1016/j.jcv.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Gavid M, Pillet S, Pozzetto B, Oriol M, Dumollard JM, Timoshenko AP, Martin C, Prades JM. Human papillomavirus and head and neck squamous cell carcinomas in the South-East of France: prevalence, viral expression, and prognostic implications. Acta oto-laryngologica. 2013;133:538–43. doi: 10.3109/00016489.2012.747221. [DOI] [PubMed] [Google Scholar]
- 18.Gillison ML, Alemany L, Snijders PJ, Chaturvedi A, Steinberg BM, Schwartz S, Castellsague X. Human papillomavirus and diseases of the upper airway: head and neck cancer and respiratory papillomatosis. Vaccine. 2012;30(Suppl 5):F34–54. doi: 10.1016/j.vaccine.2012.05.070. [DOI] [PubMed] [Google Scholar]
- 19.Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:612–9. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 20.Ryerson AB, Peters ES, Coughlin SS, Chen VW, Gillison ML, Reichman ME, Wu X, Chaturvedi AK, Kawaoka K. Burden of potentially human papillomavirus-associated cancers of the oropharynx and oral cavity in the US, 1998-2003. Cancer. 2008;113:2901–9. doi: 10.1002/cncr.23745. [DOI] [PubMed] [Google Scholar]
- 21.Blomberg M, Nielsen A, Munk C, Kjaer SK. Trends in head and neck cancer incidence in Denmark, 1978-2007: focus on human papillomavirus associated sites. International journal of cancer Journal international du cancer. 2011;129:733–41. doi: 10.1002/ijc.25699. [DOI] [PubMed] [Google Scholar]
- 22.Plummer M, de Martel C, Vignat J, Ferlay J, Bray F, Franceschi S. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob Health. 2016;4:e609–16. doi: 10.1016/S2214-109X(16)30143-7. [DOI] [PubMed] [Google Scholar]
- 23.Hill C, Doyon F. [The frequency of cancer in France: mortality trends since 1950 and summary of the report on the causes of cancer] Bulletin du cancer. 2008;95:5–10. doi: 10.1684/bdc.2008.0559. [DOI] [PubMed] [Google Scholar]
- 24.OFDT. Séries statistiques alcool. [acessed 12/01/2017]; http://www.ofdt.fr/statistiques-et-infographie/seriesstatistiques/alcool-evolution-des-quantites-consommees-par-habitant/
- 25.Richard JB, Palle C, Guignard R, Nguyen-Thanh V, Beck F, Arwidson P. La consommation d'alcool en France en 2014. In: INPES, editor. Evolutions. [acessed 12/01/2017]. http://www.inpes.sante.fr/CFESBases/catalogue/pdf/1632.pdf. [Google Scholar]
- 26.Hill C. Trends in tobacco smoking and consequences on health in France. Preventive medicine. 1998;27:514–9. doi: 10.1006/pmed.1998.0319. [DOI] [PubMed] [Google Scholar]
- 27.Guignard R, Beck F, Wilquin JL, Andler R, Nguyen-Thanh V, Richard JB, et al. La consommation de tabac en France et son évolution : résultats du Baromètre santé 2014. Bull Epidémiol Hebd. 2015:281–8. [Google Scholar]
- 28.Hill C, Laplanche A. Tabagisme et mortalité : aspects épidémiologiques. Bulletin Epidémiologique Hebdomadaire. 2003;22–23:98–100. [Google Scholar]
- 29.Koch WM, Lango M, Sewell D, Zahurak M, Sidransky D. Head and neck cancer in nonsmokers: a distinct clinical and molecular entity. The Laryngoscope. 1999;109:1544–51. doi: 10.1097/00005537-199910000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Radoi L, Menvielle G, Cyr D, Lapotre-Ledoux B, Stucker I, Luce D, group Is Population attributable risks of oral cavity cancer to behavioral and medical risk factors in France: results of a large population-based case-control study, the ICARE study. BMC Cancer. 2015;15:827. doi: 10.1186/s12885-015-1841-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lubin JH, Purdue M, Kelsey K, Zhang ZF, Winn D, Wei Q, Talamini R, Szeszenia-Dabrowska N, Sturgis EM, Smith E, Shangina O, et al. Total exposure and exposure rate effects for alcohol and smoking and risk of head and neck cancer: a pooled analysis of case-control studies. Am J Epidemiol. 2009;170:937–47. doi: 10.1093/aje/kwp222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parkin DM, Muir CS, Whelan SL, Gao Y-T, Ferlay J, Powell J. Cancer Incidence in Five Continentsed. VI LYON: IARC Scientific Publications; 1992. [Google Scholar]
- 33.Guily JL, Clavel C, Okais C, Pretet JL, Beby-Defaux A, Agius G, Birembaut P, Jacquard AC, Leocmach Y, Soubeyrand B, Riethmuller D, et al. Human papillomavirus genotype distribution in tonsil cancers. Head & neck oncology. 2011;3:6. doi: 10.1186/1758-3284-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.D'Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, Koch WM, Westra WH, Gillison ML. Case-control study of human papillomavirus and oropharyngeal cancer. The New England journal of medicine. 2007;356:1944–56. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 35.Heck JE, Berthiller J, Vaccarella S, Winn DM, Smith EM, Shan'gina O, Schwartz SM, Purdue MP, Pilarska A, Eluf-Neto J, Menezes A, et al. Sexual behaviours and the risk of head and neck cancers: a pooled analysis in the International Head and Neck Cancer Epidemiology (INHANCE) consortium. International journal of epidemiology. 2010;39:166–81. doi: 10.1093/ije/dyp350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chancellor JA, Ioannides SJ, Elwood JM. Oral and oropharyngeal cancer and the role of sexual behaviour: a systematic review. Community Dent Oral Epidemiol. 2016 doi: 10.1111/cdoe.12255. [DOI] [PubMed] [Google Scholar]
- 37.Veluire M, Brasnu D. [Evolution of sexual behaviour in France and emergence of new head and neck cancers] Bulletin du cancer. 2011;98:1185–92. doi: 10.1684/bdc.2011.1454. [DOI] [PubMed] [Google Scholar]
- 38.de Sanjose S, Diaz M, Castellsague X, Clifford G, Bruni L, Munoz N, Bosch FX. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. The Lancet Infectious diseases. 2007;7:453–9. doi: 10.1016/S1473-3099(07)70158-5. [DOI] [PubMed] [Google Scholar]
- 39.Bouvier AM, Belot A, Manfredi S, Jooste V, Uhry Z, Faivre J, Duport N, Grabar S, French network of cancer registries F Trends of incidence and survival in squamous-cell carcinoma of the anal canal in France: a population-based study. European journal of cancer prevention : the official journal of the European Cancer Prevention Organisation. 2015 doi: 10.1097/CEJ.0000000000000163. [DOI] [PubMed] [Google Scholar]