Abstract
Offspring of hypertensive pregnancies are at increased risk of developing hypertension in adulthood. In the neonatal period they display endothelial cell dysfunction and altered microvascular development. MicroRNAs, as important endothelial cellular regulators, may play a role in this early endothelial dysfunction. Therefore we identified differential microRNA patterns in endothelial cells from offspring of hypertensive pregnancies and determined their role in postnatal vascular cell function. Studies were performed on human umbilical vein endothelial cell (HUVECs) samples from 57 pregnancies. Unbiased RNA-sequencing identified 30 endothelial-related microRNAs differentially expressed in HUVECs from hypertensive compared to normotensive pregnancies. Quantitative reverse transcription PCR (RT-qPCR) confirmed a significant higher expression level of the top candidate, miR-146a. Combined miR-146a targeted gene expression and pathway analysis revealed significant alterations in genes involved in inflammation, angiogenesis and immune response in the same HUVECs. Elevated miR-146a expression level at birth identified cells with reduced ability for in vitro vascular tube formation, which was rescued by miR-146a inhibition. In contrast, miR-146a overexpression significantly reduced vascular tube formation in HUVECs from normotensive pregnancies. Finally, we confirmed that mir146a levels at birth predicted in vivo microvascular development during the first three postnatal months. Offspring of hypertensive pregnancy have a distinct endothelial regulatory microRNA profile at birth, which is related to altered endothelial cell behaviour, and predicts patterns of microvascular development during the first three months of life. Modification of this microRNA profile in vitro can restore impaired vascular cell function.
Keywords: Hypertensive pregnancy, postnatal vasculogenesis, microRNAs
Introduction
Offspring of hypertensive pregnancies display changes in endothelial function from early in life1 and are at an increased risk for hypertension in adulthood.2–4 Circulating endothelial cells5 and umbilical vein derived endothelial cells6 from neonates born to hypertensive pregnancies have altered cellular angiogenic capacity at birth. Furthermore, the poorer the function of the cells in vitro the more likely the infant is to lose microvascular dermal density during the first postnatal months, as their circulation remodels.6 Microvascular dermal rarefaction has been linked to higher blood pressure in young adults born to pregnancy complications7 and a primary abnormality in microvascular development presents a plausible mechanistic pathway to explain increased hypertensive risk for these offspring.8
MicroRNAs are a class of non-coding RNAs that are important regulators of cellular function.9 In recent years, several microRNAs have been shown to control aspects of endothelial cell behaviour such as migration, proliferation, apoptosis and vasculogenesis10 with certain microRNAs identified to be pro-angiogenic and others anti-angiogenic.11–17 Previous studies have successfully demonstrated that alteration of endothelial microRNAs, using a knock-down approach, can have dramatic impacts on endothelial proliferation and tube formation in vitro.13, 14 Stress conditions such as inflammation can also alter the regulation of microRNAs.16 It is thus plausible that in utero stress exposure related to hypertensive pregnancies may programme alterations in offspring microRNA profile. Therefore, we hypothesized endothelial cells from hypertensive pregnancy offspring would display a different pattern of microRNA expression compared to those derived from normotensive pregnancies. Furthermore, these patterns would predict vasculogenic capacity of endothelial cells as well as the in vivo development of the microvascular network in the neonate during the first three months of their postnatal life.
Methods
Study cohort and sample collection
The data that support the findings of this study are available from the corresponding author upon reasonable request. Mothers being cared for by Oxford University Hospitals NHS Foundation Trust between 2013 and 2015 were identified by their clinical care team and invited to take part in the Oxford Cardiovascular Tissue Bioresource programme, coordinated by the Oxford Cardiovascular Clinical Research Facility and NHS Blood and Transplant. A clinical recruitment team approached mothers prior to delivery to seek consent for donation of tissue aiming for on average two to three participants every month. Mothers with either hypertensive or normotensive pregnancies were identified and approached in parallel to ensure balanced recruitment during a month. Rate of recruitment was controlled to ensure adequate time for sample preparation, including cord processing, cell isolation and cell maintenance. Umbilical cords were collected immediately after delivery by a dedicated research cord collection team. All cords were processed within 12 hours of delivery. Human umbilical vein endothelial cells (HUVECs) were isolated and stored according to standard operating procedures [see Supplementary Methods]. HUVECs were cultured in endothelial basal medium (EGM-2) supplemented with the EGM-2 bullet kit (Cat # CC-3162, Lonza, UK). All cell cultures were maintained in humidified 5% CO2 at 37oC. All RNA expression analysis was performed using cells from passage 1, whereas tube formation assays were performed with cells from passage 2 or 3.
Pregnancy history including blood pressure levels were recorded for each participant from maternity records. Hypertensive pregnancies, including pregnancy-induced hypertension and preeclampsia, were defined according to the International Society for the Study of Hypertension in Pregnancy guidelines (definitions are available in the Supplementary Materials). Normotensive pregnancy was also confirmed from case records and if there was subsequent evidence of problems during pregnancy such as fetal growth restriction or glucose intolerance samples were not included in analysis. The measurement of microvascular measures at birth and three months of age in the infants has been reported previously6 (detailed in Supplementary Materials). All participants provided written consent with collection and subsequent experimental use of samples in accordance with approvals from appropriate National Research Ethics Committees [09/H0606/68, 07/H0606/148, 15/SC/0027 and 11/SC/0230].
RNA-sequencing
Total RNA (1-5µg) isolated from HUVECs (n=8) was processed using the NEBNext® Small RNA Library Prep Set for Illumina® (Cat. # E7330L) according to manufacturer recommendations. The microRNA component of the library was enriched and quantified by real-time qPCR. Sequencing was performed as 50bp single read on a GAIIx according to Illumina specifications using Cluster Generation Kit v4 and Sequencing Kit v4 (details are provided in the Supplementary Methods). Quality control was performed to determine the proportion of reads mapping to genes and variation within the dataset. The data were normalised using the R/BioConductor ‘edgeR_2.99.0’ package.
Reverse transcription (RT) and Polymerase chain reaction (PCR) reaction
TaqMan microRNA reverse transcription kit (Applied Biosystems, USA, Cat # 4366596) was used for cDNA synthesis according to the manufacturer’s instructions. Expression of hsa-miR-146a in HUVECs was quantified using the microRNA TaqMan assay (Applied Biosystems, assay ID 000468). Each PCR reaction was performed in three technical replicates and expressed as fold change using the 2-ΔΔCt method.18 Expression of miR-146a was normalized to the endogenous control RNU44 (Applied Biosystems, assay ID 001094).
Gene expression and network analysis
Total RNA of HUVECs was extracted using QIAGEN RNeasy Mini Kit (QIAGEN, Germany) according to the manufacturer’s instructions. RNA quality and abundance were assessed using a Nanodrop ND-1000 spectrophotometer (Nanodrop Technologies, USA). Gene expression microarray analysis was conducted using Illunima HT-12v4 expression bead-chip arrays according to the manufacturer’s protocol. The raw gene expression data were analysed using the R/BioConductor ‘limma_3.26.9’ package. Variance-stabilisation and normalisation (VSN) algorithm was used for data normalisation. Differential gene expression was determined using t-tests/ANOVA analysis.
Using the experimentally validated microRNA-target interactions database (miRTarBase, http://mirtarbase.mbc.nctu.edu.tw), the expression level of the microRNA-targeted genes was extracted from the gene expression array results. We conducted a network enrichment analysis using Metacore™ based on combination of the selected microRNA-targeted genes and the expression results from the array experiment. Statistical significance was corrected using Bonferroni adjustment for the total number of genes in each network.
Tube formation and cell proliferation
Angiogenic capacity was assessed by performing tube formation using growth factor-reduced Matrigel (BD Biosciences, UK). Cell proliferation was assessed using the CyQUANT NF Cell Proliferation kit (Life Technologies, USA) according to the manufacturer’s instruction (detailed in Supplementary Methods).
Cell transfection
HUVECs were plated in a 6-well plate at 1x105 cells per well overnight. Cells were transfected with hsa-miR-146a-5p mimic (10 nM), or hsa-miR-146a-5p hairpin inhibitor (80 nM), or respective non-targeting controls (control #1, Dharmacon, USA) for 24 hours using Lipofectamine RNAiMAX (Cat # 13778030, ThermoFisher, USA) according to the manufacturer’s instructions. Cells were then replaced with antibiotic-free media for additional 48 hours and collected for functional assays. Transfections were visualised and optimised with fluorescently labelled mimic and inhibitor controls (Dharmacon). Transfection efficiency was over 90%. The transfection of HUVECs with hsa-miR-146a inhibitor and mimic were confirmed by quantitative reverse transcription PCR (qRT-PCR) and immunoblotting was used to confirm alterations in a downstream protein level (details are provided in the Supplementary Methods.
In vivo microvascular imaging
Imaging of the axillary small vessel network was performed with Side Stream Dark Field (SSDF) imaging (Microscan, Microvision Medical, The Netherlands), as previously reported for neonates.19 Measurements were performed after birth and again at three months, on the same side, in a temperature-controlled room, with the infant at rest, either in their mother’s arms or in a crib (details are provided in the Supplementary Methods).
Statistical analysis
Statistical analyses were conducted using SPSS Version 22 and GraphPad Prism 6.0. Comparisons between continuous variables were performed using a two-tailed, independent samples t-test for normally distributed variables, and Mann-Whiney U test for non-normally distributed data. Levels of variables across the distribution of non-normally distributed variables were analysed with the cohort ranked into thirds or tenths of the distribution. Comparison of categorical variables was performed using a Chi-Square test. Bivariable and multivariable regression models were performed using a forced entry method. Statistically significant variables from bivariable regression analysis were then included in a multivariable regression analysis. Pearson’s correlation was recorded for bivariable regression analysis. Standardized coefficients were used in the multiple regression analysis. Results are presented as mean±standard deviation for normally distributed continuous variables, median (minimum, maximum) for non-normally distributed variables, and the number of observations (yes/no) with the percentage in each group for categorical variables. P-values ≤ 0.05 were considered statistically significant.
Results
Cohort characteristics
Samples from 57 pregnancies were used. Maternal and offspring demographic characteristics in the normotensive and hypertensive groups are presented in Table 1. Normotensive and hypertensive pregnancy groups were matched for maternal age, with no differences in smoking and alcohol consumption at time of pregnancy. Maternal body mass index, proportion of caesarean section, and blood pressure during pregnancy were significantly higher in the hypertensive mothers. Infants in the hypertensive group were on average two weeks more premature with a lower birthweight Z score. There were no differences in offspring sex distribution, head circumference and Apgar score at five minutes between the two groups.
Table 1. Characteristics of Cohort.
| Parameters | Normotensive (n=32) | Hypertensive (n=25) |
|---|---|---|
| Mother | ||
| Maternal age, years | 33±4 | 33±5 |
| BMI at booking, kg/m2 | 23.6±3.4 | 28.1±6.5† |
| Folic acid, n (%) | 23 (79) | 22 (88) |
| Smokers, n (%) | 7 (21) | 7 (27) |
| Alcohol this pregnancy, n (%) | 3 (9) | 3 (12) |
| Bleeding in early pregnancy, n (%) | 3 (9) | 4 (15) |
| Caesarean section, n (%) | 5 (15) | 17 (71)‡ |
| Booking sBP, mmHg | 110.5±10.7 | 118.9±18.5* |
| Booking dBP, mmHg | 66.9±8.8 | 71.7.5±11.4 |
| Late gestation sBP, mmHg | 111.2±10.5 | 124.5±16.6 ‡ |
| Late gestation dBP, mmHg | 65.1±7.7 | 78.6±11.7‡ |
| Highest sBP, mmHg | 116.7±11.4 | 143.5±19.7 ‡ |
| Highest dBP, mmHg | 70.8±8.3 | 90.4±10.7‡ |
| Offspring | ||
| Gestation age, weeks | 39±1.7 | 37±2.7‡ |
| Birthweight, grams | 3372.6±581.7 | 2782.3±774.7† |
| Birth weight z-score | 0.36±0.8 | -0.44±1.0† |
| Males, n (%) | 18 (55) | 13 (48) |
| Head circumference, cm | 174.3±7.8 | 175.7±9.4 |
| Apgar score (5mins) | 9.9±0.2 | 9.7±1.0 |
Values as mean±standard deviation unless stated otherwise. sBP, systolic blood pressure; dBP,diastolic blood pressure. BMI, body mass index;. * p<0.05; † p<0.01; ‡ p<0.001 by unpaired t-test.
MicroRNA profiles in HUVECs from normotensive and hypertensive pregnancy offspring
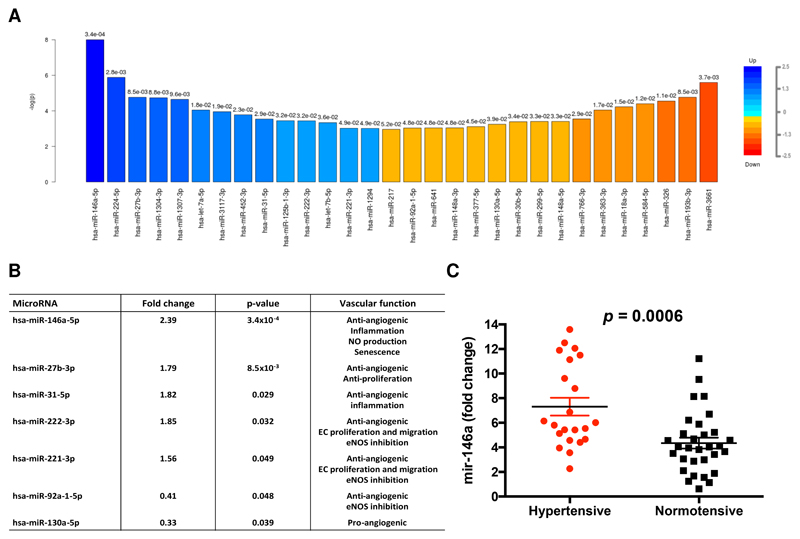
We selected eight samples (four hypertensive and four normotensive) (Supplementary Table S1), matched by maternal age and gestational age, based on a predefined RNA sequencing experimental design for RNA-sequencing analysis with enrichment in microRNA profile to investigate the microRNA profile at birth. Thirty significantly altered microRNAs were identified between hypertensive and normotensive groups (p<0.05) (Figure 1A). Based on a literature review,20 we identified seven of these differentially expressed miRNAs (miR-146a, -27b, -31, -222 and -221, -130 and -92) in the hypertensive pregnancy cohort as having previously been reported to be regulators of endothelial responses (Supplementary Table S2 and Figure 1B). After false discovery rate adjustment (Bonferroni correction based on the 20 pre-selected microRNA targets), miR-146a remained significantly upregulated with a 2.39-fold higher level in hypertensive HUVECs compared to the normotensive samples. We therefore took miR-146a forward as a candidate microRNA for validation and downstream functional investigation.
Figure 1. MicroRNA profiles in HUVECs from normotensive and hypertensive pregnancy offspring.
(A) Top 30 differently expressed microRNAs in human umbilical vein endothelial cells (HUVECs) derived from hypertensive and normal pregnancies (n=8). Fold change is indicated by colour gradient, with darker colours representing higher fold change, where blue represents upregulation of microRNA targets and orange represents downregulation of microRNA targets in the comparison. Numbers above the fold change colour bars represent the p-value obtained from the t-test comparison between groups. (B) Validation in RNA sequencing of previously reported microRNAs in endothelial cells. EC, endothelial cell; NO, nitric oxide; eNOS, endothelial nitric oxide synthase. (C) miR-146a expression (qRT-PCR) in HUVECs isolated from hypertensive pregnancy (n=25) and normotensive pregnancy (n=32). Both groups were compared with a baseline, i.e. miR-146a level measured in a peripherals blood sample of a healthy individual; p-value is obtained by unpaired t-test.
Validation of miR-146a in hypertensive pregnancy offspring
We confirmed (using qRT-PCR) in the full cohort of 57 participants that the level of miR-146a in hypertensive HUVECs was 1.74-fold higher than in the normotensive group (p=0.0006); miR-146a measured in a peripheral plasma sample was used as the reference sample for the comparison of hypertensive and normotensive samples (Figure 1C). We then studied other maternal and fetal risk factors that varied between hypertensive and normotensive pregnancy to determine whether there were any potential confounders in the association between hypertensive pregnancy and miR-146a fold change (Supplementary Table S3). In bivariable regression analysis, gestational age trended towards an inverse correlation with miR-146a (coefficient B =-0.24, p=0.08). Therefore we included gestational age, along with hypertensive pregnancy and maternal age as independent variables in a multivariable regression model. This analysis demonstrated that hypertensive pregnancy was an independent predictor of miR-146a level, with gestational age showing no association with miR-146a (coefficient B=0.01, p=0.96). (Table 2).
Table 2. Multivariable regression coefficients for maternal and fetal risk factors and miR-146a.
| Parameters | Coefficient B (miR-146a fold change) |
p-value |
|---|---|---|
| Maternal hypertension during pregnancy | 3.25 | 0.002 |
| Maternal age (years) | -0.13 | 0.14 |
| Gestational age (weeks) | 0.01 | 0.96 |
B-unstandardized coefficient with 95% confidence interval, bold represents p < 0.05.
miR-146a targeted pathway analysis in endothelial cells from hypertensive pregnancy offspring
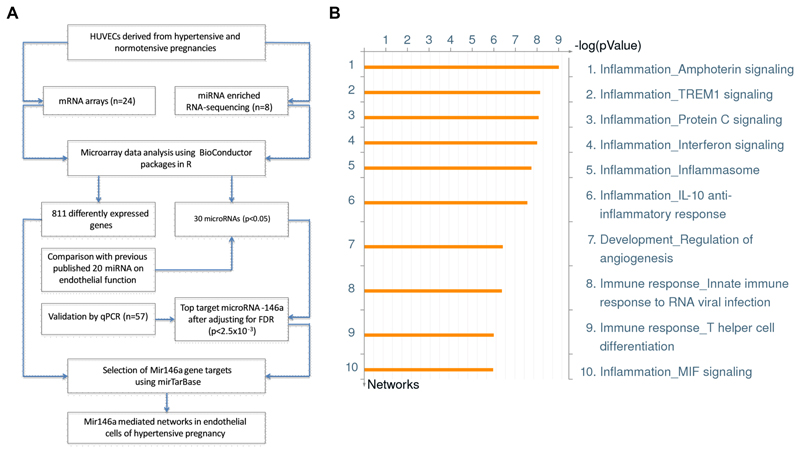
To further determine the role of miR-146a to cellular function in endothelial cells from hypertensive pregnancies, we explored evidence of variation in the gene expression profile related to known targets for miR-146a using gene expression array data in the same cohort based on samples collected from 12 hypertensive and 12 normotensive mothers. After normalization, ANOVA comparative analysis identified 811 significantly differentially expressed genes (p<0.05). As the aim of this analysis was to focus on miR-146a-targeted genes, we limited the investigation to 97 targets, which were previously validated and extracted from the miRTarBase database (http://mirtarbase.mbc.nctu.edu.tw), with details in the Methods. Among the 97 selected miR-146a gene targets, 67 targets were detected in the expression array results (Supplementary Table S4). Gene enrichment network analysis using Metacore™ was performed using the combined miR-146a targeted gene list and gene expression profile. The combined approach has been shown to be instrumental in detecting group effects in microRNA expression and related target gene sets.21 A summary of the miR-146a targeted selection process is shown in Figure 2A. Using this selection process, 44 networks were identified with statistically significant associations (p<0.05) after correcting for the total number of genes in each pathway (Supplementary Figure S1). Among all miR-146a targeted networks, the top 10 networks relate to inflammation, angiogenesis and immune response (Figure 2B). Network objects are listed in Figure S2 showing the total number of networks that each object is involved in and ranking of each object among all network maps.
Figure 2. miR-146a targeted pathway analysis in endothelial cells.
(A) Schematic workflow to identify miR-146a targeted pathways using a combined mRNA and microRNA approach in hypertensive pregnancy. (B) Ranking of miR-146a targeted enrichment networks in vascular development. Network enrichment analysis was performed using Metacore™.
miR-146a regulates endothelial tube formation in offspring of hypertensive pregnancy
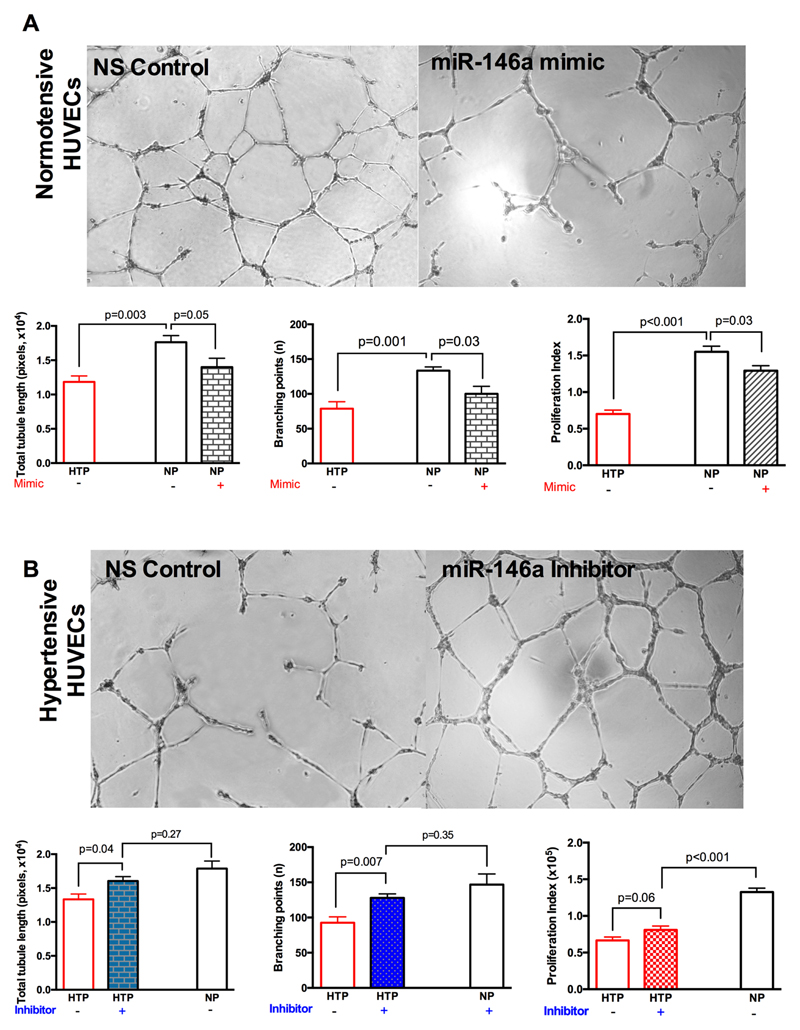
To establish whether manipulation of miR-146a level could directly influence the vascular phenotype of cells from offspring of hypertensive pregnancy, we transfected HUVECs derived from hypertensive and normotensive pregnancies with miR-146a mimic and hairpin inhibitor for 24 hours, with the average transfection efficiency greater than 90%. Subsequent qRT-PCR in HUVECs confirmed a significant increase in miR-146a level after transfection with miR-146a mimic (the effect of miR-146a mimic on a downstream target protein was confirmed by immunoblotting) and a 68% reduction in miR-146a following transfection with miR-146a hairpin inhibitor (Supplementary Figure S3).
Functionally, overexpression of miR-146a reduced the total tubule length (17,627±958 versus 13,995±1295; p=0.05) and number of branching points (133±5 versus 100±11, p=0.03) in normal HUVECs, although similar responses were not apparent in already dysfunctional HUVECs derived from hypertensive pregnancies (Figure 3A). By contrast, inhibition of miR-146a restored the total tubule length (13,339±789 versus 16,064±644; p=0.04) and branching points (93±8 versus 128±3, p=0.008) in the hypertensive group only, indicating that inhibition of miR-146a in HUVECs derived from hypertensive pregnancy, to some extent, is capable of recovering angiogenic capacity compared to cells from the normotensive group. Indeed, tube formation was no longer significantly different between these two groups (p=0.26) (Figure 3B). Similar to the tube formation, overexpression of miR-146a in HUVECs from hypertensive pregnancies resulted in reduced cell proliferation that was ameliorated by miR-146a inhibition (66,593±4,680 versus 80,970± 5,247; p=0.06, Figure 3).
Figure 3. miR-146a regulates endothelial tube formation in HUVECs.
(A) and (B) Representative images of HUVECs in growth factor-reduced matrigel after transfection with miR-146a mimic, miR-146a inhibitor or non-targeting control. Quantification of tubule formation (total tubule length and branching points) and proliferation index of HUVECs is summarised in respective bar graphs (n=10). NS, non-targeting control; NP, normotensive pregnancy; HTP, hypertensive pregnancy. p-value is obtained by unpaired t-test.
Association of miR-146a expression with in vitro and in vivo vessel formation
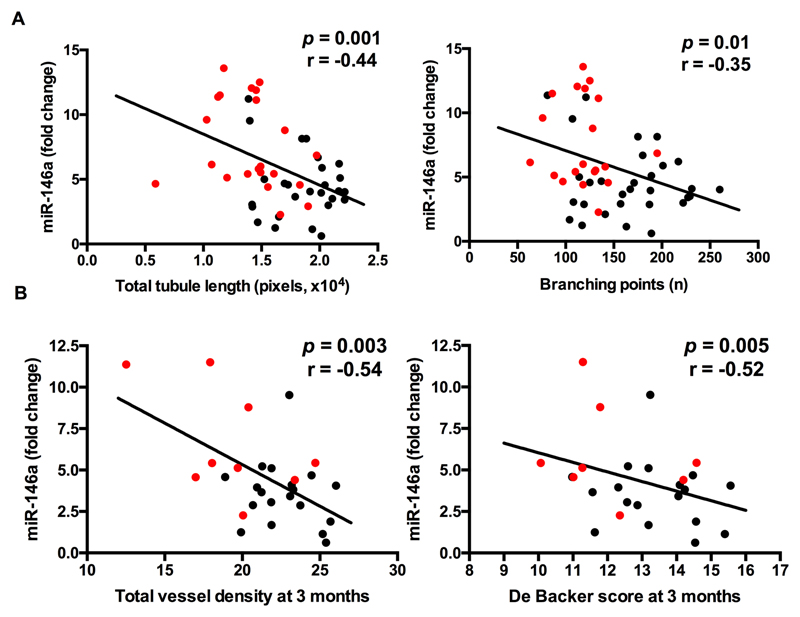
To determine whether levels of miR-146a were relevant to the in vivo and in vitro microvascular responses observed in offspring born to hypertensive pregnancy, we correlated levels with endothelial capacity for tube formation and proliferation in 49 participants. The level of miR-146a expression was negatively associated with total tubule length (p=0.001), branch points (p=0.01) and cell proliferation (p=0.01) (Figure 4A and Supplementary Figure S4). Further, there was a graded association between miR-146a levels and dermal microvascular density in the same individuals (10 hypertensive and 18 normotensive pregnancies) at three months postnatal age (Figure 4B), with higher miR-146a levels related to reduced microvascular density at three months (p=0.003 for total vessel density; p=0.005 for De Backer score).
Figure 4. Association of miR-146a expression with in vitro and in vivo vessel formation.
(A) Correlation of miR-146a level with in vitro tubule network measurements (total tubule length and branching points) using paired samples from the same infants (n=49). HUVECs were extracted from umbilical cords within 12 hours after delivery. microRNAs were measured in HUVECs at passage 1, while tubule measurements were measured in HUVECs at passage 2 and 3 (B) Correlation of miR-146a level with in vivo microvasculature at three months postnatal using paired samples from the same infant (n= 28). miR-146a levels were obtained from qRT-PCR. DB, De Backer score.
Discussion
Our study shows that endothelial cells from neonates born following a hypertensive pregnancy have significantly altered expression levels of microRNAs compared to equivalent cells from offspring of normotensive pregnancies. Specific microRNAs known to be relevant to endothelial cell phenotype/function and, in particular miR-146a, differ significantly in endothelium of hypertensive pregnancy offspring, with associated changes in the expression level of their gene targets. These differences in the microRNA profile are also associated with an impaired in vitro endothelial cell tubulogenesis phenotype in offspring of hypertensive pregnancies, which can be induced by miR-146a overexpression and rescued by miR-146a inhibition. Finally, we demonstrate that microRNA expression profile at birth in the HUVECs of an offspring can predict the pattern of microvascular development in that neonate over the next three months of life. Although the molecular mechanism through which miR-146a modulates vasculogenesis has not been explored, these results support a regulatory role of microRNAs in endothelial dysfunction seen in offspring of hypertensive pregnancy.
The early postnatal period is considered a critical developmental window for the microvasculature. Our previous work demonstrated that endothelial cellular behaviour at birth predicts early postnatal microvascular remodeling.6 Post transcriptional regulation of gene expression by microRNAs is known to be important to several endothelial phenotypes20 and, therefore, we were able to increase the efficiency of our study by using prior knowledge from the literature to focus on candidate endothelial microRNAs. A drawback of this approach is that it did not allow us to probe for potentially relevant novel microRNA targets. Nevertheless, the seven validated microRNAs have previously been linked with changes in angiogenesis and endothelial cell tubulogenesisis and were confirmed in our study.11, 13–17, 22 Our findings are therefore consistent with previous evidence for these microRNAs and, for the first time, implicates them as mediators of the anti-angiogenic state previously reported in endothelial colony forming cells5, 23 and umbilical-derived endothelial cells6 collected in neonates born after a hypertensive pregnancy.
The greatest difference in microRNA expression levels was for miR-146a, a microRNA that has previously been studied in relation to angiogenesis in cancer,24, 25 innate immune signalling12, 26 and inflammatory pathways.22, 27 MiR-146a is upregulated, as part of a feedback loop, on activation of a pro-inflammatory transcriptional programme related to NF-kB (nuclear factor kappa-light-chain-enhancer of activated B cells) signalling.26, 28 After removal of pro-inflammatory cytokines, elevated levels of miR-146a are sustained for several days, suggesting the maintenance of a prolonged ‘inflammatory memory’.22 Using gene expression profiling in endothelial cells from normal and hypertensive offspring we confirmed variation in miR-146a target pathways related to inflammation. Hypertensive pregnancies, particularly when severe, such as in preeclampsia, which are known to induce a pro-inflammatory state in the mother.29 It is therefore plausible that the elevated levels of miR-146a levels in the neonate reflect a persistent upregulation following exposure of the fetus to an inflammatory insult during pregnancy. However, other studies suggest the fetus is relatively protected from the adverse maternal circulating milieu,30 except in the most severe pregnancy complications with associated fetal growth restriction. This was not a predominant finding in our participant population and we found miR-146a to have anti-angiogenic properties, in contrast to the pro-angiogenic miR-146a response seen during inflammation.25 HUVECs exposed to a major inflammatory insult such as lipopolysaccharide (LPS)29 or when grown in an adverse metabolic state, such as seen in diabetes28, can be induced to increase miR146a expression and enhance their tubule formation.
Our cells were maintained in a neutral environment and differences in miR-146a were already evident at baseline. Endothelial colony forming cells (ECFCs) from patients with coronary artery disease similarly have increased levels of miR-146a and exhibit reduced angiogenic capacity.31 Consistent with our observations, inhibition of miR-146a restores endothelial tubulogensis in ECFCs from patients with coronary artery disease, via a distinct RhoJ-dependent mechanism.31 miR-146a also inhibits neoformation of blood vessels in a model of choroidal lesions and blocking miR-146a leads to longer tube formation in an ex vivo aortic ring angiogenic assay.14 An equivalent, anti-angiogenic impact of miR-146a has been associated with other pregnancy complications.14 Halkein et al showed exosomal miR-146a levels are increased in peripartum cardiomyopathy and myocardial uptake results in apoptosis and reduced angiogenesis.14 Furthermore, overexpression of miR-146a was found to repress proliferation and apoptosis in vitro.
We grouped results from offspring born to both pregnancy induced hypertension and preeclampsia as we have previously shown these pregnancy complications are both associated with differences in endothelial cell behaviour in the neonates6. However, the sample size was not sufficient to determine whether there are graded effects between offspring born to these two distinct hypertensive pregnancy complications. Maternal miR-146a levels are also altered during pregnancy, in a dose dependent manner, in response to tobacco smoke exposure32 33. Therefore, we undertook a regression analysis to understand whether other pregnancy factors such as maternal BMI or fetal factors such as gestational age or growth restriction may be confounders for the association we found between hypertensive pregnancy and offspring endothelial microRNA levels. This indicated the main predictor of miR-146a levels in the neonate was a history of hypertensive pregnancy rather than other factors such as preterm birth. Small group differences in maternal BMI were evident, both in the smaller RNA-sequencing cohort and full cohort. However, we found no relationship between maternal BMI and miR-146a levels in our cohort, suggesting the relationship between hypertensive pregnancies and miR-146a in the offspring is independent of maternal BMI. Nevertheless, we did not have information on some factors such as maternal metabolic function and, given the differences in maternal BMI between study groups, future studies will be needed to assess how metabolic alterations and lifestyle alter miR-146a level. It has recently been suggested that offspring born to normotensive pregnancies, but whose mother has a history of hypertensive pregnancy, may also be predisposed to hypertension,34 thus, it will be interesting to see whether altered microRNA profiles are seen in circulating endothelial cells at other points in life or in siblings of affected families. Future studies will then be able to assess the relative contribution of family history of hypertension to these alterations in endothelial phenotype in the offspring.
Expression array analysis was performed in cells at passage 1 whereas in vitro tube formation assays was performed with cells from passage 2 or 3. It has previously been shown that there is a slight shift in microRNA expression from passage 1 to 2 or 335 and therefore this may have weakened any association between expression levels and the in vitro assay. Furthermore, microRNA146a levels were validated using the delta-delta Ct method for quantification, which makes a number of assumptions that are addressed using other approaches such as Cq quantification36. Nevertheless, multiple quality control steps were taken to ensure high quality PCR reactions and we showed rescue of vasculogenic potential by inhibition of miR-146a. It will be of interest to explore in experimental models whether miR146a can also alter postnatal microvascular development in vivo. Our data in humans are necessarily associative and we cannot prove causality for associations between endothelial capacity and microvascular remodelling. However, the graded nature of the association suggests that the endothelial cell phenotype is a biomarker at birth that usefully predicts patterns of vascular development. It is possible manipulation of microRNA profile during a critical postnatal window of development could have long term benefits for vascular development and, hence, alter risk for later development of hypertension. As microRNAs tend to have multiple gene targets and are interdependent, it is possible the effects on tubulogenesis involve multiple pathways that can be targeted distinctly, and experimental models will be of value to explore molecular signals.
Perspectives
This study has shown for the first time that endothelial cells from neonates born after a hypertensive pregnancy have a distinct microRNA profile associated with an altered endothelial phenotype. The most upregulated microRNA was miR-146a and its levels predict both in vivo and in vitro vascular phenotypes seen in hypertensive pregnancy offspring. Furthermore, inhibition of miR-146a resulted in rescue of the anti-angiogenic state. These results suggest microRNAs are central to the abnormal vascular state observed in offspring of hypertensive pregnancy.
Supplementary Material
Novelty and Significance.
What is New?
Neonates born following hypertensive pregnancies have a distinct endothelial regulatory microRNA profile compared to offspring of normotensive pregnancies.
The most altered microRNA, microRNA-146a, predicts microvascular development in early postnatal life.
What is Relevant?
Offspring of hypertensive pregnancies have reduced microvascular density from early postnatal life and are at increased risk of hypertension by young adulthood.
Abnormal microvascular development increases risk for hypertension, and may therefore be an important target for cardiovascular risk reduction.
Summary.
The microRNA expression pattern in umbilical endothelial cells in offspring of hypertensive pregnancies is altered and relates with a more rapid loss of in vivo microvasculature in early postnatal life. Modification of microRNAs could improve microvascular formation and reduced hypertension risk.
Acknowledgments
We are grateful to all the pregnant woman and babies who participated in this study. We thank the High-Throughput Genomics Group at the Wellcome Trust Centre for Human Genetics (funded by Wellcome Trust grant reference 090532/Z/09/Z) for the generation of the gene expression and RNA-sequencing data.
Sources of Funding
This work was supported by grants to Professor Paul Leeson from the British Heart Foundation (FS/06/024 and FS/11/65/28865). Support was also provided by the NIHR Oxford Biomedical Research Centre, and by grants to Professor Suzanne Watt by NHS Blood and Transplant and the National Institute of Health Research under its Programme Grants Scheme (RP-PG-0310-1003 and RP-PG-0310-1001).
Footnotes
Conflicts of Interest
None.
Financial Disclosures
None.
References
- 1.Lazdam M, de la Horra A, Pitcher A, Mannie Z, Diesch J, Trevitt C, Kylintireas I, Contractor H, Singhal A, Lucas A, Neubauer S, et al. Elevated blood pressure in offspring born premature to hypertensive pregnancy: Is endothelial dysfunction the underlying vascular mechanism? Hypertension. 2010;56:159–165. doi: 10.1161/HYPERTENSIONAHA.110.150235. [DOI] [PubMed] [Google Scholar]
- 2.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: Systematic review and meta-analysis. Bmj. 2007;335:974. doi: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis EF, Lazdam M, Lewandowski AJ, Worton SA, Kelly B, Kenworthy Y, Adwani S, Wilkinson AR, McCormick K, Sargent I, Redman C, et al. Cardiovascular risk factors in children and young adults born to preeclamptic pregnancies: A systematic review. Pediatrics. 2012;129:e1552–1561. doi: 10.1542/peds.2011-3093. [DOI] [PubMed] [Google Scholar]
- 4.Davis EF, Lewandowski AJ, Aye C, Williamson W, Boardman H, Huang RC, Mori TA, Newnham J, Beilin LJ, Leeson P. Clinical cardiovascular risk during young adulthood in offspring of hypertensive pregnancies: Insights from a 20-year prospective follow-up birth cohort. BMJ open. 2015;5:e008136. doi: 10.1136/bmjopen-2015-008136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ligi I, Simoncini S, Tellier E, Vassallo PF, Sabatier F, Guillet B, Lamy E, Sarlon G, Quemener C, Bikfalvi A, Marcelli M, et al. A switch toward angiostatic gene expression impairs the angiogenic properties of endothelial progenitor cells in low birth weight preterm infants. Blood. 2011;118:1699–1709. doi: 10.1182/blood-2010-12-325142. [DOI] [PubMed] [Google Scholar]
- 6.Yu GZ, Aye CY, Lewandowski AJ, Davis EF, Khoo CP, Newton L, Yang CT, Al Haj Zen A, Simpson LJ, O'Brien K, Cook DA, et al. Association of maternal antiangiogenic profile at birth with early postnatal loss of microvascular density in offspring of hypertensive pregnancies. Hypertension. 2016;68:749–759. doi: 10.1161/HYPERTENSIONAHA.116.07586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lewandowski AJ, Davis EF, Yu G, Digby JE, Boardman H, Whitworth P, Singhal A, Lucas A, McCormick K, Shore AC, Leeson P. Elevated blood pressure in preterm-born offspring associates with a distinct antiangiogenic state and microvascular abnormalities in adult life. Hypertension. 2015;65:607–614. doi: 10.1161/HYPERTENSIONAHA.114.04662. [DOI] [PubMed] [Google Scholar]
- 8.Davis EF, Newton L, Lewandowski AJ, Lazdam M, Kelly BA, Kyriakou T, Leeson P. Pre-eclampsia and offspring cardiovascular health: Mechanistic insights from experimental studies. Clin Sci (Lond) 2012;123:53–72. doi: 10.1042/CS20110627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morales Prieto DM, Markert UR. Micrornas in pregnancy. J Reprod Immunol. 2011;88:106–111. doi: 10.1016/j.jri.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Wu F, Yang Z, Li G. Role of specific micrornas for endothelial function and angiogenesis. Biochem Biophys Res Commun. 2009;386:549–553. doi: 10.1016/j.bbrc.2009.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonauer A, Carmona G, Iwasaki M, Mione M, Koyanagi M, Fischer A, Burchfield J, Fox H, Doebele C, Ohtani K, Chavakis E, et al. Microrna-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science. 2009;324:1710–1713. doi: 10.1126/science.1174381. [DOI] [PubMed] [Google Scholar]
- 12.Chan EK, Ceribelli A, Satoh M. Microrna-146a in autoimmunity and innate immune responses. Ann Rheum Dis. 2013;72(Suppl 2):ii90–95. doi: 10.1136/annrheumdis-2012-202203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y, Gorski DH. Regulation of angiogenesis through a microrna (mir-130a) that down-regulates antiangiogenic homeobox genes gax and hoxa5. Blood. 2008;111:1217–1226. doi: 10.1182/blood-2007-07-104133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halkein J, Tabruyn SP, Ricke-Hoch M, Haghikia A, Nguyen NQ, Scherr M, Castermans K, Malvaux L, Lambert V, Thiry M, Sliwa K, et al. Microrna-146a is a therapeutic target and biomarker for peripartum cardiomyopathy. J Clin Invest. 2013;123:2143–2154. doi: 10.1172/JCI64365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poliseno L, Tuccoli A, Mariani L, Evangelista M, Citti L, Woods K, Mercatanti A, Hammond S, Rainaldi G. Micrornas modulate the angiogenic properties of huvecs. Blood. 2006;108:3068–3071. doi: 10.1182/blood-2006-01-012369. [DOI] [PubMed] [Google Scholar]
- 16.Suarez Y, Wang C, Manes TD, Pober JS. Cutting edge: Tnf-induced micrornas regulate tnf-induced expression of e-selectin and intercellular adhesion molecule-1 on human endothelial cells: Feedback control of inflammation. J Immunol. 2010;184:21–25. doi: 10.4049/jimmunol.0902369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ye J, Wu X, Wu D, Wu P, Ni C, Zhang Z, Chen Z, Qiu F, Xu J, Huang J. Mirna-27b targets vascular endothelial growth factor c to inhibit tumor progression and angiogenesis in colorectal cancer. PLoS One. 2013;8:e60687. doi: 10.1371/journal.pone.0060687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative pcr and the 2(-delta delta c(t)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Genzel-Boroviczeny O, Strotgen J, Harris AG, Messmer K, Christ F. Orthogonal polarization spectral imaging (ops): A novel method to measure the microcirculation in term and preterm infants transcutaneously. Pediatr Res. 2002;51:386–391. doi: 10.1203/00006450-200203000-00019. [DOI] [PubMed] [Google Scholar]
- 20.Yamakuchi M. Micrornas in vascular biology. Int J Vasc Med. 2012;2012 doi: 10.1155/2012/794898. 794898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Artmann S, Jung K, Bleckmann A, Beissbarth T. Detection of simultaneous group effects in microrna expression and related target gene sets. PLoS One. 2012;7:e38365. doi: 10.1371/journal.pone.0038365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng HS, Sivachandran N, Lau A, Boudreau E, Zhao JL, Baltimore D, Delgado-Olguin P, Cybulsky MI, Fish JE. Microrna-146 represses endothelial activation by inhibiting pro-inflammatory pathways. EMBO Mol Med. 2013;5:949–966. doi: 10.1002/emmm.201202318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Munoz-Hernandez R, Miranda ML, Stiefel P, Lin RZ, Praena-Fernandez JM, Dominguez-Simeon MJ, Villar J, Moreno-Luna R, Melero-Martin JM. Decreased level of cord blood circulating endothelial colony-forming cells in preeclampsia. Hypertension. 2014;64:165–171. doi: 10.1161/HYPERTENSIONAHA.113.03058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang WT, Cen WL, He RQ, Xie Y, Zhang Y, Li P, Gan TQ, Chen G, Hu XH. Effect of mir146a5p on tumor growth in nsclc using chick chorioallantoic membrane assay and bioinformatics investigation. Mol Med Rep. 2017;16:8781–8792. doi: 10.3892/mmr.2017.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu K, Pan Q, Zhang X, Kong LQ, Fan J, Dai Z, Wang L, Yang XR, Hu J, Wan JL, Zhao YM, et al. Mir-146a enhances angiogenic activity of endothelial cells in hepatocellular carcinoma by promoting pdgfra expression. Carcinogenesis. 2013;34:2071–2079. doi: 10.1093/carcin/bgt160. [DOI] [PubMed] [Google Scholar]
- 26.Taganov KD, Boldin MP, Chang KJ, Baltimore D. Nf-kappab-dependent induction of microrna mir-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A. 2006;103:12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang K, He YS, Wang XQ, Lu L, Chen QJ, Liu J, Sun Z, Shen WF. Mir-146a inhibits oxidized low-density lipoprotein-induced lipid accumulation and inflammatory response via targeting toll-like receptor 4. FEBS Lett. 2011;585:854–860. doi: 10.1016/j.febslet.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 28.Bhaumik D, Scott GK, Schokrpur S, Patil CK, Campisi J, Benz CC. Expression of microrna-146 suppresses nf-kappab activity with reduction of metastatic potential in breast cancer cells. Oncogene. 2008;27:5643–5647. doi: 10.1038/onc.2008.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lazdam M, Davis EF, Lewandowski AJ, Worton SA, Kenworthy Y, Kelly B, Leeson P. Prevention of vascular dysfunction after preeclampsia: A potential long-term outcome measure and an emerging goal for treatment. J Pregnancy. 2012;2012 doi: 10.1155/2012/704146. 704146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boutsikou T, Mastorakos G, Kyriakakou M, Margeli A, Hassiakos D, Papassotiriou I, Kanaka-Gantenbein C, Malamitsi-Puchner A. Circulating levels of inflammatory markers in intrauterine growth restriction. Mediators Inflamm. 2010;2010 doi: 10.1155/2010/790605. 790605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang TY, Tsai WC, Huang TS, Su SH, Chang CY, Ma HY, Wu CH, Yang CY, Lin CH, Huang PH, Cheng CC, et al. Dysregulation of endothelial colony-forming cell function by a negative feedback loop of circulating mir-146a and -146b in cardiovascular disease patients. PLoS One. 2017;12:e0181562. doi: 10.1371/journal.pone.0181562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maccani MA, Avissar-Whiting M, Banister CE, McGonnigal B, Padbury JF, Marsit CJ. Maternal cigarette smoking during pregnancy is associated with downregulation of mir-16, mir-21, and mir-146a in the placenta. Epigenetics. 2010;5:583–589. doi: 10.4161/epi.5.7.12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsochandaridis M, Nasca L, Toga C, Levy-Mozziconacci A. Circulating micrornas as clinical biomarkers in the predictions of pregnancy complications. Biomed Res Int. 2015;2015 doi: 10.1155/2015/294954. 294954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu GZ, Leeson P. Hypertension: Hypertension in pregnancy: A risk factor for the whole family? Nat Rev Nephrol. 2017;13:326–327. doi: 10.1038/nrneph.2017.54. [DOI] [PubMed] [Google Scholar]
- 35.Kuosmanen SM, Kansanen E, Sihvola V, Levonen AL. Microrna profiling reveals distinct profiles for tissue-derived and cultured endothelial cells. Sci Rep. 2017;7 doi: 10.1038/s41598-017-11487-4. 10943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, et al. The miqe guidelines: Minimum information for publication of quantitative real-time pcr experiments. Clin Chem. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.